Abstract
Objectives:
This study is aimed to identify the expression of Notch family proteins in placentas from patients with early-onset severe preeclampsia.
Study Design:
The expression of Notch family proteins in placentas was investigated by immunohistochemistry, Western blotting, and real-time reverse transcription–polymerase chain reaction (RT-PCR).
Results:
The profile of distribution of all Notch family proteins in placentas from patients with early-onset severe preeclampsia is similar to that in normal placentas. All Notch family proteins are expressed in placental trophoblasts. Moreover, Notch1 and Jagged1 (Jag1) are detected in placental endothelial cells. Real-time RT-PCR showed that messenger RNA levels of Notch2 and Delta-like4 (Dll4) in placentas from patients with early-onset severe preeclampsia are lower than that of normal placentas. Western blotting showed a significant increase in Notch3 expression and a significant decrease in Notch2 expression in placentas from patients with early-onset severe preeclampsia relative to those in normal placentas.
Conclusion:
The results suggest that Notch2 and Notch3 may play some roles in the pathogenesis of preeclampsia.
Keywords: Notch proteins, placenta, severe preeclampsia, early onset
Introduction
Preeclampsia (PE) is a major complication of pregnancy and leads to increased perinatal morbidity and mortality worldwide. Its incidence is 2% to 8%.1 It is a pregnancy-specific syndrome with de novo development of concurrent hypertension and proteinuria, which sometimes progresses into a multiorgan cluster of varying clinical features.2
Notch signaling is evolutionarily conserved and exerts critical functions during cell fate determination, differentiation, and many other biological processes.3 Notch functions as a receptor. There are 4 Notch receptors (Notch1-4) and 5 ligands (Jag1 and 2 and Dll1, 3, and 4) in the mammalian Notch-signaling pathway.3 Most of Notch receptors at the cell surface are heterodimeric, single-pass transmembrane molecules,4 and all of the ligands are transmembrane-type proteins.5 The extracellular domains of both Notch and its ligands contain multiple epidermal growth factor (EGF) repeats. Notch can exert its biological functions by canonical and noncanonical pathways. The canonical Notch pathway is activated when Notch ligands bind to the EGF repeats 11 to 12 and 24 to 29 of the Notch extracellular domain (NECD) from adjacent cells, followed by sequential cytoplasmic cleavage of Notch intracellular domain (NICD).6–8 The liberated NICD translocates to the nucleus, where NICD interacts with the DNA-binding transcription factor CBF-1, Suppressor of Hairless, Lag-2 (CSL), which results in the transcriptional activation of Notch-targeting genes including the hairy and enhancer of split (HES) and HES-related proteins.9 Noncanonical Notch signaling is CSL independent and can be either ligand dependent or independent.10
The results from published studies demonstrate that Notch-signaling pathway is essential for the development of placenta. Defects in the Notch receptor-ligand pathway have adverse effect on the placentation.11 The poor early placentation is especially associated with early-onset preeclampsia (<34 weeks)2 during which cytotrophoblasts play an important role. Other studies also show that there are differences in expression of some Notch receptors and ligands between placentas from patients with preeclampsia complicated placentas and normal ones.12,13 To the best of our knowledge, no study has comprehensively investigated the expression of all known ligands and receptors of the mammalian Notch members in placentas from patients with early-onset severe preeclampsia. We hypothesize that there are additional differences in Notch member expression between placentas from patients with early-onset severe preeclampsia and normal ones in addition to the differences that had been detected. Given that placentas complicated by preeclampsia express decreased levels of Notch proteins,12,13 we further hypothesize that other Notch proteins may also be expressed at lower levels in placentas from patients with early-onset severe preeclampsia.
Materials and Methods
This study included 11 women with early-onset severe preeclampsia and 11 normal-term pregnant women (control group) without medical problems. All women underwent cesarean section. The control group was selected randomly. The gestational age at birth in women with early-onset severe preeclampsia ranged from 24+4 to 33+6 weeks. Early-onset severe preeclampsia is diagnosed when it occurred between 20 and 34 weeks of gestation that new-onset hypertension greater than 140/90 mm Hg and proteinuria greater than 0.3 g/24 h complicated by one or more of the following: systolic pressure ≥160 mm Hg, diastolic pressure ≥110 mm Hg, proteinuria ≥2 g/24 h or +++ on dipstick, oliguria <60 mL for 2 successive hours or <500 mL/24 h, epigastric or liver pain, headache, blurred vision, and pulmonary edema.14–16 Cesarean section was performed on normal pregnant women at 37+4 to 39+2 weeks of gestation. All participants participated in the study voluntarily and signed informed consent forms. All placental samples were obtained in less than 15 minutes after cesarean section. Placental samples (about 0.5 cm3) were collected from the center part of the placenta within 3 cm from the umbilical cord and tissues with infarct, fibrosis, and calcification were excluded. Sections contained the entire wall of the placenta covering the portion with or without amniotic membrane and the decidua basalis. The samples with amniotic membrane and the decidua basalis were fixed in 10% buffered formaldehyde for immunohistochemistry. And the samples free of amniotic membrane and decidua basalis were stored long term at −20°C for Western blotting and real-time RT-PCR.
