Abstract
Cilia are present across most eukaryotic phyla and have diverse sensory and motility roles in animal physiology, cell signalling and development. Their biogenesis and maintenance depend on vesicular and intraciliary (intraflagellar) trafficking pathways that share conserved structural and functional modules. The functional units of the interconnected pathways, which include proteins involved in membrane coating as well as small GTPases and their accessory factors, were first experimentally associated with canonical vesicular trafficking. These components are, however, ancient, having been co-opted by the ancestral eukaryote to establish the ciliary organelle, and their study can inform us about ciliary biology in higher organisms.
The cellular innovations that differentiated the last eukaryotic common ancestor (LECA) from prokaryotes were key to the diversification of life, as exemplified by the emergence of metazoans. Thomas Cavalier-Smith argues for approximately 60 key innovations linked to eukaryogenesis1. It may not surprise an enlightened cell biologist that half are directly related to endomembranes, as well as the cytoskeleton and associated transport machinery. Quite fittingly, centrioles and cilia are also among these innovations.
Centrioles act as microtubule organizing centres (MTOCs) for cell organization and division, and are the foundation (when matured into a basal body) from which cilia are built2-4. The cilium is an organelle with a microtubule-based axoneme which is conserved in most extant protists, and is present in most vertebrate cell types5. Motile cilia (also known as flagella) provide motility to cells and gametes, or propel fluids across cell surfaces6. The ancestral cilium was not only capable of movement but probably also possessed sensory properties still in use by motile cilia7. Indeed, the intrinsic ability of cilia to act as cellular antennae would eventually be exploited fully in metazoans, where many cell types evolved to have immotile (primary) cilia8,9. Loss of motility facilitated the diversification of ciliary structures and functions. Primary cilia such as those found in the brain or olfactory epithelium are typically rod or whip shaped, but other specialized cilia, for example found in vertebrate rod and cone photoreceptors, have elaborate distal ciliary segments10,11. The functional plasticity of primary cilia as sensory organelles has been further harnessed in metazoans and vertebrates, to modulate multiple signalling pathways (including those of Hedgehog, Wnt and receptor tyrosine kinases), and play essential roles in development8,9,12,13.
Cilia are therefore relevant to understanding eukaryotic cell homeostasis, tissue physiology and development, and an ever-expanding number of human disorders classified as ciliopathies9,14,15. Here, we discuss how the biogenesis, function and maintenance of cilia depend on shared functional modules and several overlapping proteins that operate in vesicular and intraciliary trafficking pathways.
Vesicular and intraflagellar trafficking pathways co-established in the ancestral eukaryote
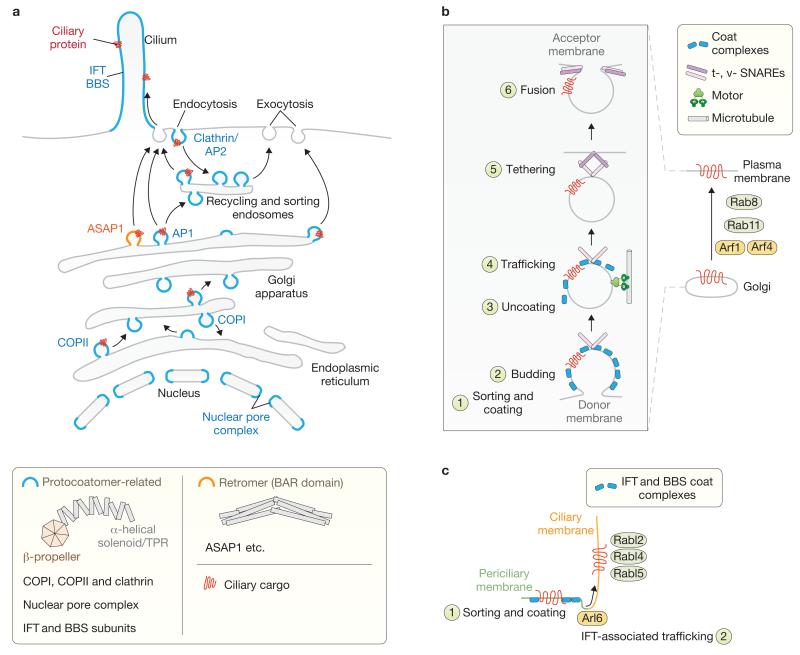
Comparative genomic and phylogenetic analyses of endomembrane-associated and vesicular trafficking constituents of extant eukaryotes reveal that the proteins were essentially all present in the ancestral eukaryote16,17. These include the COPI and COPII coatomers and clathrin and adaptin complexes, which coat vesicles and employ various small GTPases to regulate trafficking between the endoplasmic reticulum (ER), Golgi and plasma membrane (Fig. 1). One conserved structural module found in membrane-coating protein complexes consists of coupled β-propeller and solenoid-repeat domains (Fig. 1a). This domain combination is unique to eukaryotes, implying it arose as a true evolutionary innovation; the exceptional presence of topologically similar proteins in bacteria exhibiting endomembranes is likely to represent a fascinating example of convergent evolution18. Nucleoporin complexes modulate membrane curvature at nuclear pores and also harbour β-propeller and α-helical domains19. Interestingly, these domains occur within single or separate polypeptides, the latter offering a possible evolutionary stepping-stone to the origin of the integrated β-propeller–solenoid membrane-associated module.
Figure 1.
Functional modules used in membrane trafficking and shaping already established in the last eukaryotic common ancestor (LECA). (a) Membrane-coating modules in the eukaryotic cell implicated in vesicular trafficking, intraflagellar transport (IFT) and nuclear pore complex (NPC) formation. Two major classes of membrane-coating or deforming systems are represented: the protocoatomer-related and the retromer (BAR-domain-containing) complexes. Notably, COPI, COPII and clathrin coats as well as NPC and IFT (BBS) subunits share an evolutionarily conserved β-propeller–solenoid/TPR structural architecture formed from one or two polypeptides. A few key vesicular and intraciliary trafficking components are noted. (b) General mechanisms of vesicular trafficking and regulation by small GTPases. Right: a host of small GTPases (for example, Rab8, Rab11, Arf1 and Arf4) mobilize membrane-associated cargo from the Golgi to the plasma membrane. Left: a more detailed schematic of membrane trafficking. The sorting of cargo and coating of vesicles (1) and budding (2) steps are shown before uncoating and motor-dependent trafficking events (3,4) that ultimately lead to the tethering of the vesicle via t- and v-SNAREs and fusion with the acceptor membrane (5,6). Not shown are various adaptors and tethers, and involvement of the exocyst complex in the final stage of trafficking. (c) Non-vesicular-based mechanism for the trafficking of a protein from the periciliary membrane into the ciliary compartment. Sorting and coating by BBS coat complexes (1) uses the small GTPase Arl6 (BBS3) and is coordinated with the IFT trafficking machinery to move proteins into the cilium (2), with the assistance of Rab-like (Rabl) small GTPases. Sorting and coating by IFT proteins probably occurs in parallel.
The genesis of a cilium in the proto-eukaryote necessitated a dedicated cargo-trafficking pathway, termed intraflagellar transport (IFT), that builds and maintains the microtubule axoneme (Box 1)4,20-23. The same β-propeller and solenoid (tetratricopeptide repeats, TPR) modules were co-opted in several IFT machinery subunits24,25 (Fig. 2). Moreover, several proteins encoded by genes mutated in Bardet-Biedl syndrome (BBS, forming the BBSome) individually harbour β-propeller or TPR domains and are likely to have co-evolved with IFT proteins to augment the versatility and specificity of ciliary cargo transport10,26-29 (Box 2).
