Abstract
Aim:
The molecular etiology of pelvic organ prolapse (POP) is complex and not well understood. We compared the expression/activity of extracellular matrix (ECM)-processing (procollagen I N-proteinase/ a disintegrin and metalloproteinase with thrombospondin motifs [ADAMTS]-2,-3,-14) and ECM-degrading (matrix metalloproteinase [MMP]-1, -2, -7, -8, -9, -12) enzymes and their natural tissue inhibitors (tissue inhibitors of metalloproteinase [TIMP]-1,-2,-3,-4) in vaginal tissues from premenopausal women with advanced POP (POP-Q stage ≥ 3) and asymptomatic controls (POP-Q = 0).
Study Design:
We sampled the anterior vaginal wall of 36 premenopausal women (17 patients with POP and 19 controls) undergoing total hysterectomy. Exclusion criteria include steroid therapy, malignancy, previous pelvic surgery, and connective tissue diseases. Total RNAs and proteins were quantified by real-time polymerase chain reaction, immunoblotting, and Luminex assay; MMPs activity was analyzed by zymography and tissue localization by immunohistochemistry.
Results:
The MMP-2 gelatinase activity as well as expression of 58-kDa isoform of ADAMTS-2 was upregulated in patients with POP, irrespective of menstrual phase status, secretory or proliferative, when compared to controls (P < .05). The TIMP-1-4 gene and TIMP-1 protein expression were significantly (P < .05) reduced, whereas protein expression of MMP-12 (pro and active forms) was significantly increased in vaginal biopsies of patients with POP in the proliferative phase of the menstrual cycle compared to corresponding controls. Analyses of MMP-12, TIMP-1, and ADAMTS-2 tissue immunostaining indicate similar localization in the vaginal specimens from control and patients with POP.
Conclusion:
Expression of ECM-remodeling proteins is altered in the vagina of premenopausal patients with severe POP. We speculate that dysregulation of MMP/TIMP complexes and ADAMTS-2 proteins may cause connective tissue defects, which result in weakened vaginal wall support and POP development.
Keywords: POP, TIMP, MMP, PNP/ADAMTS
Introduction
Pelvic organ prolapse (POP) is a global health problem affecting women in the reproductive and postmenopausal years. The overall lifetime prevalence of POP is 30.8%,1 with a projected overall increase to 46% in the next 40 years.2 Structurally, female pelvic organs are supported by pelvic floor muscles, cardinal and uterosacral ligaments, and the endopelvic fascia.3 These structures are mainly comprised of connective tissue, which contains fibroblastic cells capable of producing substantial amounts of extracellular matrix (ECM) molecules, including structural proteins (collagens and elastin), substrate adhesion molecules (fibronectin, laminins), and proteoglycans. The integrity of the pelvic floor is maintained mostly by fibrillar ECM components, collagen, and elastin.
Procollagens are the precursors of mature collagen molecules. Two major enzymes are responsible for processing of several types of procollagen proteins. Procollagen-C-proteinase (PCP, also known as BMP-1) and procollagen I N-proteinase (PNP, also known as a disintegrin and metalloproteinase with thrombospondin motif [ADAMTS]) cleave the C-terminal and N-terminal propeptide chains of amino acids from different ends of the procollagen molecule, correspondingly. This process creates a mature collagen molecule able to assemble into fibrils in the extracellular space. We have previously reported that caucasian premenopausal women with POP show differential expression of PCP/BMP-1 enzyme in vaginal tissue compared with age-matched asymptomatic controls.4 Extracellular PNP family that cleaves the N-propeptides of procollagens I, II, III in vivo consists of ADAMTS-2 and its homologs ADAMTS-3 and -145 which have not been studied in the human vagina.
The ECM is a dynamic structure, undergoing continuous remodeling through the synthesis/deposition of its composite proteins and the restricted and tightly controlled proteolytic breakdown. Proteolytic enzymes capable of degrading ECM proteins fall into 3 groups: (1) serine proteinases (ie, neutrophil elastase); (2) cysteine proteinases (cathepsins L, S, and K), and (3) matrix metalloproteinases (MMPs).6 Collagenases (ie, MMP-1,-8) cleave the mature triple helical collagen at a single peptide bond in each of the 3 chains, yielding 2 fragments. The product of this cleavage is susceptible to rapid degradation into amino acids by 2 gelatinases (MMP-2 and MMP-9) which is critically important for the loss of strength of fibrous collagen and hence loss of tissue integrity. The MMP-12 (also known as macrophage metalloelastase) can degrade a broad spectrum of ECM components.7,8 Interestingly, we have recently reported the presence of tissue macrophages in the human vagina.9 The activity of MMPs is neutralized by a family of proteins called tissue inhibitors of metalloproteinase (TIMP-1, -2, -3, -4).10 The TIMPs bind with high affinity (in a 1:1 molar ratio) to the catalytic site of active MMPs, preventing their activation.
It is the delicate balance between production and degradation of ECM proteins in connective tissue that is critical to the pelvic floor integrity. Numerous publications have associated POP development in pre- and postmenopausal women with defective ECM synthesis9,11 and activated degradation of collagen and elastin fibers.12–14 Therefore, the main goal of this case–control study was to investigate MMPs, ADAMTSs, and TIMPs changes in vaginal tissue of a homogenous group of premenopausal Caucasian patients with POP and asymptomatic control women. Since reproductive hormones can modulate the turnover of pelvic floor connective tissue,15–17 we have controlled our study groups according to the stage of the menstrual cycle. We hypothesized that proteinase expression and activity would be altered in vaginal tissue of younger women with POP when compared to age-matched asymptomatic control women. Using real-time polymerase chain reaction (PCR) and immunoblotting analysis, we investigated (1) the gene/protein expression profile of multiple proteolytic enzymes (MMPs and ADAMTSs) and their tissue inhibitors (TIMPs) and (2) examined MMPs’ activity using gelatine zymography in the vaginal tissues of premenopausal women. Tissue localization of differentially regulated proteins was studied by immunohistochemistry (IHC). We believe that these data provide new insight into the molecular mechanisms underlying the development of POP in younger women.
Materials and Methods
Patient Selection
The Research Ethics Board of Mount Sinai Hospital, Toronto, approved this study. Premenopausal caucasian women undergoing vaginal hysterectomy for POP ≥stage 3 (uterine ± vaginal) by POP-Q were identified as “patients,” while asymptomatic women with POP-Q of stage 0 (uterine + vaginal) undergoing abdominal hysterectomy for reasons other than prolapse were identified as “controls.” We rationalized that stage 0 is the “gold standard” for normal pelvic support. Women with a history of gynecologic malignancy, endometriosis, connective tissue disorders, emphysema, previous pelvic surgery, and those on steroid therapy were excluded. The initial gynecologic examination was performed by the first author (M.A.) and by the gynecology team at Mount Sinai Hospital during regular activities. The first author obtained written informed consent, confirmed the POP staging of all participants, and collected clinical data on the day of the surgical procedure. Patients were examined in a lying position with a referred full bladder and asked to perform the Valsalva maneuver (described in4). The descensus of the vaginal compartments was measured at the maximum straining point using a centimeter scale ruler. Total vaginal length was measured at rest under POP reduction with a vaginal speculum. Afterward, straining examination in the standing position confirmed the full extent of the POP.4 Only tissues from premenopausal women having regular menstrual cycles over the preceding 12 months were analyzed. Phase of the menstrual cycle was confirmed by a histology report on the uterine specimens in all patients (n = 17) and controls (n = 19).