Immunohistochemistry
Sections, 3 μm in thickness, were deparaffinized in xylene, rehydrated in a decreasing alcoholic row, and boiled in 0.01 mol/L citrate buffer (pH 6.0) for 2 minutes in a microwave. Endogenous peroxidase activity was blocked using hydrogen peroxide (3%). And then incubation with fetal calf serum was performed to reduce nonspecific binding for 20 minutes at 37°C. The slides were incubated with the primary antibodies overnight at 4°C. The primary antibodies are listed below: Notch1 (1:200; Santa Cruz, Dallas, Texas; Cat.No. sc-6014), Notch2 (1:400; Cell Signal Technology, Boston, Massachusetts; Cat.No. 4530p), Notch3 (1:200; Santa Cruz, Cat.No. sc-5593), Notch4 (1:200; Santa Cruz, Cat.No. sc-5594), Jag1 (1:200; Santa Cruz, Cat.No. sc-8303), Jag2 (1:200; Santa Cruz, Cat.No. sc-5604), Dll1 (1:100; R&D, Minneapolis, Minnesota; Cat.No. MAB18181), and Dll4 (1:100; R&D, Cat.No. MAB1506). After 3 rinses with phosphate-buffered saline (PBS), the slides were incubated with peroxidase-conjugated secondary antibodies (peroxidase-conjugated Affinipure goat anti-rabbit immunoglobulin G [IgG] 1:1000, Cat.No. 99635; peroxidase-conjugated Affinipure goat anti-mouse IgG 1:1000, Cat.No. 100320; and peroxidase-conjugated Affinipure rabbit anti-goat IgG 1:1000, Cat.No. 67164, all antibodies from Jackson ImmunoResearch, West Grove, Pennsylvania) for 60 minutes at 37°C. The slides were washed 3 times by PBS. Afterward, 3,3-diaminobenzidin (DAB) was used for visualization. Nuclei were counterstained with hematoxylin. Isotype-matched antibody (mouse IgG2B, R&D and rabbit IgG; Santa Cruz) was used as the negative control for the primary antibodies. The slides stained by anti-CD34 primary antibody were considered as positive controls. After washing with distilled water, the slides were mounted with neutral resins. Immunohistochemical staining was observed and evaluated with Nikon Eclipse 50i (Nikon, Japan).
The slides were evaluated according to the following morphological criteria, which is in agreement with that given by Herr et al.17 Syncytiotrophoblasts are located at the surface of placental villi, and there are no border among the syncytiotrophoblast cells with smaller and darker nuclei compared to the cytotrophoblast cells. Cytotrophoblast is a single discontinuous row just beneath the syncytiotrophoblast layer in the term placental villi. Stroma cells are longitudinal cells with protrusions. Endothelial cells are spindle-shaped cells at the inner surface of the blood vessels.
RNA Extraction and Quantitative Real-Time RT-PCR Analysis
Total RNAs of placentas were isolated with TRIzol reagent (Life technologies, Carlsbad, California). RNA purity, yield, and integrity were determined spectrophotometrically. Reverse transcription was performed using the Reverse Transcriptase kit (Promega; Madison, Wisconsin) following manufacturer’s instructions. The RT-PCR was conducted for several Notch receptor and their ligand genes with the ABI Prism 7900HT detection system (Applied Biosystems, Foster City, California) using SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan). Primers for targeted genes are listed in Table 1. Negative controls were performed without complementary DNA in the reaction mixture. The results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression. The relative quantification of target gene was performed with standard curve or comparative cycle threshold (CT) method.
Table 1.
