Abstract
Bone morphogenetic protein (BMP) cytokine is known to regulate ovulation, as BMP-6 null mice exhibit a decrease in the number of ovulatory follicles without effect on either the morphology or the dynamics of follicular development. In the present study, the role of BMP-6 in ovulatory process was investigated using human granulosa-lutein cells (GCs). Granulosa-lutein cells, obtained from in vitro fertilization patients, were cultured with BMP-6 followed by RNA extraction. The neutrophil–chemotactic activity of the supernatant of cultured GC was investigated. Bone morphogenetic protein 6 significantly increased growth-regulated oncogene α (GRO-α) messenger RNA (mRNA) and protein expression in GC. In the neutrophil–chemotaxis assay, the GC supernatant cultured with BMP-6 attracted more neutrophils than control samples, which was negated with anti-GRO-α neutralizing antibody. Bone morphogenetic protein 6 also suppressed the relative expression of the protease inhibitors, secretory leukocyte peptidase inhibitor, and whey acid protein 14 mRNA in GC. Bone morphogenetic protein 6 might play a role in ovulation by increasing the accumulation of neutrophils in the ovulatory follicle and suppressing the effect of protease inhibitors.
Keywords: BMP, ovulation, neutrophil
Introduction
Multiple processes, including follicular development, ovulation, and luteinization, determine female fertility. Each process is influenced by numerous factors, including gonadotropins and intraovarian factors.1 It has been postulated that local factors regulate the sensitivity of follicular somatic cells to gonadotropins and are therefore considered to be essential to normal ovarian physiology.2 Among the intraovarian factors, bone morphogenetic proteins (BMPs), members of the transforming growth factor β superfamily, have been implicated in the local regulation of ovarian follicular development in many species.2,3 We have reported that human BMP cytokines are involved in folliculogenesis and luteinization.4–7 In mice, BMP cytokines are also known to regulate ovulation, as BMP-6 or BMP-15 null mice exhibit a decrease in the number of ovulatory follicles without effect on either the morphology or the dynamics of follicular development.8,9
During the ovulatory process, degradation of the extracellular matrix (ECM) at the follicular apex is a key event. The matrix metalloproteinases and their inhibitors, tissue inhibitors of metalloproteinases , have been postulated to play a critical role in the ECM remodeling.10 Neutrophils are also believed to actively participate in ECM remodeling by secreting proteases, supported by the observation that ovulation rate was reduced in the neutrophil-depleted rats.11,12
For attracting neutrophils to ovulatory follicles, chemokines, such as interleukin (IL) 8 and growth-regulated oncogene α (GRO-α) derived from the ovary, are reported to play key roles.13 It has also been demonstrated that luteinizing hormone (LH) is the main factor for inducing these chemokines.13 In addition, other stimuli, including inflammatory cytokines and hypoxia, are also known to regulate chemokines in the ovary.13–16
Where neutrophils are migrated, the activity of proteases secreted from these neutrophils is regulated by protease inhibitors. These inhibitors are members of the whey acid protein (WAP) family, including secretory leukocyte peptidase inhibitor (SLPI).17 Whey acid protein family members are known to play their roles via inhibiting neutrophil proteases,18 however, the role of the WAP family in human ovarian function is still unknown. In a pilot study using microarray technique, we found that among WAP family, SLPI and WAP 14 were suppressed by BMP cytokines.
In the present study, we used human granulosa-lutein cells (GCs) and investigated whether BMP-6 might play a role in human ovulation. Specifically, we focused on the relationship of BMP-6 to leukocytes. We examined the effect of BMP-6 on the induction of GRO-α, a chemokine that attracts neutrophils, and on the regulation of WAP family members that regulate neutrophil function.
Materials and Methods
Reagents and Materials
Hyaluronidase, fetal bovine serum (FBS), Dulbecco Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F12), and antibiotics (mixture of penicillin, streptomycin, and amphotericin B) were purchased from Sigma (St Louis, Missouri). Recombinant human BMP-6 and GRO-α neutralizing antibody were purchased from R&D Systems (Minneapolis, Minnesota).
