Abstract
Background
Berberis vulgaris is a well known plant with traditional herbal medical history. The aims of this study was to bioscreen and compare the in vitro biological activity (antioxidant, cholinergic, antidaibetic and the anticancer) of barberry crude extract and berberine active compound.
Methods
The effect of B. vulgaris extract and berberine chloride on cellular thiobarbituric acid reactive species (TBARS) formation, diphenyle–α-picrylhydrazyl (DPPH) oxidation, cellular nitric oxide (NO) radical scavenging capability, superoxide dismutase (SOD), glutathione peroxidase (GPx), acetylcholinesterase (AChE) and α-gulcosidase activities were spectrophotometrically determined. On the other hand, the effect of extract and berberine as anticancer was estimated on three different cell lines which were MCF-7, HepG-2, and Caco-2 cells by using neutral red uptake assay which compared with control normal cells (PBMC).
Results
Our results showed that barberry crude extract contains 0.6 mg berberine/mg crude extract. Barberry extract showed potent antioxidative capacity through decreasing TBARS, NO and the oxidation of DPPH that associated with GPx and SOD hyperactivation. Inhibitory effect of berberis crude extract on α-glucosidase was more potent than that of berberine chloride, while both had the same AChE inhibitory effect. Besides, different concentrations of both berberine chloride and barberry ethanolic extract showed to have no growth inhibitory effect on normal blood cells (PBMC). Otherwise, both berberine chloride and barberry ethanolic extract showed to have inhibitory effect on the growth of breast, liver and colon cancer cell lines (MCF7, HepG2 and CACO-2, respectively) at different incubation times starting from 24 hrs up to 72 hrs and the inhibitory effect increased with time in a dose dependant manner.
Conclusion
This work demonstrates the potential of the barberry crude extract and its active alkaloid, berberine, on suppressing lipid peroxidation, suggesting a promising use in the treatment of hepatic oxidative stress, Alzheimer and idiopathic male factor infertility. Beside, berberis vulgaris ethanolic extract is safe non-toxic extract as it was not inhibit the growth of PBMC that can induce cancer cell death that could return to its powerful antioxidant activity.
Keywords: Bioscreening, DPPH, Acetylcholinesterase, α-glucosidase, Breast cancer, Hepatoma, CACO-2, PBMC
Background
As it is increasingly believed now that traditional medicines become more popular worldwide, there is accumulating evidence suggesting medicinal plants are unlimited reservoirs of drugs. The amazing structural diversity among their active components makes them a useful source of novel therapeutics. Researchers with interest in natural products have intensified their efforts towards scientific evaluation of traditional medicines. The World Health Organization (WHO) estimates that herbal medicine is still the most common source for primary health care of about 75-80% of the world's population, mainly in the developing countries, because of better cultural acceptability, better compatibility with the human body and fewer side effects [1].
Berberis vulgaris is a shrub in the family Berberidaceae, native to central and southern Europe, northwest Africa and western Asia. It grows in a variety of soils, though is primarily cultivated in cooler regions [2-4]. Its fruit is an oblong red berry 7–10 mm long and 3–5 mm broad, ripening in late summer or autumn; it is edible but very sour, and rich in vitamin C. Barberry is extensively used as food additive and its juice is recommended to cure cholecytitis [5].
Barberry has a long history of use in traditional eastern and western herbalism [6].
Berberis vulgaris as well as other berberine (BER) containing plants [7] are used medicinally in virtually all-traditional medical systems, and have a history of usage in Ayurvedic, Iranian and Chinese medicine dating back at least 3,000 years [8]. Ancient Egyptians used barberry fruit with fennel seeds to ward off pestilent fevers [6]. Indian ayurvedic physicians used barberry in the treatment of dysentery and traditional Iranian medicine uses its fruit as a sedative [6,9]. In northern Europe barberry was used to treat gall bladder and liver problems, while it was used in the treatment of abnormal uterine bleeds and rheumatism in Russia and Bulgaria [10,11]. In North America, the Eclectics used barberry for treatment of malaria and as a general tonic [12]. Also, the American Indians found it effective in improving appetite and used its dried fruit as a gargle [13,14].
Phytochemical analysis of root, stem or bark extract of B. vulgaris demonstrated the presence of protoberberines and bisbenzyl-isoquinoline alkaloids (berbamine, tetrandrine and chondocurine, (Table 1) for which anti-inflammatory and immuno-suppressive activities have also been well established [15]. Medicinal properties for all parts of the plant have been reported, including tonic, antimicrobial, antiemetic, antipyretic, antipruritic, antioxidant, anti-inflammatory, hypotensive, antiarrhythmic, sedative, antinociceptive, anticholinergic and cholagogue actions, and it has been used in some cases like cholecystitis, cholelithiasis, jaundice, dysentery, leishmaniasis, malaria and gall stones [16] (Table 2). Furthermore, BER, an isoquinoline alkaloid and the major ingredient of this plant, has been used for treating diarrhea and gasterointestinal disorders for a long time [17,18]. It has multiple pharmacological effects including; antimicrobial activity against 54 microorganisms [19-21], inhibition of intestinal ion secretion and smooth muscle contraction, inhibition of ventricular tachyarrhythmia, reduction of inflammation, stimulation of bile secretion and bilirubin discharge [22]. In spite of extensive applications and numerous properties, the mechanism of action in most of its effects is not exactly clear. Some of these properties may occur due to antihistaminic and anticholinergic effects.
Table 1.
Compounds isolated from berberis vulgaris
| Compound | Nature | Compound | Nature |
|---|---|---|---|
| Aromoline |
Alkaloid |
Oxyberberine |
Alkaloid |
| Berbamine |
Alkaloid |
Oxycanthine |
Alkaloid |
| Berberine |
Alkaloid |
Palmatine |
Alkaloid |
| Berlambine |
Alkaloid |
Quercentin |
Flavonoid |
| Bervulcinc |
Alkaloid |
Rutin |
Flavonoid |
| Columbamine |
Alkaloid |
(-)-tejedine |
Alkaloid |
| Hydroxycanthine |
Alkaloid |
Yatronizine |
Alkaloid |
| Isocorydine | Alkaloid |
Table 2.