Notch Receptors and Ligands Primer Pairs for RT-PCR Analysis.
| Gene | Primers |
|---|---|
| Notch1 | 5′-GAGGCGTGGCAGACTATGC-3′ |
| 5′-CTTGTACTCCGTCAGCGTGA-3′ | |
| Notch2 | 5′-CCTTCCACTGTGAGTGTCTGA-3′ |
| 5′-AGGTAGCATCATTCTGGCAGG-3′ | |
| Notch3 | 5′-TGGCGACCTCACTTACGACT-3′ |
| 5′-CACTGGCAGTTATAGGTGTTGAC-3′ | |
| Notch4 | 5′-TGTGAACGTGATGTCAACGAG-3′ |
| 5′-ACAGTCTGGGCCTATGAAACC-3′ | |
| Dll1 | 5′-GATTCTCCTGATGACCTCGCA-3′ |
| 5′-TCCGTAGTAGTGTTCGTCACA-3′ | |
| Dll4 | 5′-GCCCTTCAATTTCACCTGGC-3′ |
| 5′-CAATAACCAGTTCTGACCCACAG-3′ | |
| Jag1 | 5′-GTCCATGCAGAACGTGAACG-3′ |
| 5′-GCGGGACTGATACTCCTTGA-3′ | |
| Jag2 | 5′-TGGGACTGGGACAACGATAC-3′ |
| 5′-AGTGGCGCTGTAGTAGTTCTC-3′ | |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| 5′-GAAGATGGTGATGGGATTTC-3′ |
Abbreviations: Dll, Delta-like; GADPH, glyceraldehyde 3-phosphate dehydrogenase; Jag, Jagged; RT-PCR, real-time reverse transcriptase–polymerase chain reaction.
Western Blot Assay
Placental samples were lysed with radio immunoprecipitation assay (RIPA: 50 mmol/L Tris, pH 7.4; 150 mmol/L NaCl; 1% Triton X-100; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 2 mmol/L sodium pyrophosphate; 25 mmol/L β-glycerophosphate; 1 mmol/L EDTA; 1 mmol/L sodium orthovanadate [Na3VO4]; and 0.5 µg/mL leupeptin) using a buffer containing 1 mmol/L phenylmethanesulfonyl fluoride (PMSF) for 30 minutes in ice. Lysates were centrifuged at 12 000 rpm for 20 minutes at 4°C. Protein concentration was determined with the bicinchoninic acid (BCA) assay. Protein samples of 40 µg were denatured for 5 minutes at 95°C and followed by separation in an NuPAGE Novex 4% to 12% Bis-Tris Midi Gel, 26 W (Invitrogen, Carlsbad, California). The separated proteins were then transferred from the gel to a BioTrace polyvinylidene difluoride (PVDF) Transfer Membrane (Pall Corporation, Port Washington, New York) for 100 minutes at 4°C. After washing, the membranes were blocked with 5% fat-free milk at room temperature for 60 minutes, and the blot was then incubated overnight at 4°C with a diluted primary antibody solution. The primary antibodies are listed as follows: Notch1 (1:200; Santa Cruz, Cat.No. sc-6014), Notch2 (1:1000; Cell Signal Technology, Cat.No. 4530S), Notch3 (1:200; Santa Cruz, Cat.No. sc-5593), Notch4 (1:200; Santa Cruz, Cat.No. sc-5594), Jag1 (1:500; Abcam, Cambridge, Massachusetts; Cat.No. ab7771), Jag2 (1:200; Santa Cruz, Cat.No. sc-5604), Dll1 (1:200; R&D, Cat.No. MAB18181), and Dll4 (1:500; Abcam, Cat.No. ab7280). The subsequent incubation with a secondary antibody horseradish peroxidase conjugate was performed at room temperature for 2 hours. The secondary antibody included anti-rabbit (1:5000; KANG CHEN, Shanghai, China; Cat.No. KC-RB-035), anti-goat (1:5000; KANG CHEN, Cat.No. KC-GT-035), or anti-mouse (1:2000; Cell Signal Technology, Cat.No. 7076). An anti-β-actin antibody (1:5000, KANG CHEN, Cat.No. KC-5G5) was used to confirm equal loading. The presence of secondary antibody bound to the membrane was detected by Immobilon Western chemiluminescent HRP substrate (Millipore, Billerica, Massachusetts) and exposure of the membrane to photographic film at room temperature for the required time. Western blot band densities were measured by ImageJ software (U.S. National Institute of Health, Betheseda, Maryland), and protein expression levels are presented as ratios of each protein band density to its corresponding internal control (β-actin) density.
Statistical Analyses
Results are expressed as mean ± standard deviation. All the data were analyzed in SPSS version17. A P value <.05 was considered significant.