Cell Culture of Human Luteinized GC
The method to obtain and culture human GC was described previously.14 Briefly, follicular fluids with GC were aspirated from patients undergoing oocyte retrieval for in vitro fertilization (IVF). The clinical indications for IVF in these patients were primarily male factor or tubal factor infertility. Patients with ovarian dysfunction were excluded from the study. The experimental procedures were approved by the institutional review board, and signed informed consent for use of GC was obtained from each patient. All of the follicular aspirates from each patient were mixed and centrifuged at 200g for 5 minutes, resuspended in phosphate-buffered saline (PBS) with 0.2% hyaluronidase, and incubated at 37°C for 30 minutes. The suspension was layered onto Ficoll-Paque and centrifuged at 150g for 20 minutes. The GCs were collected from the interphase, washed with PBS, and cultured in DMEM/F12 media supplemented with 5% FBS and antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin, and 250 ng/mL amphotericin B) for 15 minutes at 37°C, in order to remove contaminating macrophages from GC. Using this method, GC remained in the supernatant while macrophages were attached to the culture dish. The collected GCs were cultured in DMEM/F12 containing 5% FBS and antibiotics in 12-well plates at a density of 2 × 105 cells/mL and kept at 37°C in a humidified 5% CO2/95% air environment. All of the GCs used for the experiments were precultured for 3 days prior to treatments. In a pilot study, we confirmed that 3 days of preculture allowed the GC to regain sensitivity to follicle-stimulating hormone stimulation.19 Media were changed at 48-hour intervals. Human GCs were cultured with or without BMP-6 (0-300 ng/mL) for up to 48 hours. Recombinant BMP-6 was dissolved in 0.1% BSA + 4 mmol/L HCl as a vehicle. The same amount of vehicle was used for a control.
Isolation, Purification, and Culture of Neutrophils
Human neutrophils were isolated from freshly drawn venous blood samples of healthy premenopausal women irrespective of the menstrual phase. The methods of neutrophil isolation were divided into 3 steps: (1) dextran sedimentation, (2) Ficoll-Paque centrifugation, and (3) lysis of contaminating red blood cells.20 In brief, the whole blood was mixed with 0.9% sodium chloride, which contained 3% dextran 500, and the mixture was allowed to settle for 30 minutes at room temperature for sedimentation of red blood cells. The supernatant was collected and centrifuged at 250g for 10 minutes. The pellet was resuspended with 8 mL PBS, layered on 5 mL Ficoll-Paque, and centrifuged at 400g for 30 minutes. To lyze contaminating red blood cells, the remaining pellet was resuspended with 0.2% sodium chloride for 30 seconds and subsequently mixed with an equal volume of 1.6% sodium chloride. The neutrophils were washed, pelleted, and resuspended at 2 × 106 cells/mL in DMEM/F12 containing 0.1% BSA. The cells were plated in 6-well plates at 2 × 106 cells/mL and incubated for 2 hours before beginning the migration assay.
Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was extracted from GC, using the RNeasy minikit (Qiagen, Hilden, Germany). Reverse transcription was performed using Rever Tra Dash (TOYOBO, Tokyo, Japan). Total RNA of 1 μg was reverse transcribed in a 20 µL volume. For the quantification of various messenger RNA (mRNA) levels, real-time polymerase chain reaction (PCR) was performed using a LightCycler (Roche Diagnostic GmbH, Mannheim, Germany), according to the manufacturer’s instructions. The PCR primer sets were designed to span introns to discriminate PCR products that might arise from possible chromosomal DNA contaminants. The primer sequences were as follows: GRO-α (NM_001511.3: 35-54 and 273-254), SLPI (NM_002046: 628-648 and 1079-1060), WAP 14 (NM_002046: 628-648 and 1079-1060), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; NM_002046: 628-648 and 1079-1060). The PCR conditions were as follows: GRO-α: 35 cycles of 95°C for 10 seconds, 63°C for 10 seconds, and 72°C for 8 seconds; SLPI: 35 cycles of 95°C for 10 seconds, 61°C for 10 seconds, and 72°C for 10 seconds; WAP 14: 35 cycles of 95°C for 10 seconds, 68°C for 10 seconds, and 72°C for 8 seconds; GAPDH: 35 cycles of 95°C for 10 seconds, 64°C for 10 seconds, and 72°C for 18 seconds. After amplification, the melting curve analysis was performed. The relative expression of each mRNA was normalized by GAPDH mRNA.
