Abstract
Recent studies have consistently found pregnancy-associated plasma protein A2 (PAPP-A2) to be upregulated in preeclamptic placentae at term. We tested whether first-trimester circulating PAPP-A2 levels differed between complicated and uncomplicated pregnancies. We measured maternal PAPP-A2 levels at 10 to 14 weeks of gestational age in 17 pregnancies resulting in small-for-gestational-age (SGA) infants, 6 which developed preeclampsia (PE), 1 which developed PE and resulted in an SGA infant, and 37 gestational age-matched controls. The concentration of the PAPP-A2 isoform corresponding to the full-length protein was significantly higher in pregnancies that developed PE (35 ng/mL) compared with those that did not (23 ng/mL; P < .044). In contrast, we found no difference in PAPP-A2 levels between pregnancies that did or did not result in an SGA infant. The upregulation of PAPP-A2 that has previously been observed in PE at term appears to begin early in pregnancy, well before the symptoms develop.
Keywords: PAPP-A2, preeclampsia, small-for-gestational-age
Introduction
Abnormal placental development is associated with a wide range of adverse gestational outcomes, including preeclampsia (PE) and intrauterine growth restriction (IUGR).1–3 Preeclampsia, a gestational syndrome characterized by acute hypertension and proteinuria in the mother, affects 5% to 7% of all pregnancies and remains a leading cause of maternal mortality for which there is presently no cure other than delivery of the fetus and the placenta.2,4 Meanwhile, IUGR resulting from placental insufficiency carries consequences such as increased risk of perinatal mortality, sepsis, and asphyxia and an increased risk of several adult-onset diseases such as type 2 diabetes, stroke, hypertension, and coronary heart disease.5 Both PE and IUGR are often, though not completely, concurrent.
The placental insufficiency that is associated with PE and IUGR has been characterized by defective remodeling of the uterine spiral arteries, coinciding with impaired invasion of extravillous trophoblast (EVT) into the maternal decidua.3 Insulin-like growth factors I and II (IGF-I and -II, respectively) stimulate the EVT migration and invasion that is key to spiral artery remodeling.6,7 The bioavailability of IGF-I and IGF-II is modulated by insulin-like growth factor-binding proteins (IGFBPs).6,8–10 Pregnancy-associated plasma protein A (PAPP-A) is a protease of IGFBP-4, IGFBP-5 and possibly IGFBP-2 and thus is expected to play a role in increasing the bioavailability of the IGFs and potentially affect processes such as EVT migration and invasion.6,11–13 Pr5 egnancy-associated plasma protein A circulates in the maternal blood at high levels during pregnancy and is already in clinical use as a first-trimester biomarker for Down syndrome, as abnormally low levels of PAPP-A early in gestation have been associated with elevated risk of chromosomal abnormalities.14 Abnormally low levels of PAPP-A are also associated with an increased risk of PE15–19 and IUGR,15–18,20 but the predictive value of PAPP-A alone for detecting these conditions is poor, with only 8% to 23% of PE cases and 11% to 24% of IUGR cases having PAPP-A serum levels below the fifth percentile.21
Pregnancy-associated plasma protein A2 (PAPP-A2) shares 45% amino acid similarity with PAPP-A and cleaves IGFBP-5 and possibly IGFBP-3, thus potentially modulating the activity of the IGFs.22–24 While the low first-trimester levels of PAPP-A are associated with an increased risk of PE, recent studies have shown PAPP-A2 to be upregulated in term placentae from pregnancies with PE and hemolytic anemia, elevated liver enzymes, and low platelet count (HELLP) syndrome,25–32 with PAPP-A2 being one of the top 5 most consistently upregulated genes in a meta-analysis of microarray studies of placentae with PE.32 Additionally, third-trimester circulating levels of PAPP-A2 have also been found to be elevated in women with PE.27
The objective of the present study was to determine whether PAPP-A2 levels in the maternal circulation are elevated in the first trimester of pregnancies that subsequently develop PE and/or result in a small-for-gestational-age (SGA) baby, compared with uncomplicated pregnancies. Understanding whether the elevation of PAPP-A2 in complicated pregnancies occurs early, near the time when placental dysfunction is developing, or later in pregnancy, in response to fully developed placental pathology, will shed light on the mechanisms underlying the associations between PAPP-A2 upregulation and PE.