Results
Distribution of Notch Receptors and Ligands in Placentas From Patients With Early-Onset Severe Preeclampsia
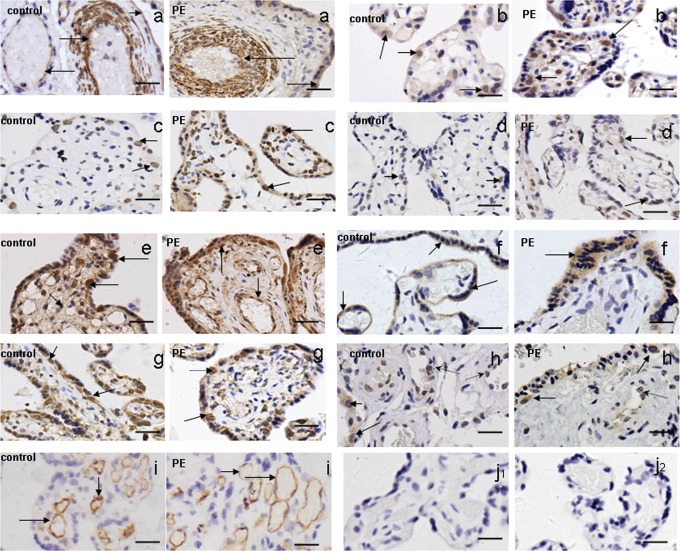
All of the Notch receptors (Notch1-4) and ligands (Jag1 and 2 and Dll1 and 4) were detected in placental villi from patients with early-onset severe preeclampsia. However, there was no significant difference in the expression profile between the abnormal placental villi and the normal-term ones. Notch1 was predominantly expressed in the endothelial cells and the vessel walls of the placental villi (Figure 1A). In addition, there was weak Notch-1 immunopositivity in the cytoplasm of syncytiotrophoblasts (Figure 1A). Notch2, 3, and 4 immunopositive cells were mainly cytotrophoblast cells (Figure 1B-D). Jag1 was detected in the cytotrophoblast cells as well as in the endothelial cells of the placental villi (Figure 1E). Jag2 was mainly found in the cytoplasm of syncytiotrophoblasts (Figure 1F). The other Notch ligands, Dll1 and Dll4, were predominantly immunopositive in the cytotrophoblast cells (Figure 1G and H), together with a faint immunopositivity for Dll4 in the endothelial cells (Figure 1H).The negative control is shown in Figure 1J, and the positive control (anti-CD34 staining) is displayed in Figure 1I.
Figure 1.
A-H, The expression profile of Notch receptors 1-4 (A-D) and its ligands Jag1 and 2 (E and F) and Dll1 and 4 (G and H) in placentas from patients with early-onset severe preeclampsia, by immunohistochemical staining. Notch1 was predominantly expressed in the endothelial cells and the vessel walls of the placental villi (A). In addition, there was weak Notch-1 immunopositivity in the cytoplasm of syncytiotrophoblasts (A). Notch 2, 3, and 4 immunopositive cells were mainly cytotrophoblast cells (B-D). Jag1 was detected in cytotrophoblast cells as well as in the endothelial cells of the placental villi (E). Jag2 was mainly found in the cytoplasm of syncytiotrophoblasts (F). Dll1 and Dll4 were predominantly immunopositive in the cytotrophoblast cells (G, H: solid arrows) together with a faint immunopositivity for Dll4 in the endothelial cells (H: dashed line arrows).The positive controls (anti-CD34 staining) was displayed in (I), and the negative control (isotype-matched control) was shown in (J1; Mouse IgG2B) and (J2; Rabbit IgG). Scale bar: 20 μm.
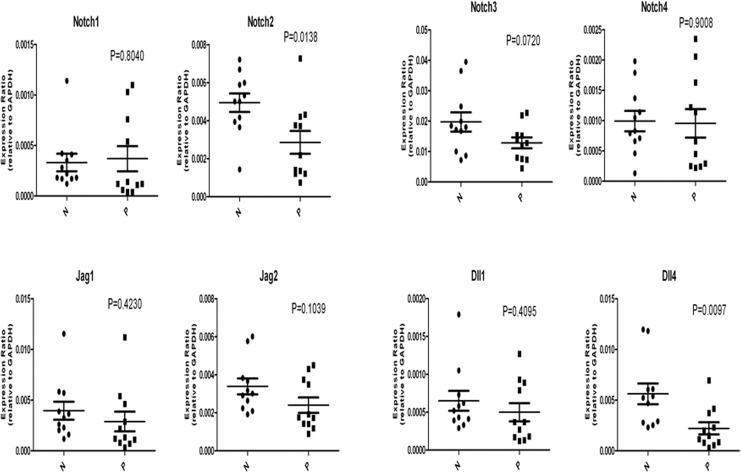
Quantitative Messenger RNA expression of Notch Receptors and Their Ligands in Placentas From Patients With Early-Onset Severe Preeclampsia
All of the specific messenger RNAs (mRNAs) of Notch receptors 1-4 and their ligands Jag1 and 2 and Dll1 and 4 were detected in the placentas from patients with early-onset severe preeclampsia (Figure 2). There was a trend for the downregulation of the mRNA expression of all Notch receptors and ligands, except for Notch1, in placentas from patients with early-onset severe preeclampsia when compared with the normal term placentas. Only the mRNA expression of Notch2 and Dll4 was significantly decreased in placentas from patients with early-onset severe preeclampsia. The mRNA expression of Notch1 was elevated in placentas from patients with early-onset severe preeclampsia, although the difference did not reach statistical significance.
