Abstract
Secretin, a classical gastrointestinal and neuroendocrine peptide, plays an important role in maintaining the body fluid balance. However, the expression and regulation of secretin in the reproductive system are still unknown. In our study, secretin is specifically expressed in the decidua on days 5 to 8 of pregnancy. Secretin expression is not detected under delayed implantation but is stimulated after estrogen activation and under artificial decidualization. Progesterone induces secretin expression in ovariectomized mice and cultured stromal cells, which is abrogated by specific LY294002. Because secretin is mainly localized in the decidua and also strongly expressed during in vitro decidualization, secretin may play a role during mouse decidualization through regulating cyclic adenosine monophosphate level.
Keywords: uterus, embryo implantation, decidualization, progesterone, secretin
Introduction
Embryo implantation is a mutual interaction between blastocyst and uterus. Successful implantation is dependent on the cellular and molecular dialogue between competent embryos and receptive uterus.1,2 Although many specific factors have been identified and characterized during embryo implantation, the molecular mechanism underlying embryo implantation still remains unknown.
Secretin belongs to the vasoactive intestinal polypeptide (VIP)/pituitary adenylate cyclase–activating polypeptide (PACAP)/glucagon-like peptide family. It is first discovered in gastrointestinal system and has multiple characterized roles in inhibiting gastric acid secretion and empty and stimulating the watery bicarbonate solution secretion from pancreas and bile duct.3 Secretin is also detected in brain regions such as brain-gut peptide, which participates in neurite outgrowth4 and neuroendocrine functions.5,6 Secretin receptor is a G-protein-coupled receptor. Secretin can bind secretin receptor with higher affinity and bind other G-protein-receptor with lower affinity, including VIP receptor, the PACAP receptor, and the glucagon-like peptide receptors. Secretin binding to secretin receptor stimulates the cyclic adenosine monophosphate (cAMP) secretion by coupling with adenylate cyclase and activates protein kinase A (PKA) pathway and mitogen-activated protein kinase (MAPK) pathway, which activate specific gene expression.7 In the field of reproductive biology, secretin stimulates electrolyte and water secretion in the epididymis.8 During mouse embryonic development, secretin is widely expressed in the brain, intestine, and heart.9 Plasma secretin concentration increases significantly in late pregnancy and reaches the highest level at 36 weeks.10 In mouse placenta, secretin level expressed specifically by the trophoblast is many times higher than that in any other tissues.11 Compared to delayed implantation, secretin expression in mouse uterus is 53-fold higher when delayed implantation is activated by estrogen treatment.12 The aim of this study was to investigate the expression and regulation of secretin in mouse uterus during early pregnancy.
Materials and Methods
Animals and Treatments
Mature mice (CD1; 24-30 g) were housed in a temperature- and light-controlled environment (12-hour light–12-hour dark) with free access to regular food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee of Xiamen University.
Timed mating of animals were conducted by placing females with fertile males or vasectomized males to induce pregnancy or pseudopregnancy (day 1 is the day of vaginal plug). From days 1 to 4, pregnancy was confirmed by recovering embryos from the oviducts or uteri. The implantation sites on day 5 were visualized through intravenous injection of 0.1 mL of 1% Chicago blue dye (Sigma, St Louis, Missouri) in saline.
Delayed implantation was induced by ovariectomizing pregnant mice at 08:30 to 09:00 am on day 4 of pregnancy and treated with daily injection of progesterone (1 mg/mouse; Sigma) from days 5 to 7. To induce embryo implantation in these mice, estradiol-17β (25 ng/mouse; Sigma) was given to these progesterone-primed mice to initiate implantation on day 7 of pregnancy. The mice were killed to collect uteri 24 hours after estrogen treatment. Delayed implantation was confirmed by flushing blastocysts from 1 horn of the uterus. The implantation sites of activated uterus were identified through intravenous injection of 0.1 mL of 1% Chicago blue dye.
To induce artificial decidualization, 1 uterine horn on day 4 of pseudopregnancy was intraluminally infused 25 μL of sesame oil (Sigma), whereas the contralateral uninjected horn served as a control. The uteri were collected on day 8 of pseudopregnancy.
To study the effects of estrogen and progesterone on secretin expression, steroid hormonal treatments were initiated 2 weeks after ovariectomy. The ovariectomized mice were injected subcutaneously with estradiol-17β (100 ng/mouse), progesterone (1 mg/mouse), or a combination of estradiol-17β and progesterone once for 24 hours, respectively. The control mice only received vehicle (sesame oil, 0.1 mL/mouse). To further study the time course of progesterone treatment on secretin, ovariectomized mice were killed 1, 3, 12, and 24 hours later after progesterone injection, respectively. For mifepristone (RU486) treatment, ovariectomized mice were injected with RU486 (0.75 mg/mouse) 1 hour before progesterone treatment. Mice were killed 24 hours later after the hormonal injection to collect uteri for RNA extraction.