Enzyme-Linked Immunosorbent Assay
The concentration of GRO-α in conditioned media was measured using a specific enzyme-linked immunosorbent assay kit (R&D). The limit of sensitivity of the kit was 31.3 pg/mL, and the intra- and interassay coefficients of variation were <5% in the assays.
Neutrophil Migration Assay
The neutrophil–chemotactic activity of the conditioned medium was measured using chemotaxicell chambers (pore size of the filter membrane, 3 μm; Kurabo, Osaka, Japan).20 The chambers were filled with 250 μL conditioned medium, and 100 μL of the cell suspension was transferred to each chemotaxicell chamber for 2 hours followed by counting migrated neutrophils. For inhibition of GRO-α-mediated recruitment, 2 μg/mL anti-GRO-α goat polyclonal antibody or control goat immunoglobulin G were added to the medium 20 minutes before the migration assay.20
Statistical Analysis
All results were shown as mean ± standard error of the mean of data from at least 4 separate experiments. Data were analyzed by Mann-Whitney test for paired comparison, and 1-way analysis of variance with post hoc test for multiple comparisons using Statview Software (SAS Institute Inc, Cary, North Carolina). A P value of less than .05 was considered statistically significant.
Results
The Effect of BMPs on GRO-α Expression in Human GC
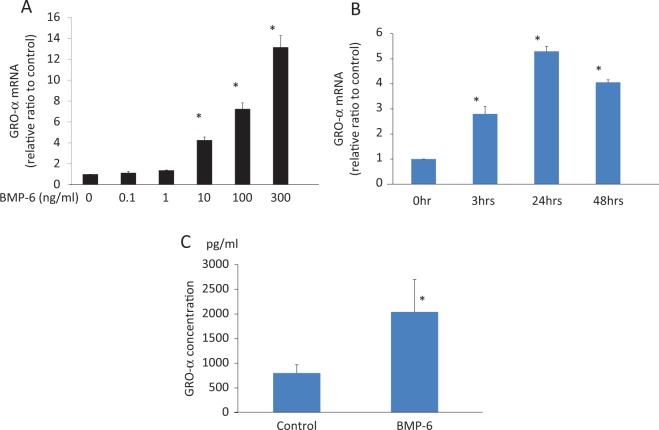
Bone morphogenetic protein 6 increased the expression of GRO-α mRNA. In a dose-finding study, BMP-6 concentrations of at least 10 ng/mL significantly increased GRO-α mRNA expression (Figure 1A). In a time course study, BMP-6 (100 ng/mL) increased GRO-α mRNA expression, with a maximum induction of GRO-α mRNA expression occurring after 24 hours of treatment (Figure 1B). As shown in Figure 1C, the basal level of GRO-α concentration was about 800 pg/mL in this culture system. Treatment with BMP-6 (100 ng/mL) for 24 hours stimulated an approximately 2.5-fold increase in GRO-α concentration in the cultured supernatant.
Figure 1.
Bone morphogenetic protein (BMP)-6-induced growth-regulated oncogene (GRO) α expression in human granulosa-lutein cells (GCs). Cultured human GCs were stimulated with BMP-6 (0-300 ng/mL) for 24 hours (A) or with BMP-6 (100 ng/mL) for 3 to 48 hours (B). Total RNA was extracted from the cells and subjected to real-time PCR to determine the GRO-α mRNA levels. Data were normalized by GAPDH mRNA levels to show the relative abundance to the control level. Data from at least 4 different experiments were combined and shown as the mean ± SEM relative to an adjusted value of 1.0 for the mean value of the control. *P < .01 (vs control). C, GRO-α concentration in cultured supernatant stimulated with BMP-6 (100 ng/mL, 24 hours) was measured using ELISA. Data from 4 different experiments were combined *P < .01 (vs control). mRNA indicates messenger RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; SEM, standard error of the mean.