The pharmacological effects of Berberis vulgaris (NAPALERT; natural products alert database)
| System | Effect | Part of plant | Preparation |
|---|---|---|---|
| Cardiovascular |
Hypotensive activity |
Dried root |
Alkaloid fraction |
| Dried fruit |
Aqueous extract |
||
| Gastrointestinal |
Gastric secretory stimulation |
Root |
Ethanol—H20 (67%) extract |
| Endocrine |
Choleretic activity in rat |
Dried root |
Total alkaloids |
| Choleretic activity |
Stem bark |
||
| Increases tone of the digestive tract and gives rise to increased and irregular peristalsis |
Dried root |
||
| Anticholinergic activity in guinea pig ileum |
Dried fruit |
Decoction |
|
| Menstruation induction effect in guinea pig |
Stem |
Ethanol (95%) extract |
|
| Uterine stimulant effect in cat, rabbit and guinea-pig |
Leaf |
Ethanol-acetone (50%) extract |
|
| Immune system |
Antibody formation suppression in mouse |
Dried root |
Alkaloid fraction |
| Antiinflammatory activity |
Root |
Alkaloid fraction |
|
| Organisms |
Complement alternative pathway inhibition |
Root |
Ethanol (l00%) extract |
| Delayed type cutaneous hypersensitivity inhibition |
Alkaloid fraction and ethanol (95%) extract Alkaloid fraction |
||
| Central nervous system |
Antipyretic activity in rat |
Dried bark |
Alkaloid fraction |
| Dried fruit |
Ethanol (95%) extract |
||
| Narcotic antagonist activity |
Dried root |
|
|
| Sedative |
Fruit |
|
|
| Renal |
Diuretic activity in rat |
Dried bark |
Alkaloid fraction |
| Other |
Toxicity assessment in mouse — LD50 = 520 0 mg/kg |
Dried root |
Alkaloid fraction |
| Toxicity assessment in mouse — LD50 = 2.6 ± 022 g/kg b w | |||
| Male reproduction |
Idiopathic ma factors due to oxidative damage |
Root | Crude methanolic (95%) extraction |
| Crude acetic acid (5%) extraction |
Among berberine multiple pharmacological actions, anti-inflammatory activity has been extensively studied [23]. Antipyretic activity of berberine sulfate has also been shown by Sabir and Bhide 1971 using a model of experimentally induced fever in rats [22]. This effect has been found to be approximately three times greater than sodium salicylate. Anti-colitic property is another pharmacological effect has been demonstrated for berberine by Zhou and Mineshita [24].
The barberry phenolic compounds include anthocyanins and carotenoid pigments [22-26]. Several pharmacological effects such as antioxidant and cytoprotective [27], inhibitory effects on capillary permeability [28] and epidermal growth factor [29] anticholinergic and antihistaminergic [27], have been demonstrated for anthocyanins and berberry fruit extract (BFE).
The aim of this study was to compare the in vitro biological activity (antioxidant, cholinergic, antidaibetic and the anticancer) of barberry crude extract and berberine active compound.
Methods
Human and animal biological samples
Human participants and their biological materials (blood) met the ethical standards for donor approval required by national regulatory bodies. Blood samples were collected from ten healthy subjects after they signed consent informed the use of their blood in this study Consents’ approval and all study protocols for animal and biological tissue samples treatment, involved in this study, were firmly subjected to ethical instructions outlined by Animal Ethics Committees (AEC) that published via The National Health and Medical Research Council (NHMRC) policies and guidelines that recommended by the Egyptian Ministry of Health and Population, High Committee Of Medical Specialties, Arab Republic of Egypt [30]. This study was permission granted from the Biomedical technology, SRAT-city and Biochemistry Department, Faculty of Science, Alexandria University, following approval of the Research Ethics Committee, Faculty of Pharmacy, Alexandria University.
Barberry, Berberis vulgaris
Barberry’s roots were purchased and authenticated by Prof. Salma El-dareir, Botany Department, Faculty of Science, Alexandria University, Egypt. Firstly, this classification was being dependent on the data about the plant published in Dargon Herbarium [31]. After classification, the plant roots were separated then dried at room temperature, powdered, sieved, and stored prior to further use. Dried barberry roots were phytochemically screened for alkaloids, phobatannins, saponnins, flavonoids, steroids, terpenoids and cardiac glycosides [1].
Barberry crude extract preparation
The dried powdery roots of barberry were exhaustively defatted with petroleum ether then dried in fresh air to evaporate the solvent. The dried roots were used to prepare the ethanolic crude extract by subjecting to steam distillation method using Soxhlet apparatus in which the powder was added in glass thimble and boiled ethanol extracted the active compounds for 8 hours. The ethanolic extract was concentrated to minimum volume using rotary evaporator at 60°C and 100 rpm (Büchi, Switzerland) then lyophilized (DISHI, DS-FD-SH10, Xi’an Heb Biotechnology Co, China) to obtain a powder extract of barberry (25%). The barberry extract powder form was kept at -20°C until subjected to further biochemical analysis.
HPLC analysis
The ethanolic extract was analyzed using HPLC (Series 500 Bio-Tek Instrument, Milano, Italy) to determine the berberine concentration in the extract. The analytes were separated by a Zorbax Eclipse XDB-C18 (250 × 4.6 mm i.d., 5 μm particle size) column (Agilent, Santa Clara, CA, USA).
The freeze-dried Berberis vulgaris extracts were dissolved in equal volume water: ethanol solution (1 mg/ml) and were filtered through a 0.22-μm syringe filter prior to HPLC analysis. The operating temperature was maintained at 30°C and the detector was operated at a wavelength of 254 nm. The mobile phase was a mixture of two solvent compositions, solvent A (deionized water) and solvent B (methanol). The program was started with 40% solvent A and 60% solvent B at a solvent flow of 0.8 ml/min and injection volume of 20 μl [32].
Preparation of berberine chloride
Berberine chloride was commercially available from Sigma-Aldrich where, different concentrations of berberine chloride were prepared by dissolved certain weights at 1 ml of 10% ethanol.
Preparation of liver homogenate
Six Balb/c mice were obtained from animal of house of medical research institute, Alexandria University. After anesthesia, liver was isolated and washed in cold saline, and then one gram of each liver was homogenized in 9 mL phosphate buffer saline. The homogenate was centrifuged at 3000 and metabolites containing supernatant was decanted for further biochemical estimations.
Biochemical assays
1. Determination of Acetylcholinesterase (AChE) activity
AChE activity was measured according to the method of Ellman et al. [33]. 130 μL phosphate buffer (0.1 M pH 7.4) were added to a mixture of 20 μL of liver homogenate and 20 μL of barberry extract (test) or organic solvent (control), then incubated for 45 min at 37°C. 5 μL of substrate ACTI (75 mM) were added, mixed well and incubated for 15 min at 37°C. 60 μL DTNB (0.32 mM) were added and left for 5 min. The absorbance was measured at 405 nm and the specific activity was calculated.
2. Determination of α-glucosidase activity
Method mentioned by Han and Srinivasan [34] was carried out with a slight modification to estimate the effect of barberry extract on α-glucosidase (EC 3.2.1.20) activity. 100 μL of barberry extract (test), organic solvents (control) or dH2O (blank) were diluted with 2.5 mL of 0.1 M phosphate buffer, pH 7.4. 100 μL of liver homogenate were added, mixed well and incubated in a water bath with the reaction mixture at 30°C for 5 min. 500 μL PNPG, 5 mM, were added and the reaction was allowed to proceed for 15 min. The reaction was stopped by the addition of 2 mL of 1 M Na2CO3. The producing color was spectrophotometrically detected at 400 nm. A unit of enzyme activity was defined as nmoles of p-nitrophenol released/min.