Tissue Collection
We used vaginal tissue for our research as earlier studies have confirmed that a correlation exists between markers of collagen metabolism and content in the endopelvic fascia, the uterosacral ligament, and vaginal tissue.18 At the time of the hysterectomy and after removal of the uterus, full-thickness vaginal tissue specimens (approximately 1 cm2) were obtained by sharp dissection down to the avascular space of loose areolar tissue of the anterior connective tissue of the vagina using Metzenbaum scissors. Adventitia was excluded from our analysis as it is easily torn during dissection. To account for variations in stretch conditions and thickness of muscularis layer throughout the vaginal length, the site of tissue collection was standardized at the anterior middle portion of the vaginal vault.19 One of the first authors (M.A. or H.K.), not blinded for the sample status, immediately received the tissue biopsies from the surgeon (M.A. or H.D.) in the operative room and further performed the biochemical assays under direct supervision of the senior author (O.S.). For RNA and protein analysis, the vaginal biopsy samples were washed in ice-cold phosphate-buffered saline, flash frozen in liquid nitrogen, and stored at −80°C. For IHC studies, the vaginal specimens were sectioned longitudinally and fixed in 4% paraformaldehyde for 48 hours.
Reverse Transcription and Real-Time PCR Protocol
Reverse transcription
RNA was extracted using TRIZOL (Gibco, Burlington, Ontario, Canada), column purified using RNeasy Mini kit (Qiagen, Mississauga, Ontario, Canada), and treated with 2.5 µL DNase I (2.73 Kunitz U/µL, Qiagen), according to the manufacturer’s instructions. The messenger RNA (mRNA) quality was checked through electrophoregram (Experion, Bio-Rad, Hercules, California). Two micrograms of RNA was reverse transcribed into complementary DNA (cDNA) in a total reaction volume of 100 µL using the TaqMan Reverse Transcription kit (ABI, Carlsbad, California). To assess for genomic DNA contamination in the RNA samples, a “RT (−) control” was used.
Real-time PCR
The primer sequences for genes of interest and housekeeping genes (Table 1) were designed using Primer Express 2.1 (ABI) to span from 2 adjacent exons and were verified for specificity by basic local alignment search tool (BLAST) analysis. The cDNA with SYBR Green Master Mix (Bio-Rad) was subjected to real-time PCR using Realplex Mastercycler (Eppendorf, Hamburg, Germany) or CFX-96 Real Time System C1000 Thermal Cycler (Bio-Rad). After PCR, a dissociation curve was constructed by increasing temperature from 65°C to 95°C to verify the specificity of PCR products. A cycle threshold (CT) mean value was recorded for each sample. Values obtained for each gene were normalized to the geometric mean of 3 housekeeping genes: β-actin gene (ACTB), TBP, and YWHAZ. Relative quantification (ΔΔCT method20) was used to compare gene expression between patients with POP and control women. The mRNA levels for patients with POP were expressed as fold change relative to the control mRNA levels (set as 1). Validation experiments were performed to ensure that the PCR efficiencies between the target genes and the housekeeping genes were approximately equal.
Table 1.
Real-Time PCR Primer Sequences of a Panel of Genes Studied and Housekeeping genes.
| Target Genes | Primer Sequences | GenBank Accession # |
|---|---|---|
| MMP1 | Forward 5’ – TACGAATTTGCCGACAGAGATG– 3’ Reverse 5’–GCCAAAGGAGCTGTAGATGTCC– 3’ | NM_002421 |
| MMP2 | Forward 5’ – GAATACCATCGAGACCATGCG– 3’ Reverse 5’–CGAGCAAAGGCATCATCCA– 3’ | NM_004530 |
| MMP7 | Forward 5’ – TCCCCCTGCATTTCAGGAA– 3’ Reverse 5’–TTCCTGGCCCATCAAATGG– 3’ | NM_002423 |
| MMP9 | Forward 5’ – CCTCGAACTTTGACAGCGACA– 3’ Reverse 5’–AATGATCTAAGCCCAGCGCGT– 3’ | NM_004994 |
| MMP12 | Forward 5’ – AAGGCCGTAATGTTCCCCA– 3’ Reverse 5’– CAGGATTTGGCAAGCGTTG– 3’ | NM_002426 |
| Elastase | Forward 5’ – AATCCACGGAATTGCCTCCTT– 3’ Reverse 5’– TCGGAGCGTTGGATGATAGAG– 3’ | NM_001972 |
| Cathepsin S | Forward 5’ – CGCGTCATCCTTCTTTCTTCC – 3’ Reverse 5’– TGTGGCCCCAGCTGTTTTT– 3’ | NM_004079 |
| Cathepsin L | Forward 5’ – TAATGGAGGCCTGGACTCTGA – 3’ Reverse 5’– CAAAGCCGGTGTCATTAGCA– 3’ | NM_001912 |
| Cathepsin K | Forward 5’ – CCCGCAGTAATGACACCCTTT – 3’ Reverse 5’–AAGCCCAACAGGAACCACACT – 3’ | NM_000396 |
| TIMP1 | Forward 5’ – TTCTGGCATCCTGTTGTTGCT– 3’ Reverse 5’–CCTGATGACGAGGTCGGAATT– 3’ | NM_003254 |
| TIMP2 | Forward 5’ – GCGTTTTGCAATGCAGATGTAG– 3’ Reverse 5’– TCTCAGGCCCTTTGAACATCTT– 3’ | NM_003255 |
| TIMP3 | Forward 5’ – CTGCTGACAGGTCGCGTCTA– 3’ Reverse 5’–GCTGGTCCCACCTCTCCAC– 3’ | NM_000362 |
| TIMP4 | Forward 5’ – TCTGAACTGTGGCTGCCAAAT Reverse 5’–AGCTTTCGTTCCAACAGCCAG– 3’ | NM_003256 |
| ADAMTS2 | Forward 5’ – GTGTGCACCTGGCAAGCATTGTTT-3’ Reverse 5’–AGCCAAACGGACTCCAAGCGC– 3’ | NM_014244.4 |
| ADAMTS3 | Forward 5’ – TGCATGGGTGACCCTCCCGA-3’ Reverse 5’–ACCGCAGGTCACTGAACACTCA– 3’ | NM_014243.2 |
| ADAMTS14 | Forward 5’ – CGCTGGATGGGACTGAGTGT-3’ Reverse 5’–CGCGAACATGACCCAAACTT-3’ | NM_139155.2 |
| YWHAZ | Forward 5’–ACTTTTGGTACATTGTGGCTTCAA – 3’ Reverse 5’ –CCGCCAGGACAAACCAGTAT – 3’ | NM_003406 |
| TBP | Forward 5’ – TGCACAGGAGCCAAGAGTGAA – 3’ Reverse 5’ – CACATCACAGCTCCCCACCA – 3’ | NM_003194 |
| ACTB | Forward 5’– ACCTTCAACACCCCAGCCATGTACG - 3’ Reverse 5’ –CTGATCCACATCTGCTGGAAGGTGG – 3’ | NM_001101 |
Abbreviations: ADAMT, A Disintegrin And Metalloproteinase with Thrombospondin Motifs; ACTB, β-actin; MMP, matrix metalloproteinase; PCR, polymerase chain reaction; TIMP, tissue inhibitors of metalloproteinases.