In Situ hybridization
Total RNAs from mouse uteri on day 8 of pregnancy were reverse transcribed and amplified with primers 5′-TCCCAGGACCCCAAGACACT and 5′-TTGATGCCAAGGACAACCAATC. The amplified fragment was cloned into pGEM-T plasmid (pGEM-T Vector System 1; Promega, Madison, Wisconsin), verified by sequencing, and amplified with the primers for T7 and SP6 to prepare the templates for labeling probes. Digoxigenin (DIG)-labeled antisense probes were transcribed in vitro using DIG RNA labeling kit (Roche Diagnostics GmbH, Mannheim, Germany). Digoxigenin-labeled sense probe was used as negative control.
As previously described,13 frozen sections (10 μm) were mounted on 3-aminopropyltriethoxy-silane (Sigma)-treated slides and fixed in 4% paraformaldehyde solution in phosphate-buffered saline. Hybridization was performed at 55°C for 16 hours. Then sections were incubated with sheep antidigoxigenin antibody conjugated to alkaline phosphatase (1:5000; Roche). The signal was visualized with the buffer containing 0.4 mmol/L 5-bromo-4-chloro-3-indolyl phosphate and 0.4 mmol/L nitroblue tetrazolium. Endogenous alkaline phosphatase activity was inhibited with 2 mmol/L levamisole (Sigma). All of the sections were counterstained with 1% methyl green. The positive signal of in situ hybridization was visualized as a dark brown color.
Isolation of Uterine Stromal Cells
Uterine stromal cells were isolated as described previously.14 Briefly, uterine horns from day 4 pregnant mice were split longitudinally, washed thoroughly with Hanks balanced salt solution (HBSS; Sigma), and digested in HBSS containing 1% (wt/vol) trypsin (Amresco, Solon, Ohio), 6 mg/mL dispase (Roche, Indianapolis, Indiana), and 1% penicillin/ streptomycin (Hyclone, Logan City, Utah). The digested uteri were shaken gently for 5 minutes to dislodge sheets of luminal epithelial cells. The remaining tissues were rinsed 3 times with HBSS and incubated in HBSS containing 0.15 mg/mL collagenase I (Invitrogen, Carlsbad, California) and 1% penicillin/streptomycin (Hyclone, Logan City, Utah) at 37°C for 30 minutes, followed by vigorously shaking for 1 minutes until the supernatant became turbid. The supernatant was then passed through a 70-μm wire gauze filter to eliminate epithelial sheets and centrifuged. The cell pellets were washed twice with HBSS and resuspended in complete medium consisting of Dulbecco Modified Eagle Medium (DMEM)-nutrient mixture F-12 Ham (DMEM-F12; Sigma) with 10% charcoal-treated fetal bovine serum (cFBS; Life Technologies, Inc, Grand Island, New York). Viable cells were determined by trypan blue exclusion, counted, and plated onto 35-mm culture dishes at the concentration of 1 × 106 cells/dish or 2 × 105/well for 24-well culture plates. The culture dishes or plates were cultured in a 5% CO2 incubator at 37°C. The medium with 2% cFBS was used for further culture after 1 hour of initial culture and changed every 24 hours.
Steroid Hormonal Treatments In Vitro
Cultured stromal cells were treated with 1 μmol/L of progesterone (Sigma) for 48 hours. For further studies, cells were pretreated with RU486 (1 mmol/L; Sigma) or Ly294002 (15 μmol/L; Cell Signaling, Boston, Massachusetts) 1 hour before progesterone treatment. Then the cells were collected 48 hours later for further analysis by real-time polymerase chain reaction (PCR) or Western blot.
Real-Time PCR
Total RNAs from mouse uteri or cultured cells were isolated using TRIzol reagent (Invitrogen), digested by RQ1 deoxyribonuclease I (Promega, Madison, Wisconsin), and reverse transcribed into complementary DNA (cDNA) with PrimeScript reverse transcriptase reagent kit (Perfect real time; TaKaRa, Dalian, China). Then cDNA was amplified using an SYBR Premix Ex Taq kit (TaKaRa; DRR041 S) on the Rotor-Gene 3000A system (Corbett Research, Mortlake, Victoria, Australia). Primer sequences used for real-time PCR were as follows: Sct (5′-TGGATGGGTCCCTGTCTCTC and 5′-TGATGCCAAGGACAACCAAT), Sctr (5′-GGTCTGGTCGGATTGGGTAG and 5′-CCTCTTCAGGAGCTGGGTGT), and rpl7 (5′-GCAGATGTACCGCACTGAGATTC and 5′-ACCTTTGGGCTTA CTCCATTGATA). The conditions used for real-time PCR were as follows: 95°C for 10 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 34 seconds. Data from real-time PCR were analyzed using the 2−ΔΔCt method.