3. Determination of Thiobarbaturic acid reactive substance (TBARS) level in induced lipid peroxidation model
Two mL of barberry extract or berberine chloride (test), the organic solvent (control) or distilled water (dH2O) (blank) were incubated with equal volume of liver homogenate for about 45 min at 37°C. In vitro tissue lipid peroxidation was induced by adding H2O2 and ferrous sulphate (FeSO4.7H2O) at a final concentration of 1 mM and 0.5 mM, respectively, in both test and control reaction mixtures. After an incubation period of about 30 min at 37°C, butylated hydroxyl toluene (BHT) was added at a final concentration of 0.02% and mixed carefully to stop the peroxidation reaction. The mixtures were centrifuged at 3000 rpm for 15 min, and then 1 mL of the resultant supernatant was mixed with 1 mL of 15 % trichloroacetic acid (TCA) followed by centrifugation at 3000 rpm for 10 min [1].
Then TBARS was determined in previous solution according to the method described by Wills [35]. 1 mL of protein free supernatant was mixed with 500 μL of 0.7% thiobarbituric acid (TBA), heated in boiling water bath for 45 min, cooled and the colour in the supernatant was detected at 532 nm. The TBARS level was calculated against a control according to the following equation: TBARS level (nmol/ml) = At / 0.156
4. Determination of Diphenyle –α-picrylhydrazyl (DPPH) radical scavenging
DPPH radical scavenging assay of the total extract was performed by using the previously established and modified methodology by Choi et al.[36]. Assays were performed in flat bottom polystyrene 96 well microtiter plates. To 100 μL of each sample (1-6 mg/ml) in EtOH 25 μL DPPH (1 mM) in ethanol was added. The resultant mixture was briefly shaken and maintained at room temperature, in the dark for 30 min. At the end of this period, the absorbance (A) of the mixture was measured at 490 nm, using ELISA. Scavenging ratio of DPPH assay calculated as follows:
5. Determination of nitric oxide scavenging activity
Nitric oxide was determined by the method described by Green et al. [37], where nitric oxide was generated from sodium nitroprusside and measured by Griess reaction. Scavenger of nitric oxide competes with oxygen leading to reduced production of nitric oxide. Sodium nitroprusside (5 mM) in phosphate buffered saline (pH 7.2), was mixed with different concentrations (1–6 mg/ mL) of the extract and incubated at 25°C. Samples from the above were reacted with Griess reagent (1% sulphanilamide, 2% phosphoric acids and 0.1% Naphthylethylendiamine hydrochloride). The absorbance of the chromophore (A) formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with naphthylethylenediamine was read at 546 nm. The scavenged ratio of NO calculated as follows:
6. Determination of SOD activity
Twenty microliters of liver homogenate supernatant (test) or buffer (reference) and 20 μl of extract or berberine chloride different concentrations were added to 1 mL buffer solution and incubated at 37°C for 45 min. 10 μl pyrogallol (20 mM in HCl, 10 mM) was added to the previous solution and the absorbance of test (At) or reference (Ar) was measured at 420 nm against air after 30 and 90 s. The percentage inhibition of pyrogallol autoxidation by supernatant was calculated according to the following equation:
The specific activity of serum SOD as ng/min/mg protein was calculated with dividing the value of SOD in ng/min/ml by protein concentration in the sample.
One unit of SOD activity is defined as the amount of enzyme which inhibits the rate of auto-oxidation of pyrogallol by 50%. From the standard curve it was found that, one unit equals 153 ng. The sample enzyme activity in U/ mg protein was obtained by diving value in ng/ min/ mg protein by 153 [38].
7. Determination of liver glutathione peroxidase (GPx) activity
Fifty microliters of liver homogenate were incubated with equal volume of different extract or berberine chloride different concentrations for 45 min at 37°C, then were added to 100 μl GSH, 100 μl cummen H2O2 and 750 μl Tris–HCl, pH 7.6 (test) and incubated at 37°C for 10 min. For control, 50 μl diluted supernatant and100 μl GSH were added to 750 μl Tris–HCl, pH 7.6, then incubated at 37°C for 10 min. One milliliter TCA was added to test and control as well as 100 μl cummen H2O2 were added to control then both were centrifuged at 3000 r.p.m. for 20 min and then the supernatants were separated off. One milliliter of supernatants was added to 2 ml Tris–HCl, pH 8.9 and 100 μl DTNB then incubated for 5 min [39]. The absorbances of test and control (Ac) were read at 412 nm against distilled water. The activity of liver GPx was calculated with the following equation;
In Vitro anticancer assay
In order to determine the safe concentrations of both barberry ethanolic extract and berberine standard that can be used for In Vitro cell culture; normal peripheral blood mononuclear cells (PBMC) were isolated from a healthy individual by Ficoll-Hypaque (density 1.077 g/L, Lonza, USA) gradient centrifugation. PBMC were collected and washed using HBSS, then cell viability and count were determined using Trypan blue exclusion test. After collecting the PBMC, cells were suspended at concentration of 1×106 cell/ml in RPMI 1640 (Lonza) 25 mM N-2-hydroxyethylpiperazine-N`-2-ethanesulfonic acid (HEPES) (Lonza), 4 mM L-glutamine (Lonza), 100U of penicillin and 100 μg streptomycin (Lonza) and 10% FBS (Lonza). In 96 well plate, 1×105 cell/well were seeded with different concentrations ranging from 0 to 100 μg/ml (20, 40, 60, 80, 100 μg/ml) of barberry ethanolic extract, or berberine standard for 72 hr at 37°C incubator with 95% humidity and 5% CO2.
The cytotoxic effect of the different concentrations of the extract and the standard were determined using the neutral red uptake assay [40]. Briefly, Neutral red working solution (80 μg/ml) (Serva, Austria) was incubated overnight at 37°C. In each well of the incubated cells, culture media was removed and 100 μl of neutral red medium were added then incubated for 3 hr to allow for vital dye incorporation into living cells. The neutral red media was removed and rapid rinsed with 150 μl HBSS. Dye was extracted from the cells by adding 150 μl extraction buffer (1% acetic acid: 50% ethanol (96%): 49% deionized H2O) followed by rapid agitation for at least 10 min on micrometer plate shaker. The extract neutral red color intensity was measured at 490 nm in a micro-titer plate reader spectrophotometer.
Using the relation between used concentrations and neutral red intensity value, IC50 of the barberry ethanolic extract and standard berberine chloride was calculated.