Western Immunoblot Analysis
The Western immunoblot technique was described in detail earlier.4 Briefly, frozen vaginal tissue samples were crushed under liquid nitrogen and homogenized in radio immunoprecipitation assay lysis buffer. Total protein concentrations were determined using BCA Protein Assay kit (Pierce, Rockford, Illinois). Protein samples (50 μg) were resolved by electrophoresis on a 10% sodium dodecyl sulphate (SDS)-polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, Massachusetts). Blots were blocked with 5% nonfat milk, incubated with the primary antibodies, washed 3 times in tris-buffered saline, and incubated with the required secondary antibody (Table 2). We used individual PVDF membranes for each protein studied; membranes were stripped and reprobed with housekeeping protein (β actin) to control the loading variations. The density of each band was measured using Image J software analysis (NIH, Bethesda, Maryland). The values of each sample were expressed as a ratio of protein of interest to housekeeping protein. We analyzed protein samples from 13 patients and 13 controls.
Table 2.
Summary of Antibodies Used in Western Immunoblot (WB) or in Immunohistochemical (IHC) Analysis.
| Antibody | Primary or Secondary | Monoclonal or Polyclonal | Dilution | WB and/or IHC | Source | Company |
|---|---|---|---|---|---|---|
| MMP12 | Primary | Polyclonal | 1:2000 | WB, IHC | Chicken | Abcam, Cambridge, Massachusetts (cat# ab1827) |
| TIMP 1 | Primary | Polyclonal | 1:1000 | WB | Mouse | RDI (Fitzgerald Industries, Concord, Massachusetts (cat# 10R-T112a) |
| TIMP1 | Primary | Monoclonal | 1:100 | IHC | Mouse | Abcam, Cambridge, Massachusetts (cat# ab1828) |
| TIMP2 | Primary | Monoclonal | 1:500 | WB, IHC | Mouse | Abcam, Cambridge, Massachusetts (cat# ab39206) |
| TIMP3 | Primary | Polyclonal | 1:1000 | WB, IHC | Rabbit | Abcam, Cambridge, Massachusetts (cat# ab39206) |
| TIMP4 | Primary | Polyclonal | 1:500 | WB, IHC | Rabbit | Abcam, Cambridge, Massachusetts (cat# ab51207) |
| ADAMTS2 | Primary | Monoclonal | 1:200 | WB, IHC | Mouse | Abcam, Cambridge, Massachusetts (cat# ab56735) |
| β-actin | Primary | Polyclonal | 1:1000 | WB | Rabbit | BioVision, Mountain View, California (cat# 3662-100) |
| α-SM actin | Primary | Polyclonal | 1:50 | IHC | Rabbit | Dako, Carpinteria, California, M0851 |
| HRP-conjugated anti-rabbit IgG | Secondary | Polyclonal | 1:3000 | WB | Donkey | GE Healthcare, Buckinghamshire, United Kingdom |
| HRP-conjugated anti-chicken IgG | Secondary | Polyclonal | 1:10000 | WB | Rabbit | Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania |
| HRP-conjugated anti-mouse IgG | Secondary | Polyclonal | 1:4000 | WB | Sheep | GE Healthcare, Buckinghamshire, United Kingdom |
| Anti-mouse IgG | Secondary | Polyclonal | 1:200 | IHC | Horse | Vector Lab-BA2000 |
| Anti-rabbit IgG | Secondary | Polyclonal | 1:200 | IHC | Goat | Vector Lab-BA1000 |
| Anti-chicken IgY | Secondary | Polyclonal | 1:1000 | IHC | Rabbit | Jackson ImmunoResearch |
Abbreviations: ADAMT, A Disintegrin And Metalloproteinase with Thrombospondin Motifs; Ig, immunoglobulin; HRP, horseradish peroxidase; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinases.
Luminex Assay
Fifty micrograms of total protein from vaginal biopsy samples were used for Luminex assay. Tissue MMPs or TIMPs levels were quantified using (1) Milliplex MAP Magnetic Bead Human MMP Panel-2 kit and (2) Human TIMP Panel-1 kit (both from Millipore). Multiplex assay was performed on Luminex 200 system and Bioplex HTF (Bio-Rad) in accordance with the manufacturer’s instructions. Standards and each sample were analyzed in duplicate. Data analysis was performed using Bio-Plex Manager, version 5.0 (Bio-Rad) and presented as concentrations (pg/mL). We analyzed protein samples from 11 patients and 9 controls.
Zymography
Gelatin zymography were used to detect the enzymatic activity of MMP-2 and MMP-9. Total protein (50 μg/lane) was mixed with an equal volume of 2× nonreducing sample buffer and loaded on a gel (Novex 10% Zymogram gelatin gel, Invitrogen; Carlspad, California). The gels were electrophoresed in Tris/Glycine/SDS running buffer and were washed twice in renaturing buffer (2.5% [vol/vol] Triton X-100) at room temperature to remove SDS. Zymograms were subsequently developed by incubation in substrate reaction developing buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 0.02% (wt/vol) NaN3, pH 8.0) overnight at 37°C with gentle shaking. Incubated gels were then stained with Coomassie Blue R250 in 10% (vol/vol) acetic acid and 30% methanol and destained briefly in the same solution without the dye. Proteolytic activities were detected by clear bands indicating lysis of the substrate. The gels were scanned and quantification of band density was carried out using Image J software analysis (NIH).
Immunohistochemistry
To verify the morphology of vaginal samples, we used α-smooth muscle actin (ACTC1) immunostaining; the expression and localization of total collagen and elastin proteins in vaginal tissues were studied using histological Masson Trichrome and Elastin Van Gieson (EVG) staining, correspondingly. Immunohistochemical staining was performed as described earlier.9 Antigen retrieval was performed by treatment in microwave for 20 minutes in 10 nmol/L sodium citrate buffer (TIMP-1) and 0.25% trypsin (ACTC1 and MMP-12) or 10 µg/mL proteinase K (ADAMTS-2) followed by blocking with serum-free protein block for 30 minutes (Dako, Carpinteria, California) and overnight incubation with primary antibodies at 4°C followed by an appropriate secondary antibodies (Table 2). For the negative controls, ChromPure nonspecific mouse immunoglobulin (IgG) Gs (Jackson Laboratories, West Grove, Pennsylvania), nonspecific rabbit IgG and nonspecific goat IgG (both Santa Cruz, Santa Cruz, California) were used at the same concentration as the primary antibodies (Table 2) and sections were also incubated with secondary antibodies in the absence of primary antibodies. Vaginal tissue sections were observed on a Leica DMRXE microscope (Leica Microsystems, Canada). A minimum of 3 fields were examined for each specimen and the representative tissue sections were photographed with Sony DXC-970 MD (Sony, Ontario, Canada) 3CCD color video camera. In all, 3 vaginal samples from patients with POP and 3 samples from asymptomatic controls were analyzed.