BOX 1. The evolutionarily conserved core IFT machinery.
The core IFT machinery (Fig. 2b) is highly conserved in ciliated organisms. Detailed phylogenetic analyses of kinesin and dynein families132,133 suggest that the LECA had a complete molecular motor toolset for mobilizing vesicles in the cytosol, moving IFT particles in the cilium and providing ciliary motility via axonemal dyneins. IFT motor-associated proteins, biochemically and genetically separable into two major complexes (IFT-A and IFT-B, with at least 6 and 14 subunits, respectively)4,23, also existed in the ancestral eukaryote. Yet, an awareness of IFT-associated component losses in specific organisms could provide useful insights into how cilia are built and function. The major IFT kinesin motors, heterotrimeric kinesin-2 and OSM-3 (Kif17), are present in all ciliated species — but, intriguingly, are absent from Plasmodium falciparum, which, like Drosophila spermatozoa, builds cilia intracellularly in an IFT-independent manner134. Similarly, the main IFT dynein motor (DYNC2H1) is present in nearly all organisms that build cilia. It is, however, absent from P. falciparum and missing in some ciliated organisms (for example, the apicomplexan Toxoplasma gondii and diatom Thalassiosira pseudonana) that nevertheless possess kinesin-2 and other IFT components. These observations raise intriguing questions regarding how functional cilia can be built without IFT, or maintained without retrograde IFT (ref. 133).
Although it is likely that most core IFT components have now been identified, other central or peripheral IFT-associated proteins may still remain to be uncovered. Recently, Tubby-like protein 3 (TULP3), a negative regulator of Hedgehog signalling, was shown to function within IFT-A in GPCR trafficking; interestingly, however, its disruption does not cause the anticipated retrograde IFT defects associated with IFT-A (ref. 135). Such IFT-associated proteins might represent ‘adaptors’ that enhance IFT selectivity. Likewise, IFT25 and IFT27 are ostensibly specific for Hedgehog signalling41,44,45, and thus missing from Drosophila and C. elegans, which lack ciliary Hedgehog signalling. Furthermore, the BBSome (Box 2) and other motors (including Kif28 (KLP-6) and the paralogues Kif7 and Kif27), or kinesin–dynein motor combinations, enhance the selectivity of ciliary transport in different cell types or conditions10,90,136.
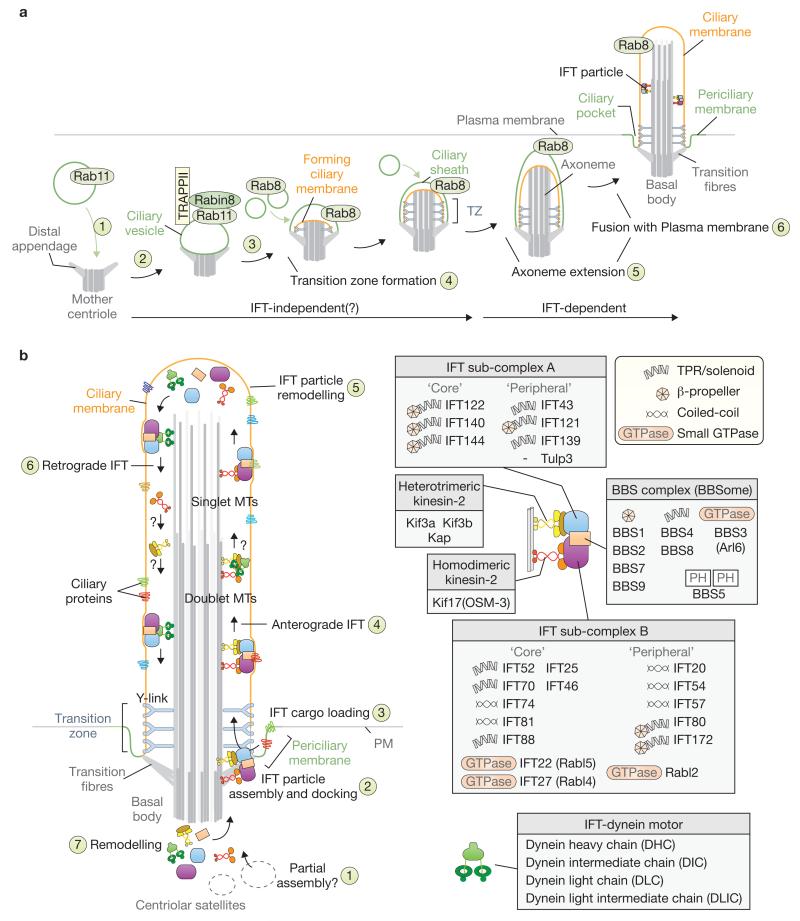
Many aspects of IFT remain equivocal. For instance, why IFT-A proteins are commonly associated with retrograde IFT in diverse organisms, whereas IFT-B components seem generally essential for anterograde trafficking10,21-23, are pressing questions. Moreover, how the IFT-A, IFT-B and BBSome modules remodel the IFT machinery (assembly and disassembly at ciliary tip and base) to engage a molecular switch from anterograde transport to retrograde transport10,21-23 is also largely unexplored. Finally, several IFT-associated small GTPases (Rabl2, Rabl4 and Rabl5) may have membrane-cargo regulatory roles (Fig. 2b), but how these function compared to their vesicle trafficking Rab counterparts remains to be investigated.
Figure 2.
Ciliary-vesicle-dependent steps of ciliogenesis, and modular organization and mechanism of the IFT machinery. (a) A pathway for ciliogenesis involves a ciliary vesicle and the small GTPases Rab8 and Rab11. The mother centriole uses distal appendages (which mature into transition fibres) to interact with a Rab11-associated ciliary vesicle (1). Rabin8 and coat protein small GTPase tethering complexes (including TRAPPII) are recruited to the ciliary vesicle (2). Rab8 is then recruited and seems to mark a Rab11-to-Rab8 switch, although this event is not clearly defined spatiotemporally (3). A transition zone (TZ) emerges (4), from which axoneme extension occurs (5), either before or following fusion of the invaginated ciliary vesicle with the plasma membrane (6). Both the transition fibres on the basal body and mature TZ help to seal the ciliary compartment, which is identifiable by the presence of Rab8. (b) Model for IFT-mediated transport. A complete anterograde IFT particle (including kinesin motor(s), IFT sub-complexes A and B, and the BBSome) assembles near or at the basal body transition fibres in association with the periciliary membrane, from components trafficked to the base of the cilium and centriolar satellites (1,2). Ciliary cargo is loaded onto IFT particles (3) and transported to the tip of the cilium (4) using one or more anterograde motors. Heterotrimeric kinesin-2 (Kin-II) is normally required for this motility, although additional kinesins may be used, including Kif17 (OSM-3). Remodelling at the tip (5) prepares the IFT machinery for retrograde transport (6), and at the base completes the IFT cycle (7). How the dynein and kinesin machineries move to the tip and back, respectively, remains unclear (shown as question marks). Cilia possess a proximal axoneme composed of doublet microtubules (MTs), and often have a distal axoneme with singlet MTs. In many C. elegans cilia, Kin-II and OSM-3 operate coordinately along doublet MTs, whereas OSM-3 acts alone in the distal segment. On the right, the composition and organization of the IFT machinery are depicted together with their various structural and functional domains (including β-propeller and TPR/solenoid motifs, small GTPases, coiled-coils and pleckstrin homology (PH) phosphatidylinositol lipid-binding module).
BOX 2. The IFT-associated BBS protein module.