Figure 2.
The messenger RNA (mRNA) expression of Notch receptor 1-4 and its ligands Jag1 and 2 and Dll1 and 4 by real-time reverse transcriptase polymerase chain reaction analysis in placentas from patients with early-onset severe preeclampsia. The relative mRNA expression of the Notch 2 and Dll4 decreased significantly in placentas of patients with early-onset severe preeclampsia compared with those of the normal-term placentas. Differences were considered to be significant with at least P < .05. P indicates placentas from patients with early-onset severe preeclampsia; N, normal term placentas.
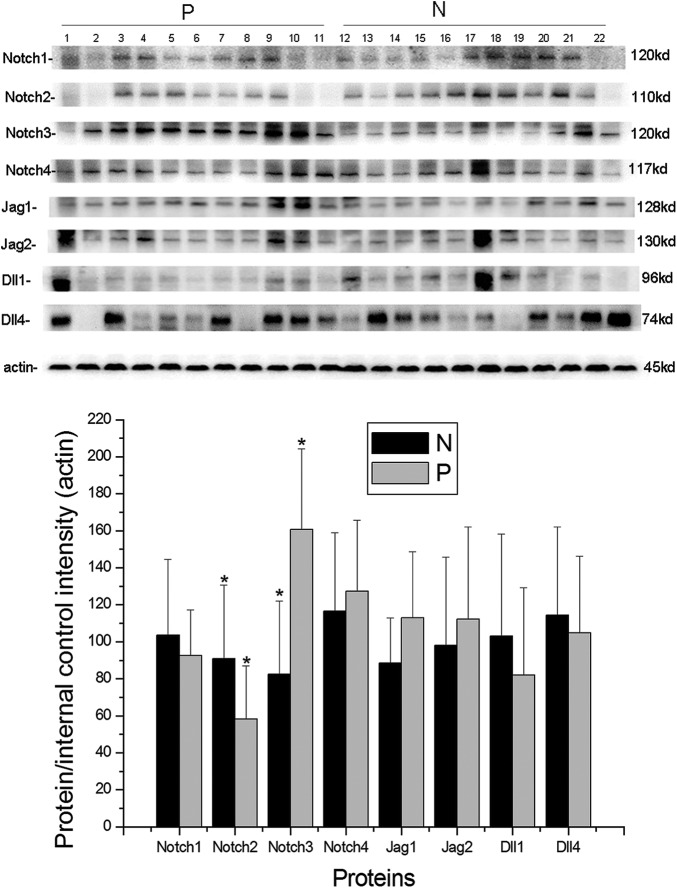
Levels of Protein Expression of Notch Receptors and Ligands in Placentas From Patients With Early-Onset Severe Preeclampsia
The level of protein expression of Notch family members was detected by Western blotting (Figure 3). The level of protein expression of Notch2 in placentas from patients with early-onset severe preeclampsia was found to be significantly lower than that in normal-term placentas (P < .05; Figure 3), while the level of protein expression of Notch3 in placentas from patients with early-onset severe preeclampsia was higher than that in the normal-term placentas (P < .01; Figure 3). The level of protein expression of other Notch receptors and ligands was similar in placentas from patients with early-onset severe preeclampsia and normal-term ones (P > .05, Figure 3).
Figure 3.
The protein expression of Notch receptors 1-4 and its ligands Jag1 and 2 and Dll1 and 4, by Western blotting in placentas of patients with early-onset severe preeclampsia. The protein expression level of Notch2 in placentas from patients with early-onset severe preeclampsia was significantly lower than that in the normal-term placentas (P < .05), while the protein expression level of Notch3 in placentas of patients with early-onset severe preeclampsia was higher than that in the normal-term placentas (P < .01). *P < .05. The error bars represent the means ± standard deviation (SD). P indicates placentas from patients with early-onset severe preeclampsia; N, normal-term placentas.
