Abstract
Background
Adjacent segment degeneration is a long-term complication of arthrodesis. However, the incidence of adjacent segment degeneration varies widely depending on the patient’s age and underlying disease and the fusion techniques and diagnostic methods used.
Questions/purposes
We determined (1) the frequency of adjacent segment degeneration and increased lordosis on imaging tests, (2) the frequency and severity of clinical sequelae of these findings, including revision surgery, and (3) the sequence of degeneration and risk factors for degeneration.
Methods
Seventy-three patients underwent anterior lumbar interbody fusion for low-grade isthmic spondylolisthesis at one institution between October 2000 and February 2002. Forty-nine (67%) of the original patients had complete radiographic and clinical followup for 10 years. CT and MRI were performed at 5 years and 10 years in all cases. The disc height, sagittal profiles, and facet and disc degeneration at adjacent levels were examined to identify radiographic and clinical adjacent segment degeneration. Mean followup was 134.2 months (range, 120–148 months).
Results
Cranial segment lordosis increased (from 14.8° to 18.5°; p < 0.001), while caudal segment lordosis changed little (from 16.4° to 17.3°). Radiographic and clinical adjacent segment degeneration occurred in 19 (38.8%) and six (12.2%) patients, respectively, and two patients (4.1%) underwent revision surgery. Patients with adjacent segment degeneration had more advanced preexisting facet degeneration than patients without adjacent segment degeneration (odds ratio: 18.6; 95% CI, 1.97–175.54, p = 0.01). Acceleration of disc and facet degeneration occurred in 4.1% and 10.2%, respectively.
Conclusions
Adjacent segment degeneration requiring surgery is rare, although radiographic adjacent segment degeneration is common after anterior lumbar interbody fusion for isthmic spondylolisthesis. The only risk factor we found was preexisting facet degeneration of the cranial segment.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence
Introduction
Low-grade isthmic spondylolisthesis can be treated by several different surgical fusion techniques, all of which aim to ensure stability, decompress the neural structures, and correct deformity [4, 17, 18, 22, 23]. Anterior lumbar interbody fusion can support the anterior column, reduce slippage, minimize posterior injury, and restore disc height to achieve indirect neural decompression [4, 5, 15, 16, 22].
Adjacent segment degeneration is a long-term complication of spinal arthrodesis. Biomechanical stress on the disc and facet joint of the adjacent segment has been suggested to be key to the development of adjacent segment degeneration [3, 12]. Adjacent segment degeneration has been studied extensively [19, 27]. Its incidence in the literature has ranged from 5.2% to 100% [27]. The wide range of the reported incidence results from differences in patient age, underlying disease, fusion technique, diagnostic criteria, and diagnostic methods used. To our knowledge, there has been no study of a group of patients with homogeneous disease at a single level treated by the same method and examined using CT and MRI.
We determined (1) the frequency of adjacent segment degeneration and increased lordosis on imaging tests, (2) the frequency and severity of clinical sequelae of these findings, including revision surgery, and (3) the sequence of degeneration and risk factors for degeneration 10 years after anterior lumbar interbody fusion with percutaneous pedicle screw fixation for treatment of low-grade isthmic spondylolisthesis.
Patients and Methods
Patient Cohort
Approval of our institutional review board for this retrospective study was obtained. Seventy-three patients were treated with anterior lumbar interbody fusion with percutaneous pedicle screw fixation for low-grade isthmic spondylolisthesis at a single institution according to an established evaluation and followup protocol from October 2000 to February 2002. The preliminary report (73 patients) after 16 months of followup was published in 2004 [22]. Of the original 73 patients, 10 were lost before our report at 5 years [15], and an additional 14 were lost to followup between 5 and 10 years; for this study, we had complete radiographic followup on 49 of the original 73 patients (67%). Complete followup included plain radiographs (including dynamic views), CT, MRI, and clinical scores. These patients underwent the following postoperative radiographic examinations: plain dynamic radiographs after 1, 3, 5, 7, and 10 years and CT and MRI after 5 and 10 years. The inclusion criteria were as follows: (1) isthmic spondylolisthesis limited to L4–L5 and L5–S1, (2) slippage of Meyerding Grade 2 or less [24], (3) only anterior lumbar interbody fusion with percutaneous pedicle screw fixation performed, and (4) followup for a minimum of 120 months. Mean followup was 134 months (range, 120–148 months). Anterior lumbar interbody fusion was performed at L4–L5 in 29 patients and at L5–S1 in 20 patients. The final fusion rate was 100% (Table 1).
Table 1.