So far, 17 different genes are linked to BBS, a ciliopathy characterized by obesity, blindness, cystic kidney disease and other clinical manifestations137,138. BBS proteins were first shown to be associated with IFT in C. elegans26,52, a finding subsequently confirmed in Chlamydomonas29. The seven most conserved BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8 and BBS9) can be isolated as a complex (the BBSome) from mammalian cells, and are recruited by the small GTPase BBS3 (Arl6) to form a coat on liposomes28,36. The latter finding is consistent with the assembly of β-propeller and TPR-domain-containing BBS subunits into COPI–COPII–clathrin-like coat complexes (Figs 1c and 2b). Additional BBS proteins (BBS6, BBS10 and BBS12), which assemble with the actin–tubulin-folding chaperonin CCT, evolved in higher metazoans to assist BBSome assembly139. A missense mutation in one BBS patient has also been found in the muscular-dystrophy-associated ubiquitin ligase TRIM32 (BBS11)140. Intriguingly, several BBS-associated proteins localize to the transition zone (known as the ciliary gate), namely BBS13 (MKS1), BBS14 (CEP290 or NPHP6), BBS16 (SDCCAG8 or NPHP10)141,142 and potentially the planar cell polarity protein Fritz (BBS15)143. Given their localization, a potential functional association between the ciliary gate and BBSome requires further exploration. Another BBSome-interacting protein, BBIP1 (also known as BBIP10 or BBS18), promotes ciliogenesis and regulates microtubule stability through tubulin acetylation, properties not seemingly common to other BBSome subunits144. Hence, there seems to be one core IFT module containing BBSome proteins (including Arl6 and potentially BBIP1) present since the dawn of eukaryotes, and other BBS proteins that perform regulatory functions.
Work in Chlamydomonas suggests that the BBSome is substoichiometric relative to IFT particles and does not influence IFT particle function or stability29. However, disruption of C. elegans BBS proteins destabilizes the entire BBSome complex, affects the cohesion between IFT-A and IFT-B sub-complexes, and causes retrograde defects20,26,83,137,145. How β-propeller and solenoid-domain-containing BBS proteins are organized with respect to the core IFT machinery and motors, influence IFT-A and IFT-B interactions, and act in IFT retrograde transport, remains unclear. A recent study implicates BBS proteins in IFT-A and IFT-B remodelling at the ciliary base and tip146, a role perhaps critical in metazoan cilia that use two anterograde kinesins in concert for IFT particle transport (Fig. 2b).
In any case, BBSome disruption leads to relatively subtle IFT and ciliary defects compared to abrogating core IFT components, consistent with the notion that BBS proteins are not generally essential for cilium formation in protists or metazoans10,26,29,36,144. Instead, the BBSome is likely to be a specialized transport adaptor for various signalling molecules, including GPCRs (Sstr3, Mchr1 and D1). LZTFL1 (BBS17), a mammalian BBSome-interacting protein, was found to regulate ciliary trafficking of BBSome components and of smoothened, a Hedgehog signalling protein147,148. Although LZTFL1 is absent from insects and nematodes, which lack cilium-dependent Hedgehog signalling, it is conserved in diverse ciliated protists (including Chlamydomonas and Trypanosoma) that have BBS proteins but lack this signalling pathway. Hence, LZTFL1 probably modulates BBS-dependent trafficking of other, as-yet unidentified, ciliary cargo. It will be interesting to investigate whether the ancestral BBS function may have been the ciliary transport of the first signal transduction system adopted by the emergent ciliary apparatus149, such as the cyclic nucleotide signalling machinery. Indeed, a possible link between BBS proteins and cGMP signalling recently emerged150.
As eukaryotes diversified, especially in metazoan lineages, gene duplication increased the complexity of membrane-trafficking regulatory components30. In particular, the Rab GTPase family has expanded the most to regulate tethering between membranes, or between membranes and the cytoskeleton or motors, both in vesicular and intraflagellar trafficking31,32.
Monosiga brevicollis, the unicellular organism closest to metazoans, encodes 25 Rabs, whereas Caenorhabditis elegans, Drosophila melanogaster and humans have 33, 54 and 115, respectively33. So far, 44 Rab sub-families are recognized in mammals, 16 of which probably originated in the LECA (ref. 33). Of these ancient sub-families, 7 are conserved across eukaryotes: Rab1, 2, 5, 6, 7, 8 and 11. Notably, Rab8 and Rab11 boast a large repertoire of vesicular trafficking functions34, and participate in ciliary assembly, with Rab8 also localizing within cilia35-38. Three LECA-associated Rab-like proteins, Rabl2, Rabl4 and Rabl5, singularly lack a membrane-targeting prenylation site and directly associate with IFT particles39-43. The crystal structure of Rabl4 (also known as IFT27), was recently shown to resemble that of Rab8 and Rab11 (ref. 44). It functions together with IFT25 — not for ciliogenesis, but rather to regulate the transport of Hedgehog signal transduction proteins in vertebrate cilia45. Intriguingly, it modulates the cell cycle in Chlamydomonas40. Rabl5 is required for cilium formation in Trypanosoma42 but not in C. elegans, where it modulates insulin signalling39. Rabl2 seems to be restricted to motile cilia, and in mammalian sperm is required for motility and fertility43. Another Rab protein traceable to the LECA, Rab23, localizes to cilia in Trypanosoma brucei as well as in mammalian cells, where it regulates the ciliary localization of Hedgehog effectors46-49. Aside from Rab8 and Rab23, mammalian Rab17 was the only other Rab uncovered in a screen for ciliogenesis50, although subtle roles for other Rabs in cilium formation, maintenance or function may have been missed.
In conjunction with Rab GTPases, several members of the ADP-ribosylation factor (Arf) and Arf-like (Arl) family of small GTPases, including Arf4, Arl3, Arl6 and Arl13b, also have varied cilium-associated functions31,32,51 (Figs 1b,c, 2b and 3). Arf4 acts in trafficking to the ciliary base, Arl3 and Arl13b are ciliary proteins with links to IFT, and Arl6 (also known as BBS3) enables the BBSome to form coats trafficked at the periciliary membrane and within cilia by IFT (refs 28,52,53). At least three of the above-mentioned small GTPases (Arl3, Arl6 and Arl13b) originated in the LECA. Other members of the Arf–Arl family (over 20 in humans and 11 in C. elegans) might have yet undiscovered ciliary functions.
Figure 3.