Western Blot
Proteins were extracted from uterine tissues by homogenization in lysis buffer containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, and complete protease inhibitor cocktail (Roche). Cultured cells were collected directly into the lysis buffer. Protein concentration was measured by BCA reagent kit (Applygen, Beijing, China). Samples were run on a 10% polyacrylamide gel and transferred onto polyvinyl difluoride membranes. After blocking with 5% nonfat dry milk in TBST (0.1% Tween 20 in Tris-buffered saline [TBS]) for 1 hour, membranes were incubated with each primary antibody for progesterone receptor A (PR-A; ab2764; Abcam, Hong Kong, China), phosphorylated activated protein kinase B (p-AKT; 4058s; Cell Signaling) or AKT (9272; Cell Signaling) overnight at 4°C. After 3 washes in TBST for 10 minutes each, membranes were incubated in goat anti-rabbit antibody conjugated with horseradish peroxidase for 1 hour followed by 3 washes in TBST for 5 minutes each. The signals were developed with the enhanced chemiluminescent kit (Applygen).
All the experiments were independently repeated at least 3 times. For in vivo experiments, at least 3 mice were used for each treatment. Data are presented as mean ± standard error of the mean. The significance of difference was assessed by 1-way analysis of variance or Student t test. P < .05 was considered statistically significant.
Results
Secretin Messenger RNA Expression During Early Pregnancy
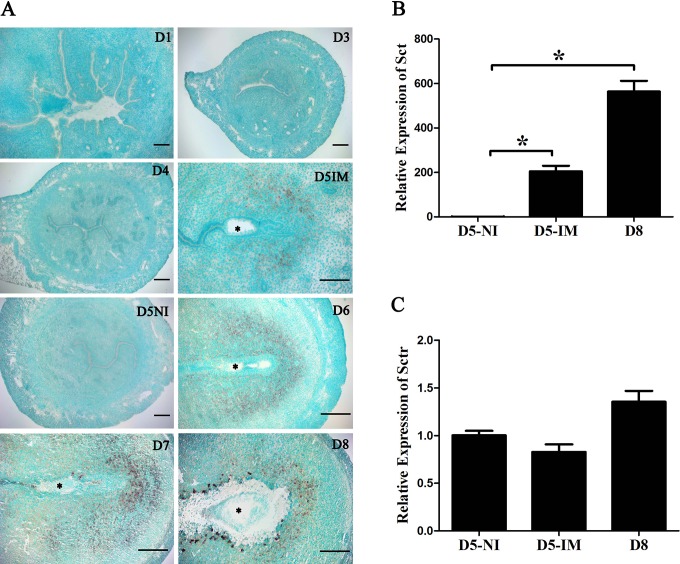
In situ hybridization was used to localize secretin messenger RNA (mRNA) expression during early pregnancy. There was no secretin mRNA signal in the uteri from days 1 to 4 of pregnancy. On day 5 of pregnancy, a weak secretin mRNA signal was detected only in the subluminal stromal cells surrounding the implanting blastocyst, and no signal was seen in the interimplantation sites. From days 6 to 8 of pregnancy, secretin mRNA signal was detected in the decidua (Figure 1A).
Figure 1.
Expression of secretin (Sct) messenger RNA (mRNA) in mouse uteri during early pregnancy. A, In situ hybridization localization of secretin mRNA in mouse uterus from days 1 (D1) to 8 (D8). Asterisk (*), embryo. B, Real-time polymerase chain reaction (PCR) analysis of Sct expression * indicates statistical significance (P < .05; error bars, standard error [SE]). C, Real-time PCR analysis of Sct receptor (Sctr) expression. D5-NI, interimplantation site on day 5; D5-IM, implantation site on day 5; D8, day 8 of pregnancy.
Real-time PCR was performed to quantify the mRNA level of secretin and secretin receptor in mouse uteri on days 5 and 8 of pregnancy. Compared with interimplantation sites on day 5, the level of secretin mRNA at the implantation sites on days 5 and 8 was significantly upregulated (Figure 1B). Although secretin receptor expression was detected by real-time PCR, there was no significant change in secretin receptor mRNA expression between days 5 and 8 of pregnancy (Figure 1C).
Secretin Expression Under Delayed and Activated Implantation
Delayed implantation was performed to see whether the uterine expression of secretin is dependent on the presence of active blastocysts. When embryo implantation was delayed by ovariectomy, there was no detectable secretin mRNA signal in the uterus. After delayed implantation was terminated by estrogen, secretin signals were detected in the stromal cells at the implantation sites (Figure 2).
Figure 2.