Methods
Samples and Control Matching
First-trimester serum samples were collected as part of unrelated first-trimester screening and stored at the Pacific Centre for Reproductive Medicine (PCRM), in Burnaby, British Columbia, Canada. For women who delivered at BC Women’s Hospital, pregnancy outcomes associated with each sample were obtained from the Provincial Health Services Authority. The use of these samples for a retrospective study was approved by both the University of British Columbia Children’s and Women’s Research Ethics Board and the Simon Fraser University Research Ethics Board.
Samples included 24 case samples, comprising 17 pregnancies resulting in SGA infants, 6 pregnancies that developed PE, and 1 pregnancy that developed PE and resulted in an SGA infant. Small-for-gestational-age was defined as birth weight within the lowest fifth percentile, and PE was defined as gestational hypertension with significant proteinuria.33 Only pregnancies resulting in singleton births with no other known pathologies (including congenital abnormalities) were included in this study. For control matching, we obtained 37 serum samples from women who experienced normal pregnancies (normotensive women who gave birth to babies with birth weights above the 10th percentile). The earliest gestational age at birth was 34 weeks. We matched case samples with controls according to gestational age at the time of first-trimester sampling as well as length of time in storage, with some case samples being matched to 2 controls and others to only 1, giving priority in matching the gestational age at the time of sampling. First-trimester PAPP-A2 levels were too low to be detected using an enzyme-linked immunosorbent assay (ELISA) adapted from Nishizawa et al27 (data not shown) and therefore were quantified using immunofluorescent Western blot.
Immunofluorescent Western Blot
Patient serum was thawed and diluted 1/8 in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin before being aliquoted and refrozen (for a total of 1 additional freeze–thaw cycle prior to assay). The PAPP-A2 protein levels in serum were quantified using a previously described Western blotting method,34,35 using wet transfer from gel to nitrocellulose membrane instead of semi-dry transfer. Twenty microliters of diluted serum samples were mixed with 5 µL of 6× sodium dodecyl sulfate (SDS) loading buffer and heated for 10 minutes at 100°C. Samples were run for 1 hour and 50 minutes through a 4% stacking and 8% separating polyacrylamide gel to separate proteins in the 100 to 250 kDa region. Wet transfer to nitrocellulose membrane (Bio-Rad, Hercules, California) was performed for 75 minutes at 100 V (constant) on the Criterion Wet Blotter System (Bio-Rad) in 3-(cyclohexylamino)-1-propane sulfonic acid buffer with 10% MeOH (Sigma Aldrich, St Louis, Missouri). Initial optimization studies established that in a range of transfer times from 30 minutes up to 2 hours, 75 minutes of wet transfer to nitrocellulose was optimal to ensure complete transfer of proteins without blow through. Nitrocellulose was then blocked using Odyssey Blocking Buffer For Quantitative Westerns (Li-Cor Biosciences, Lincoln, Nebraska) for 1 hour before overnight incubation with 1:500 polyclonal goat anti-human PAPP-A2 antibody (R&D Systems, Minneapolis, Minnesota; AF1668) in the same blocking buffer with 0.1% Tween-20 (Sigma Aldrich). Membranes were washed 5 times with sterile PBS containing 0.1% Tween-20 (PBST) at room temperature before being incubated in a solution containing 1:10 000 fluorescently labeled IRDye 800 secondary antibody (Li-Cor Biosciences) diluted in blocking buffer, 0.1% Tween-20 and 0.1% SDS for 45 minutes in the dark. The membranes were washed again for 5 minutes each in PBST at room temperature, rinsed with filter-sterilized PBS, and scanned with an Odyssey Infrared imaging system.
Quantification
Pregnancy-associated plasma protein A2 patient serum concentrations were derived from the optical density values of PAPP-A2-immunospecific bands and a standard curve of recombinant PAPP-A2 protein (R&D Systems; 1668-ZN) included on the same blot. Optical density values for each of the PAPP-A2-immunospecific bands were calculated with Odyssey Infrared Imaging System Application Software (version 2.1.12) using rectangles of the same size and shape for all bands selected by an observer who was blind to the outcome of the samples. Pregnancy-associated plasma protein A2 immunospecificity was determined via comparison of identical membranes developed either with polyclonal goat anti-human PAPP-A2 antibody or with polyclonal goat anti-human immunoglobulin G as the primary antibody (Supplemental Figure 1). Pregnancy-associated plasma protein A2-specific bands are present at ∼290, ∼250, and ∼130 kDa (the expected size of a splice variant of PAPP-A2), consistent with previous findings.23,27,35
Each sample was measured in triplicate, with each replicate run on a separate gel. Each gel contained its own standard curve of recombinant PAPP-A2 protein (5 wells, 20 - 1.25 ng/mL) and 3 to 4 case–control groups, that is, case samples were always run adjacent to their controls (Figure 1). Each case–control group replicate was run on 3 separate gels and was run adjacent to different case–control groups in each replicate. Group position on the gel always varied between replicates to control for potential gel edge effects.