Radiographic and clinical changes between preoperative and 10-year followup examinations
| Variable | Preoperative | 10-year followup | p value |
|---|---|---|---|
| SL (°)* | 14.4 ± 7.4 | 18.1 ± 6.3 | 0.02 |
| WL (°)* | 42.1 ± 14.7 | 52.4 ± 9.2 | < 0.001 |
| Slippage (%)* | 19.9 ± 8.7 | 6.9 ± 5.4 | 0.003 |
| DH (mm)* | 7.3 ± 2.6 | 12.6 ± 2.4 | < 0.001 |
| Cr-SL (°)* | 14.8 ± 6.3 | 18.6 ± 6.3 | < 0.001 |
| Ca-SL (°)* | 16.4 ± 7.2 | 17.7 ± 7.9 | 0.65 |
| Cr-DH (mm)* | 12.0 ± 1.8 | 12.0 ± 1.8 | 0.89 |
| Ca-DH (mm)* | 11.2 ± 2.2 | 11.3 ± 2.5 | 0.74 |
| Cr-FA (°)* | |||
| L4–L5 | 36.2 ± 10.7 | 36.8 ± 11.5 | 0.69 |
| L5–S1 | 44.8 ± 10.1 | 46.5 ± 10.5 | 0.63 |
| Ca-FA (°)* | |||
| L4–L5 | 48.1 ± 10.3 | 46.6 ± 10.2 | 0.36 |
| Cr-FD ≥ 2 (number of patients) | 10 | 24 | 0.02 |
| Ca-FD ≥ 2 (number of patients) | 4 | 17 | 0.03 |
| Cr-DD ≥ 3 (number of patients) | 35 | 40 | 0.04 |
| Ca-DD ≥ 3 (number of patients) | 18 | 23 | 0.02 |
| VAS score (points)* | |||
| Back | 7.5 ± 2.0 | 3.2 ± 2.3 | 0.01 |
| Leg | 6.3 ± 2.4 | 2.1 ± 2.5 | 0.01 |
| ODI (%)* | 65.9 ± 18.0 | 18.2 ± 15.0 | 0.005 |
| Satisfaction rate (%) | 83.1 ± 15.3 | ||
| Fusion rate (%) | 100 | ||
* Values are expressed as mean ± SD; SL = segmental lordosis; WL = whole lumbar lordosis; DH = disc height; Cr = cranial segment; Ca = caudal segment; FA = facet angle; FD = facet degeneration grade; DD = disc degeneration grade; ODI = Oswestry Disability Index.
Procedure
Anterior lumbar interbody fusion procedures were performed using the mini-laparotomic retroperitoneal approach as previously described [22]. After discectomy, a cage (polyetheretherketone or Fidgi cage) was carefully placed at the affected level as an interbody device containing allograft bone chips in all cases. After the completion of anterior lumbar interbody fusion, all percutaneous pedicle screws were inserted under fluoroscopy.
Clinical Evaluation
Clinical outcomes were assessed by a VAS (0–10 points) for pain and by the Oswestry Disability Index (ODI) (0–100%) for function. Subjective surgical satisfaction rate was assessed by asking the patient the following question “How satisfied were you with this operation?”
Radiographic Evaluation
Radiographic measurements were performed blindly by two neurosurgeons (KCC, HKS) not involved with the surgeries. Total L1–L5 lumbar lordosis and the segmental angle, angular motion, and degree of translation were measured for the operative and adjacent segments. Fusion was defined as the presence of trabecular osseous continuity and less than 4° mobility between segments on a flexion and extension radiography and CT scan. Disc height was calculated as an average of anterior and posterior disc height [6]. Disc degeneration grading on MRI was based on Pfirrmann grade by observing the T2-weighted image at the midsagittal plane [30]. Facet degeneration, which was examined using the grading system proposed by Weishaupt et al. [34], was classified into four grades (0–3) and compared according to the width of the joint space, osteophyte formation, hypertrophy of the articular bone erosion, and subchondral cyst observed on the CT image. We assessed the intra- and interobserver agreement of the grading of disc degeneration on MRI and facet degeneration on CT using kappa coefficients. Both of these grades showed high intraobserver agreement, with kappa coefficients for disk and facet degeneration grading of 0.86 and 0.91 for Observer 1 and 0.82 and 0.94 for Observer 2, and high interobserver agreement, with kappa coefficients of 0.84 and 0.85, respectively, for disc and facet degeneration grading. Facet angles were measured between the faced line and midsagittal line on axial CT scan using the method of Karacan et al. [14]. The mean of the right and left facet angles was reported as the facet angle. The facet joint violation of cranial segment was examined using CT scan. A facet joint was considered violated if the screw or screw head was clearly within the facet joint [4]. Radiographic adjacent segment degeneration was diagnosed according to the following criteria: (1) olisthesis (anterolisthesis or retrolisthesis of greater than 4 mm), (2) greater than 10% loss of disc height, (3) angular motion of greater than 10° between adjacent bodies on flexion and extension radiographs, (4) osteophyte formation of greater than 3 mm, (5) disc herniation or spinal stenosis by CT or MRI, (6) change of disc degeneration of Grade 2 or greater, (7) change of facet arthropathy of Grade 2 or greater, (7) scoliosis, or (8) compression fracture [7, 17, 20, 25, 27, 29, 32]. Clinical adjacent segment degeneration referred to the development of new clinical symptoms that corresponded to radiographic adjacent segment degeneration, a VAS pain score of 6 or more for the back or legs, an ODI score of more than 40%, or if surgery was required.