Neutrophil Migration Assay
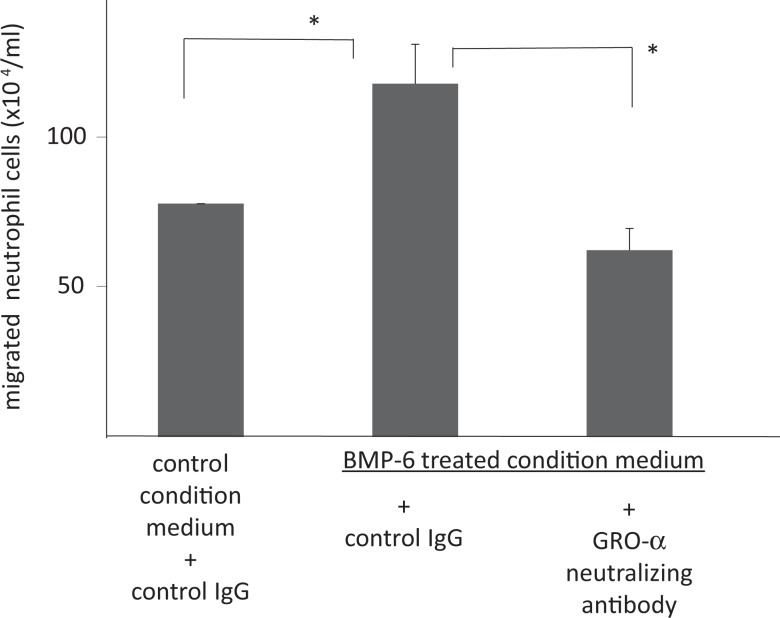
A neutrophil migration assay of cultured supernatant was performed to investigate the effect of GRO-α derived from GC. There was no effect of neutrophil migration observed with stimulation of BMP-6 itself (data not shown). As shown in Figure 2, the GC supernatant cultured with BMP-6 attracted more neutrophils compared with the control in which GCs were cultured for 24 hours without BMP-6. Addition of an excess amount of anti-GRO-α-neutralizing antibody negated the effect of BMP-6 on cultured GC, indicating that GRO-α may mediate the effect of BMP-6 on attracting neutrophils.
Figure 2.
The role of bone morphogenetic protein (BMP) 6 in neutrophil migration. Conditioned media obtained from granulosa-lutein cell (GC) cultures were used for a neutrophil migration assay. Granulosa-lutein cells were cultured with or without BMP-6 (100 ng/mL) for 24 hours. The neutrophil–chemotactic activity of the conditioned medium was measured using chemotaxicell chambers. The chambers were filled with 250 μL conditioned medium, and 100 μL of neutrophil cell suspension was transferred to each chemotaxicell chamber for 2 hours followed by counting migrated neutrophils. In some experiments, 2 μg/mL antigrowth-regulated oncogene (GRO) α antibody or control goat IgG was added to the medium 20 minutes before the migration assay. The numbers of neutrophils were shown. Data from 6 different experiments were shown as the mean ± SEM. *P < .01 (vs control). IgG indicates immunoglobulin G; SEM, standard error of the mean.
The Effect of BMPs on WAP14 and SLPI mRNA Level
To investigate the regulation of WAP14 and SLPI, which are regulatory factors of neutrophils, human GCs were cultured with BMP-6 (100 ng/mL) for 24 hours. Bone morphogenetic protein 6 stimulation resulted in a 50% and 80% decrease in the mRNA expression of SLPI and WAP14, respectively (P < .05; Figure 3).
Figure 3.
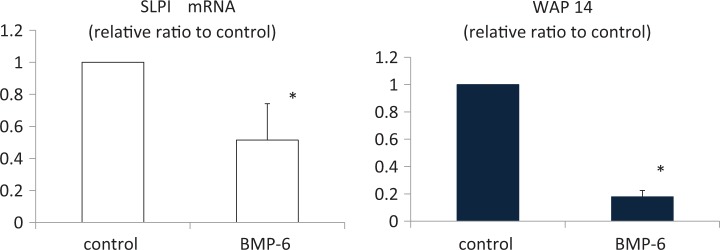
Bone morphogenetic protein (BMP) 6 suppressed secretory leukocyte peptidase inhibitor (SLPI) and whey acid protein (WAP) 14 mRNA expression in cultured human GC. Cultured human granulosa-lutein cells (GCs) were stimulated with BMP-6 (100 ng/mL) for 24 hours. Total RNA was extracted from the cells and subjected to real-time PCR to determine the expression of SLPI and WAP14. Data were normalized by GAPDH mRNA levels to show the relative abundance. Data from 4 different experiments were combined and shown as the mean ± SEM relative to an adjusted value of 1.0 for the mean value of the each control. *P < .05 (vs control). PCR indicates polymerase chain reaction; mRNA, messenger RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PCR, polymerase chain reaction; SEM, standard error of mean.