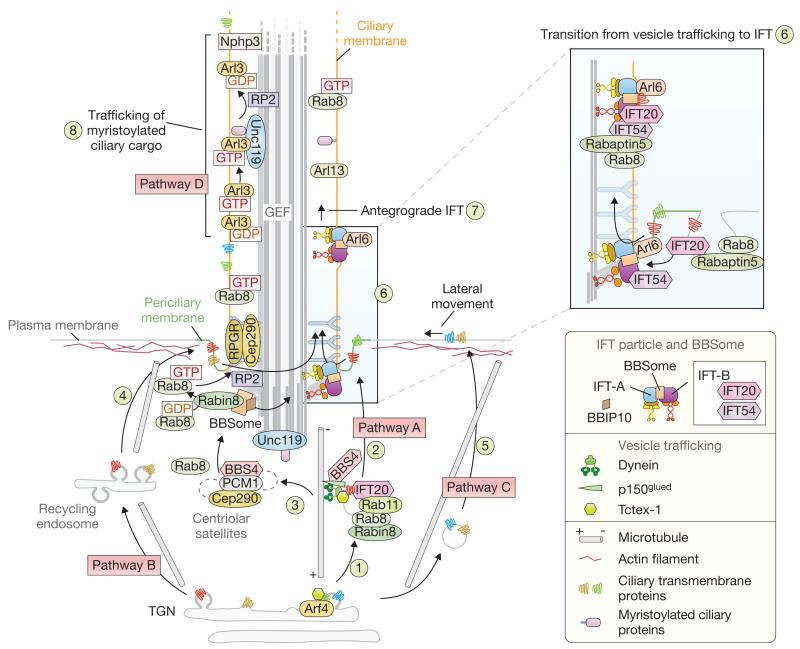
Model depicting ciliary cargo trafficking pathways. Cilium-bound, membrane-associated proteins are initially sorted at the trans-Golgi network (TGN) before being delivered to the subapical (pericentriolar) region. There is evidence for at least four possible transport pathways. Pathway A involves direct targeting to the periciliary membrane. At the TGN, Arf4 regulates the budding of vesicles containing membrane-associated ciliary proteins, and Tctex-1 (Dynlt1) binds to a ciliary targeting sequence on the cytoplasmic domains of a cargo protein (for example, rhodopsin) to promote dynein motor coupling (1). Additional regulatory components, including Rab8, Rab11, Asap1 and FIP3 (not shown), and at least one IFT protein (IFT20), may also ride on the pericentriolarly directed transport vesicles (2). Centriolar satellites associated with the basal body (3) may act as a way station for ciliary trafficking components (for example, BBS4) and ciliary proteins (transition-zone-associated RPGR and Cep290, which are required for Rab8 trafficking to cilia). The recycling endosome (4), which is important for basal body migration and early ciliogenesis, may represent an intermediary trafficking step in pathway B. Some ciliary-bound proteins may use a third route (pathway C) and be delivered to the apical plasma membrane before lateral movement into the periciliary membrane area (5). The switch from vesicular trafficking to intraflagellar trafficking may involve direct interactions between IFT54 (also known as MIP-T3, Elipsa and DYF-11) and IFT20 (both part of IFT sub-complex B), as well as the Rab8-binding protein Rabaptin5 (6, inset). IFT-associated cargoes are then moved into the cilium by anterograde transport (7). Finally, a fourth potentially discrete mechanism (pathway D) employing Unc119, Arl3 and RP2 ensures the trafficking of myristoylated cargo (for example, Nphp3 and G proteins) into the cilium (8).
Different regulators evolved to fine-tune the spatiotemporal activities of Rab, Arf and Arl GTPases, including GTP exchange factors (GEFs) and GTPase activating proteins (GAPs). For example, the Rab8 GEF Rabin8 interacts with proteins required for ciliary trafficking and ciliogenesis, including the BBSome subunit BBS1 and basal body protein Cep164 (refs 36,54). Rabaptin5, a Rab4 and Rab5 effector, interacts with Rab8 and is required for ciliogenesis in zebrafish37 (Fig. 3). This suggests that the LECA-associated Rab4 and Rab5 proteins may also have ciliary functions. Interestingly, the endocytic Rab5 protein is not required for ciliogenesis per se but balances exocytosis at the periciliary membrane to maintain ciliary membrane homeostasis55. Probing the phylogenetic distribution of GTPase regulators may illuminate possible ciliary roles; for instance, the Arl3 GAP termed retinitis pigmentosa 2 (RP2), which supports G protein trafficking to cilia56,57 (Fig. 3), stems from the LECA.
IFT therefore seems to be an extension of a vesicular trafficking pathway that not only shares structural-functional modules, but also effectors and molecular mechanisms. The central elements of the two interconnected trafficking routes were present in the ancestral eukaryote, and expansion of family members probably refined the pathways in organisms and cell types bearing functionally distinct cilia. Comparative genomics aimed at identifying evolutionarily conserved genes associated uniquely with ciliated organisms could help uncover novel ciliary trafficking components25,58. For example, although not directly linked to trafficking, the DnaJ-domain-containing small GTPase RJL displays a phylogenetic profile strongly suggestive of being motile-cilium-associated59. Indeed, the Trypanosoma orthologue localizes near the ciliary base60.
A vesicular-trafficking-associated pathway guides the early steps of ciliogenesis
Ciliogenesis is generally described as centrosome maturation to a basal body that moves to and docks with the cell membrane, followed by axoneme extension61 (Fig. 2a). But how did this pathway emerge? A cilium-anchoring basal body is likely to have evolved from a primitive MTOC used during cell division2,5. MTOC-directed vesicular trafficking of signalling proteins to a membrane patch could have inaugurated a ciliary precursor capable of more efficient signal transduction5,24. Such an evolutionary intermediate has long been lost and is hypothetical, although a remarkably similar cellular arrangement evolved independently to create the immune synapse, the site of signalling between non-ciliated T cells and antigen-presenting cells62.
In various vertebrate and mammalian cell types, the basal body, joined to a so-called ciliary vesicle at the centriole distal end, migrates from its perinuclear position to the membrane before complete axoneme elongation63. Consistent with the use of a membrane trafficking pathway, recent live-imaging studies in RPE-1 cells suggest a dynamic targeting of the Rab8 GEF (Rabin8) to Rab11-positive recycling endosome (post-Golgi) vesicles by the TRAPII vesicle-tethering complex to the centriole distal end38. Rab8 is then recruited to the putative ciliary vesicle and defines the emergent ciliary membrane during axoneme elongation (Fig. 2a). A potentially key regulator in this process is Rabaptin5, which binds Rab8 and two IFT proteins, IFT20 and MIP-T3 (also termed DYF-11 and Elipsa), at the basal body37. The Rabaptin5–Rab8–IFT functional coupling may help IFT particle assembly at the site of ciliogenesis, facilitating the transition between vesicular and intraciliary trafficking (Fig. 3).
Remarkably, immune synapse formation also depends on IFT20 and Rab11, as well as on Unc119, a protein recently implicated together with Arl3 and RP2 in ciliary trafficking56,62,64 (Fig. 3). Assembling the immune synapse involves remodelling the actin cytoskeleton around an MTOC that moves to the membrane, along with polarization of the Golgi and recycling endosome to direct secretion and endocytosis65. Notably, actin is also implicated in basal body migration66 and, as discussed below, the recycling endosome may represent an important junction in ciliary trafficking. The parallels between cilium and immune synapse biogenesis and function are therefore striking62.
We pondered that basal-body–ciliary-vesicle trafficking was established in the LECA because of the mutually exclusive functions of the ‘cytosolic’ and ‘membrane-associated’ MTOC in cell division and cilium formation, respectively. However, unlike in metazoans cells, which almost invariably shed their cilium to liberate centrioles for cell division, most protists undergo cytokinesis with their basal bodies engaged with the ciliary apparatus. Where ciliary resorption occurs before cell division, as in Chlamydomonas reinhardtii, centrioles remain closely juxtaposed to the plasma membrane and are unlikely to require a ciliary vesicle for ciliogenesis67. Intriguingly, however, the basal-body–ciliary-vesicle migration observed in vertebrate cells (including photoreceptors68) occurs during ciliated gamete formation in the multicellular fungus Allomyces arbusculus69. This suggests that the basal-body–ciliary-vesicle pathway is ancient, with roots in opisthokonts (the lineage that includes fungi and metazoans). Evolution of a ‘cytoplasmic’ centrosome probably facilitated the control of asymmetric cell divisons, cell polarity and cilium formation — all key for the genesis of metazoans2.
Basal-body–ciliary-vesicle trafficking is therefore relevant to primary cilium formation, and defects in this pathway are linked to disease70. The pathway also beckons us to investigate how centrioles migrate, whether along the membrane or from a more central cellular position; how the basal body distal end interacts with the ciliary vesicle or plasma membrane; and lastly, how the site of cilium outgrowth is established. Investigating the role of proteins found at distal appendages — which mature into basal-body-anchoring transition fibres — will help answer these questions.