Statistical Analysis
We performed statistical analyses using SPSS® for Windows® (Version 14.0; SPSS Inc, Chicago, IL, USA). Depending on the variables, intergroup differences were analyzed using Fisher’s exact test or Mann-Whitney U test. Logistic regression analysis was used to analyze the assumed risk factors of facet degeneration, disc degeneration, degree of lordosis, disc height, and facet angle. The result was considered statistically significant if the probability value was less than 0.05.
Results
Adjacent segment degeneration was common in this patient population. While 30 patients showed no adjacent segment degeneration (Fig. 1), radiographic adjacent segment degeneration occurred in 19 (38.8%), and clinical adjacent segment degeneration occurred in six (12.2%) (Fig. 2). Adjacent segment degeneration affected the cranial segment in 16 patients (84.2%) and the caudal segment in three (15.8%). Cranial segment increases in lordosis were more pronounced than caudal segment lordotic changes. Total L1–L5 lordosis and segmental lordosis increased from a mean ± SD of 42.1° ± 14.7° and 14.4° ± 7.5° to 52.4° ± 9.2° and 18.1° ± 6.3°, respectively (Table 1). Cranial segmental lordosis increased significantly from 14.8° ± 6.3° to 18.6° ± 6.3° (p < 0.001). However, caudal segmental lordosis changed little (16.4° ± 7.2° to 17.7° ± 7.9°; p = 0.65).
Fig. 1A–C.
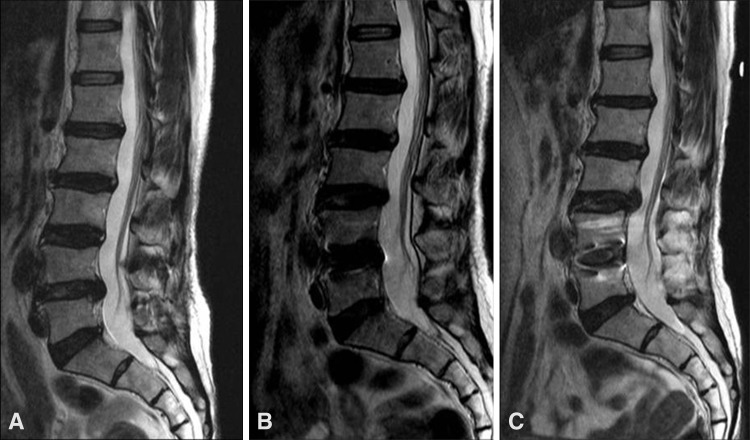
Images illustrate the case of a 46-year-old woman who underwent anterior lumbar interbody fusion at L4–L5 and experienced no adjacent segment degeneration at followup. Relative to (A) the preoperative MRI findings, no adjacent segment degeneration was seen after (B) 5 or (C) 10 years.
Fig. 2A–C.
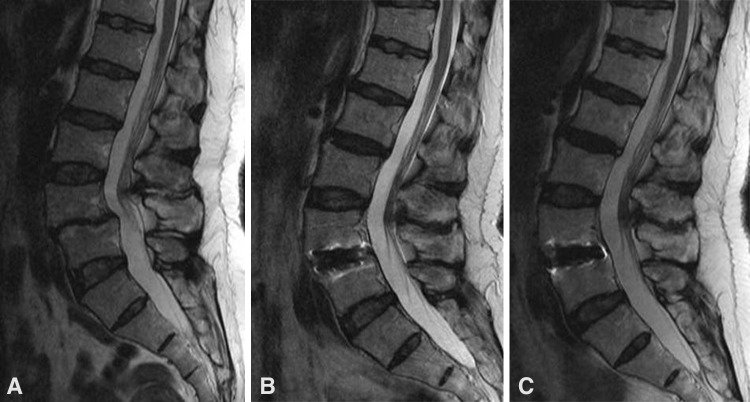
Images illustrate the case of a 55-year-old woman who underwent anterior lumbar interbody fusion and experienced clinical adjacent segment degeneration at followup. Compared with the (A) initial findings, disc herniation of the cranial segment continued to enlarge after (B) 5 and (C) 10 years. The patient complained of back and left leg pain at the 10-year followup examination. She underwent two epidural steroid injections and took medications.