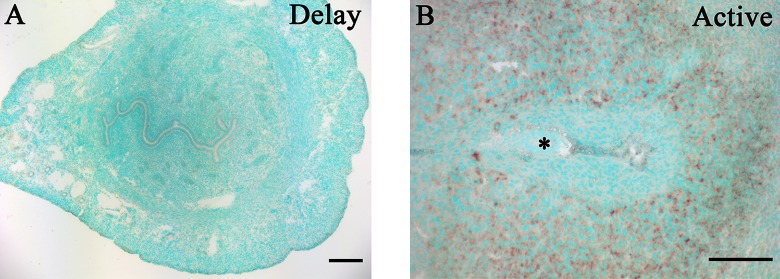
In situ hybridization of secretin messenger RNA (mRNA) in mouse uteri under delayed implantation. A, Mouse uterus under delayed implantation. Delayed implantation was induced by ovariectomizing pregnant mice at 08:30 to 09:00 am on day 4 of pregnancy and treated with daily injection of progesterone (1 mg/mouse; Sigma) at 09:00 from days 5 to 7. The mice were killed to collect uteri at 09:00 am on day 8 of pregnancy. B, Mouse uterus after delayed implantation was activated by estrogen treatment. To induce embryo implantation in these mice, estradiol-17β (25 ng/mouse; Sigma) was given to these progesterone-primed mice to initiate implantation on day 7 of pregnancy. The mice were killed to collect uteri 24 hours after estrogen treatment. Asterisk (*), embryo.
Secretin Expression Under Artificial Decidualization
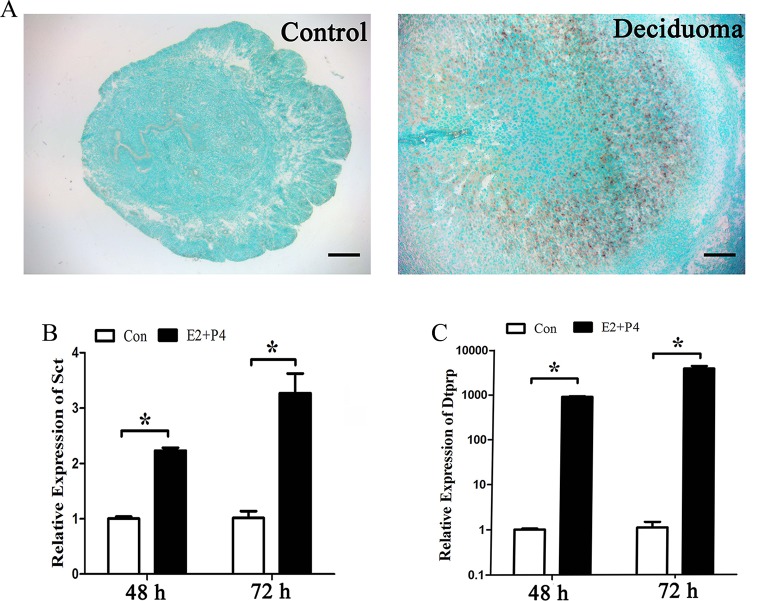
Artificially induced decidualization was performed to determine whether secretin is expressed in deciduoma. There was no detectable secretin mRNA signal in the control uterine horn. Under artificially induced decidualization, secretin mRNA signal was strongly detected in the decidualized cells (Figure 3A).
Figure 3.
Expression of secretin (Sct) messenger RNA (mRNA) under decidualization. A, In situ hybridization of Sct expression under artificial decidualization. B, Real-time polymerase chain reaction (PCR) analysis of Sct expression under in vitro decidualization for 48 and 72 hours, respectively. C, Real-time PCR of decidual/trophoblast prolactin-related protein (Dtprp) expression under in vitro decidualization for 48 and 72 hours, respectively. E2, estrogen; P4, progesterone; *, statistical significance (P < .05; error bars, standard error [SE]).
Secretin Expression During In Vitro Decidualization
Because decidual/trophoblast prolactin-related protein (Dtprp) is a reliable marker for in vitro decidualization in mice,15 real-time PCR was performed to examine Dtprp level under in vitro decidualization. After mouse stromal cells were induced for decidualization with both estrogen and progesterone for 48 or 72 hours, secretin expression significantly increased (Figure 3B). The Dtprp expression was also significantly induced from 48 to 72 hours during in vitro decidualization (Figure 3C).