Figure 1.
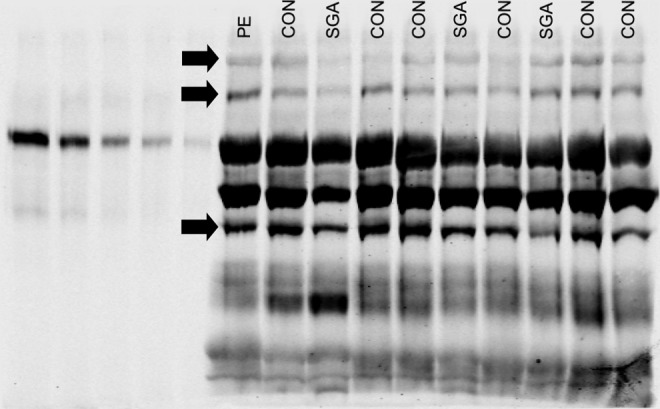
A representative Western blot of the 21 that were run to quantify each case–control group in triplicate. Solid arrows show PAPP-A2 immunospecific bands that were quantified in this analysis at 290, 250, and 130 kDa (top to bottom). Lanes 1 to 5: PAPP-A2 protein standard (20-1.25 ng/mL); lanes 6 to 15: 4 groups of cases and matched controls. PAPP-A2 indicates pregnancy-associated plasma protein A2.
Pregnancy-associated plasma protein A levels in maternal serum were quantified using the DELFIA(R) Xpress PAPP-A immunoassay kit (PerkinElmer, Waltham, MA) and were converted to multiples of the median (MoM) using LifeCycle Elipse (PerkinElmer).
Statistical Analyses
The optical densities of the 290, 250, and 130 kDa PAPP-A2 immunospecific bands were converted to serum concentration in nanogram per milliliter for each replicate using the standard curve included on the same gel/blot. For each band, all 3 replicate values for each sample were analyzed using a general linear model including the effects of sample identity, blot, and lane on the gel. In these analyses, the sample identity term was highly significant for the 290, 250, and 130 kDa PAPP-A2 bands (P < .0001 for each), indicating that the variation between samples was significantly greater than the variation among triplicate values. These analyses yielded 1 least squares mean for each sample, and these least squares means were used for further analyses. Deidentified information regarding gestational age at time of sampling, ethnicity, maternal age, weight, and parity was used to test whether these factors influence first-trimester PAPP-A2 levels among women who went on to have uncomplicated pregnancies. To determine whether the first-trimester PAPP-A2 levels differ between uncomplicated controls, pregnancies with PE and/or pregnancies resulting in SGA, each of the 3 PAPP-A2 reactive bands at 290, 250, and 130 kDa was analyzed by a general linear model including the effects of PE (yes/no) and SGA (yes/no).
Results
Biological Factors Affecting PAPP-A2 in Uncomplicated Pregnancy
There were no significant differences in maternal age, weight, ethnicity, parity, or sampling variables (gestational age on draw date of sample or total freeze time of sample) between the women who developed PE and/or gave birth to SGA infants and controls (Table 1). Among our controls 5 were East Asian women, 1 Asian woman and 31 caucasian women (Table 1). In all, 16 were nulliparous, 20 were parous, and 1 woman was of uncertain parity (Table 1).
Table 1.