The types of adjacent segment degeneration present were six retrolisthesis (Fig. 3), five disc herniation, five aggravation of facet arthropathy (Fig. 4), three anterolisthesis, two spinal stenosis, two aggravation of disc degeneration, and one instability. Adjacent segment degeneration was first observed after 3 years in three patients, after 5 years in seven, after 7 years in four, and after 10 years in six. The adjacent segment degeneration had progressed since the 5-year followup in four patients: angular motion had progressed to retrolisthesis in three and the disc herniation had enlarged in one. The six patients with clinical adjacent segment degeneration had mean final VAS scores for back and leg pain of 5.2 and 6.2, respectively, compared with 3.2 and 2.1 for all patients. Their ODI score was 33.3% (versus 18.2% overall) and their satisfaction rate was 65.2% (versus 83.1% overall). Two patients (4.1%) underwent decompression surgery to treat clinical adjacent segment degeneration after 5 years (Table 2).
Fig. 3A–C.
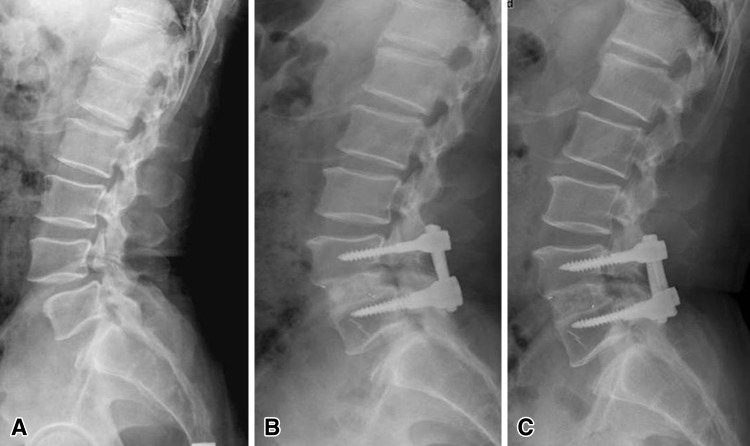
Images illustrate the case of a 51-year-old man who underwent anterior lumbar interbody fusion at L4–L5 and experienced radiographic adjacent segment degeneration at followup. (A) A preoperative lateral radiograph shows isthmic spondylolisthesis at L4–L5 and retrolisthesis of the cranial segment progressing after (B) 5 and (C) 10 years.
Fig. 4A–E.
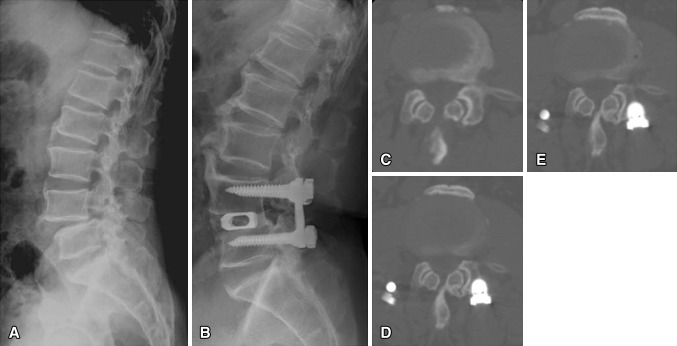
Images illustrate the case of a 63-year-old man who underwent anterior lumbar interbody fusion at L4–L5 and experienced radiographic adjacent segment degeneration at followup. (A) A preoperative radiograph shows isthmic spondylolisthesis at L4–L5. (B) A 10-year followup radiograph shows solid fusion and degenerative changes. Compared with the (C) preoperative CT findings, progressive facet degeneration of the cranial segment was evident (D) 5 and (E) 10 years later.
Table 2.
Summary of clinical adjacent segment degeneration
| Patient | Age (years) | Sex | Fusion level | Cranial/ caudal | Disease | Symptom-free interval (months) | Revision surgery |
|---|---|---|---|---|---|---|---|
| 1 | 60 | Male | L4–L5 | Cranial | Foraminal stenosis | 120 | No |
| 2 | 59 | Female | L5–S1 | Cranial | Foraminal stenosis | 96 | No |
| 3 | 55 | Female | L4–L5 | Cranial | Herniated nucleus pulposus | 120 | No |
| 4 | 44 | Male | L5–S1 | Cranial | Herniated nucleus pulposus | 60 | Yes |
| 5 | 48 | Female | L5–S1 | Cranial | Anterolisthesis | 82 | No |
| 6 | 49 | Female | L4–L5 | Caudal | Herniated nucleus pulposus | 60 | Yes |
Facet degeneration had progressed in 29 (59.2%) patients and disc degeneration had progressed in 15 (31.6%) (p = 0.024). Acceleration (change to Grade 2 or more) of disc and facet degeneration occurred in two (4.1%) and five (10.2%) patients, respectively. Facet degeneration worsened in 22 and eight patients during the first and second 5-year intervals of followup, respectively. Disc degeneration worsened in nine patients in the first 5 years and in 14 patients in the second 5 years (Table 3).