Statistical Analysis
Unpaired comparison between expression/activity of MMPs, ADAMTS-2, TIMPs genes, and proteins in patients with POP and asymptomatic controls was performed using Wilcoxon signed-rank test (Prism version 4.02). Fisher exact test was used to compare demographic variables between the groups. The level of significance was set at P < .05. Results are reported as mean ± standard error of the mean (SEM).
Results
Vaginal tissue biopsy samples were obtained from 17 patients with POP and 19 controls that fit our selection criteria and were included in the study. In all, 10 controls and 8 patients with POP were in the secretory phase of the menstrual cycle and 9 patients and 9 controls in the proliferative phase. Study groups were matched for age (43.5 vs 44.9 years), race (caucasian), and body mass index (BMI; 27.5 vs 28.7). The mean parity, family history of POP, and incidence of stress urinary incontinence (SUI) was significantly higher in patients with POP compared to controls (P = .01, .01, and .007, respectively; Table 3). No significant difference was noted regarding smoking habits between the 2 study groups.
Table 3.
Demographic Data of the Study Groups.
| Study Groups | Group 1 | Group 2 |
|---|---|---|
| Premenopausal Patients | Premenopausal Control | |
| n | 17 | 19 |
| Mean age | 43 ( ± 8) | 46.4 ( ± 8) |
| Mean BMI | 27.13 ( ± 17.3) | 28.53 ( ± 14.4) |
| Mean Parity | 2.81 (1,4) | 1.8 (0,3)a |
| SUI | 64% | 29%a |
| Family History POP (%) | 50% | 12%a |
| Stage of POP (n, %) | III C (n = 14, 87%) IV C (n = 3, 13%) | Stage 0 (19) |
Abbreviations: BMI, body mass index; POP, pelvic organ prolapse; SUI, Stress urinary incontinence.
aindicates statistical difference between groups 1 and 2.
Fisher’s exact test; level of significance: P value <.05.
Gene Expression Profile
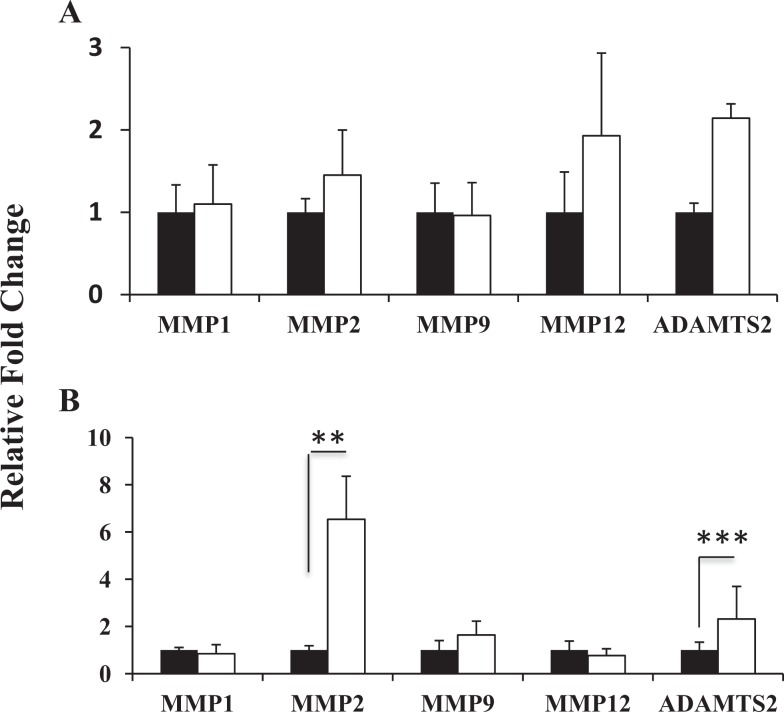
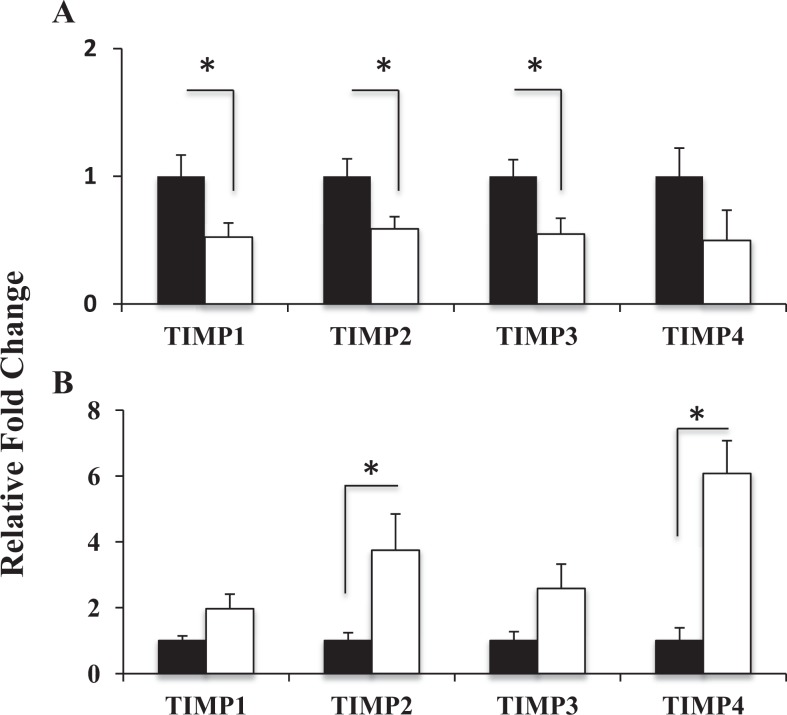
MMP-1, -2, -7, -8, -9, -12, elastase, cathepsins S, L, K, ADAMTS-2, -3, -14; and TIMP-1, -2, -3, -4 expressions were studied in all tissue samples collected (see Table 4). The mRNA levels of neutrophil elastase, cathepsins S, L, and K were very low and did not change between the 2 groups (data not shown). Neither MMP-7 nor MMP-8 was detected in either premenopausal patients with POP or controls. This was not due to poor quality of mRNA, which was confirmed by the consistent CT levels of the 3 different housekeeping genes across all samples. There was a significant (P < .05) increase in MMP-2 mRNA levels in vaginal tissue of patients with POP in the secretory phase of the menstrual cycle compared to controls (Figure 1A). During the proliferative phase, MMP-12 transcript level was increased in diseased tissues; however, the difference did not reach significance (not significant, P = .06; Figure 1A); there was no difference in MMP-12 mRNA between patients with POP and controls in vaginal tissues from the secretory phase. The MMP-1 and MMP-9 transcripts were detectable in both the study groups but were not affected by prolapse. The MMP-1 expression was lower than other protease genes. The ADAMTS-2 mRNA levels were increased in both the groups of patients with severe POP compared to controls, independent of the phase of the menstrual cycle, secretory or proliferative (Figure 1, P = .002 and P = .06, correspondingly). ADAMTS-3 and ADAMTS-14 transcript levels were very low in patients with POP and were not detected in vaginal biopsies of the control group. Importantly, gene expression of the whole TIMPs family (TIMP-1-4) in vaginal tissue of patients with POP showed a significant reduction (Figure 2A, P < .05) during the proliferative phase of the menstrual cycle compared to controls; however TIMP-2 and TIMP-4 mRNA were significantly increased during the secretory phase (Figure 2B).