Several players have emerged as being important. Cep164 is localized to distal appendages and is essential for their formation, as well as interaction with the ciliary vesicle and Rab8 effector, Rabin8 (ref. 54). ODF2, also found at appendages, is required for their formation and ciliogenesis, and directly interacts with Rab8 and Rab11 (refs 50,71). Four novel distal appendage proteins necessary for ciliogenesis were recently uncovered — Cep89, Cep83 (CCDC41), SCLT1 and FBF1 — one of which, Cep83, enables interactions between the centriole and the ciliary vesicle and membrane72.
Docking and assembly of IFT particles in association with transition fibres and periciliary membrane is conserved in Chlamydomonas, C. elegans and vertebrate cells73-75 (Fig. 2b). There, OFD1 (oral-facial-digital syndrome 1) helps recruit IFT88 and may provide an assembly site for IFT particles76. Another player, Ttbk2 (tau tubulin kinase 2), regulates the removal of the centrosomal protein CP110, which caps the mother centriole, and also helps recruit IFT proteins — presumably key steps in initiating axoneme extension72,77 (Fig. 2a). Ttbk2, a microtubule plus-end tracking protein, and another protein sharing this activity, Cep104, can be isolated with CP110, highlighting a likely coordination between instigating axoneme extension and microtubule growth78.
Hence, distal appendages functionally interact not only with the ciliary vesicle and membrane trafficking machinery but also IFT components to help create the ciliary compartment. A key challenge will be to understand how proteins associated with the appendages, ciliary vesicle and emerging axoneme (including the transition zone, or ‘ciliary gate’61,75,79; Fig. 2a) are coupled to ‘canonical’ downstream vesicle transport and exocytosis players — for example, actin cytoskeleton, myosin V, exocyst complex and Cdc42 (refs 66,80) — to assist in basal body migration and membrane docking and fusion of the ciliary vesicle at the intended site of cilium outgrowth.
The intraflagellar transport pathway
Once the basal body and forming transition zone associates with the plasma membrane, either directly or via a ciliary vesicle, the axoneme elongates61,63,75 (Fig. 2a). The discovery of IFT by the Rosenbaum lab81 ushered in a new era of understanding how the axoneme is assembled and maintained, and how ciliary protein composition can be modulated dynamically. The core of the IFT machinery consists of kinesin-2 and cytoplasmic dynein motors that alternate between anterograde and retrograde motility, respectively, and the two multi-protein sub-complexes IFT-A and IFT-B (refs 20-23,82). These components are widely conserved across ciliated eukaryotes, having arisen in the LECA (Box 1). Another IFT module, the BBSome (refs 29,36,83) (Box 2), is not universally conserved in ciliated organisms, but is present in most eukaryotic clades36 and is therefore also primeval. Key structural and mechanistic features of the IFT machinery are presented in Fig. 2b and Boxes 1 and 2.
There are ostensibly two primary purposes to IFT. The first is to build and maintain ciliary axonemes by trafficking structural components such as tubulin and, if necessary, motile cilium-specific machinery. The second is mobilizing sensory and signalling components in and out of the cilium. Hao and colleagues84 recently provided evidence that IFT transports tubulin building blocks, by following GFP-tagged C. elegans α- and β-tubulins (TBA-5 and TBB-4) with time-lapse microscopy and kymograph analyses. Fluorescence recovery after photobleaching (FRAP) experiments also revealed that tubulins first concentrate at the ciliary tip (and doublet microtubule ends), corroborating elegant studies demonstrating that newly incorporated tubulin and radial spokes proteins assemble at pre-existing Chlamydomonas ciliary tips85. Studies in Drosophila also indicate a requirement for IFT in tubulin transport, and more specifically, implicates kinesin-2 (KLP64D) and IFT-B (OSEG2, OSM1, IFT172) subunits — both involved in anterograde transport — but not OSEG1 (DAF-10, IFT122), an IFT-A subunit linked to retrograde transport. Evidence for IFT-dependent transport of radial spokes and the outer dynein arm subunit also exists86-88 — thus, core components of both motile and non-motile axonemes may require IFT for their assembly.
Given the close interaction between IFT particles and the ciliary membrane81,89, how the IFT machinery interacts with ‘soluble’ cargoes remains largely unexplored. Binding to IFT sub-complexes A or B, or to kinesin itself, could occur within the ciliary matrix. Indeed, evidence exists that kinesins directly transport dynein machinery in preparation for retrograde transport90. The IFT subunits IFT74 and IFT81 were recently shown to form a specific tubulin-binding module91. Also possible is the peripheral association of soluble components with membrane, as suggested by work of Stephens and colleagues on tubulin92.
Do signalling components depend on IFT-mediated transport? Very few are known to directly bind IFT-associated machinery (for example, IFT20 binds opsins93, and IFT proteins associate with cGMP-dependent protein kinase94), but several depend on IFT for dynamic ciliary localization. A few IFT proteins are implicated in transporting Hedgehog signalling components — Patched (Ptch1), Smoothened (Smo), Sufu, Gli2 and Gli3 (ref. 8). IFT25 and IFT27 may represent specific adaptors, not being required for building cilia and absent from invertebrates devoid of cilium-dependent Hedgehog signalling45. Interestingly, the BBSome also participates in Hedgehog signalling, with BBS1 directly interacting with Ptch1 and Smo proteins95. Furthermore, several G-protein-coupled receptors (GPCRs), including Sstr3, Htr6, Mchr1 and dopamine receptor 1 (D1), utilize a conserved ciliary targeting sequence (CTS) recognized by multiple BBS subunits to modulate dynamic ciliary localization27,28,96,97. Notably, the CTS of Sstr3 is sufficient for ciliary targeting in a BBSome- and Arl6 (BBS3)-dependent fashion28. An intriguing aspect of IFT–BBS-mediated ciliary transport is its apparent bidirectionality; for example, mammalian D1 is removed from cilia in response to environmental cues and agonist binding, potentially by direct binding to BBS5 (ref. 97), and several signal transduction proteins accumulate in a Chlamydomonas BBS mutant29.
But most of the evidence for IFT-dependent ciliary trafficking is indirect. The first visualization of IFT-driven motility for a non-structural ciliary protein, in 2005, was obtained in C. elegans for OSM-9 and OCR-2, the transient receptor potential vanilloid (TRPV) channels associated with multiple sensory functions98. The Chlamydomonas orthologue of a calcium channel implicated in polycystic kidney disease (PKD2) was subsequently shown to require IFT for ciliary motility99. There may be technical difficulties in observing IFT for cargo proteins. Compared to core IFT or BBS proteins, movement may be less robust and/or saltatory, as observed with C. elegans TBA-5 and TBB-4, as well as Chlamydomonas PKD2. Substoichiometic amounts of fluorescently tagged cargo protein on each IFT ‘train’ probably results in a low signal-to-noise ratio. A recent study, using FRAP and shRNA-knockdown of kinesin-II in a heterologous cell line or photoreceptor cell, suggests IFT-dependent movement of opsin — known to occur at a rate of thousands of molecules per minute — but does not unambiguously show it100.