Table 3.
Changes in the degrees of facet and disc degeneration in the first and second 5-year intervals
| Degeneration | Number of patients | ||
|---|---|---|---|
| First 5 years | Second 5 years | 10 years | |
| Facet degeneration (≥ 1 grade) | 22 (44.9%) | 8 (16.3%) | 29 (59.2%) |
| Accelerated facet degeneration (≥ 2 grades) | 4 (8.2%) | 1 (2%) | 5 (10.2%) |
| Disc degeneration (≥ 1 grade) | 9 (18.4%) | 14 (28.6%) | 15 (31.6%) |
| Accelerated disc degeneration (≥ 2 grades) | 1 (2%) | 1 (2%) | 2 (4.1%) |
Of the parameters examined, only preexisting facet degeneration differed significantly between patients with and without adjacent segment degeneration. The patients with adjacent segment degeneration exhibited more advanced preoperative facet degeneration (Grade 2 or more) of the cranial segment than the patients without adjacent segment degeneration (p = 0.03) (Table 4). The odds ratio for adjacent segment degeneration was 18.6 (95% CI, 1.97–175.54; p = 0.01) with facet degeneration of Grade 2 versus Grade 0. Only the cranial segment facet degeneration at 10-year followup differed significantly between the two groups (p = 0.02) (Table 5).
Table 4.
Comparison of the preoperative characteristics of patients with and without adjacent segment degeneration
| Variable | Patients with adjacent segment degeneration | Patients without adjacent segment degeneration | p value |
|---|---|---|---|
| Number of patients | 19 | 30 | |
| Age (years)* | 50.7 ± 9.4 | 48.9 ± 9.4 | 0.38 |
| Sex (male/female) (number of patients) | 6/13 | 8/22 | 0.65 |
| Level (number of patients) | |||
| L4–L5 | 10 | 19 | 0.46 |
| L5–S1 | 9 | 11 | |
| PI (°)* | 57.4 ± 9.9 | 54.6 ± 11.5 | 0.27 |
| SS (°)* | 29.9 ± 15.3 | 29.0 ± 20.7 | 0.70 |
| T-score* | 0.18 ± 1.0 | −0.13 ± 1.6 | 0.12 |
| Z-score* | 0.9 ± 1.1 | 0.7 ± 1.4 | 0.45 |
| SL (°)* | 13.7 ± 7.4 | 14.7 ± 7.5 | 0.35 |
| WL (°)* | 36.7 ± 17.1 | 42.4 ± 11.9 | 0.38 |
| Cr-SL (°)* | 14.6 ± 5.1 | 14.9 ± 7.0 | 0.77 |
| Ca-SL (°)* | 16.8 ± 6.7 | 16.1 ± 7.7 | 0.68 |
| Slippage (%)* | 19.9 ± 8.0 | 19.9 ± 9.2 | 0.64 |
| DH (mm) * | 7.1 ± 2.2 | 7.4 ± 2.9 | 0.96 |
| Cr-DH (mm)* | 11.8 ± 1.6 | 12.1 ± 2.0 | 0.89 |
| Ca-DH (mm)* | 10.7 ± 2.2 | 11.6 ± 2.1 | 0.15 |
| Cr-FA (°)* | |||
| L4–L5 | 31.6 ± 11.6 | 39.3 ± 10.2 | 0.07 |
| L5–S1 | 43.5 ± 10.9 | 45.8 ± 10.5 | 0.63 |
| Ca-FA (°)* | |||
| L4–L5 | 48.8 ± 8.9 | 47.8 ± 11.4 | 0.36 |
| Cr-FD < 2 (number of patients) | 12 | 27 | 0.03 |
| Cr-FD ≥ 2 (number of patients) | 7 | 3 | |
| Ca-FD < 2 (number of patients) | 8 | 13 | 0.33 |
| Ca-FD ≥ 2 (number of patients) | 2 | 2 | |
| Cr-DD < 3 (number of patients) | 4 | 10 | 0.35 |
| Cr-DD ≥ 3 (number of patients) | 15 | 20 | |
| Ca-DD < 3 (number of patients) | 3 | 8 | 0.69 |
| Ca-DD ≥ 3 (number of patients) | 7 | 11 | |
* Values are expressed as mean ± SD; PI = pelvic incidence, SS = sacral slope; SL = segmental lordosis; WL = whole lumbar lordosis; Cr = cranial segment; Ca = caudal segment; DH = disc height; FA = facet angle; FD = facet degeneration grade; DD = disc degeneration grade.
Table 5.