Table 4.
Description of the Main Functions of the Studied ECM-Remodeling Proteins.
| Nomenclature Name | Name | Protein Function |
|---|---|---|
| MMP-1 | Interstitial collagenase | Degradation of collagen I, II, III |
| MMP-2 | Gelatinase A | Degradation of denatured collagens (gelatin) |
| MMP-7 | Stromelysin (matrilysin 1) | Degradation of proteoglycans, fibronectin, elastin, involved in wound healing |
| MMP-8 | Neutrophil collagenase | Cleavage of interstitial collagens |
| MMP-9 | Gelatinase B | Degradation of denatured collagens |
| MMP-12 | Macrophage Elastase | Degrades soluble and insoluble elastin |
| Elastase | Neutrophil Elastase | Degrades soluble and insoluble elastin |
| Cathepsin S | Lysosomal cystein protease (papain family) | Degrades elastin, laminin, fibronectin, some collagens and proteoglycans |
| Cathepsin L | Lysosomal cystein protease (papain family) | Degrades collagen, elastin and alpha-1 protease inhibitor, a major controlling element of Neutrophil Elastase activity. |
| Cathepsin K | Lysosomal cystein protease | Degrades collagen, elastin and gelatin, expressed predominantly in osteoclasts |
| TIMP-1 | Tissue inhibitor of MMPs | Inhibitory role against most of the known MMPs |
| TIMP-2 | Tissue inhibitor of MMPs | Critical role in inhibiting protease activity in tissues undergoing remodeling of ECM; interacts with MMP2 |
| TIMP-3 | Tissue inhibitor of MMPs | Modulation of MMPs function |
| TIMP-4 | Tissue inhibitor of MMPs | Modulation of MMPs function |
| ADAMTS2 | Procollagen-N-Peptidase | Cleavage of N-terminal pro-peptide chain of procollagen molecule |
| ADAMTS3 | Procollagen-N-Peptidase | Procollagen II N-peptidase activity in cartilage |
| ADAMTS14 | Procollagen-N-Peptidase | Procollagen I N-peptidase activity in tendon |
Abbreviations: ADAMT, A Disintegrin And Metalloproteinase with Thrombospondin Motifs; ECM, extracellular matrix; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinases.
Figure 1.
Real-time quantitative RT-PCR analyses of MMP-1, -2, -9, -12 and ADAMTS-2 mRNA levels in vaginal tissues from premenopausal women with POP (empty bars) and asymptomatic control (black bars) on the proliferative (A) and secretory (B) phase on the menstrual cycle. The results shown are the mean ± SEM. A significant difference is indicated by * (p<0.05). Total RNA from 17 POP patients and 19 controls was analysed.
Figure 2.
Real-time quantitative RT-PCR analysis of TIMPs family gene expression in vaginal tissue of premenopausal women with severe POP (empty bars) and premenopausal asymptomatic controls (black bars) on the proliferative (A) and secretory (B) phase on the menstrual cycle. The results represent the mean ± SEM. A significant difference is indicated by * (P<0.05). Total RNA from 17 POP patients and 19 controls was analysed.
Protein Expression Profile
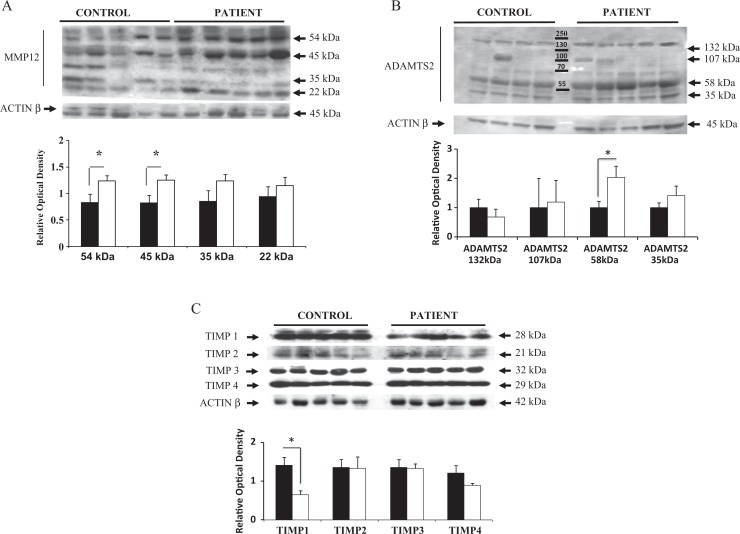
Next, we examined whether there was correlation between the genes that were altered in both patients with POP and controls (MMP-2, -9, -12, ADAMTS-2, and TIMP-1, -2, -3, -4) with their corresponding protein expression levels. First, immunoblotting analysis was performed using an antibody that specifically recognized MMP-12 protein in vaginal extracts from patients with POP and controls in the proliferative phase of the menstrual cycle. It was reported that a 54-kDa proform of MMP-12 is quickly activated to the 45-kDa form and breaks down to a cascade of active forms, ending with the common 22-kDa isoform.9 , 21 Assay results consistently showed a pattern of 4 different bands of appropriate molecular weight (54, 45, 35, and 22 kDa) in vaginal tissue sample bands (Figure 3A). Densitometric analysis of the different MMP-12 isoforms revealed a significant increase in the expression of the 54-kDa proform and the 45-kDa active form in vaginal biopsy samples from patients with POP (P < .05). The 35-kDa and 22-kDa active forms of MMP-12 were also increased in vaginal samples of patients with POP compared to controls; however, the difference was not significant (Figure 3A). Second, ADAMTS-2 protein expression was examined in both study groups. We detected 4 bands of appropriate molecular weight: ADAMTS-2 isoform I: 132 kDa (pro-form) and 107 kDa (active form) and ADAMTS-2 isoform II: 58 kDa (pro-form) and 35 kDa (active form; Figure 3B). A similar significant increase in the expression of the 58-kDa form of ADAMTS-2 was found in the vaginal tissues of patients with POP compared to asymptomatic controls in both the proliferative (P < .01, shown in Figure 3B) and the secretory phase (data not shown) of the menstrual cycle.
Figure 3.
Representative Western immunoblot and densitometric analysis of (A) MMP-12, (B) ADAMTS-2, (C) TIMPs family proteins detected in vaginal biopsies from premenopausal patients with POP (empty bar) and asymptomatic controls (black bar). A: The expected 54 kDa MMP-12 (pro-form) and 45 kDa, 35 kDa, and 22 kDa forms were detected in all samples from women in the proliferative phase of the menstrual cycle. Expression of the 54 kDa and 45 kDa forms of MMP-12 were significantly elevated. B: Four bands representing ADAMTS-2 isoform 1 (132-107 kDa) and ADAMTS-2 isoform 2 (58-35 kDa) were detected in the human vaginal tissues during proliferative and secretory phase of menstrual cycle. 58 kDa protein representing ADAMTS-2 isoform 2 was increased in POP patients as compared to asymptomatic controls in both groups; (C) TIMP-1, -2, -3, -4 were all expressed in the in vaginal tissues of patients with POP and controls in both study groups. Densitometric analysis indicates that only TIMP-1 expression was significantly reduced in POP patients from the proliferative phase group. The results shown are the mean ± S.E.M. A statistically significant difference is indicated by *(p<0.05). Protein samples from 13 POP patients and 13 controls were analyzed.