Regulation of Ovarian Steroid Hormones on Secretin Expression
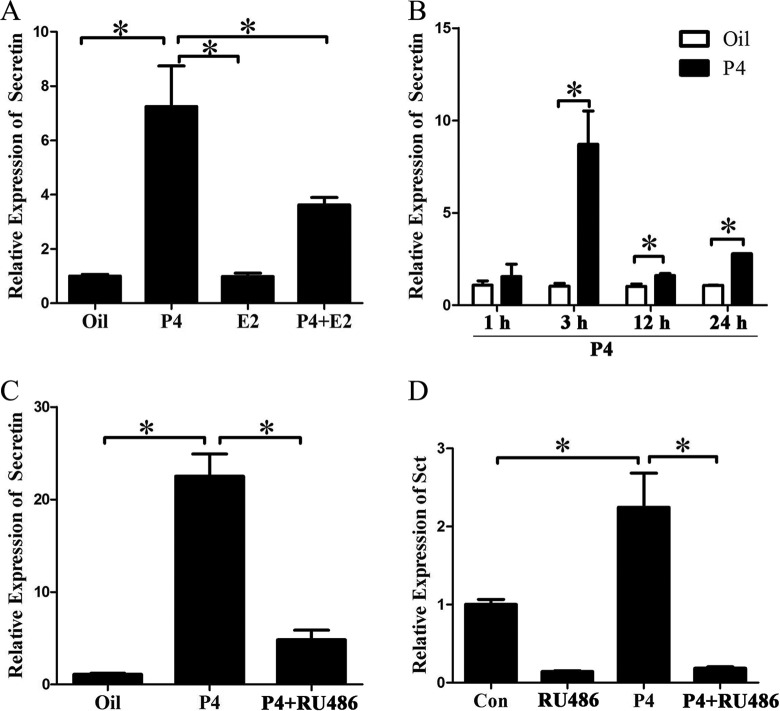
Because estrogen and progesterone are essential for mouse embryo implantation, ovariectomized mice were used to examine whether secretin expression is regulated by estrogen or progesterone. Real-time PCR showed that estrogen had no effect on secretin expression and progesterone significantly increased secretin expression. Compared with progesterone treatment, secretin expression was slightly weaker with estrogen and progesterone cotreatment, indicating that estrogen had an antagonistic effect on progesterone upregulation of secretin expression (Figure 4A). Then ovariectomized mice were treated with progesterone for different time points. Secretin expression was upregulated in the uterus by progesterone at 1, 3, 12 and 24 hours, respectively. The highest level was reached at 3 hours after progesterone treatment (Figure 4B). RU486, a specific antagonist for progesterone receptor, was used to determine whether progesterone upregulation of secretin expression is mediated through progesterone receptor. Progesterone-stimulated secretin expression was significantly reduced by RU486 (Figure 4C). In cultured stromal cells, secretin expression was also induced by progesterone treatment, which was significantly inhibited by RU486 pretreatment (Figure 4D).
Figure 4.
Progesterone regulation on secretin expression in vivo and in vitro. A, Real-time polymerase chain reaction (PCR) analysis of secretin messenger RNA (mRNA) level after ovariectomized mice were treated with progesterone (P4), estradiol-17β (E2), or a combination of progesterone and estradiol-17β (P4 + E2), respectively. B, Real-time PCR analysis of secretin mRNA level after ovariectomized mice were treated with progesterone for 1, 3, 12, and 24 hours, respectively. C, Real-time PCR analysis of secretin mRNA level in the uteri of ovariectomized mice injected with oil, P4, or P4 plus mifepristone (RU486) for 24 hours. The ovariectomized mice were injected subcutaneously with P4 (1 mg/mouse) once. For RU486 treatment, ovariectomized mice were injected with RU486 (0.75 mg/mouse) 1 hour before P4 treatment. The mice were killed to collect uteri after 24 hours. D, Real-time PCR of secretin expression after stromal cells were treated with P4, RU486, or P4 plus RU486 for 48 hours. Cultured stromal cells were treated with 1 μmol/L of progesterone (Sigma) for 48 hours or cells were pretreated with RU486 (1 mmol/L, Sigma) 1 hour before progesterone treatment. Then, the cells were collected 48 hours later. E2 indicates estrogen; P4, progesterone; *, statistical significance (P < .05; error bars, standard error [SE]).
Progesterone Induction on Secretin Expression Through PI3K/AKT Pathway
In mouse uterus, PI3K/AKT pathway is induced by progesterone through progesterone receptor.16 Therefore, we first examined the expression of PR-A and p-AKT in the uteri on day 8 of pregnancy and under artificial decidualization. On day 8 of pregnancy, the protein level of both PR-A and p-AKT was higher at implantation sites than that at the interimplantation sites. Under artificial decidualization, the protein level of PR-A and p-AKT was also higher in the deciduoma than that in the control (Figure 5A). Because both PR-A and p-AKT were present in mouse decidua, we hypothesized that PI3K/AKT may be involved in progesterone regulation on secretin expression. Ly294002, a specific PI3K inhibitor, was used to test this possibility. When stromal cells were treated with progesterone, p-AKT level was significantly upregulated (Figure 5B). However, progesterone-induced secretin expression was obviously abrogated by Ly294002 treatment (Figure 5C).
Figure 5.