Comparison of the postoperative changes between patients with and without adjacent segment degeneration
| Variable | Patients with adjacent segment degeneration | Patients without adjacent segment degeneration | p value |
|---|---|---|---|
| SL (°)* | 17.2 ± 6.6 | 18.1 ± 7.6 | 0.47 |
| WL (°)* | 48.7 ± 10.5 | 53.6 ± 7.4 | 0.07 |
| Cr-SL (°)* | 19.8 ± 5.4 | 18.0 ± 6.7 | 0.12 |
| Ca-SL (°)* | 16.9 ± 8.5 | 17.6 ± 7.7 | 0.58 |
| DH (mm)* | 12.6 ± 1.6 | 12.5 ± 2.6 | 0.89 |
| Cr-DH (mm)* | 11.9 ± 1.6 | 11.9 ± 1.9 | 0.79 |
| Ca-DH (mm)* | 10.7 ± 1.9 | 12.0 ± 2.4 | 0.45 |
| Cr-FA (°)* | |||
| L4–L5 | 33.7 ± 10.5 | 38.5 ± 12.2 | 0.18 |
| L5–S1 | 45.6 ± 10.9 | 47.3 ± 11.9 | 0.72 |
| Ca-FA (°)* | |||
| L4–L5 | 47.1 ± 9.2 | 46.3 ± 11.3 | 0.34 |
| Cr-FD < 2 (number of patients) | 5 | 20 | 0.02 |
| Cr-FD ≥ 2 (number of patients) | 14 | 10 | |
| Ca-FD < 2 (number of patients) | 3 | 7 | 1.00 |
| Ca-FD ≥ 2 (number of patients) | 7 | 12 | |
| Cr-DD < 3 (number of patients) | 2 | 4 | 1.00 |
| Ca-FD ≥ 3 (number of patients) | 17 | 26 | |
| Ca-DD < 3 (number of patients) | 2 | 4 | 1.00 |
| Ca-DD ≥ 3 (number of patients) | 8 | 15 | |
| Cranial facet violation by screw (number of patients) | 8 (42.1%) | 16 (53.3%) | 0.09 |
| VAS score (points)* | |||
| Back | 3.3 ± 3.3 | 3.1 ± 2.3 | 0.89 |
| Leg | 1.4 ± 2.7 | 2.5 ± 2.9 | 0.11 |
| ODI (%)* | 17.2 ± 15.5 | 18.9 ± 16.3 | 0.41 |
| Satisfaction rate (%)* | 84.6 ± 17.6 | 82.1 ± 16.6 | 0.37 |
* Values are expressed as mean ± SD; SL = segmental lordosis; WL = whole lumbar lordosis; DH = disc height; Cr = cranial segment; Ca = caudal segment; FA = facet angle; FD = facet degeneration grade; DD = disc degeneration grade; ODI = Oswestry Disability Index.
Complications related to anterior lumbar interbody fusion occurred in six patients (8.2%): three iliac vein injuries, two wound hematoma, and one deep vein thrombosis. Complications related to the percutaneous pedicle screw fixation included two cortical wall violations and three screw malpositions.
Discussion
Adjacent segment degeneration is a long-term complication that can require surgery and affect clinical outcomes [31, 35]. Biomechanical alteration to the facet load and intradiscal pressure of the mobile segment are responsible for this degeneration [3, 12]. Although it has been extensively studied, the incidence of adjacent segment degeneration has a wide range and risk factors are inconsistent due to different patient populations, surgical techniques, and diagnostic methods. In this study, we studied changes in the adjacent segment in patients with homogeneous disease at a single level with long-term followup using CT and MRI. We determined (1) the frequency of adjacent segment degeneration and increased lordosis on imaging tests, (2) the frequency and severity of clinical sequelae of these findings, including revision surgery, and (3) the sequence of degeneration and risk factors for degeneration.
This study had several limitations. First, it was a retrospective review with a relatively small sample size. For 10-year followup, it had a high dropout rate (33%) and we must consider our results as a best-case scenario. Second, there was no comparison with a control group (age-matched normal population). Therefore, adjacent segment degeneration cannot be distinguished clearly from the aging process. Third, this study did not include patient factors such as weight or smoking.
The incidence of radiographic adjacent segment degeneration in the literature ranges from 5.2% to 100% [27]. Our incidence of radiographic adjacent segment degeneration was 38.8%. Adjacent segment degeneration occurred much more frequently in the cranial segment than in the caudal segment. Cranial adjacent segment degeneration was associated with instability, including retrolisthesis, anterolisthesis, and angulation, while caudal adjacent segment degeneration, due to L5–S1 anatomic stability, was associated with advanced disc degeneration and foraminal stenosis with disc degeneration. Anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation gained lumbar lordosis restoring disc height. We observed that the cranial segment tilted backward (hyperextension) with gain of lordosis. Local hyperextension is sufficient to posteriorly plane the upper spine. This generates an increase in stress on posterior structures, exposing them to the risk of retrolisthesis, which may result in accelerated facet joint arthritis [2]. The patients with adjacent segment degeneration had more sagittally oriented angles than the patients without adjacent segment degeneration in the cranial segment only in L4–L5 fusion, although the difference was not statistically significant.