Discussion
This study shows that all of the mammalian Notch receptors (Notch1-4) and ligands (Jag1 and 2 and Dll1 and 4) were expressed at the levels of transcription and translation in placental villi from patients with early-onset severe preeclampsia except Dll3. Dll3 was excluded from the present study due to its mutation in the human Dll3 gene, which resulted in spondylocostal dysostosis, affecting the axial skeletal system.17 The mRNA levels of Notch2 and Dll4 were significantly decreased in placentas from patients with early-onset severe preeclampsia when compared to those of normal-term placentas. The level of Notch2 protein expression in placentas from patients with early-onset severe preeclampsia was significantly lower, whereas the level of Notch3 protein expression was higher than that in the normal-term placentas.
We found that most of the Notch receptors and ligands were expressed by cytotrophoblasts, suggesting that Notch-signaling pathway may be involved in the functions of cytotrophoblasts such as proliferation, differentiation, and invasion. Moreover, it is possible that the Notch members may regulate each other’s function since many Notch proteins coexpressed in cytotrophoblasts. Because the investigation of the role of Notch-signaling pathway in cytotrophoblast functions is beyond the scope of this study, we plan to do so in the near future.
In the current study, significant decreases in Notch2 and Dll4 mRNA expression were found in placentas from patients with early-onset severe preeclampsia relative to normal-term placentas. The protein expression of Notch members was evaluated by Western blot analysis. The data indicate that the protein level of Notch2 was significantly decreased in placentas from patients with early-onset severe preeclampsia, whereas that of Notch3 was significantly increased. In addition, since our finding on the protein expression levels of Notch3 is inconsistent with our hypothesis that Notch members are expressed at lower levels in preeclampsia-complicated placentas, we repeated Western blot assays 3 times and the results were highly reproducible. The opposite results from RT-PCR and Western blot assays may be due to (1) the differences in the translational rate of Dll4 and Notch3 mRNAs and/or (2) the different stability of Dll4 and Notch3 proteins. It is noted that such inconsistent results from RT-PCR and Western blot assays have been observed for Notch members in a previous study.18
As shown in Figure 1, Notch1-4, Jag1 and 2, and Dll1 and 4 were detected in placental villi of the 2 groups, which is consistent with another study.17 There was no significant difference in the staining pattern between the 2 groups. But the expression profile of some proteins in this study is seemingly at variance compared with the findings of the previous studies.13,17,19 Notch1 was not detected in cytotrophoblasts in the present study and in the study by Hunkapiller et al,11 while it was observed in the cytotrophoblasts by other studies.13,17,19 The current study revealed that cytotrophoblast cells were stained by Notch2, 3, and 4; Jag1; and Dll1 and 4, whereas only Notch1 and 3 and Dll1 were detected by Herr and coworkers.17 In the present study, Jag1 was immunostained in cytotrophoblasts, and our results are in agreement with those of the previous studies.13,19 We also found that Jag1 was expressed in the endothelial cells of placentas, consistent with the finding from a previous study.17 However, 2 other previous studies failed to detect Jag1 expression in the endothelial cells of placentas.13,19 Moreover, we found that Jag2 was present in the cytoplasm of syncytiotrophoblasts, but a previous study demonstrated that Jag2 was not expressed in syncytiotrophoblasts.17 Possible explanations for these differences include the heterogeneity of the placental tissues, the different antibodies used in the studies, different experimental systems, and different races.