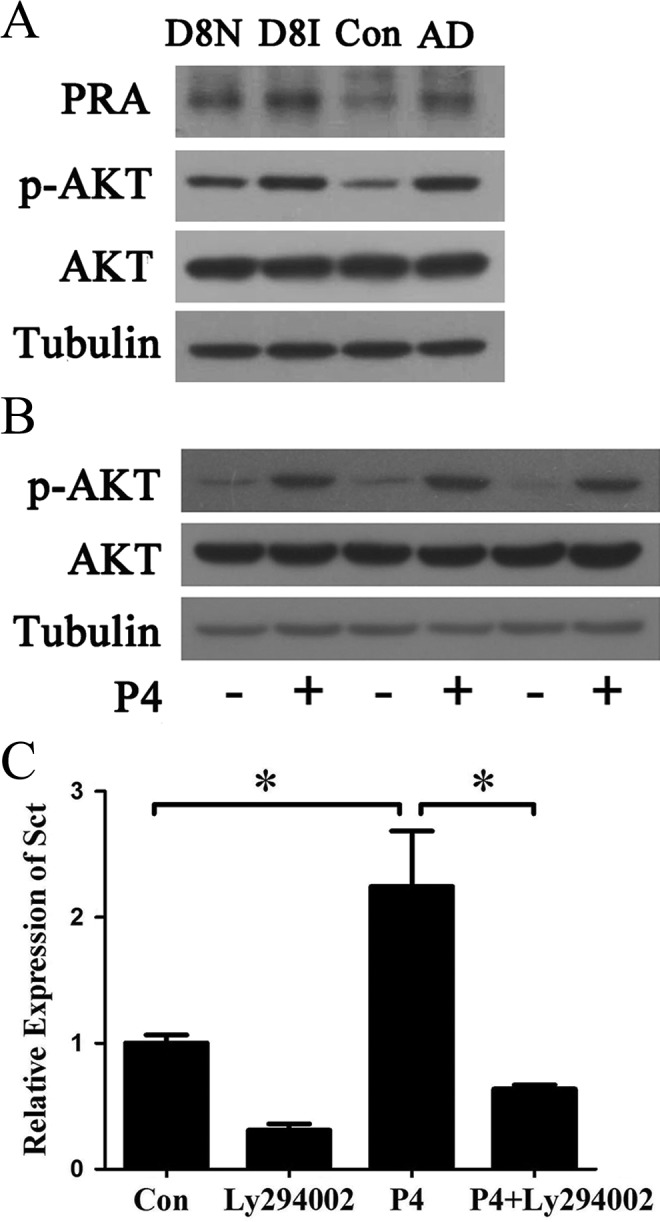
The involvement of phosphorylated activated protein kinase B (p-AKT) in progesterone-induced secretin expression. A, Western blot analysis on the levels of progesterone receptor A (PR-A), phosphorylated AKT (p-AKT), and total AKT (AKT) in the uteri on day 8 of pregnancy and in the deciduoma under artificial decidualization. B, Western blot analysis on the levels of p-AKT and AKT after stromal cells were treated with progesterone (P4) for 48 hours. C, Real-time polymerase chain reaction (PCR) analysis of secretin expression after stromal cells were treated with Ly294002, P4, or P4 plus Ly294002 (P4 + Ly294002) for 48 hours. In P4 + Ly294002-treated group, stromal cells were pretreated with 15 μmol/L Ly294002 for 1 hour before progesterone treatment. * indicates statistical significance (P < .05; error bars, standard error [SE]).
Discussion
Progesterone Regulates Secretin Expression Through PI3K/AKT Pathway
In our study, secretin is specifically localized in the decidua on days 5 to 8 of pregnancy and highly expressed in mouse stromal cell under in vitro decidualization. Secretin expression at implantation sites is regulated by active blastocysts. Our previous study also showed that secretin is strongly expressed in mouse uterus when delayed implantation was activated by estrogen.12
Progesterone signaling is critical for the initiation and maintenance of decidualization.17 The actions of progesterone are mediated through intracellular progesterone receptor. Both progesterone receptor knockout mice and progesterone receptor A knockout mice display the failure of decidualization.18,19 In this study, progesterone could significantly promote secretin expression in ovariectomized mouse uterus and in cultured stromal cells, while secretin expression is attenuated by progesterone receptor antagonist RU486, suggesting that progesterone-regulated secretin expression is mediated by progesterone receptor. It has been reported that progesterone significantly induces the phosphorylation of AKT, and the expression of phosphorylated AKT is significantly elevated in mouse and human decidua.16,20 In our study, both PR-A and p-AKT are upregulated at implantation sites on day 8 of pregnancy and in the deciduoma under artificial decidualization. Because the upregulation of secretin by progesterone is blocked by Ly294002, a specific PI3K inhibitor, p-AKT signaling should be involved in progesterone regulation on secretin expression.
Possible Function of Secretin During Decidualization
Secretin and secretin receptor axis trigger the activation of intracellular secondary messenger systems, such as cAMP.21 Cyclic AMP is a strong inducer of decidualization during early pregnancy in humans.22 In the mouse, uterine concentration of cAMP elevates following blastocyst- and artificially induced decidualization.23–26 Delayed implantation can be activated by cAMP.27 Alkaline phosphatase is a reliable marker for mouse and rat decidualization.28 Prostoglandin E2-induced alkaline phosphatase activity is at least partially mediated by cAMP.29 In PC12 cells, secretin induces neurite outgrowth by activating the mitogen-activated protein kinases (MAPK) pathway.4 The MAPK signaling pathway is involved in endometrial cell decidualization because the inhibition of MAPK phosphorylation by PD98059 impairs the extent of decidualization.30 Secretin may be essential for mouse decidualization through regulating cAMP level.