Finally, we detected TIMP-1, TIMP-2, TIMP-3, and TIMP-4 in the vaginal specimens as specific bands at 28 kDa, 21 kDa, 32 kDa, and 29 kDa, respectively. There was a significant reduction in TIMP-1 protein in patients with POP compared to asymptomatic controls during the proliferative phase of the menstrual cycle (see Figure 3C); however, the expression of TIMP-2, -3, and -4 proteins was similar between controls and patients with POP. The expression of all TIMP proteins did not change between patients with POP and controls in the vaginal samples of premenopausal women in the secretory phase of the menstrual cycle.
We additionally analyzed protein tissue lysates extracted from vaginal biopsy samples of patients with POP and control women by high-throughput approach, bead-based 3-plex MMP kit (MMP-1, -2, -9), and 4-plex TIMP 1-4 kit (Millipore) on the Luminex 200 platform. The MMP-1 (fibroblast collagenase) protein expression was barely detectable (2-10 pg in 50 μg of total protein) in the vaginal tissue of premenopausal women, which mirrored the low transcript levels of the MMP-1 gene. The MMP-2 protein expression levels were not significantly different between patients with POP and controls both in proliferative phase (2871 ± 1805 in controls vs 5179 ± 2012 pg in patients with POP, P = .089) and secretory phase (6368 ± 4130 in controls vs 8659 ± 4168 pg in patients with POP, P = .158) of menstrual cycle. Expression of MMP-9 was lower than MMP-2 during proliferative phase (1189 ± 400 pg in controls vs 1951 ± 1753 pg in patients with POP, P = .35) and secretory phase (2194 ± 2556 pg in controls vs 1818 ± 752 pg in patients with POP, P = .23). The average protein expression of TIMP-2 in human vaginal tissues (12 319 ± 4665 pg, range 6355-2526 pg) was 10 times higher than TIMP-1 (1236 ± 449 pg in 50μg of total protein, range 622-2394 pg) and TIMP-3 (1179 ± 733 pg, range 445-2691 pg). The TIMP-4 expression was very low in human vagina (25.8 ± 14.2 pg/mg in 50 μg of total protein, range 8.2-51.8 pg).
Gelatinase Activity
Enzymatic activity was examined by zymography in vaginal samples from patients with POP and asymptomatic controls. Gelatinase activity corresponding to proform and active forms of both, MMP-2 and MMP-9, was detected. The MMP-2 activity was observed at 68 kDa (latent proform) and 62 kDa (active form); MMP-9 activity was found at 92 kDa (latent pro-form) and 87 kDa (active form; Figure 4). Densitometric analysis of the detected bands revealed a significant increase in gelatinase activity of MMP-2 (both latent and active forms) in vaginal biopsy samples of patients with POP during proliferative phase (Figure 4A) and secretory phase of menstrual cycle (Figure 4B) compared to controls; MMP-9 activity was similar between the 2 groups.
Figure 4.
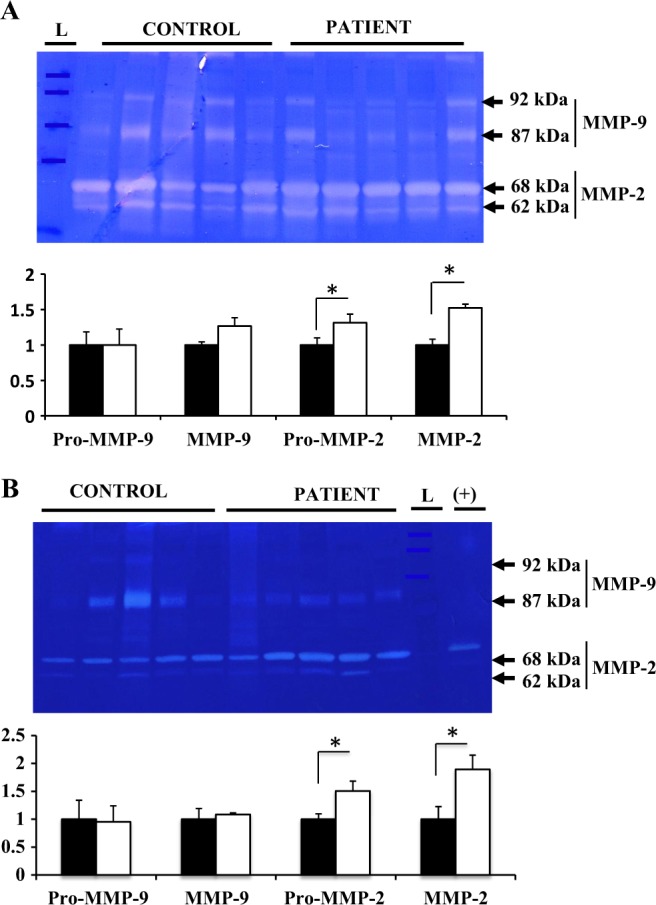
Representative gelatin zymography and densitometric analysis of MMP-2 and MMP-9 gelatinase activity in vaginal tissues of women with severe POP (white bar) and control asymptomatic women (black bar) in the proliferative (A) and secretory (B) phase of the menstrual cycle. MMP-2 activity is observed predominantly at 68 kDa (latent pro-form) and 62 kDa (active form); MMP-9 activity is detected at 92 kDa (latent pro-form), and 87 kDa (active form). The results shown are the mean density of MMP-2 and MMP-9 ± S.E.M. “L” represents protein ladder, “(+)” represents positive control (human decidual conditioned media). A significant difference is indicated by * (p<0.05). Protein samples from 13 POP patients and 14 controls were analyzed.
Immunohistochemical Localization of TIMP-1, MMP-12, and ADAMTS-2
Masson trichrome, EVG staining, and immunolabeling with ACTC1 confirmed that all collected vaginal samples from premenopausal patients with POP and premenopausal controls included the stratified squamous epithelium (SSE), lamina propria (LP), and muscularis (MUS) layers (Supplemental Figures 1 –3). The SSE was seen as a thin mucosal layer, overlying a loose connective tissue layer of lamina propria, containing fibroblast cells, elastin, collagen, and multiple blood vessels. The vaginal muscular layer consists primarily of smooth muscle cells and vascular complexes with smaller amounts of collagen and elastin. Our visual observation indicates that in women with POP, the muscularis layer was organized in discrete, tightly packed bundles that were oriented in circular and longitudinal directions; these bundles were smaller in asymptomatic controls.
The specific immunostaining using anti-MMP-12, anti-TIMP-1, and anti-ADAMTS-2 antibodies was performed on full-thickness vaginal wall samples (n = 3/group) to establish tissue localization of proteins that showed differential expression by quantitative immunoblot analysis. Our results show that immunolabeling with anti-MMP-12 antibodies was detected in the SM fibers of the muscularis layer and in the vascular SM cells. The MMP-12 protein was also detected in the SSE layer, mainly at the pericellular and cytoplasmic areas of epithelial cells. The TIMP-1 protein was expressed in SSE vaginal layer and vaginal tissue fibroblasts but not in the SM cells of the muscularis layer. The ADAMTS-2 proteins were expressed by vaginal tissue fibroblasts and macrophage-like cells. Importantly, a large number of macrophage-like cells, that also displayed a strong MMP-12 and TIMP-1 immunostaining, were scattered in the LP and MUS around blood vessels. According to our visual observation of the limited number of tissue specimens the distributions of positive cells were similar in vaginal tissue of patients with POP and asymptomatic controls (Supplemental Figure 4).