Discussion
A recent study by Sugiura et al demonstrated that the intraovarian cytokine, BMP-6, plays a role in ovulation. In this study, BMP-6 null mice exhibited fewer ovulations than control mice.8 Moreover, BMP-6 administration was shown to increase the number of ovulated oocytes in mice.21 In the present study, we investigated the effect of BMP-6 in human GC on ovulation-related events.
A key event during the ovulatory process is the degradation of the matrix at the follicular apex, where the leukocytes accumulate to secrete proteases.12 Therefore, chemokines that attract neutrophils to ovulatory follicles play a key role in ovulation.12,13 Immunohistochemistry studies have revealed that chemokine GRO-α is localized to theca layers and granulosa layers and that GRO-α was found in approximately 10-fold higher concentrations in follicular fluid than in blood plasma from the same patients.22 It has also been reported that in human GC, GRO-α is induced by LH/human chorionic gonadotropin stimulation, IL-1, and tumor necrosis factor α.16,22
The present study showed that BMP-6 induced GRO-α mRNA and protein in GC. Also, in the in vitro chemoattractant study using GC supernatant, the number of neutrophils attracted was increased with a culture media, which was stimulated with BMP-6, and this effect was abolished with anti-GRO-α-neutralizing antibody. In a previous study, we demonstrated that BMP-6 is expressed in GC of healthy follicles but not atretic follicles.5 Therefore, healthy preovulatory follicles might have higher amounts of GRO-α. This is consistent with the finding that the neutrophil–chemotactic activity in follicular fluid of IVF patients was significantly higher in conceptual cycles than in nonconceptual cycles.23
Migrated neutrophils release proteases for broad substrate specificity, such as elastin, collagen, proteoglycan, fibronectin, laminin, and other ECM proteins,24 resulting in the degradation of follicular wall. The activity of these proteases is regulated by the WAP family.17,18,25 The WAP family is characterized by a 4-disulfide structural domain and consists of 18 members, including SLPI and elafin.25
In the present study, we found that BMP-6 might augment the action of neutrophils by reducing the expression of WAP family members, SLPI and WAP14, in GC. In the mouse model of polycystic ovary (PCO), which is induced with excessive administration of estradiol valerate, the expression of SLPI in the ovary is higher than in control mice.26 Given that PCO leads to anovulatory status, one could hypothesize that higher SLPI might suppress ovulation by inhibiting the effect of neutrophils.
In conclusion, we found that BMP-6, an intraovarian cytokine, might be involved in the ovulatory process of human GC. Although BMP-6 null mice exhibit a decrease in ovulation, there have been no reports of BMP-6 abnormalities in humans. Therefore, further investigation is needed to prove this hypothesis.