Maternal and Sample Variables Examined in First-Trimester Serum.a
| Control (n = 37 Including 2 Preterm) | PE (n = 6 Including 1 Preterm) | PE/SGA (n = 1) | SGA (n = 17) | |
|---|---|---|---|---|
| Maternal age at draw date, years | 34.3 ± 0.6 | 36.7 ± 1.3 | 36.0 | 34.9 ± 0.6 |
| Maternal weight, kg | 64.4 ± 2.0 | 62.9 ± 2.7 | 61.0 | 59.9 ± 2.9 |
| Gestional age on draw date, weeks | 11.8 ± 0.1 | 12.4 ± 0.4 | 11.7 | 11.6 ± 0.2 |
| Freeze time, months | 42.4 ± 1.2 | 42.0 ± 4.3 | 46.0 | 41.2 ± 2.6 |
| % Parous | 54 | 33 | 100 | 24 |
| Ethnicity | ||||
| Caucasian/Asianb/East Asian | 31/1/5 | 3/1/2 | 1/0/0 | 9/0/8 |
| Birth weight, g | ||||
| Including preterm(s) | 3468 ± 81.7 | 3138 ± 321.0 | c | c |
| Excluding preterm(s) | 3551 ± 60.8 | 3426 ± 174.5 | 2180 | 2633 ± 31.1 |
| Gestional age at delivery, weeks | ||||
| Including preterm(s) | 39.1 ± 0.3 | 37.5 ± 0.8 | c | c |
| Excluding preterm(s) | 39.4 ± 0.2 | 38.2 ± 0.6 | 37.0 | 40.0 ± 0.2 |
| Mean PAPP-A2, ng/mL | ||||
| Including preterm(s) | 23.3 ± 2.0 | 35.2 ± 2.8 | c | c |
| Excluding preterm(s) | 22.2 ± 1.6 | 35.7 ± 3.4 | 19.8 | 23.7 ± 2.6 |
| MoM PAPP-A | 1.20 ± 0.1 | 1.17 ± 0.3 | 1.14 | 0.76 ± 0.1 |
Abbreviations: MoM, multiples of the median; PAPP-A, pregnancy-associated plasma protein A; PAPP-A2, pregnancy-associated plasma protein A2; PE, preeclampsia; SGA, small-for-gestational-age.
aValues represented as mean ± standard error of the mean.
bFor example, India, Pakistan, and Bangladesh.
cThe PE/SGA and SGA sample sets contained no preterms.
Among controls, there were no correlations between the concentrations of any of the PAPP-A2 isoforms or PAPP-A MoMs and gestational age at the time of first-trimester sampling or maternal age or weight at the time of first-trimester sampling, whether or not preterm births (prior to 37 weeks, but at least 34 weeks, of which there were 2) were included (P > .05 in all cases). Similarly, there was no difference in PAPP-A2 or PAPP-A concentrations between women who had not previously given birth and those who had, whether or not preterm births were included (P > .05 in all cases). Excluding pregnancies that resulted in preterm births, birth weight was positively correlated with the levels of the 290-kDa PAPP-A2 isoform (r = .379, P < .02) and the PAPP-A MoMs (r = .343, P < .03) but not with levels of the 250- or 130-kDa isoforms of PAPP-A2 (r = .078, P > .6 and r = −.176 and P > .25, respectively). Additionally, significant correlations were found between PAPP-A MoMs and the 290-kDa PAPP-A2 isoform (r = .375, P < .02), as well as between PAPP-A MoM and the 250-kDa PAPP-A2 isoform (r = .399, P < .01), but not between PAPP-A MoM and the 130-kDa PAPP-A2 isoform (r = −.126, P > .4). There was no significant correlation between the 290- and 250-kDa PAPP-A2 isoforms (r = .254, P > .1), the 290- and the 130-kDa PAPP-A2 isoforms (r = .227, P > .15), or the 250 and 130 kDa PAPP-A2 isoforms (r = .208, P > .15).
Relationship Between PAPP-A2 and the Development of PE and/or SGA
In a general linear model including the effects of PE (yes/no) and SGA (yes/no), first-trimester 250-kDa PAPP-A2 serum concentration was significantly higher in women who subsequently developed PE than in those who did not (F = 4.25, P < .044) but was not different between women who did or did not give birth to SGA infants (F = 0.04, P > .844). Very similar results were obtained if preterm births, that is, prior to 37 weeks, but at least 34 weeks were excluded (excluded samples included 1 preterm PE sample plus its 2 gestational-age-matched controls, of which 1 was preterm and 1 was nonpreterm as well as 1 other preterm control plus its matched nonpreterm SGA case sample; data not shown). This difference was also significant when analyzed by nonparametric tests (Wilcoxon Z = 2.43, P = .015; Kruskal-Wallis χ2 = 5.97, P = .015). Levels of the 250-kDa isoform of PAPP-A2 were approximately 51% higher in women who developed PE compared to controls, with a mean serum concentration of 35 ng/mL in PE pregnancies compared to 23 ng/mL in controls (Table 1; Figure 2). The concentrations of 290 and 130 kDa PAPP-A2 did not differ between pregnancies that did or did not subsequently develop PE or that did or did not result in an SGA infant (P > .05 in all cases). First-trimester PAPP-A MoMs were significantly lower in women who subsequently gave birth to SGA babies than in those who did not (F = 6.64, P < .01) but were not different between women who did or did not develop PE (F = 0.01, P > .9). The difference between SGA pregnancies and controls was significant if preterm births were excluded and/or nonparametric tests were used (data not shown). Pregnancy-associated plasma protein A MoMs were approximately 37% lower in women with SGA infants (0.76) than that of controls (1.20, Table 1; Figure 3).