The incidence of clinical adjacent segment degeneration ranges from 5.2% to 18.5% in the literature. Our incidence was 12.2%, and 4% of those patients underwent revision surgery for this condition. Aiki et al. [1] reported 7.7% clinical adjacent segment degeneration requiring reoperation at minimum 2-year followup. Gillet [11] reported 20% adjacent segment degeneration requiring reoperation after a minimum of 5 years. According to the survival analysis by Ghiselli et al. [10], clinical adjacent segment degeneration requiring surgery was 36.1% at 10 years. Another survival analysis obtained annual incidence rates of clinical adjacent segment degeneration ranging from 0.6% to almost 4% per year [19]. Time to second operation for clinical adjacent segment degeneration ranges from 5.2 to 7.1 years [1, 31]. Our revision surgeries were performed 5 years after initial surgery.
Many potential risk factors for adjacent segment degeneration have been described [19, 27]. These risk factors can be divided into patient-associated and surgery-related factors. Surgery-related factors are posterior lumbar interbody fusion, fusion length, injury to the facet joint of the adjacent segment, and sagittal alignment. Patient-related factors are old age, female sex, menopause, and preexisting degeneration. As all of our patients underwent anterior lumbar interbody fusion at the same institution, we examined only patient-associated factors. Patients with isthmic spondylolisthesis are relatively younger than those with degenerative spinal disease requiring fusion and maintain preoperative lumbar lordosis with minimal adjacent segment degeneration. Preexisting facet degeneration was the only risk factor we found for adjacent segment degeneration. Furthermore, preexisting facet degeneration in a hyperextended cranial segment may accelerate degeneration and increase the incidence of adjacent segment degeneration. Progression of preexisting degenerative changes is a cause of adjacent segment degeneration [13]. Some authors have suggested that an already-degenerated disc is associated with development of adjacent segment degeneration [26]. In a study of 1069 patients, Lee et al. [21] also suggested that preexisting facet degeneration was the only risk factor for adjacent segment degeneration after fusion, similar to our result. Cranial inferior facet damage can potentially contribute to adjacent segment degeneration [27]. Percutaneous pedicle screws violate the cranial facet joint more often than traditional instrumented screws [28]. Our incidence of violation of the cranial facet joint was 49%. However, cranial facet joint violation between the patients with and without adjacent segment degeneration was not significantly different.
In our study, disc degeneration had progressed in 31.6% of patients at the 10-year followup examination, although disc height had changed little. Facet degeneration had progressed in 59.2% of patients. Aging is associated with disc degeneration and facet joint arthritis [9]. Both Penta et al. [29] and Wai et al. [33] reported that the incidence of degenerative changes observed on long-term followup after anterior lumbar interbody fusion is similar to that in the normal population. These studies were performed in patients who had undergone noninstrumented anterior lumbar interbody fusion for mainly discogenic back pain, and the mean age was relatively young (45 years at final followup). Facet degeneration accelerated 10.2% and disc degeneration accelerated 4.1%. Furthermore, more facet degeneration occurred during the first 5 years of the followup period than during the second 5 years. Fugiwara et al. [9] reported that facet arthrosis on MRI appears to precede disc degeneration. They also showed that facet joint arthritis affected segmental instability [9]. Eubanks et al. [8] suggested that facet degeneration follows disc degeneration and that facet osteophytosis appears early in the degenerative process, preceding vertebral rim osteophytosis. It is not clear whether facet degeneration precedes disc degeneration or vice versa. It seems the degenerative process appears early in the facet joint. Then, facet arthrosis may accelerate disc degeneration.
Radiographic adjacent segment degeneration occurred commonly in our patients 10 years after anterior lumbar interbody fusion for isthmic spondylolisthesis. However, adjacent segment degeneration requiring surgery was rare. Only preexisting cranial facet degeneration was found to be associated with development of adjacent segment degeneration.
Acknowledgments
The authors thank B. H. Kim BS and J. Y. Lim BA for their contribution to this study. We also thank the Wooridul Foundation for supporting costs associated with followup data collection, including MRI, CT, and radiography.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Seoul Wooridul Spine Hospital, Seoul, Korea.