Additionally, cAMP can induce the phosphorylation of water channels and translocation through PKA or MAPK pathway.31,32 Aquaporins (AQPs) are a family of membrane channel proteins that enhance bulk water transport and have been showed distinct uterine expression patterns during implantation and maintained water homeostasis of endometrial cells during decidualization.33 The AQP7, an aquagly-ceroporin-facilitated glycerol transport, shows a specific expression in the decidual zone during the decidualization process, suggesting that the increased requirement in glycerol utilization supports energy during decidualization.34 However, the effect of secretin on regulation of AQP7 still needs further verification. Saline drinking and dehydration can also stimulate secretin expression.35,36 Secretin itself is recognized as a dipsogenic hormone for controlling water intake and reabsorption.37 There is an alteration in volume and sodium homeostasis and osmoregulation during human pregnancy.38 It is possible that secretin may play an important role in maintaining homeostasis during decidualization.
Compared with murine placental hormones, secretin is more strongly and specifically expressed in differentiated trophoblast cells and spongiotrophoblasts than that in maternal decidua.11 Moreover, secretin and secretin receptor are located in the developing fetal regions.9,39 Thus, it is very likely that secretin acts either on placentogenesis or on tissues of the developing fetus. Secretin and secretin receptor-deficient mice have been generated, respectively. However, these mice appear normal and fertile, except for the impairment in synaptic plasticity of the hippocampus and social behavior, an abolishment or much reduced angiotensin II osmoregulatory functions.40–41 It seems that the loss of secretin or secretin receptor in response to normal physiological status is not critical, or their functions are compensated by other genes of peptide family in the uterus. However, whether lack of secretin or secretin receptor has any effects on reproduction in response to hypertonicity stimulation needs to be confirmed.
In our study, the considerable and specific expression of secretin is located at implantation sites during days 5 to 8 of pregnancy. The regulation of progesterone on secretin may be mediated by PI3K-AKT-signaling pathway. Secretin may play a role during mouse decidualization via regulating cAMP level.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Basic Research Program of China (2011CB944402) and National Natural Science Foundation of China (30930013, 31071276, and 31272263).
References
- 1. Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. [DOI] [PubMed] [Google Scholar]
- 3. Chu JY, Yung WH, Chow BK. Secretin: a pleiotrophic hormone. Ann N Y Acad Sci. 2006;1070:27–50. [DOI] [PubMed] [Google Scholar]
- 4. Kim HS, Yumkham S, Kim SH, et al. Secretin induces neurite outgrowth of PC12 through cAMP-mitogen-activated protein kinase pathway. Exp Mol Med. 2006;38(1):85–93. [DOI] [PubMed] [Google Scholar]
- 5. Chu JY, Lee LT, Lai CH, et al. Secretin as a neurohypophysial factor regulating body water homeostasis. Proc Natl Acad Sci U S A. 2009;106(37):15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu JY, Yung WH, Chow BK. Endogenous release of Secretin from the hypothalamus. Ann N Y Acad Sci. 2006;1070:196–200. [DOI] [PubMed] [Google Scholar]
- 7. Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20(3):460–466. [DOI] [PubMed] [Google Scholar]
- 8. Chow BK, Cheung KH, Tsang EM, Leung MC, Lee SM, Wong PY. Secretin controls anion secretion in the rat epididymis in an autocrine/paracrine fashion. Biol Reprod. 2004;70(6):1594–15999. [DOI] [PubMed] [Google Scholar]
- 9. Siu FK, Sham MH, Chow BK. Secretin, a known gastrointestinal peptide, is widely expressed during mouse embryonic development. Gene Expr Patterns. 2005;5(3):445–451. [DOI] [PubMed] [Google Scholar]
- 10. Holst N, Jenssen TG, Burhol PG, Maltau JM. Plasma secretin concentrations during normal human pregnancy, delivery, and postpartum. Br J Obstet Gynaecol. 1989;96(4):424–427. [DOI] [PubMed] [Google Scholar]
- 11. Knox K, Leuenberger D, Penn AA, Baker JC. Global hormone profiling of murine placenta reveals Secretin expression. Placenta. 2011;32(11):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su RW, Lei W, Liu JL, et al. The integrative analysis of microRNA and mRNA expression in mouse uterus under delayed implantation and activation. PLoS One. 2010;5(11):e15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni H, Sun T, Ding NZ, Ma XH, Yang ZM. Differential expression of microsomal prostaglandin e synthase at implantation sites and in decidual cells of mouse uterus. Biol Reprod. 2002;67(1):351–358. [DOI] [PubMed] [Google Scholar]