Figure 2.
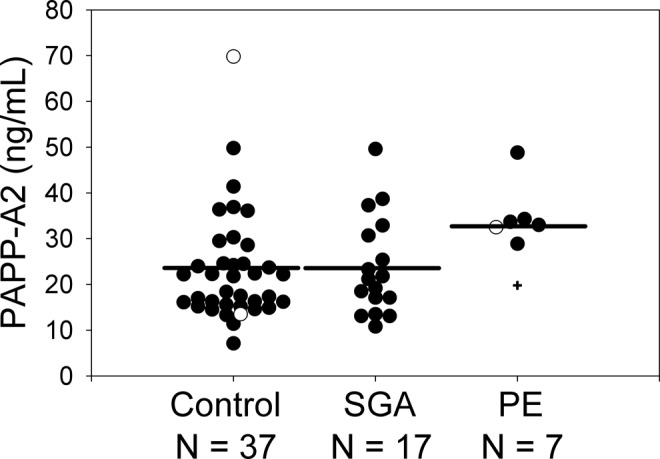
First-trimester maternal serum levels of 250 kDa pregnancy-associated plasma protein A2 (PAPP-A2; measured via immunofluorescent Western blot) were significantly elevated in PE compared with control but not in PE/SGA or SGA, regardless of whether or not preterm samples (prior to 37 weeks, but at least 34 weeks, indicated by open symbols) were included. One sample (plus symbol) was from a pregnancy that subsequently developed PE and resulted in an SGA baby. Horizontal lines represent the least squares means from a general linear model including the effects of PE and SGA. PE indicates preeclampsia; SGA, small-for-gestational-age.
Figure 3.
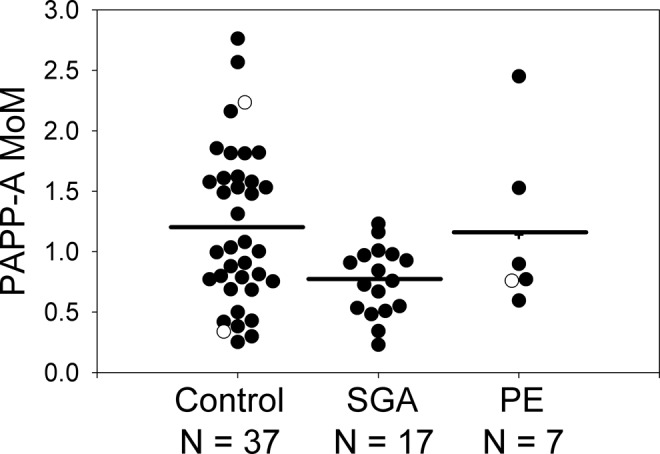
First-trimester levels of PAPP-A (measured via the DELFIA(R) Xpress PAPP-A immunoassay kit) were significantly lower in pregnancies resulting in an SGA infant compared with controls but not in PE/SGA or PE, regardless of whether or not preterm samples (prior to 37 weeks, but at least 34 weeks, indicated by open symbols) were included. One sample (plus symbol) was from a pregnancy that subsequently developed PE and resulted in an SGA baby. Horizontal lines represent the least squares means from a general linear model including the effects of PE and SGA. PAPP-A indicates pregnancy-associated plasma protein A; PE, preeclampsia; SGA, small-for-gestational-age.
Discussion
This study measured the IGFBP protease PAPP-A2 in serum samples collected in the first trimester of pregnancy from women who subsequently gave birth to SGA infants and/or developed PE. Concentrations of the 250-kDa isoform of PAPP-A2 were significantly higher in pregnancies that subsequently developed PE but not in those that resulted in SGA infants.