Following the same method selected concentrations of both barberry ethanolic extract and standard berberine chloride were used to examine their effect on different cancer cell lines. In a 96-well tissue culture plates, MCF-7, HepG-2, Caco-2 and EL4 cells were plated each in its respective culture media, at a density of 3000 cells/well, 11000 cells/well, 6000 cell/well and 15000 cells/well, respectively. Cells were left to adhere by incubation for 24 hr at 37°C, 95% humidity and 5% CO2. Following that the selected concentration of the ethanolic extract or standard was added and cell viability was measure at 24 hr intervals for maximum of 72 hr using neutral red uptake assay as previously described.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). The differences were considered to be statistically significant at P < 0.05. Statistical analyses were performed using Primer of Biostatistics program V5 for analysis of the unpaired Student's t-test and one-way analysis of variation (ANOVA).
Results
Preliminary phytochemical screening of barberry’s roots revealed the presence of alkaloids, flavonoids, saponin, phenolic contents, terpenoids and cardiac glycosides. However, steroid and phobatannins were not detected. The percentage of alkaloids, flavonoids, saponin and total phenolic content were 4, 1.9, 0.35 g/ 100 gm plant tissue and 100 mg/ml of ethanolic extract, respectively (Table 3). Table 3 and Figure 1 showed that 1 mg of berberis ethanolic extract contains 0.6 mg berberine active compound.
Table 3.
Quantitative phytochemical screening of barberry roots and Berberine concentration in Berberis vulgaris crude extract
| Extract | Berberice concentration (mg/mg extract) |
|---|---|
| Ethanolic extract | 0.62 |
Figure 1.
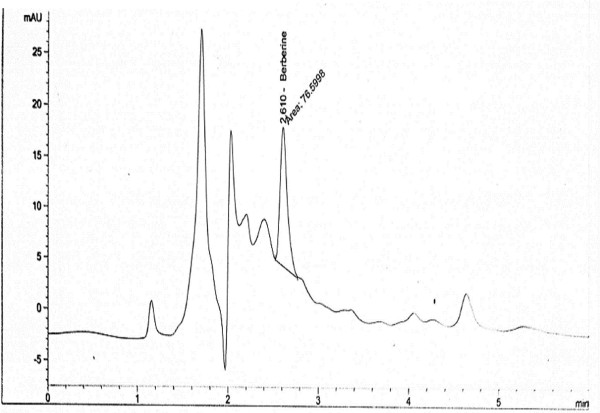
Berberis vulgaris ethanolic extract HPLC chart.
Figure 2 showed that Berberis vulgaris and berberine chloride different concentrations exerted the same AChE inhibitory ability in percentage (%) at p < 0.05, this inhibitory effect was increased as the concentration of Berberis vulgaris and berberine chloride increased.
Figure 2.
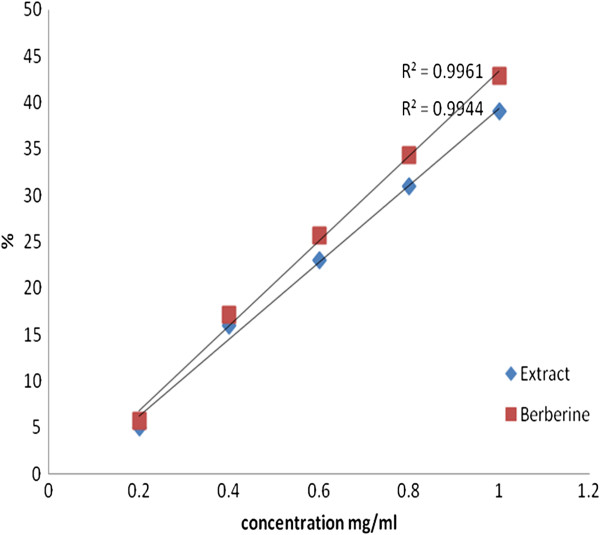
Inhibitory effect percentage of Berberine chloride and Berberis vulgaris toward AChE.
Both Berberis vulgaris and berberine chloride had α-glucosidase inhibitory effect but the effect of Berberis crude extract was more potent than that of berberine chloride as shown in Figure 3. Furthermore, this inhibitory effect was directly proportional with that ingredient concentration at p < 0.05.
Figure 3.
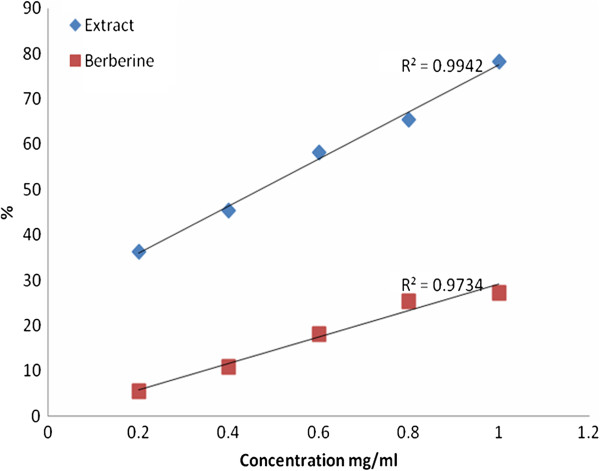
Inhibitory effect percentage of Berberine chloride and berberis vulgaris toward glucosidase.
The antioxidants effect of Berberis vulgaris crude extract and berberine chloride were tabulated in Table 4. Our results showed that both crude extract and active ingredient had powerful antioxidants properties as they inhibited the production of TBARS, NO and the oxidation of DPPH that associated with GPx and SOD hyperactivation. These biochemical properties were exerted in a concentration dependent manner where TBARS production decreased from 9 ± 0.3 nmol/g to 4 ± 1.1 nmol/g as the concentration of berberis crude extract increased from 0.2 mg/ml to 1 mg/ml. The same pattern and the same inhibitory effect were shown with berberine chloride different concentrations. Both berberis extract and berberine chloride different concentrations ranged from 0.2-1 mg/ml lowered NO level in range from 16 to 25%, respectively, at p < 0.05. Furthermore, the same tested concentrations of B. vulgaris or berberine chloride inhibited the DPPH oxidation in range from 13 to 46% than control level at p < 0.05. On the other hand, the GPx and SOD activities increased in the range of 10-70% and 55-270%, respectively, when the concentration of Berberis vulgaris or berberine chloride increased from 0.2 mg/ml to 1 mg/ml as comparing with control levels.
Table 4.