Discussion
It is well known that reproductive organs are influenced by ovarian hormones during the menstrual cycle, a process that diminishes after menopause. It was shown by others that overall collagen metabolism is activated in the proliferative phase, while elastin metabolism changes are more pronounced in the secretory phase of the menstrual cycle.21,22 Thus, the goal of this cross-sectional case-control study was to examine potential changes in the proteins responsible for the integrity of vaginal tissue structure in patients with POP and age-matched women before menopause. We included premenopausal women similar in age, race, hormonal status, and POP-Q stage in our study groups. Our results summarized in Table 5 (1) confirm that in patients with advanced POP, there is a higher expression of the ECM-degrading protein, MMP-2, during the secretory phase and (2) higher MMP-12, paralleled with a decrease in TIMP-1 expression during proliferative phase; (3) MMP-2 activity and the expression of ADAMTS-2/PNP, one of the enzymes responsible for mature collagen processing, were altered in patients with POP, independent of the menstrual cycle phase. Altogether, our current findings and our previous studies 23 suggest that ovarian hormones can modulate vaginal ECM components of women affected by POP.
Table 5.
Summary of the ECM-Remodeling Protein Changes Between Secretory and Proliferative Phases for Patients With POP Versus Control Asymptomatic Women.
| ECM Remodeling Proteins | ADAMTS2 | MMP-2 | MMP-9 | MMP-12 | TIMP-1 | TIMP-2 | TIMP-3 | TIMP-4 |
|---|---|---|---|---|---|---|---|---|
| Proliferative (POP vs control) |
|
|

|
|

|

|

|

|
| Secretory (POP vs control) |
|
|

|

|

|

|

|

|
Abbreviations: ADAMTS, A Disintegrin And Metalloproteinase with Thrombospondin Motifs; ECM, extracellular matrix; MMP, matrix metalloproteinase; POP, pelvic organ prolapse; TIMP, tissue inhibitors of metalloproteinases.
The MMPs are secreted or anchored to the cell surface and their potential physiological substrates include all membrane and ECM proteins. Typically, MMPs and other proteases (cathepsins) are not expressed in normal healthy tissues but can be detected during repair or remodeling processes or in diseased and inflamed tissue. We believe that this may be a reason why the transcript levels of neurtophil elastase, cathepsins, MMP-8 (neutrophil collagenase) and MMP-7 (also known as matrilysin) that degrades proteoglycans, fibronectin, elastin, and all involved in wound healing, were practically undetectable in both the study groups. Our preliminary IHC studies indicate the absence of the vaginal tissue neutrophils in both the groups (data not shown). Additionally, we were not able to detect a difference in MMP-1 (also known as collagenase 1) expression between patients with POP and controls in vaginal tissues from the homogenous group of premenopausal women. This was in contrast to immunostaining results presented by Dviri and colleagues in a mixed group of pre- and postmenopausal women, which showed an increase in MMP-1 in the POP group (consisting mostly of older patients) when compared to the younger controls.12 Our recently published data indicate that the molecular mechanisms underlying POP development in asymptomatic control women after menopause are different from the ones observed in asymptomatic control women before menopause; we discovered that the phase of the menstrual cycle can play an important role in the expression of vaginal MMPs of healthy women.23 Specifically, we have shown that MMP-1 transcript levels are significantly decreased during the proliferative phase of the menstrual cycle in control women when compared to the secretory phase and were further downregulated in the vagina of postmenopausal controls.
The present study revealed an increase in MMP-12 (macrophage metalloelastase) protein expression in vaginal tissues of patients with POP during the proliferative phase. The MMP-12 can degrade a broad spectrum of ECM components, including soluble and insoluble elastin. It also plays an indirect role in tissue destruction by degrading the serine proteinase inhibitor α1-antitrypsin, which altogether potentiates neutrophil elastase activity and results in further destruction of elastin24 negatively impacting ECM stability. The lack of regeneration in functional elastic fibers of adults is a major problem and once this regenerative ability is lost, the restoration of normal function is not possible.25 In addition, by degrading elastin, MMP-12 can generate elastin-derived peptides that are chemotactic for monocytes.26 We speculate that products of ECM degradation could attract monocytes to the pelvic floor tissues from the local vasculature, where they differentiate into tissue macrophages. We have recently reported that CD68-positive resident tissue macrophages were widely distributed in human vaginal samples.9 Our current immunohistological data shows that in addition to smooth muscle cells of the vaginal muscularis layer expressing MMP-12, there were numerous macrophage-like MMP-12-positive cells surrounding blood vessels. Further studies are needed to confirm their cellular authenticity. Macrophages are capable of producing other proteases, TIMPs, some angiogenic factors, and multiple inflammatory cytokines, all of which could change the microenvironment of the tissue. Recent analysis of single MMP-mutant mice reveals an important function of MMPs in regulating tissue response to environmental challenges, such as wounding, infection, and inflammation (reviewed in27,28). It is possible that the development of POP could be related to an abnormal repair of the injured tissue after the stress of vaginal delivery. We suggest therefore that upregulation of MMP-12 may play a role in the repair process in pelvic tissues of women with POP.
We also detected a significant increase in MMP-2 gelatinase activity in the vaginal tissue of women with POP, which may lead to reduced connective tissue strength and a progression of disease. Our results agree with previous studies showing significantly increased MMP-2 in the uterosacral ligament of women with POP paralleled with a dramatic decrease in collagen type I and type III.29,30 Other groups reported an increase in active MMP-9 in full-thickness vaginal biopsies of patients with POP relative to control women.12,14,31 In this study, however, we did not detect a difference in MMP-9 expression and activity in premenopausal women due to a high variability between samples.
To examine the regulatory mechanisms underlying the complex process of ECM degradation, we studied the expression of the whole family of TIMPs, major physiological inhibitors of MMPs, in vaginal tissue of women with POP and asymptomatic controls. We recently reported that TIMP-1 gene was upregulated in vaginal tissue samples of control premenopausal women in the proliferative (estrogen dominated) phase compared to the secretory (estrogen/progestogen regulated) phase of the menstrual cycle.23 Now we detected a significant reduction in TIMP-1 protein expression in premenopausal patients with POP versus controls from the proliferative group. As ovarian hormones may control vaginal collagen metabolism, these data may indicate that the estrogen-dominated environment (which was protective against collagen degradation via MMP1/TIMP1 homeostasis in asymptomatic women23) has failed in patients with POP. Similar to our results obtained in the premenopausal group of women, Chen and colleagues32 detected a decrease in TIMP-1 but not of TIMP-2 or TIMP-3 expression in vaginal tissue of mixed pre- and postmenopausal patients with SUI and POP. This may indicate that the TIMP-1 decrease is not influenced by age or menopause status of patients with POP and that TIMP-1 deficiency is a feature consistently present in the deficient (prolapsed) pelvic floor tissue.