Pregnancy-Associated Plasma Protein A2 Immunospecific Bands
Of the 3 bands immunospecific for PAPP-A2, the levels of the 250 kDa protein were significantly elevated in samples from pregnant women who subsequently developed PE (but not SGA). This band is the size expected for the longer isoform, and has been previously found to be present at higher levels in the serum of pregnant women than that in nonpregnant women using the same antibody.35 In contrast, neither the 130- nor 290-kDa isoforms were associated with the development of PE or SGA. We speculate that the 290 kDa band represents a glycosylated or otherwise modified variant of the same protein, as glycosylation has been shown to add between 40 and 50 kDa to the molecular weight of PAPP-A2 in recombinant protein (http://www.sinobiological.com/PDF/10528-H08H.pdf). However, because the 290-kDa band did not differ between complicated and uncomplicated pregnancies, we did not investigate this further. The 130-kDa band represents a known splice variant of PAPP-A2.23,35 Previous work has found PAPP-A2 proteolytic activity against IGFBP-5 in pregnancy plasma but only among proteins greater than 150 kDa, suggesting that the 130 kDa splice variant of PAPP-A2 does not contribute to this proteolytic activity.36
Pregnancy-Associated Plasma Protein A2 and PE
Pregnancy-associated plasma protein A2 is consistently upregulated in placentae at term in pregnancies with PE and HELLP,25–32 and also circulates at higher levels in late gestation in women with PE.27 The late-gestation upregulation of PAPP-A2 has been suggested to be a mechanism to compensate for the abnormal placentation by increasing IGFBP proteolysis to raise local IGF levels to promote fetoplacental growth.37 Placental hypoxia, known to occur in PE, has been hypothesized as a mechanism by which PAPP-A2 might be upregulated in placental pathologies, since hypoxia upregulates PAPP-A2 messenger RNA and protein expression in BeWo cells and late-gestation placental explants,31,38 but this has not been examined in the first-trimester tissue. The present study suggests that upregulation, whatever the cause, occurs early in complicated pregnancies and therefore may mitigate developmental abnormalities that might otherwise have more severe effects.
While a growing number of studies have found associations between PE and PAPP-A2 at term, we know of only 1 study that examined circulating PAPP-A2 concentrations early in gestation described in a meeting abstract.39 Our observation of elevated levels of circulating PAPP-A2 in the first trimester of pregnancy in women who subsequently develop PE is consistent with this study,39 but in contrast with the frequent findings of lower first-trimester PAPP-A circulating levels in pregnancies that develop PE15–17,19,40 and other complications.14–17,20 Although we found the PAPP-A2 levels to be elevated in pregnancies that subsequently developed PE, PAPP-A concentrations were significantly lower in pregnancies that resulted in an SGA baby, consistent with previous work. This finding suggests that while PAPP-A and PAPP-A2 are both IGFBP proteases, they have different functions and/or regulation and therefore play different roles in adverse gestational outcomes. As a result, the potential of first-trimester PAPP-A and PAPP-A2 levels to jointly predict PE deserves further attention.
Pregnancy-Associated Plasma Protein A2 and SGA
Levels of PAPP-A2 were not found to be significantly different in pregnancies resulting in SGA infants compared with controls. However, previous work has shown PAPP-A2 to be elevated in maternal circulation and upregulated in term placentae from pregnancies with IUGR.41 A potential explanation for this discrepancy is that, in our study, SGA was defined as simply having birth weights below the lowest fifth percentile, whereas Whitehead et al examined pregnancies complicated by IUGR that required delivery before 34 weeks with antenatal evidence of uteroplacental insufficiency (asymmetrical growth + abnormal umbilical artery Doppler velocimetry, ±oligohydramnios or abnormal fetal vessel velocimetry).41 Accordingly, it is possible that elevation of PAPP-A2 may not be specific to PE and may be elevated in cases of preterm IUGR as well.
Our sample sizes are small, limiting our ability to adjust for potential confounding effects. However, the observation that the elevation in PAPP-A2 levels was significant despite this small sample size, even though the well-established association between PAPP-A levels and PE was not significant, suggests that the dysregulation of PAPP-A2 may be substantial and consistent. This is the first report of elevated first-trimester PAPP-A2 in PE, which warrants further investigation with expanded sample sizes as well as the development of a more sensitive PAPP-A2 ELISA or perhaps other proteomic techniques to improve and streamline quantification.