Effect of berberine chloride and berberis on cellular prooxidants/antioxidants status
| mg/rn | Tears | DPPH | NO | Gpx | SOD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
||||||||||
| Extract |
Berberine |
Extract |
Berberine |
Extract |
Berberine |
Extract |
Berberine |
Extract |
Berberine |
|
| 0 |
12 ± 2.5 |
22 ± 5.1 |
60 ± 12 |
0.3 ± 0.02 |
2.5 ± 0.2 |
|||||
| 0.2 |
9 ± 0.3 |
8 ± 0.7 |
19 ± 2.1 |
19 ± 0.1 |
50 ± 1.1 |
55 ± 1 |
0.33 ± 0.0 1 |
0.39 ± 0.0 5 |
3.9 ± 0.1 |
4 ± 0.3 |
| 0.4 |
7 ± 0.7 |
6 ± 0.1 |
17.1.1 |
16 ± 0.1 |
50 ± 2.1 |
53 ± 2.1 |
0.38 ± 0.0 3 |
0.42 ± 0.0 3 |
5 ± 0.3 |
5.1 ± 0.5 |
| 0.6 |
4.5 ± 0.4 |
5.3 ± 0.6 |
15 ± 0.9 |
14 ± 0.3 |
48 ± 1.9 |
49 ± 0.2 |
0.43 ± 0.0 2 |
0.48 ± 0.0 2 |
6.6 ± 0.2 |
6 ± 0.6 |
| 0.8 |
4.4 ± 0.8 |
4.5 ± 0.3 |
12.3 ± 0.5 |
12 ± 1.1 |
46 ± 2 |
46 ± 0.2 |
0.49 ± 0.0 2 |
0.52 ± 0,0 1 |
8.9 ± 0.4 |
9 ± 0.4 |
| 1 | 4 ± 1.1 | 4 ± 0.6 | 12 ± 2.3 | 11 ± 1.6 | 45 ± 9 | 43 ± 8.1 | 0.5 ± 0.05 | 0.55 ± 0.0 5 | 9.2 ± 0.9 | 10 ± 0.5 |
The cytotoxicity study that was done on PBMC for both of b. vulgaris extract and berberine chloride, the main alkaloid constituent, showed that both of them had not any mentioned cellular toxicity while they had a significant proliferatory effect. As, different concentrations (20, 40, 60, 80 and 100 μg/ml) of both berberine chloride and barberry ethanolic extract showed to have inhibitory effect on normal blood cells (PBMC) growth rate maintenance, in contrary they slightly stimulated the proliferation of PBMC (Figure 4) especially after incubation for 72 hrs. At the same time as indicated in Figure 5, concentrations starting from 1 μg/ml up to 100 μg/ml of both berberine chloride and barberry ethanolic extract showed to have inhibitory effect on the growth of breast, liver and colon cancer cell lines (MCF7, HepG2 and CACO-2, respectively) at different incubation times starting from 24 hrs up to 72 hrs and the inhibitory effect increased with time in a dose dependant manner. It was interest to notice that with time the inhibitory dose of both berberine chloride and barberry ethanolic extract increased with time in case of normal cells (PBMC) and decreased dramatically with time in case of cancer cells (Table 5).
Figure 4.
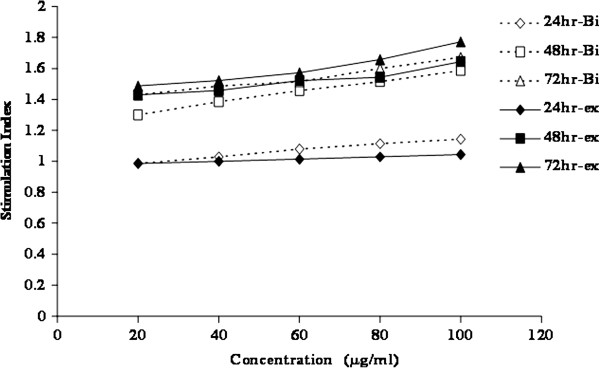
Effect of different concentrations of Berberine chloride (Bi) andBerberis vulgarisextract(ex)on proliferation of normal peripheral blood mono nuclear cells (PBMC) at 24, 48 and 72 hrs. Data were represented as stimulation index (SI).
Figure 5.
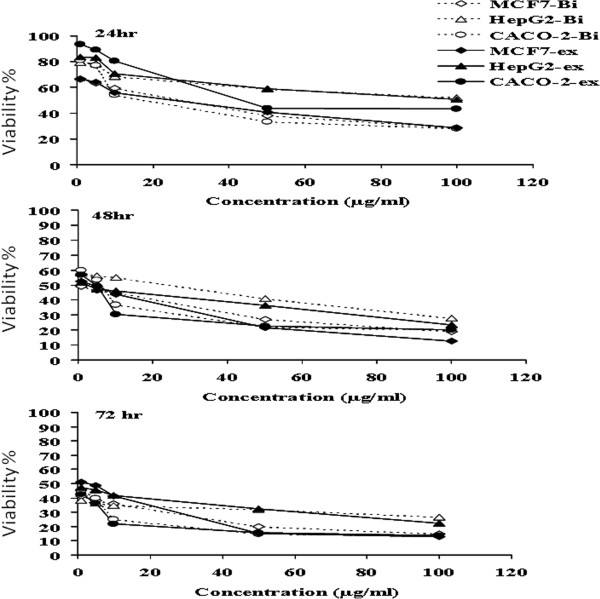
Effect of Berberine chloride (Bi) andBerberis vulgarisextract(ex)on viability of three human cancer cell lines. Different concentration of both Berberine chloride and berberis vulgaris were incubated with 105 cells /ml of breast cancer (MCF7), Liver Cancer (HepG2) and Colon cancer (CACO-2) cell lines (ATTC, see Methods) for 24, 48 and 72 hours, cell viability were evaluated by using neutral red cell stainig.
Table 5.
Inhibition concentrations of both Berberine chloride and Berberis vulgaris that can inhibit the growth of 50% of normal and cancer cells at different incubation time
| 11250 (mg/mi) | ||||||
|---|---|---|---|---|---|---|
| |
Berberine |
Berheris vuigaris
|
||||
| |
24 |
48 |
72 |
24 |
48 |
72 |
| PBMC |
0.66649 |
5.568 |
883 .994 |
0.90 19 |
6.05 1 |
2.546 |
| MCF7 |
0.01593 |
0.00443 |
0.00195 |
0.01561 |
0.00454 |
0.00397 |
| Rep 02 |
0.06586 |
0.0 1149 |
0.00 173 |
0.06805 |
0.00555 |
0.00408 |
| CAC 0-2 | 0.0 1764 | 0.005 1 | 0.00 183 | 0.04996 | 0.00384 | 0.00 144 |
Discussion
The phytochemical constituents of B. vulgaris act in synergism to increase barberry's bioactivity such as antioxidant, antimicrobial, anticholinergic, anti-diabetic, etc. [41]. In our previously published work, we mentioned that B. vulgaris ethanolic extract has a competitive AChE inhibitory ability suggesting its use to alleviate over activity of AChE in dementia patients [42]. In agreement with this finding, our data showed the same inhibitory effect toward AChE enzyme. This inhibitory effect may be returned to the presence of berberine in ethanolic crude extract where it represented 60% of its ingredients. Berberine binds AChE active site as it is acts as competitive inhibitor that leads to enzyme conformational change and increases entropy value. AChE is mainly present in the central nervous system and its principle role is to catalyze the hydrolysis of the neurotransmitter acetylcholine (ACh) to choline. This process can return an activated cholinergic neuron back to its resting state. The pathogenesis of AD is linked to a deficiency in the brain ACh [32]. Thus, AChE is an important pathogenic factor of AD and most pharmacological studies for screening agent to combat AD has been focused on AChE inhibitors to alleviate cholinergic deficit and improve neurotransmission [43].