Cobellis et al found using Western blot method that there was a significant downregulation of Notch1 and Notch4 in placentas with preeclampsia13; while in the present study, the protein expression of Notch1 and Notch4 was similar between placentas with preeclampsia and normal-term placentas. In another study, immunoreactivity of Notch1, 2, and 4 and Jag2 revealed significant downregulation in placentas with preeclampsia12; whereas in our study, only the protein expression of Notch2 showed decrease in the preeclampsia group. The difference between these 2 articles and the present article may attribute to the different gestational age of the samples. The gestational age of the samples with preeclampsia in the study by Cobellis et al ranged from 35 to 40 weeks,13 while in the current study it was less than 34 weeks of gestation. In a similar way, the gestational age of specimens with preeclampsia ranged from 35 to 40 weeks in the study by Sahin and coworkers.12 In the study by Sahin and coworkers, the samples were from basal plate and chorionic plate of the placentas,12 whereas the specimens in the current study were excluded from basal plate and chorionic plate of the placentas. Moreover, the protein levels of Notch members in the current study were determined by Western blot analysis. In contrast, Sahin and coworkers investigated the expression of Notch members by immunohistochemistry method.12
To our knowledge, our study is the first one to indicate the elevated protein level of Notch3 in placentas from patients with early-onset severe preeclampsia compared to the normal-term placentas. We have provided the evidence that Notch3 is the first one of Notch family whose protein expression level increased in placentas with preeclampsia. The role of Notch3 in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) has been thoroughly investigated over the last decade.20 In addition, it has been demonstrated that Notch3 takes some roles in cancer.21,22 Given that there is a significant difference in Notch3 protein expression between placentas from patients with early-onset severe preeclampsia and normal-term placentas, it is implied that Notch3 may play some role in the pathogenesis of early-onset severe preeclampsia. Previous studies have demonstrated that Notch3 is involved in some cell functions. It can promote proliferation and inhibit apoptosis of ErbB2-negative breast tumor cells through the activation of the canonical Notch pathway.23 In addition, Notch3 is capable of regulating the differentiation and proliferation of esophageal squamous cell.24 Rahman et al revealed that proliferation of KFr13 and KFr13Tx cells was decreased and apoptosis was induced when Notch3 was inactivated by γ-secretase inhibitor or small interfering RNA.21 Other studies also provided the evidences that Notch3 plays some role in cell proliferation and apoptosis.25–27 Our experimental data, together with the aforementioned studies, imply that Notch3 may regulate the proliferation, apoptosis, or differentiation of placental cytotrophoblast cells, which are dysregulated in preeclampsia28–30 and thus play important roles in the pathophysiological mechanism of early-onset severe preeclampsia. However, the exact roles of Notch3 in early-onset severe preeclampsia remain to be elucidated. Similarly, Notch2 can also regulate cell proliferation, apoptosis, and differentiation.31–33 Thus, Notch2 may also be implicated in the pathophysiological mechanism of early-onset preeclampsia since its protein expression was significantly decreased in placentas with preeclampsia compared with normal placentas. It remains to be demonstrated whether Notch2 and Notch3 interact, since they are coexpressed in the placental cytotrophoblast cells.
There are some possible limitations in our study. First, this study was limited by the small sample sizes. Second, the gestational age between the 2 groups is inconsistent. If the gestational age in the control group is consistent with that in patient group, it should be less than 34 weeks of gestation. However, the women, who delivered before 34 weeks of gestation, always had complications and abnormalities, such as preterm labor, placental abruption, and so on. These complications may affect the expression of Notch members. Thus, the normal-term control was chosen for this current study. Moreover, it was revealed that Notch3 expression was elevated in placentas of third trimester relative to that in the placentas of first trimester,17 which mirrors the reality of this current study. Unfortunately, there were no results available for comparison of Notch2 expression between first-trimester and third-trimester placentas because Notch2 receptor primers displayed 2 peaks in the first derivative of melting curve.17
In conclusion, the present study demonstrates increased protein expression of Notch3 and decreased Notch2 in placentas of patients with early-onset severe preeclampsia compared to normal-term placentas. The results imply that the Notch-signaling pathway, especially Notch3 and Notch2 mediating Notch-signaling pathway, may play an important role in the pathogenesis of early-onset severe preeclampsia. Further studies are required to elucidate the exact mechanism of the Notch-signaling pathway in the pathophysiological mechanism of preeclampsia.
Acknowledgment
The authors would like to thank Xu Feng for critically reviewing the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China , No. 81170589/H0420. And this work was supported by Science and Technology Commission of Shanghai Municipality, China, No. 11495803800, Grants from Shanghai Health Bureau Key Disciplines and Specialties Foundation, Shanghai Education Commission Key Disciplines Foundation, Key Discipline Project of Renji Hospital, Shanghai Jiaotong University School of Medicine.