Conclusion
We observed elevated first-trimester levels of PAPP-A2 in the maternal circulation in pregnancies that developed PE but not in those that developed SGA. The findings presented here suggest that the elevation in PAPP-A2 expression in PE that has consistently been observed at term actually begins early in pregnancy and so is not simply a response to fully developed placental pathology. This result underlines the need for further research into the functions of PAPP-A2 early in pregnancy.
Supplementary Material
Acknowledgments
The authors thank Jina Byun of PCRM for sample processing, members of Alison Kermode’s laboratory, in particular Gholamreza Babajani, for invaluable advice, Devin De Zwaan for technical help, Zoë Hodgson for logistical support, and Alex Beristain and Tim Beischlag for their guidance.
Authors’ Note: The research for this study was conducted at the Simon Fraser University, Burnaby, British Columbia, Canada
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by a Canadian Institutes of Health Research Master's Award (Frederick Banting and Charles Best Canada Graduate Scholarships) to EJC, a Nelly Auersperg award from the Women’s Health Research Institute of BC to JKC and AG, and an NSERC Discovery Grant to JKC.
Supplemental Material: Supplemental Figure 1 is available at http://rs.sagepub.com/supplemental.
References
- 1. Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet. 2003;64(2):96–103. [DOI] [PubMed] [Google Scholar]
- 2. Kinzler WL, Vintzileos AM. Fetal growth restriction: a modern approach. Curr Opin Obstet Gynecol. 2008;20(2):125–131. [DOI] [PubMed] [Google Scholar]
- 3. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. [DOI] [PubMed] [Google Scholar]
- 4. von Dadelszen P, Magee L. What matters in preeclampsia are the associated adverse outcomes: the view from Canada. Curr Opin Obstet Gynecol. 2008;20(2):110–115. [DOI] [PubMed] [Google Scholar]
- 5. Godfrey KM. The role of the placenta in fetal programming—a review. Placenta. 2002;23(suppl A):S20–S27. [DOI] [PubMed] [Google Scholar]
- 6. Nayak NR, Giudice LC. Comparative biology of the IGF system in endometrium, decidua, and placenta, and clinical implications for foetal growth and implantation disorders. Placenta. 2003;24(4):281–296. [DOI] [PubMed] [Google Scholar]
- 7. Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2011;589(pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278(6):E967–E976. [DOI] [PubMed] [Google Scholar]
- 9. Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14(4):176–181. [DOI] [PubMed] [Google Scholar]
- 10. Murphy LJ. Insulin-like growth factor-binding proteins: functional diversity or redundancy? J Mol Endocrinol. 1998;21(2):97–107. [DOI] [PubMed] [Google Scholar]
- 11. Giudice LC, Conover CA, Bale L, et al. Identification and regulation of the IGFBP-4 protease and its physiological inhibitor in human trophoblasts and endometrial stroma: evidence for paracrine regulation of IGF-II bioavailability in the placental bed during human implantation. J Clin Endocrinol Metab. 2002;87:2359–2366. [DOI] [PubMed] [Google Scholar]
- 12. Boldt HB, Kjaer-Sorensen K, Overgaard MT, et al. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity. J Biol Chem. 2004;279:38525–38531. [DOI] [PubMed] [Google Scholar]
- 13. Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. [DOI] [PubMed] [Google Scholar]
- 14. Brambati B, Macintosh MC, Teisner B, et al. Low maternal serum levels of pregnancy associated plasma protein A (PAPP-A) in the first trimester in association with abnormal fetal karyotype. Br J Obstet Gynaecol. 1993;100:324–326. [DOI] [PubMed] [Google Scholar]
- 15. Bersinger NA, Odegard RA. Second- and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational age pregnancies. Acta Obstet Gynecol Scand. 2004;83:37–45. [PubMed] [Google Scholar]
- 16. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446–1451. [DOI] [PubMed] [Google Scholar]
- 17. Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–1767. [DOI] [PubMed] [Google Scholar]
- 18. Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25:949–953. [DOI] [PubMed] [Google Scholar]
- 19. Zwahlen M, Gerber S, Bersinger NA. First trimester markers for pre-eclampsia: placental vs. non-placental protein serum levels. Gynecol Obstet Invest. 2007;63:15–21. [DOI] [PubMed] [Google Scholar]