B. vulgaris inhibited α-glucosidase enzyme activity provides an effective way for diabetes treatment. The inhibition of α -glucosidase activity is one of therapeutic approaches for reducing postprandial hyperglycemia. α-Glucosidase inhibitor is effective in delaying absorption of carbohydrates and suppressing postprandial hyperglycemia which contribute to the decrease in hemoglobin A1C (HbA1c). The decreasing of HbA1c could reduce the incidence of chronic vascular complication in diabetic patients [44].
Both of TBARS and DPPH assays besides SOD and GPx activities give a complete picture about the total antioxidants capacity of the B. vulgaris ethanolic extracts. It is important to determine the relative antioxidant capacity of the extract since free radicals and oxidants in the body commonly cause damage to aqueous-based cellular structures and organelles as well as lead to peroxidation of lipids [45]. Berberis vulgaris ethanolic extract was a potent inhibitor for hepatocytes' lipid peroxidation induced by Fe2+ and H2O2. This finding was proved previously on acidic and methanolic barberry extracts [1], where berberine and barberry crude extract showed a significant reductive ability and radicals scavenging effects, especially on hydroxyl and DPPH radicals. Also, they have the ability to increase SOD and GPx activities. It is reported that the most polar solvent dissolving several compounds of different polarities such as acids, sugars or glycosides which may be contributed to the total phenolic content of the extract and represented the highly antioxidant properties. Depending on this we speculated that acetic acid extract preparations exhibited appreciable antioxidantive activity against the generation of cellular oxidized lipid particles [1].
Nitric oxide is an essential bioregulatory molecule required for several physiological processes like neural signal transmission, immune response, control vasodilatation and control of blood pressure. However, the elevation of the NO results in several pathological conditions, including cancer [46]. The plant/plant products may have the property to counteract the effect of NO formation and in turn may be of considerable interest in preventing the ill effects of excessive NO generation in vivo[46]B. vulgaris extract reduced the generation of NO in vitro in a concentration dependent manner. The implications of these findings may be very important for human health, since this herb has been used in several countries from ancient times. Further, the high scavenging activity may also help to arrest the chain of reactions initiated by excess generation of NO, that are detrimental to the human health as excess NO is known to damage the immune system.
Chemotherapeutic drugs generally have low safety despite of their high efficacy. This might be due to toxicity and side effects that are usually associated with almost all types of chemotherapeutic medicine, besides the generated resistance to this group of therapeutic drugs which makes it more difficult to the patients. Between 1982 and 2002, more than half of the new anti-cancer chemical compounds were derived directly or indirectly from natural resources either microorganisms, plant or both [47,48]. Berberine as an isoquinoline alkaloid isolated from different types of plants is well recognized for its assorted pharmacological actions, for example berberine purified from Coptidis Rhizoma (Huanglian) is used in Chinese medicine for heat dissipation and detoxification [49], moreover, berberine purified from members of family Berberidaceae is well known for its anti-inflammatory and immunosuppressive capacity beside other pharmacological activities [50-56]. Berberine is also recognized for its anti-cancer activity [56-64]. For assessment of anticancer effect of certain compounds, cytotoxicity on primary normal cell culture must be formed to calculate the safe dose. Several types of primary cells can be used; one of them is PBMC [65]. Furthermore, we based on neutral red assay for cytotoxicity study as it is reported that neutral red and the MTT assay being the most sensitive in detecting cytotoxic events compared to the LDH leakage and the protein assay [66]. Moreover, it is well known that berberine shifted the balance between Th2 and Th1, increased the production of IL 12 and altered the cytokines profile [67]. So, it mimics PHA response and in such cases PHA did not used to make cell stimulation because it could cause cell death due to cell overstimulation [68,69].
In the present study, the anti-cancer activity of berberine chloride and total ethanolic extract from B. vulgaris was examined, in which this herb as member of the family Berberidaceae has not been identified before for its anti-cancer activity. At first, the dose for this in vitro testing was determined using healthy PBMC, and as indicated in Figure 3, for berberine chloride and B. vulgaris ethanolic extracts, both are slightly stimulate immune cells and the safety margin concentrations of both berberine chloride and B. vulgaris ethanolic extracts are significantly high (IC50 =0.66649, 5.568 and 883.994 mg/ml after incubation at 24, 48 and 72 hours). Followed by examining the effect of 1, 5, 10, 50 and 100 μg/ml of both berberine chloride and b. vulgaris extracts on viability of breast, hepatic, colon and cervix cancer cell lines after incubation for 24, 48, and 72 hours. The cell viability of all cancer cell line used in this study was decreased significantly and in a dose dependent manner with both berberine chloride and berberis vulgaris ethanolic extracts (Figure 5 and Table 4). This data are in agreement with the published data about other members of family berberidaceae[56,58-60,64]. The mode of action of both berberine chloride and b. vulgaris ethanolic extracts were not determined during the course of the present study, but in another study performed in our laboratory (data not shown) that p53 expression was increased due to treatment with both berberine chloride and b. vulgaris ethanolic extracts. This might explain that b. vulgaris ethanolic extract can induce cancer cell death (apoptosis) through this mode of action. On the other hand, the antioxidant activity might play a major role in increasing efficiency of such extracts to kill cancer cells and protect normal cells.
Conclusion
This work demonstrates the potential of the bioactive ingredients of barberry on suppressing lipid peroxidation, suggesting a promising use in the treatment of hepatic oxidative stress, Alzheimer and idiopathic male factor infertility. Besides, berberis vulgaris ethanolic extract can induce cancer cell death that could return to its powerful antioxidant activity. Although the significant curative potential of b. vulgaris against many diseases, it didn’t deposited in a publicly available herbarium yet. Thus, for further experimental investigations, protocol for ingredients’ extraction mentioned in methods section is recommended to be followed. Furthermore, the plant has been registered in several herbarium and we use this as reference, also we purchased the root from USA market so how come we register it in our herbarium as it is not grown in Egypt but we will register the extract after we finish complete toxicological study [31,70,71].
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors designed the study, collected the data, performed the techniques employed in the study. GAD, AEA and EAM made the interpretation of statistical analyses and wrote the paper with input from all the authors who each approved the final version. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Abeer E Abd El-Wahab, Email: abdelwahababeer@gmail.com.