References
- 1. Leitner Y, Harel S, Geva R, Eshel R, Yaffo A, Many A. The neurocognitive outcome of IUGR children born to mothers with and without preeclampsia. J Matern Fetal Neonatal Med. 2012;25(11):2206–2208. [DOI] [PubMed] [Google Scholar]
- 2. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010; 376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 3. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 4. Fortini ME, Bilder D. Endocytic regulation of Notch signaling. Curr Opin Genet Dev. 2009;19(4):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90(2):271–280. [DOI] [PubMed] [Google Scholar]
- 7. Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998; 393(6683):382–386. [DOI] [PubMed] [Google Scholar]
- 8. De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–522. [DOI] [PubMed] [Google Scholar]
- 9. Talora C, Campese AF, Bellavia D, et al. Notch signaling and diseases: an evolutionary journey from a simple beginning to complex outcomes. Biochim Biophys Acta. 2008;1782(9):489–497. [DOI] [PubMed] [Google Scholar]
- 10. Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22(5):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunkapiller NM, Gasperowicz M, Kapidzic M, et al. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138(14):2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahin Z, Acar N, Ozbey O, Ustunel I, Demir R. Distribution of Notch family proteins in intrauterine growth restriction and hypertension complicated human term placentas. Acta Histochem. 2011;113(3):270–276. [DOI] [PubMed] [Google Scholar]
- 13. Cobellis L, Mastrogiacomo A, Federico E, et al. Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell Tissue Res. 2007;330(3):527–534. [DOI] [PubMed] [Google Scholar]
- 14. National high blood pressure education program working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 1990;163(5 pt 1):1691–1712. [DOI] [PubMed] [Google Scholar]
- 15. Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A. Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG. 2005;112(1):89–96. [DOI] [PubMed] [Google Scholar]
- 16. Kovo M, Schreiber L, Ben-Haroush A, Gold E, Golan A, Bar J. The placental component in early-onset and late-onset preeclampsia in relation to fetal growth restriction. Prenat Diagn. 2012;32(7):632–637. [DOI] [PubMed] [Google Scholar]
- 17. Herr F, Schreiner I, Baal N, Pfarrer C, Zygmunt M. Expression patterns of Notch receptors and their ligands Jagged and Delta in human placenta. Placenta. 2011;32(8):554–563. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Fan H, Ma Y, et al. Notch1 is a 5-fluorouracil resistant and poor survival marker in human esophagus squamous cell carcinomas. PLoS One. 2013;8(2):e56141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Falco M, Cobellis L, Giraldi D, et al. Expression and distribution of notch protein members in human placenta throughout pregnancy. Placenta. 2007;28(2-3):118–126. [DOI] [PubMed] [Google Scholar]
- 20. Rinnoci V, Nannucci S, Valenti R, et al. Cerebral hemorrhages in CADASIL: report of four cases and a brief review. J Neurol Sci. 2013;330(1-2):45–51. [DOI] [PubMed] [Google Scholar]
- 21. Rahman MT, Nakayama K, Rahman M, et al. Notch3 overexpression as potential therapeutic target in advanced stage chemoresistant ovarian cancer. Am J Clin Pathol. 2012;138(4):535–544. [DOI] [PubMed] [Google Scholar]
- 22. Pancewicz J, Nicot C. Current views on the role of Notch signaling and the pathogenesis of human leukemia. BMC Cancer. 2011;11:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamaguchi N, Oyama T, Ito E, et al. NOTCH3 signaling pathway plays crucial roles in the proliferation of ErbB2-negative human breast cancer cells. Cancer Res. 2008;68(6):1881–1888. [DOI] [PubMed] [Google Scholar]
- 24. Ohashi S, Natsuizaka M, Yashiro-Ohtani Y, et al. NOTCH1 and NOTCH3 coordinate esophageal squamous differentiation through a CSL-dependent transcriptional network. Gastroenterology. 2010;139(6):2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez-Nino MD, Ortiz A. Notch3 and kidney injury: never two without three. J Pathol. 2012;228(3):266–273. [DOI] [PubMed] [Google Scholar]
- 26. Raimondi L, Ciarapica R, De Salvo M, et al. Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21(Cip1) expression and hampers tumour cell growth in vitro and in vivo. Cell Death Differ. 2012;19(5):871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serafin V, Persano L, Moserle L, et al. Notch3 signalling promotes tumour growth in colorectal cancer. J Pathol. 2011;224(4):448–460. [DOI] [PubMed] [Google Scholar]
- 28. Crocker I. Gabor Than award lecture 2006: pre-eclampsia and villous trophoblast turnover: perspectives and possibilities. Placenta. 2007;28 (suppl A):S4–S13. [DOI] [PubMed] [Google Scholar]
- 29. Prusac IK, Zekic Tomas S, Roje D. Apoptosis, proliferation and Fas ligand expression in placental trophoblast from pregnancies complicated by HELLP syndrome or pre-eclampsia. Acta Obstet Gynecol Scand. 2011;90(10):1157–1163. [DOI] [PubMed] [Google Scholar]
- 30. Vargas A, Toufaily C, LeBellego F, Rassart E, Lafond J, Barbeau B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci. 2011;18(11):1085–1091. [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Yang C, Zhang S, Li Y, Chen J. Notch2 inhibits proliferation of chronic myeloid leukemia cells. Oncol Lett. 2013;5(4):1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin H, Gong W, Zhang C, Wang S. Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. Onco Targets Ther. 2013;6:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dill MT, Tornillo L, Fritzius T, et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57(4):1607–1619. [DOI] [PubMed] [Google Scholar]