- 20. Cowans NJ, Spencer K. First-trimester ADAM12 and PAPP-A as markers for intrauterine fetal growth restriction through their roles in the insulin-like growth factor system. Prenat Diagn. 2007;27:264–271. [DOI] [PubMed] [Google Scholar]
- 21. Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010;30(4):293–308. [DOI] [PubMed] [Google Scholar]
- 22. Farr M, Strube J, Geppert HG, Kocourek A, Mahne M, Tschesche H. Pregnancy-associated plasma protein-E (PAPP-E). Biochim Biophys Acta. 2000;1493(3):356–362. [DOI] [PubMed] [Google Scholar]
- 23. Page NM, Butlin DJ, Lomthaisong K, Lowry PJ. The characterization of pregnancy associated plasma protein-E and the identification of an alternative splice variant. Placenta. 2001;22(8-9):681–687. [DOI] [PubMed] [Google Scholar]
- 24. Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276(24):21849–21853. [DOI] [PubMed] [Google Scholar]
- 25. Buimer M, Keijser R, Jebbink JM, et al. Seven placental transcripts characterize HELLP-syndrome. Placenta. 2008;29(5):444–453. [DOI] [PubMed] [Google Scholar]
- 26. Sitras V, Paulssen RH, Gronaas H, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30(5):424–433. [DOI] [PubMed] [Google Scholar]
- 27. Nishizawa H, Pryor-Koishi K, Suzuki M, et al. Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients. Mol Hum Reprod. 2008;14(10):595–602. [DOI] [PubMed] [Google Scholar]
- 28. Winn VD, Gormley M, Paquet AC, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paiva P, Whitehead C, Saglam B, Palmer K, Tong S. Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia. J Clin Endocrinol Metab. 2011;96(11):E1807–E1815. [DOI] [PubMed] [Google Scholar]
- 30. Varkonyi T, Nagy B, Fule T, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32 suppl:S21–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macintire K, Tuohey L, Ye L, et al. PAPPA2 is increased in severe early onset pre-eclampsia and upregulated with hypoxia [published online March 14, 2013]. Reprod Fertil Dev. 2013. doi:10.1071/RD12384. [DOI] [PubMed] [Google Scholar]
- 32. Dopierala JA, Smith GC, Charnock-Jones SD. Meta-analysis of pre-eclampsia microarray data. Reprod Sci. 2013;20(3 suppl):262A.22773407 [Google Scholar]
- 33. von Dadelszen P, Menzies JM, Payne B, Magee LA; PIERS (Pre-eclampsia Integrated Estimate of RiSk) Study Group. Predicting adverse outcomes in women with severe pre-eclampsia. Semin Perinatol. 2009;33(3):152–157. [DOI] [PubMed] [Google Scholar]
- 34. Wagner PK, Christians JK. Altered placental expression of PAPPA2 does not affect birth weight in mice. Reprod Biol Endocrinol. 2010;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang J, Qiu Q, Haider M, Bell M, Gruslin A, Christians JK. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J Endocrinol. 2009;202(3):337–345. [DOI] [PubMed] [Google Scholar]
- 36. Yan X, Baxter RC, Firth SM. Involvement of pregnancy-associated plasma protein-A2 in insulin-like growth factor (IGF) binding protein-5 proteolysis during pregnancy: a potential mechanism for increasing IGF bioavailability. J Clin Endocrinol Metab. 2010;95(3):1412–1420. [DOI] [PubMed] [Google Scholar]
- 37. Christians JK, Gruslin A. Altered levels of insulin-like growth factor binding protein proteases in preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010;30(9):815–820. [DOI] [PubMed] [Google Scholar]
- 38. Wagner PK, Otomo A, Christians JK. Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo). Reprod Biol Endocrinol. 2011;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kramer A, Hoffman C, Lynch A, Winn VD. Maternal serum PAPP-A2 levels are elevated in early onset preeclampsia at time of diagnosis and in early gestation. Reprod Sci. 2012;19(3 suppl):87A. [Google Scholar]
- 40. Poon LC, Stratieva V, Piras S, Piri S, Nicolaides KH. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11-13 weeks. Prenat Diagn. 2010;30(3):216–223. [DOI] [PubMed] [Google Scholar]
- 41. Whitehead CL, Walker SP, Ye L, et al. Placental specific mRNA in the maternal circulation are globally dysregulated in pregnancies complicated by fetal growth restriction. J Clin Endocrinol Metab. 2013;98(3):E429–E436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