Doaa A Ghareeb, Email: d.ghareeb@yahoo.com.
Eman EM Sarhan, Email: eman.sarhan@yahoo.com.
Marwa M Abu-Serie, Email: marwaelhedaia@yahoo.com.
Maha A El Demellawy, Email: meldemellawy@gmail.com.
References
- El-Sayed M, Ghareeb DA, Sarhan EM, Khalil AA. Therapeutic Bio-screening of the bioactive extracted ingredients of Berberis vulgaris, berberine. FPSB. 2011;13:63–68. [Google Scholar]
- Harden GJ, editor. Flora of New South Wales, Volume 1. Kensington: NSW University Press; 2000. [Google Scholar]
- Grieve M. 1931. A Modern Herbal. London: Tiger Books International; 1994. [Google Scholar]
- Arayne MS, Sultana N, Bahadur SS. The berberis story: Berberis vulgaris in therapeutics. Pakistan J Pharmacol Sci. 2007;13(1):83–92. [PubMed] [Google Scholar]
- Zargari A. Medicinal Plants. Tehran: Tehran University Press; 1983. 1:68. [Google Scholar]
- Chevallier A. The Encyclopedia of Medicinal Plants. St Leonards: Dorling Kindersley; 2001. [Google Scholar]
- Souri EAG, Dehmobed-Sharifabadi A, Nazifi A, Farsam H. Antioxidant activity of sixty plants from Iran. Iranian J Pharm Res. 2004;13:55–59. [Google Scholar]
- Timothy CBN, Gregory S, Kelly ND. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;13:94–103. [Google Scholar]
- Kunwar RM, Nepal BK, Kshhetri HB, Rai SK, Bussmann RW. Ethnomedicine in Himalaya: a case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J Ethnobiol Ethnomed. 2006;13:27. doi: 10.1186/1746-4269-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;13(3):222–225. doi: 10.1002/ptr.1661. [DOI] [PubMed] [Google Scholar]
- Ivanovska N, Philipov S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int J Immunopharmacol. 1996;13(10):553–561. doi: 10.1016/S0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, Berberine. Phytotherapy Research 2008. Published online at Wiley InterScience. Adv Physiol Educ. doi:10.1002/ptr.2399 ( http://www.interscience.wiley.com) [DOI] [PubMed]
- Mills S, Bone K. Principals and Practice of Phytotherapy. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- Bone K. A Clinical Guide to Blending Liquid Herbs: herbal formulations for the individual patient. St Louis, Missouri: Churchill Livingstone; 2003. [Google Scholar]
- Li SY, TheBS LL, Seow WK, Thong YH. Anti-inflammatory and immuno-suppressive properties of the bis-benzylquinolones: in-vitro comparisons of tetrandrine and berbamine. Int J Immunopharmacol. 1989;13:395–441. doi: 10.1016/0192-0561(89)90086-6. [DOI] [PubMed] [Google Scholar]
- Khosrokhavar R, Ahmadiani ASF. Antihistaminic and Anticholinergic Activity of Methanolic Extract of Barberry Fruit (Berberis vulgaris) in the Guinea- Pig Ileum. J Med Plants. 2010;13:99–105. doi: 10.1016/s0378-8741(98)00122-6. [DOI] [PubMed] [Google Scholar]
- Akhter MH. SMaBN: Possible mechanism of antidiarrheal effect of berberine. Indian J Med Res. 1979;13:233–241. [PubMed] [Google Scholar]
- Rabbani GHBT, Knight J, Sanyal SC, Alam K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholera. J Infect Dis. 1987;13:979–984. doi: 10.1093/infdis/155.5.979. [DOI] [PubMed] [Google Scholar]
- Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimidrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;13:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. New York: Wiley Publishers; 1996. pp. 66–67. [Google Scholar]
- Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;13:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- Sabir M. Study of some pharmacological actions of berberine. Indian J Physiol Pharmacol. 1971;13:111–132. [PubMed] [Google Scholar]
- Kuo C, Chi C, Liu T. The anti-inflammatory potential of berberine in-vitro and in-vivo. Cancer Lett. 2004;13:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;13(3):822–829. [PubMed] [Google Scholar]
- KF L. In: Adverse Effects of Herbal Drugs. Pagm KK, Hansel R, Chandler RF, editor. Berlin: Springer Verlag; 1994. Berberine; pp. 97–98. [Google Scholar]
- Shamsa F, Ahmadiani A, Khosrokhavar R. Antihistaminic and anticholinergic activity of berberry fruit (Berberis vulgaris) in the guinea-pig ileum. J Ethnopharmacol. 1999;13:161–166. doi: 10.1016/S0378-8741(98)00122-6. [DOI] [PubMed] [Google Scholar]
- Tomosaka H, Salim AA, Keller WJ, Chai H, Kinghorn AD. Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry) Phytother Res. 2008;13:979–981. doi: 10.1002/ptr.2443. [DOI] [PubMed] [Google Scholar]
- Cohen-Boulakia F. In-vivo sequential study of skeletal muscle capillary permeability in diabetic rats. Metabolism. 2000;13:880–885. doi: 10.1053/meta.2000.6754. [DOI] [PubMed] [Google Scholar]
- Meiers S. The anthocyanidins cyaniding and delphinidin are potent inhibitors of the epidermal growth factor receptor. J Agric Food Chem. 2000;13:958–962. doi: 10.1021/jf0009100. [DOI] [PubMed] [Google Scholar]
- http://www.mohp.gov.eg/default.aspx#, Egyptian Ministry of Health and Population, Arab Republic of Egypt, 9/5/2013, 11:30 PM.
- http://www.dragonherbarium.com/bulk-herbs-spices/bulk-herbs-b/barberry-root-bark-cs-wc-berberis-vulgaris.