Expression of ADAMTS-2/PNP proteins was altered in the vaginal tissues of patients with premenopausal POP. This may result in the formation of anomalous ECM fibers in women with POP disease, which may impair the functional integrity of the pelvic floor connective tissue. The cumulative effect of these changes in ADAMTS-2, MMP-2, and TIMP-1 may contribute to the weakened pelvic floor tissues of patients with POP, which culminate in the development of prolapse.
The strength of our study is the strictly homogeneous patient population, regarding age, ethnicity, and the hormonal status. We acknowledge, however, that our study has limitations. Specifically, the 2 study groups were not matched for parity, a known factor in POP formation. We were not able to analyze the protein level of all samples used for mRNA quantification due to the size of the vaginal tissue biopsies, sometimes just enough for RNA quantification. Moreover, because of scarce samples we completed IHC only in a small number of vaginal tissue specimens from patients with POP and asymptomatic controls to localize the proteins involved in the process of ECM homeostasis.
Conclusions
This comprehensive study analyzed multiple MMPs, TIMPs, and ADAMTSs in human vaginal tissue from premenopausal women and suggest an association between increased MMPs/decreased TIMPs and POP development. We speculate that dysregulation of the finely tuned balance of ECM synthesis/degradation, paralleled with changes in mature collagen fiber composition, may compromise the quality of connective tissue in women leading to prolapse. Our data indicate that the protective effect of ovarian hormones on ECM-regulating enzymes that was detected in asymptomatic women during the proliferative phase as compared to the secretory phase of menstrual cycle23 was lost in patients with POP, possibly due to acquired effects of POP or risk factors associated with increased mechanical loading of the pelvic floor that can cause POP. Further studies are needed to understand whether the changes we detected in the prolapsed vaginal tissues are the cause or the effect of POP.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Authors’ Note: May Alarab and Hala Kufaishi have contributed equally in this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study’s funding was provided by the Physician’s Services Inc. Foundation (PSI) and the Research Fund, Department of Obstetrics and Gynecology at Mount Sinai Hospital, University Health Network, Toronto, Ontario, Canada.
Supplemental Material: Supplemental Figures 1-4 are available at http://rs.sagepub.com/supplemental.
Reference
- 1. Samuelsson EC, Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 pt 1):299–305. [DOI] [PubMed] [Google Scholar]
- 2. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278–1283. [DOI] [PubMed] [Google Scholar]
- 3. Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25(4):723–746. [DOI] [PubMed] [Google Scholar]
- 4. Bortolini MA, Shynlova O, Drutz HP, et al. Expression of Bone Morphogenetic Protein-1 in vaginal tissue of women with severe pelvic organ prolapse. Am J Obstet Gynecol. 2011;204(6):544–548. [DOI] [PubMed] [Google Scholar]
- 5. Le Goff C, Somerville RP, Kesteloot F, et al. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development. 2006;133(8):1587–1596. [DOI] [PubMed] [Google Scholar]
- 6. Werb Z, Banda MJ, McKerrow JH, Sandhaus RA. Elastases and elastin degradation. J Invest Dermatol. 1982;79(suppl 1):154s–159s. [DOI] [PubMed] [Google Scholar]
- 7. Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol. 2003;138(1-2):106–114. [DOI] [PubMed] [Google Scholar]
- 8. Wu L, Tanimoto A, Murata Y, et al. Matrix metalloproteinase-12 gene expression in human vascular smooth muscle cells. Genes Cells. 2003;8(3):225–234. [DOI] [PubMed] [Google Scholar]
- 9. Alarab M, Bortolini MA, Drutz H, Lye S, Shynlova O. LOX family enzymes expression in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Int Urogynecol J. 2010;21(11):1397–1404. [DOI] [PubMed] [Google Scholar]
- 10. Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12(11):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao BH, Zhou JH. Decreased expression of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral ligaments of postmenopausal women with pelvic organ prolapse. J Obstet Gynaecol Res. 2012;38(6):925–931. [DOI] [PubMed] [Google Scholar]
- 12. Dviri M, Leron E, Dreiher J, Mazor M, Shaco-Levy R. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):113–117. [DOI] [PubMed] [Google Scholar]
- 13. Moon YJ, Choi JR, Jeon MJ, Kim SK, Bai SW. Alteration of elastin metabolism in women with pelvic organ prolapse. J Urol. 2011;185(5):1786–1792. [DOI] [PubMed] [Google Scholar]
- 14. Strinic T, Vulic M, Tomic S, Capkun V, Stipic I, Alujevic I. Matrix metalloproteinases-1, -2 expression in uterosacral ligaments from women with pelvic organ prolapse. Maturitas. 2009;64(2):132–135. [DOI] [PubMed] [Google Scholar]
- 15. Chung DJ, Bai SW. Roles of sex steroid receptors and cell cycle regulation in pathogenesis of pelvic organ prolapse. Curr Opin Obstet Gynecol. 2006;18(5):551–554. [DOI] [PubMed] [Google Scholar]
- 16. Roshanravan SM, Dreves P, Word RA. Estrogen regulates elastogenesis and processing of Procollagen and Lysyl Oxidases in the vaginal wall. Reprod Sci. 2010;17(3):158A.19805552 [Google Scholar]
- 17. Zbucka-Kretowska M, Marcus-Braun N, Eboue C, et al. Expression of estrogen receptors in the pelvic floor of pre- and post-menopausal women presenting pelvic organ prolapse. Folia Histochem Cytobiol. 2011;49(3):521–527. [DOI] [PubMed] [Google Scholar]
- 18. Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG. 2006;113(1):39–46. [DOI] [PubMed] [Google Scholar]
- 19. Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. [DOI] [PubMed] [Google Scholar]
- 20. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 21. Chen B, Wen Y, Zhang Z, Wang H, Warrington JA, Polan ML. Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. Am J Obstet Gynecol. 2003;189(1):89–97. [DOI] [PubMed] [Google Scholar]
- 22. Chen B, Wen Y, Wang H, Polan ML. Differences in estrogen modulation of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-1 expression in cultured fibroblasts from continent and incontinent women. Am J Obstet Gynecol. 2003;189(1):59–65. [DOI] [PubMed] [Google Scholar]
- 23. Shynlova O, Bortolini M, Alarab M. Genes responsible for vaginal extracellular matrix metabolism are modulated by women's reproductive cycle and menopause. Int Braz J Urol. 2013;39(2):257–267. [DOI] [PubMed] [Google Scholar]
- 24. Nenan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005;100(suppl 1):167–172. [DOI] [PubMed] [Google Scholar]
- 25. Culav EM, Clark CH, Merrilees MJ. Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther. 1999;79(3):308–319. [PubMed] [Google Scholar]
- 26. Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66(4):859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. [DOI] [PubMed] [Google Scholar]
- 29. Gabriel B, Watermann D, Hancke K, et al. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):478–482. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Guess M, Datar A, et al. Knockdown of Hoxa11 in vivo in the uterosacral ligament and uterus of mice results in altered collagen and matrix metalloproteinase activity. Biol Reprod. 2012;86(4):100. [DOI] [PubMed] [Google Scholar]
- 31. Moalli PA, Jones IS, Meyn LA, Zyczynski HM. Risk factors associated with pelvic floor disorders in women undergoing surgical repair. Obstet Gynecol. 2003;101(5 pt 1):869–874. [DOI] [PubMed] [Google Scholar]
- 32. Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(2):80–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.