- 14. Hu SJ, Ren G, Liu JL, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283(34):23473–23484. [DOI] [PubMed] [Google Scholar]
- 15. Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294(2):445–456. [DOI] [PubMed] [Google Scholar]
- 16. Lei W, Feng XH, Deng WB, et al. Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J Biol Chem. 2012;287(19):15174–15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptor-alpha-deficient mice. Endocrinology. 1999;140(6):2704–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 19. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 20. Toyofuku A, Hara T, Taguchi T, Katsura Y, Ohama K, Kudo Y. Cyclic and characteristic expression of phosphorylated Akt in human endometrium and decidual cells in vivo and in vitro. Hum Reprod. 2006;21(5):1122–1128. [DOI] [PubMed] [Google Scholar]
- 21. Sans MD, Sabbatini ME, Ernst SA, D'Alecy LG, Nishijima I, Williams JA. Secretin is not necessary for exocrine pancreatic development and growth in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G791–G798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55(5):795–810. [DOI] [PubMed] [Google Scholar]
- 23. Vilar-Rojas C, Castro-Osuna G, Hicks JJ. Cyclic AMP and cyclic GMP in the implantation site of the rat. Int J Fertil. 1982;27(1):56–59. [PubMed] [Google Scholar]
- 24. Leroy F, Vansande J, Shetgen G, Brasseur D. Cyclic AMP and the triggering of the decidual reaction. J Reprod Fertil. 1974;39(1):207–211. [DOI] [PubMed] [Google Scholar]
- 25. Rankin JC, Ledford BE, Baggett B. Early involvement of cyclic nucleotides in the artificially stimulated decidual cell reaction in the mouse uterus. Biol Reprod. 1977;17(4):549–554. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy TG. Prostaglandin E2, adenosine 3′:5′-cyclic monophosphate and changes in endometrial vascular permeability in rat uteri sensitized for the decidual cell reaction. Biol Reprod. 1983;29(5):1069–1076. [DOI] [PubMed] [Google Scholar]
- 27. Holmes PV, Bergstrom S. Induction of blastocyst implantation in mice by cyclic AMP. J Reprod Fertil. 1975;43(2):329–332. [DOI] [PubMed] [Google Scholar]
- 28. Finn CA, Hinchliffe JR. Reaction of the mouse uterus during implantation and deciduoma formation as demonstrated by changes in the distribution of alkaline phosphatase. J Reprod Fertil. 1964;8:331–338. [DOI] [PubMed] [Google Scholar]
- 29. Yee GM, Kennedy TG. Prostaglandin E2, cAMP and cAMP-dependent protein kinase isozymes during decidualization of rat endometrial stromal cells in vitro. Prostaglandins. 1993;46(2):117–138. [DOI] [PubMed] [Google Scholar]
- 30. Vallejo G, Mestre-Citrinovitz AC, Monckedieck V, Grümmer R, Winterhager E, Saragüeta P. Ovarian steroid receptors and activated MAPK in the regional decidualization in rats. Biol Reprod. 2011;84(5):1063–1071. [DOI] [PubMed] [Google Scholar]
- 31. Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta. 2006;1758(8):1100–1105. [DOI] [PubMed] [Google Scholar]
- 32. Chu JY, Cheng CY, Lee VH, Chan YS, Chow BK. Secretin and body fluid homeostasis. Kidney Int. 2011;79(3):280–287. [DOI] [PubMed] [Google Scholar]
- 33. Richard C, Gao J, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology. 2003;144(4):1533–1541. [DOI] [PubMed] [Google Scholar]
- 34. Peng H, Zhang Y, Lei L, et al. Aquaporin 7 expression in postimplantation mouse uteri: a potential role for glycerol transport in uterine decidualization. Fertil Steril. 2011;95(4):1514–1517. [DOI] [PubMed] [Google Scholar]
- 35. Lee VH, Lam IP, Choi HS, Chow BK, Lee LT. The estrogen-related receptor alpha upregulates Secretin expressions in response to hypertonicity and angiotensin II stimulation. PLoS One. 2012;7(6):e39913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee VH, Lee LT, Chu JY, et al. An indispensable role of Secretin in mediating the osmoregulatory functions of angiotensin II. FASEB J. 2010;24(12):5024–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng SS, Yung WH, Chow BK. Secretin as a neuropeptide. Mol Neurobiol. 2002;26(1):97–107. [DOI] [PubMed] [Google Scholar]
- 38. Davison JM, Lindheimer MD. Volume homeostasis and osmoregulation in human pregnancy. Baillieres Clin Endocrinol Metab. 1989;3(2):451–472. [DOI] [PubMed] [Google Scholar]
- 39. Siu FK, Sham MH, Chow BK. The prenatal expression of secretin receptor. Ann N Y Acad Sci. 2006;1070:561–565. [DOI] [PubMed] [Google Scholar]
- 40. Yamagata T, Urano H, Weeber EJ, Nelson DL, Nishijima I. Impaired hippocampal synaptic function in secretin deficient mice. Neuroscience. 2008;154 (4):1417–1422. [DOI] [PubMed] [Google Scholar]
- 41. Nishijima I, Yamagata T, Spencer CM, et al. Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior. Hum Mol Genet. 2006;15(21):3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]