- Qadir S, Han J, Ji H, Chung H, Ahn J, Lee H. Effect of different extraction protocols on anticancer and antioxidant activities of berberis koreana bark extracts. J Biosci Bioeng. 2009;13:331–339. doi: 10.1016/j.jbiosc.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ellman G, Andres V, Featherstone RA. New and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;13:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Han YN, Srinivasan VR. Purification and characterization of beta-glucosidase of Alcaligenesfaecalis. J Bacteriol. 1969;13:1355–1363. doi: 10.1128/jb.100.3.1355-1363.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1965;13(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, King YC, Leroy P, Siest G, Wellman M. Differential role of CYP2E1 binders and isoniazid on CYP2E1 protein modification in NADPHdependent microsomal oxidative reactions: FR scavenging ability of isoniazid. Free Radic Res. 2002;13:893–904. doi: 10.1080/1071576021000005339. [DOI] [PubMed] [Google Scholar]
- Green LC, Glogowski J. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal Biochem. 1982;13:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;13:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Paglia E, Valentine N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clinic Med. 1967;13:158–169. [PubMed] [Google Scholar]
- Repetto G, Zurita J. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;13:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Wongbutdee J. physiological effects of berberine. Thai Pharm Health Sci J. 2008;13:78–83. [Google Scholar]
- Ghareeb D, Newarry A, El-Rashidy F, Hussein H, Ali A. Efficacy of natural extracts of Ginkgo biloba and barberry and a synthetic derivative of genistein (ipriflavone), as acetylcholinesterase inhibitors, comparative study with Aricept® effect. J Biochem Biotechnol. 2010;13:15–20. [Google Scholar]
- Muñoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr Med Chem. 2008;13:2433–2455. doi: 10.2174/092986708785909067. [DOI] [PubMed] [Google Scholar]
- Yibchok-anun S, Jittaprasatsin W, Somtir D, Bunlunara W, Adisakwattana S. Insulin secreting and a-glucosidase inhibitory activity of Coscinium fenestratum and postprandial hyperglycemia in normal and diabetic rats. J Med Plants Res. 2009;13(9):646–651. [Google Scholar]
- Bijvoet A, Pieper F, Vliet M, Boer H, Ploeg A, Verbeet M, Reuser A. Recombinant human acid α-glucosidase: high level production in mouse milk, biochemical characteristics, correction of enzyme deficiency in GSDII KO mice. Hum Mol Genet. 1998;13:1815–1824. doi: 10.1093/hmg/7.11.1815. [DOI] [PubMed] [Google Scholar]
- Frei B. Natural antioxidants in human health and disease. San Diego: Academic Press; 1994. [Google Scholar]
- Jagetia G, Baliga M, Babu K. The Evaluation of Nitric Oxide Scavenging Activity of Certain Herbal Formulations in vitro: A Preliminary Study. Phytother Res. 2004;13:561–565. doi: 10.1002/ptr.1494. [DOI] [PubMed] [Google Scholar]
- Tan W, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;13:27–43. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;13:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Ong ES, Woo SO, Yong YL. Pressurized liquid extraction of berberine and aristolochic acids in medicinal plants. J Chromatogr A. 2000;13:57–64. doi: 10.1016/S0021-9673(00)00914-6. [DOI] [PubMed] [Google Scholar]
- Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi J, Kim JK, Bae YS, Won MH. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008;13(11):1527–32. doi: 10.1002/ptr.2527. [DOI] [PubMed] [Google Scholar]
- Yoo KY, Hwang IK, Lim BO, Kang TC, Kim DW, Kim SM, Lee HY, Kim JD, Won MH. Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol Pharm Bull. 2006;13(4):623–628. doi: 10.1248/bpb.29.623. [DOI] [PubMed] [Google Scholar]
- Cho BJ, Im EK, Kwon JH, Lee KH, Shin HJ, Oh J, Kang SM, Chung JH, Jang Y. Berberine inhibits the production of lysophosphatidylcholine-induced reactive oxygen species and the ERK1/2 pathway in vascular smooth muscle cells. Mol Cells. 2005;13(3):429–434. [PubMed] [Google Scholar]
- Musumeci R, Speciale A, Costanzo R, Annino A, Ragusa S, Rapisarda A, Pappalardo MS, Iauk L. Berberis aetnensis C. Presl. extracts: antimicrobial properties and interaction with ciprofloxacin. Int J Antimicrob Agents. 2003;13(1):48–53. doi: 10.1016/S0924-8579(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Chung JG, Chen GW, Hung CF, Lee JH, Ho CC, Ho HC, Chang HL, Lin WC, Lin JG. Effects of berberine on arylamine N-acetyltransferase activity and 2-aminofluorene-DNA adduct formation in human leukemia cells. Am J Chin Med. 2000;13(2):227–238. doi: 10.1142/S0192415X00000271. [DOI] [PubMed] [Google Scholar]
- Ivanovska N, Philipov S, Hristova M. Influence of berberine on T-cell mediated immunity. Immunopharmacol Immunotoxicol. 1999;13(4):771–86. doi: 10.3109/08923979909007141. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;13(2):227–33. doi: 10.1016/S0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- Mahata S, Bharti AC, Shukla S, Tyagi A, Husain SA, Das BC. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol Cancer. 2011;13:39. doi: 10.1186/1476-4598-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park SY, Shin I, Han W, Noh DY. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine. 2010;13(6):436–740. doi: 10.1016/j.phymed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Wang GY, Lv QH, Dong Q, Xu RZ, Dong QH. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J Asian Nat Prod Res. 2009;13(3):219–28. doi: 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- Ho YT, Yang JS, Lu CC, Chiang JH, Li TC, Lin JJ, Lai KC, Liao CL, Lin JG, Chung JG. Berberine inhibits human tongue squamous carcinoma cancer tumor growth in a murine xenograft model. Phytomedicine. 2009;13(9):887–890. doi: 10.1016/j.phymed.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN, Chu SC. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;13(25):10437–10445. doi: 10.1021/jf071868c. [DOI] [PubMed] [Google Scholar]
- Lin SS, Chung JG, Lin JP, Chuang JY, Chang WC, Wu JY, Tyan YS. Berberine inhibits arylamine N-acetyltransferase activity and gene expression in mouse leukemia L 1210 cells. Phytomedicine. 2005;13(5):351–358. doi: 10.1016/j.phymed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition of activator protein 1 activity by berberine in human hepatoma cells. Planta Med. 1999;13(4):381–387. doi: 10.1055/s-2006-960795. [DOI] [PubMed] [Google Scholar]
- Jenny M, Wondrak A, Zvetkova E, Tram N, Phiz P5, Schennach H, Culig Z, Ueberall F, Fuchs D. Crinum Latifolium Leave Extracts Suppress Immune Activation Cascades in Peripheral Blood Mononuclear Cells and Proliferation of Prostate Tumor Cells. Sci Pharm. 2011;13:323–335. doi: 10.3797/scipharm.1011-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotakis G, Timbrell A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;13(2):171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kim T, Kang B, Cho D, Kim S. Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4+ T cells from a Th2 to a Th1 response. Immunology. 2003;13(3):407–414. doi: 10.1046/j.1365-2567.2003.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny T, Keen C, Schmitz H, Gershwin M. Immune Effects of Cocoa Procyanidin Oligomers on Peripheral Blood Mononuclear Cells. Exp Biol Med. 2007;13:293–300. [PubMed] [Google Scholar]
- Kashyap R, Husain A, Morey S, Panchbhai M, Deshpande P, Purohit H, Taori G, Daginawala H. Assessment of immune response to repeat stimulation with BCG vaccine using in vitro PBMC model. J Immune Based Ther Vaccin. 2010;13:3. doi: 10.1186/1476-8518-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://plants.usda.gov/core/profile?symbol=bevu.
- http://clarysageherbarium.com/herbs-etc./bulk-herbs/b.