How the IFT machinery transports presumptive cargo, and its similarities or differences with vesicular trafficking, therefore remains largely undetermined. Direct visualization of moving cargo, for example, using single-molecule imaging101, will be important to answer this question. For example, how do IFT particles traffic from the periciliary membrane-transition fibre region across the transition zone, which functions as a ‘ciliary gate’ or membrane diffusion barrier61,80? One possibility is that in addition to interacting with membrane-associated cargo, IFT particles assemble lipid rafts that facilitate bulk-like transport into, and potentially out of, the cilium10. Where is cargo released? Just past the transition zone, or at the ciliary tip, as with structural components? The context of IFT should be informative. Whereas vesicles present a curved membrane surface, the ciliary membrane has much reduced and inversed curvature. Ciliary coating proteins would be likely to adopt a different geometry for membrane interaction. Indeed, the BBSome forms coat complexes on purified liposomes, without deforming the membrane like COPI and COPII (ref. 28). Similar experiments have yet to be reported for core IFT proteins. Structural information on BBS and IFT protein complexes (the latter work being underway82) will help shed light on the relative topology of β-propeller–solenoid scaffolds compared to those used for vesicular trafficking and nuclear pore formation. Such structural analyses will also help to unravel the arrangement of IFT proteins within detailed electron tomography images obtained of IFT ‘trains’89. Further mechanistic questions regarding IFT are outlined in Box 2.
Vesicular and membrane trafficking for ciliary proteins
Coat formation is an ancient conserved mechanism employed for the sorting of cargo proteins to be transported from a donor to an acceptor membrane30 (Fig. 1). In mammalian cells, coat assembly is initiated when inner coat proteins (for example, adaptor protein complexes) recognize specific sorting signals on the cytoplasmic domains of proteins. The YXXΦ motif, for example, enables the sorting of proteins targeted to the basolateral membrane of polarized cells, and internalization of endocytic receptors from the plasma membrane. Subsequent interactions with outer coat components (for example, clathrin and COPs) completes the assembly102.
Coat assembly provides an effective means of concentrating membrane cargoes into patches and inducing local membrane deformation through insertion of amphipathic helices into the lipid bilayer. Arf family small GTPases, including Arf1 and Arf3, sense membrane curvature and regulate the formation and budding of coat–cargo complexes. Vesicles pinched from donor membranes then travel using cytoskeleton-associated molecular motors. When encountering their target membranes, specific tethering and docking protein interactions (often involving Rabs) permit cargo delivery into recipient membranes through SNARE-mediated membrane fusion103. Coats either disassemble after vesicles emanate from the donor membrane, or remain on transport carriers (perhaps partially), and participate in protein–protein, protein–cytoskeleton and/or vesicle-targeting membrane interactions102. Conserved modules and general mechanisms of vesicular trafficking are depicted schematically in Fig. 1.
Intuitively, proteins destined for the cilium must first be sorted at the trans-Golgi network (TGN), and traffic to the basal body or (sub)apical region of polarized cells. Here, additional sorting is likely to occur, to segregate these proteins from those destined to reside in the apical plasma membrane. At least four trafficking routes seem possible before ciliary entry (Fig. 3): direct transport to and fusion with the periciliary membrane (pathway A); use of a recycling endosome as a way station (pathway B); trafficking to the plasma membrane followed by lateral diffusion to the periciliary membrane (pathway C); and finally, a fourth route involving Unc119–RP2–Arl3 is also emerging (pathway D).
The trans-Golgi network as an initial sorting facility
Apical sorting at the TGN does not involve canonical coat formation. Instead, cells seem to utilize various signals, including glycosphingolipids, glycans or protein motifs on the exoplasmic (transmembrane) domains, as apical determinants104. Interestingly, although present in most basolateral membrane proteins, few cytoplasmic sorting sequences have been identified in apical membrane proteins104. In sharp contrast, almost all ciliary targeting signals (CTS) identified so far have been mapped to the cytoplasmic domain96,105-107.
Rhodopsin, which localizes to the ciliary photoreceptor outer segment108, is a prototypal protein carrying cytoplasmic apical sorting signals. A VxPx motif present in its cytoplasmic C-terminus, initially identified as a mutational hotspot for human retinitis pigmentosa, is critical for its targeting to the outer segment109,110. So far, two proteins, Tctex-1 (Dynlt1) and Arf4, are implicated in sorting rhodopsin at the TGN. Both bind directly to its C-terminus in a VxPx-dependent fashion111,112. At the TGN, Arf4, Rab11 and associated proteins (the Arf GAP Asap1 and Rab11-interacting/effector protein FIP3) regulate the budding of rhodopsin-bearing transport carriers112. Tctex-1, a light chain subunit of cytoplasmic dynein, connects rhodopsin-bearing vesicles with the motor, permitting translocation on microtubule tracks to the apical poles111,113 (Fig. 3). A similar VxPx motif sufficient for ciliary targeting is found on the cytoplasmic domains of polycystin-1 and polycystin-2 (PKD2)106,107.
IFT20 also binds the C-terminus of rhodopsin. However, the interaction is VxPx-independent114, suggesting that IFT20 is not part of the key sorting machinery. Instead, it probably serves as an adaptor (together with the IFT protein MIP-T3 (Elipsa) and Rabaptin5–Rab8 (ref. 37)) for recruiting other IFT components before incorporation on to IFT trains (Fig. 3 inset); indeed, the IFT20 interaction with opsin is detected in both the cytoplasm and as part of the IFT particle93. Additional IFT-associated proteins downstream of the TGN may interact with apically directed transport carriers; this includes BBS4, which interacts with the dynactin (dynein activator) subunit p150glued and is required for opsin outer segment localization115,116.
Trafficking directly to the periciliary membrane
A plausible route for ciliary proteins (including rhodopsin) may involve vesicular transport targeted specifically to the periciliary membrane (Fig. 3, pathway A). Various aforementioned players are likely to participate in cargo selection, vesicle budding and trafficking steps, including Arf4, Asap1 (FIP3), Rab8–Rabin8 and Rab11 (ref. 117). Similarly to canonical vesicular trafficking, exocyst, tethering complexes and SNARE fusion machineries probably promote vesicle fusion with the periciliary membrane. A recent study showed that NDR2, involved in a canine ciliopathy (retinal degeneration), may play a role in this latter process by phosphorylating Rabin8 and triggering its switch in association with phosphatidylserine-containing vesicles to the exocyst component Sec15 (ref. 118); whether this is also critical for cilium formation or maintenance remains unclear.
The recycling endosome as a possible second sorting checkpoint
An emerging view argues that many newly synthesized apical surface proteins follow an indirect transport route involving the recycling endosome before reaching their distal destination119. The recycling endosome is a highly dynamic endocytic compartment in which proteins moving toward their target membranes are concentrated and segregated from other components in narrow tubules120. In polarized MDCK (Madin-Darby canine kidney) cells, recycling endosomes localize proximal to the basal body121. Despite their names, recycling endosomes seem to serve as intermediate compartments at the crossroads of several intracellular trafficking pathways, including the TGN-to-apical-surface and apical-surface-to-basolateral-surface routes119. The recycling endosome may provide a sorting intermediate compartment wherein proteins are partitioned between the cilium and apical plasma membrane (Fig. 3, pathway B).
Supporting this notion, vesicles containing cilia-targeted fibrocystin were found in the recycling endosome105. Moreover, several recycling-endosome-localized membrane transport components — including Rab8–Rabin8, Rab11, Rab17 and its GAP TBC1D7, TBC1D30 (a RabGAP), Evi5like (Rab23 GAP), the exocyst complex protein Sec10, and TRAPPII complex — are linked to ciliary assembly35,36,38,50,106,122-126. At the recycling endosome, Rab11–GTP interacts with Rabin8 and stimulates its GEF activity toward Rab8, which probably promotes targeting to the ciliary base35,38,127 (Fig. 3).