Acknowledgments
We thank Dr Heather M. Martinez for her helpful discussion and critical reading of the article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan , Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. [DOI] [PubMed] [Google Scholar]
- 2. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. [DOI] [PubMed] [Google Scholar]
- 3. Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. [DOI] [PubMed] [Google Scholar]
- 4. Shi J, Yoshino O, Osuga Y, et al. Growth differentiation factor 3 is induced by bone morphogenetic protein 6 (BMP-6) and BMP-7 and increases luteinizing hormone receptor messenger RNA expression in human granulosa cells. Fertil Steril. 2012;97(4):979–983. [DOI] [PubMed] [Google Scholar]
- 5. Shi J, Yoshino O, Osuga Y, et al. Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin beta subunits, and anti-Mullerian hormone in human granulosa cells. Fertil Steril. 2009;92(5):1794–1798. [DOI] [PubMed] [Google Scholar]
- 6. Shi J, Yoshino O, Osuga Y, et al. Bone morphogenetic protein-2 (BMP-2) increases gene expression of FSH receptor and aromatase and decreases gene expression of LH receptor and StAR in human granulosa cells. Am J Reprod Immunol. 2011;65(4):421–427. [DOI] [PubMed] [Google Scholar]
- 7. Shi J, Yoshino O, Osuga Y, Nishii O, Yano T, Taketani Y. Bone morphogenetic protein 7 (BMP-7) increases the expression of follicle-stimulating hormone (FSH) receptor in human granulosa cells. Fertil Steril. 2010;93(4):1273–1279. [DOI] [PubMed] [Google Scholar]
- 8. Sugiura K, Su YQ, Eppig JJ. Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse? Biol Reprod. 2010;83(6):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. [DOI] [PubMed] [Google Scholar]
- 10. Goldman S, Shalev E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front Biosci. 2004;9:2474–2483. [DOI] [PubMed] [Google Scholar]
- 11. Brannstrom M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. [DOI] [PubMed] [Google Scholar]
- 12. Brannstrom M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol. 2002;57(1-2):47–60. [DOI] [PubMed] [Google Scholar]
- 13. Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update. 2000;6(1):1–15. [DOI] [PubMed] [Google Scholar]
- 14. Yoshino O, Osuga Y, Koga K, et al. Upregulation of interleukin-8 by hypoxia in human ovaries. Am J Reprod Immunol. 2003;50(4):286–290. [DOI] [PubMed] [Google Scholar]
- 15. Kawano Y, Furukawa Y, Fukuda J, Matsumoto H, Yuge A, Narahara H. The effects of platelet-activating factor on the secretion of interleukin-8 and growth-regulated oncogene alpha in human immortalized granulosa cell line (GC1a). Am J Reprod Immunol. 2007;58(5):434–439. [DOI] [PubMed] [Google Scholar]
- 16. Oral E, Seli E, Bahtiyar MO, Jones EE, Arici A. Growth-regulated alpha expression in human preovulatory follicles and ovarian cells. Am J Reprod Immunol. 1997;38(1):19–25. [DOI] [PubMed] [Google Scholar]
- 17. Scott A, Weldon S, Taggart CC. SLPI and elafin: multifunctional antiproteases of the WFDC family. Biochem Soc Trans. 2011;39(5):1437–1440. [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson TS, Roghanian A, Simpson AJ, Sallenave JM. WAP domain proteins as modulators of mucosal immunity. Biochem Soc Trans. 2011;39(5):1409–1415. [DOI] [PubMed] [Google Scholar]
- 19. Erickson GF, Garzo VG, Magoffin DA. Insulin-like growth factor-I regulates aromatase activity in human granulosa and granulosa luteal cells. J Clin Endocrinol Metab. 1989;69(4):716–724. [DOI] [PubMed] [Google Scholar]
- 20. Takamura M, Osuga Y, Izumi G, et al. Interleukin-17A is present in neutrophils in endometrioma and stimulates the secretion of growth-regulated oncogene-alpha (Gro-alpha) from endometrioma stromal cells. Fertil Steril. 2012;98(5):1218–1224 e1211–1212. [DOI] [PubMed] [Google Scholar]
- 21. Park SS, Park MJ, Joo BS, Joo JK, Son JB, Lee KS. Improvement of ovarian response and oocyte quality of aged female by administration of bone morphogenetic protein-6 in a mouse model. Reprod Biol Endocrinol. 2012;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karstrom-Encrantz L, Runesson E, Bostrom EK, Brannstrom M. Selective presence of the chemokine growth-regulated oncogene alpha (GROalpha) in the human follicle and secretion from cultured granulosa-lutein cells at ovulation. Mol Hum Reprod. 1998;4(11):1077–1083. [DOI] [PubMed] [Google Scholar]
- 23. Herriot DM, Warnes GM, Kerin JF. Pregnancy-related chemotactic activity of human follicular fluid. Fertil Steril. 1986;45(2):196–201. [DOI] [PubMed] [Google Scholar]
- 24. Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. [DOI] [PubMed] [Google Scholar]
- 25. Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008;29(9):444–453. [DOI] [PubMed] [Google Scholar]
- 26. Park JJ, Bae CS, Choi BD, et al. Induction of secretory leukocyte protease inhibitor (SLPI) in estradiol valerate (EV) induced polycystic ovary. Arch Pharm Res. 2011;34(8):1389–1397. [DOI] [PubMed] [Google Scholar]