References
- 1.Aiki H, Ohwada O, Kobayashi H, Hayakawa M, Kawaguchi S, Takebayashi T, Yamashita T. Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci. 2005;10:490–495. doi: 10.1007/s00776-005-0919-3. [DOI] [PubMed] [Google Scholar]
- 2.Barrey C, Roussouly P, Perrin G, Le Huec JC. Sagittal balance disorders in severe degenerative spine: can we identify the compensatory mechanisms? Eur Spine J. 2011;20(suppl 5):626–633. doi: 10.1007/s00586-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CS, Cheng CK, Liu CL, Lo WH. Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Med Eng Phys. 2001;23:483–491. doi: 10.1016/S1350-4533(01)00076-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Zhao J, Xu H, Liu A, Yuan J, Wang C. Technical factors related to the incidence of adjacent superior segment facet joint violation after transpedicular instrumentation in the lumbar spine. Eur Spine J. 2008;17:1476–1480. doi: 10.1007/s00586-008-0776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi KC, Ahn Y, Kang BU, Jang JH, Kim KK, Shin YH, Choi JO, Lee SH. Failed anterior lumbar interbody fusion due to incomplete foraminal decompression. Acta Neurochir (Wien). 2011;153:567–574. doi: 10.1007/s00701-010-0876-2. [DOI] [PubMed] [Google Scholar]
- 6.Dabbs VM, Dabbs LG. Correlation between disc height narrowing and low-back pain. Spine (Phila Pa 1976). 1990;15:1366–1369. doi: 10.1097/00007632-199012000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 8.Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop Relat Res. 2007;464:184–189. doi: 10.1097/BLO.0b013e3181583d4e. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, Saotome K. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–450. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86:1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338–345. doi: 10.1097/00024720-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Yoganandan N, Maiman DJ, Pintar FA. Internal and external responses of anterior lumbar/lumbosacral fusion: nonlinear finite element analysis. J Spinal Disord Tech. 2008;21:299–304. doi: 10.1097/BSD.0b013e31812e6276. [DOI] [PubMed] [Google Scholar]
- 13.Guigui P, Lambert P, Lassale B, Deburge A. [Long-term outcome at adjacent levels of lumbar arthrodesis] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1997;83:685–696. [PubMed] [Google Scholar]
- 14.Karacan I, Aydin T, Sahin Z, Cidem M, Koyuncu H, Aktas I, Uludag M. Facet angles in lumbar disc herniation: their relation to anthropometric features. Spine (Phila Pa 1976). 2004;29:1132–1136. doi: 10.1097/00007632-200405150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Choi WG, Lee SH. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis: minimum 5-year follow-up. Spine J. 2010;10:404–409. doi: 10.1016/j.spinee.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Lee KY, Lee SH, Lee HY. Which lumbar interbody fusion technique is better in terms of level for the treatment of unstable isthmic spondylolisthesis? J Neurosurg Spine. 2010;12:171–177. doi: 10.3171/2009.9.SPINE09272. [DOI] [PubMed] [Google Scholar]
- 17.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauber S, Schulte TL, Liljenqvist U, Halm H, Hackenberg L. Clinical and radiologic 2–4-year results of transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Spine (Phila Pa 1976). 2006;31:1693–1698. doi: 10.1097/01.brs.0000224530.08481.4e. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence BD, Wang J, Arnold PM, Hermsmeyer J, Norvell DC, Brodke DS. Predicting the risk of adjacent segment pathology after lumbar fusion: a systematic review. Spine (Phila Pa 1976). 2012;37:S123–S132. doi: 10.1097/BRS.0b013e31826d60d8. [DOI] [PubMed] [Google Scholar]
- 20.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, Lee MY. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–1643. doi: 10.1007/s00586-009-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J. 2004;4:644–649. doi: 10.1016/j.spinee.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Madan S, Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 2002;27:1536–1542. doi: 10.1097/00007632-200207150-00011. [DOI] [PubMed] [Google Scholar]
- 24.Meyerding H. Spondylolisthesis: surgical treatments and results. Surg Gynecol Obstet. 1932;54:371–377. [Google Scholar]
- 25.Miyakoshi N, Abe E, Shimada Y, Okuyama K, Suzuki T, Sato K. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine (Phila Pa 1976). 2000;25:1837–1842. doi: 10.1097/00007632-200007150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Nakai S, Yoshizawa H, Kobayashi S. Long-term follow-up study of posterior lumbar interbody fusion. J Spinal Disord. 1999;12:293–299. doi: 10.1097/00002517-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 28.Park Y, Ha JW, Lee YT, Sung NY. Cranial facet joint violations by percutaneously placed pedicle screws adjacent to a minimally invasive lumbar spinal fusion. Spine J. 2011;11:295–302. doi: 10.1016/j.spinee.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995;20:743–747. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 30.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Phillips FM, Carlson GD, Bohlman HH, Hughes SS. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord. 2000;13:432–437. doi: 10.1097/00002517-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine (Phila Pa 1976). 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 33.Wai EK, Santos ER, Morcom RA, Fraser RD. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2006;31:1952–1956. doi: 10.1097/01.brs.0000228849.37321.a8. [DOI] [PubMed] [Google Scholar]
- 34.Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28:215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]
- 35.Whitecloud TS 3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine (Phila Pa 1976). 1994;19:531–536. [DOI] [PubMed]