Sorting at or near the periciliary membrane
The final destination of cilium-bound cargoes is the periciliary membrane, where entry into the organelle is either IFT-dependent or -independent. Although the periciliary membrane can be reached directly or via the recycling endosome, some proteins (for example, smooth-ened128) delivered to the apical membrane may diffuse laterally to reach this site (Fig. 3, pathway C). Other components targeted to cilia may temporarily be held in centriolar satellites closely associated with the basal body (for example, Rab8, BBS4 and the transition zone protein Cep290) (Fig. 3).
Several lines of evidence suggest that the BBSome is a bona fide component of the sorting machinery required for targeting several GPCRs (including SSTR3) to cilia (Figs 1c and 3 inset; see also Box 2). Most BBSome components are enriched in the basal body and centriolar satellite region, and their binding to the SSTR3 CTS (AX[S/A]XQ) is needed for trafficking the GPCR (refs 27,28). Collectively, the studies on BBS proteins suggest a compelling model for ciliary transport: the BBSome decodes sorting signals of specific cargoes, forms coats using the small GTPase Arl6 (BBS3), and assembles onto IFT trains10,26-29,83. The membrane cargoes are ‘dragged’ across the diffusion barrier through an as-yet unidentified mechanism (Fig. 3, inset). However, the BBSome is unlikely to be the sole sorting machinery accounting for ciliary trafficking. Core IFT components (Box 1) and the Unc119–RP2–Arl3 ciliary transport system (reviewed by in ref. 129) undoubtedly also play key roles, and, together with the BBSome, account for targeting proteins to the ciliary compartment.
A complex ciliary trafficking pathway only starting to yield its secrets
The very existence and essence of the LECA strongly depended on the emergence of vesicle and intraciliary trafficking pathways employing related structural and functional modules. Ultimately, the evolution of metazoans and their complex traits, such as neuron-based olfaction and vision, also benefited from the interplay between the two cellular pathways. An integrated approach to studying cilium-associated trafficking seems necessary: not only components, but also mechanisms underlying vesicular trafficking, cilium assembly and intraflagellar transport should be studied and compared. The conserved nature of the processes means that novel components and mechanisms can even be uncovered in non-ciliated cells. For example, characterizing the formation and operation of the vertebrate proto-cilium-like immune synapse could provide valuable insights relevant to ciliogenesis or cilium function62, as does studying the Saccharomyces cerevisiae orthologues of cilia-associated Rabs (Sec4p (Rab8) and Sec2p (Rabin8)) in vesicular trafficking.
At the same time, different ciliated metazoan cells such as photoreceptors have distinct functions, and defining the nature of the trafficking components and mechanisms that generate their unique functionality is essential. Such research may help uncover the molecular aetiology of ciliopathies. For instance, it is unclear why one particular isoform of Arl6 (BBS3) seems to have a photoreceptor-specific function130.
Finally, manipulating ciliary trafficking, formation and function could be effective in targeting numerous clinical ailments, including PKD and obesity. Heralding such a possibility, a recent study identified small molecules that selectively affect smoothened (a Hedgehog signalling protein) cilia-related trafficking, and these could thus be employed as anti-tumour compounds131.
ACKNOWLEDGEMENTS
The field of cilium trafficking has grown tremendously in the last few years and the authors apologize for not covering all relevant studies due to space restrictions. M.R.L. acknowledges funding from the Canadian Institutes of Health Research (CIHR; grant MOP-123527) and a senior scholar award from Michael Smith Foundation for Health Research (MSFHR). C.H.S. is funded by NIH-EY11307, NIH-EY016805, Research To Prevent Blindness, and Starr Stem Cell Foundation.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Ching-Hwa Sung, Margaret M. Dyson Vision Research Institute, Department of Ophthalmology, Weill Medical College of Cornell University, 1300 York Avenue, New York, New York 10065, USA.
Michel R. Leroux, Department of Molecular Biology and Biochemistry, Simon Fraser University, 8888 University Drive, Burnaby, British Columbia V5A 1S6, Canada. leroux@sfu.ca
References
- 1.Cavalier-Smith T. Predation and eukaryote cell origins: a coevolutionary perspective. Int. J. Biochem. Cell Biol. 2009;41:307–322. doi: 10.1016/j.biocel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- 3.Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella – back to the future! Biol. Cell. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy S. The motile cilium in development and disease: emerging new insights. Bioessays. 2009;31:694–699. doi: 10.1002/bies.200900031. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr. Top. Dev. Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Sung CH, Chuang JZ. The cell biology of vision. J. Cell Biol. 2010;190:953–963. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen ST, Clement CA, Satir P, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J. Pathol. 2012;226:172–184. doi: 10.1002/path.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation. 2012;83:S49–55. doi: 10.1016/j.diff.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med. Genet. C Semin. Med. Genet. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N. Engl. J. Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field MC, Dacks JB. First and last ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr. Opin. Cell Biol. 2009;21:4–13. doi: 10.1016/j.ceb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Field MC, Sali A, Rout MP. Evolution: On a bender — BARs, ESCRTs, COPs, and finally getting your coat. J. Cell Biol. 2011;193:963–972. doi: 10.1083/jcb.201102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santarella-Mellwig R, et al. The compartmentalized bacteria of the planctomycetes–verrucomicrobia–chlamydiae superphylum have membrane coat-like proteins. PLoS Biol. 2010;8:e1000281. doi: 10.1371/journal.pbio.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 20.Blacque OE, Cevik S, Kaplan OI. Intraflagellar transport: from molecular characterisation to mechanism. Front. Biosci. 2008;13:2633–2652. doi: 10.2741/2871. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 22.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J. Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83:S12–22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 25.Avidor-Reiss T, et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 26.Blacque OE, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl Acad. Sci. USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin H, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechtreck KF, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 2007;120:2977–2985. doi: 10.1242/jcs.013250. [DOI] [PubMed] [Google Scholar]
- 31.Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol. Cell. 2011;103:209–221. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Ling K, Hu J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J. Cell Biochem. 2012;113:2201–2207. doi: 10.1002/jcb.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diekmann Y, et al. Thousands of rab GTPases for the cell biologist. PLoS Comput. Biol. 2011;7:e1002217. doi: 10.1371/journal.pcbi.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peranen J. Rab8 GTPase as a regulator of cell shape. Cytoskeleton. 2011;68:527–539. doi: 10.1002/cm.20529. [DOI] [PubMed] [Google Scholar]
- 35.Knodler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 37.Omori Y, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 38.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl Acad. Sci. USA. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schafer JC, et al. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J. Cell Sci. 2006;119:4088–4100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- 40.Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Fan ZC, Williamson SM, Qin H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PLoS ONE. 2009;4:e5384. doi: 10.1371/journal.pone.0005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adhiambo C, Blisnick T, Toutirais G, Delannoy E, Bastin P. A novel function for the atypical small G protein Rab-like 5 in the assembly of the trypanosome flagellum. J. Cell Sci. 2009;122:834–841. doi: 10.1242/jcs.040444. [DOI] [PubMed] [Google Scholar]
- 43.Lo JC, et al. RAB-like 2 has an essential role in male fertility, sperm intra-flagellar transport, and tail assembly. PLoS Genet. 2012;8:e1002969. doi: 10.1371/journal.pgen.1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhogaraju S, Taschner M, Morawetz M, Basquin C, Lorentzen E. Crystal structure of the intraflagellar transport complex 25/27. EMBO J. 2011;30:1907–1918. doi: 10.1038/emboj.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keady BT, et al. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev. Cell. 2012;22:940–951. doi: 10.1016/j.devcel.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boehlke C, et al. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J. Cell Sci. 2010;123:1460–1467. doi: 10.1242/jcs.058883. [DOI] [PubMed] [Google Scholar]
- 47.Lumb JH, Field MC. Rab23 is a flagellar protein in Trypanosoma brucei. BMC Res. Notes. 2011;4:190. doi: 10.1186/1756-0500-4-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev. Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Y, et al. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat. Genet. 2004;36:989–993. doi: 10.1038/ng1414. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J. Cell Biol. 2010;189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt KN, et al. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 2012;199:1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan OI, et al. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr. Biol. 2012;22:451–460. doi: 10.1016/j.cub.2012.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright KJ, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz N, Novoselova TV, Wait R, Hardcastle AJ, Cheetham ME. The X-linked retinitis pigmentosa protein RP2 facilitates G protein traffic. Hum. Mol. Genet. 2012;21:863–873. doi: 10.1093/hmg/ddr520. [DOI] [PubMed] [Google Scholar]
- 58.Li JB, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 59.Elias M, Archibald JM. The RJL family of small GTPases is an ancient eukaryotic invention probably functionally associated with the flagellar apparatus. Gene. 2009;442:63–72. doi: 10.1016/j.gene.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Dos Santos GR, et al. The GTPase TcRjl of the human pathogen Trypanosoma cruzi is involved in the cell growth and differentiation. Biochem. Biophys. Res. Commun. 2012;419:38–42. doi: 10.1016/j.bbrc.2012.01.119. [DOI] [PubMed] [Google Scholar]
- 61.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finetti F, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorska MM, Liang Q, Karim Z, Alam R. Uncoordinated 119 protein controls trafficking of Lck via the Rab11 endosome and is critical for immunological synapse formation. J. Immunol. 2009;183:1675–1684. doi: 10.4049/jimmunol.0900792. [DOI] [PubMed] [Google Scholar]
- 65.Brown AC, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 2011;9:e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawe HR, Farr H, Gull K. Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 2007;120:7–15. doi: 10.1242/jcs.03305. [DOI] [PubMed] [Google Scholar]
- 67.Johnson UG, Porter KR. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J. Cell Biol. 1968;38:403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedmak T, Wolfrum U. Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol. Cell. 2011;10:449–466. doi: 10.1042/BC20110034. [DOI] [PubMed] [Google Scholar]
- 69.Renaud FL, Swift H. The development of basal bodies and flagella in Allomyces arbusculus. J. Cell Biol. 1964;23:339–354. doi: 10.1083/jcb.23.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;9:448. doi: 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S. The centrosome regulates the Rab11-dependent recycling endosome pathway at appendages of the mother centriole. Curr. Biol. 2012;22:1944–1950. doi: 10.1016/j.cub.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanos BE, et al. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27:163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 74.Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J. Cell Biol. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams CL, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goetz SC, Liem KFJ, Anderson KV. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151:847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang K, et al. A Proteome-wide screen for mammalian SxIP motif-containing microtubule plus-end tracking proteins. Curr. Biol. 2012;22:1800–1807. doi: 10.1016/j.cub.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 79.Garcia-Gonzalo FR, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizuno N, Taschner M, Engel BD, Lorentzen E. Structural studies of ciliary components. J. Mol. Biol. 2012;422:163–180. doi: 10.1016/j.jmb.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ou G, et al. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol. Biol. Cell. 2007;18:1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hao L, et al. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 2011;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J. Cell Biol. 1992;119:1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hou Y, et al. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 2008;183:313–322. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pigino G, et al. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao L, Efimenko E, Swoboda P, Scholey JM. The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PLoS ONE. 2011;6:e20995. doi: 10.1371/journal.pone.0020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhogaraju S, et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341:1009–1012. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephens RE. Tubulin and tektin in sea urchin embryonic cilia: pathways of protein incorporation during turnover and regeneration. J. Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- 93.Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol. Biol. Cell. 2011;22:921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum. Mol. Genet. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of Ciliary Localization Sequences within the Third Intracellular Loop of G Protein-Coupled Receptors. Mol. Biol. Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Domire JS, et al. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol. Life Sci. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin H, et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 99.Huang K, et al. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trivedi D, Colin E, Louie CM, Williams DS. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric Kinesin-2. J. Neurosci. 2012;32:10587–10593. doi: 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye F, et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 103.Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 105.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ward HH, et al. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell. 2011;22:3289–3305. doi: 10.1091/mbc.E11-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geng L, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 108.Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J. Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sung CH, Schneider BG, Agarwal N, Papermaster DS, Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl Acad. Sci. USA. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxyterminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 112.Mazelova J, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tai AW, Chuang JZ, Sung CH. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J. Cell Biol. 2001;153:1499–1510. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abd-El-Barr MM, et al. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Int. J. Biochem. Cell Biol. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim JC, et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 117.Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31:4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiba S, Amagai Y, Homma Y, Fukuda M, Mizuno K. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 2013;32:874–885. doi: 10.1038/emboj.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J. Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maxfield FR, McGraw TE. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 121.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prigent M, et al. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–1121. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffmeister H, et al. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J. Cell Biol. 2011;192:631–645. doi: 10.1083/jcb.201007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaplan OI, et al. The AP-1 clathrin adaptor facilitates cilium formation and functions with RAB-8 in C. elegans ciliary membrane transport. J. Cell Sci. 2010;123:3966–3977. doi: 10.1242/jcs.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwarz N, Hardcastle AJ, Cheetham ME. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Res. 2012;75:2–4. doi: 10.1016/j.visres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 130.Pretorius PR, et al. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet. 2010;6:e1000884. doi: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu VM, Chen SC, Arkin MR, Reiter JF. Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis. Proc. Natl. Acad. Sci. USA. 2012;109:13644–13449. doi: 10.1073/pnas.1207170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wickstead B, Gull K. A “holistic” kinesin phylogeny reveals new kinesin families and predicts protein functions. Mol. Biol. Cell. 2006;17:1734–1743. doi: 10.1091/mbc.E05-11-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wickstead B, Gull K. Dyneins across eukaryotes: a comparative genomic analysis. Traffic. 2007;8:1708–1721. doi: 10.1111/j.1600-0854.2007.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Briggs LJ, Davidge JA, Wickstead B, Ginger ML, Gull K. More than one way to build a flagellum: comparative genomics of parasitic protozoa. Curr. Biol. 2004;14:R611–R612. doi: 10.1016/j.cub.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 135.Mukhopadhyay S, et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malicki J. Who drives the ciliary highway? Bioarchitecture. 2012;2:111–117. doi: 10.4161/bioa.21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell. Mol. Life Sci. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J. Clin. Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seo S, et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc. Natl Acad. Sci. USA. 2010;107:1488–1493. doi: 10.1073/pnas.0910268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiang AP, et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proc. Natl Acad. Sci. USA. 2006;103:6287–6292. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leitch CC, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 142.Schaefer E, et al. Mutations in SDCCAG8/NPHP10 cause Bardet-Biedl syndrome and are associated with penetrant renal disease and absent polydactyly. Mol. Syndromol. 2011;1:273–281. doi: 10.1159/000331268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim SK, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loktev AV, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev. Cell. 2008;15:854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 146.Wei Q, et al. The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 2012;14:950–957. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seo S, et al. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marion V, et al. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet-Biedl syndrome with situs inversus and insertional polydactyly. J. Med. Genet. 2012;49:317–321. doi: 10.1136/jmedgenet-2012-100737. [DOI] [PubMed] [Google Scholar]
- 149.Johnson JL, Leroux MR. cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends Cell Biol. 2010;20:435–444. doi: 10.1016/j.tcb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Mok CA, et al. Mutations in a guanylate cyclase GCY-35/GCY-36 modify Bardet-Biedl syndrome-associated phenotypes in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002335. doi: 10.1371/journal.pgen.1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]