Abstract
Matrix metalloproteinase (MMP) 9 plays an important role in the degradation of the extracellular matrix in fetal membranes, and pathological activation of MMP-9 can lead to preterm birth. In nongestational tissues, modulation of histone deacetylases (HDACs) regulates MMP-9 expression. The aim of this study was to determine whether class I to III HDACs regulate MMP-9 expression and activity in primary amnion cells. Class I and II HDAC regulation of MMP-9 was assessed using the general class I and II HDAC inhibitors (HDACi) trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA), the class I HDACi MS-275, and the class II HDACi MC1568. Class III HDAC regulation of MMP-9 was assessed using the SIRT1 activators resveratrol and SRT1720 as well as SIRT1 small interfering RNA (siRNA). Primary amnion epithelial cells were incubated with 1 ng/mL interleukin (IL) 1β in the absence or presence of 0.3 μmol/L TSA, 5 μmol/L SAHA, 2.5 μmol/L MS-275, 2.5 μmol/L MC1568, 50 μmol/L resveratrol, or 10 μmol/L SRT1720 for 20 hours. We found that the class I and II HDACi TSA and SAHA and the class II HDACi MC1568 significantly decreased IL-β-induced MMP-9 gene and pro-MMP-9 expression in primary amnion cells. There was, however, no effect of the class I HDACi MS-275 on IL-β-induced MMP-9 expression. On the other hand, inhibition of class III HDAC SIRT1 using siRNA significantly augmented IL-1β-induced MMP-9, and SIRT1 activation using resveratrol and SRT1720 inhibited IL-1β-induced MMP-9 expression. In summary, class I to III HDACs differentially regulate inflammation-induced MMP-9 expression in primary amnion cells.
Keywords: fetal membranes, histone deacetylation, MMP-9, SIRT1
Introduction
Preterm birth is one of the most significant health care issues globally; 9.6% of all births in 2005 were preterm.1 Being born early is the leading direct cause of early neonatal death, responsible for approximately 1 million annual neonatal deaths.2 Survivors of preterm birth have greatly increased rates of long-term disabilities including cerebral palsy, intellectual handicap, and chronic lung disease requiring oxygen.3 Not only do these chronic diseases cause enormous financial and emotional burden on the family they also present a challenge to finite health care resources.4
Of clinical significance, in 20% to 25% of preterm birth is a result of prelabor rupture of membranes (PROMs).5 What causes the membranes to weaken and thus rupture is not known; however, an area of altered morphology has been observed in fetal membranes obtained from the along the tear line after term labor.6 Similarly, less extensive changes have also been observed in fetal membranes lying over the cervix at term in the absence of labor7–11 and after preterm birth.12 In addition to changes in morphology and structure, these fetal membranes also exhibit increased apoptosis and collagen-degrading enzymes including matrix metalloproteinase (MMP)-9.13 The MMP-9 has an ascribed role in mediating degradation of the extracellular matrix (ECM) in both normal and pathologic conditions, such as PROM.14,15 Given the important role for MMP-9 in the rupture of fetal membranes and thus preterm birth, it is essential to fully understand the mechanisms surrounding MMPs in order to develop effective therapeutic strategies.
Histone acetylation and deacetylation play important roles in gene expression. Acetylation of core histones by histone acetyltransferases leads to unwinding of DNA, which subsequently allows transcription factors and RNA polymerase II to switch on gene transcription. Conversely, deacetylation of core histones is generally associated with transcriptional repression. There are 18 human histone deacetylases (HDACs) and they are grouped into 4 classes and 2 families: the “classical” and the silent information regulator 2-related protein (sirtuin; SIRT) families. The classical HDACs consist of the following groups: class I (1-3, 8), class II (4-7, 9, 10), and class IV (11). Class III HDACs belongs to the SIRT family, which contains 7 members (SIRT1-7). They have no sequence resemblance to members of the classical family; they are NAD+-dependent protein deacetylases.
There is now increasing evidence that many prolabor genes, such as interleukin (IL) 1β, IL-6, tumor necrosis factor α (TNF-α), and cyclooxygenase 2 (COX-2) are regulated by HDAC inhibitor (HDACi).16–18 In addition, our own studies on SIRT1 and SIRT6 also reveal a functional role in regulating proinflammatory cytokines in fetal membranes.19,20 However, whether class I and class II HDACs and the class III HDAC SIRT1 also regulate MMP-9 expression and activity in human fetal membranes is not known. Thus, in this study, we used human primary amnion cells stimulated with IL-1β to determine the effect of (1) general class I and II HDACi trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA), (2) class I-specific HDACi MS-275; (3) class II-specific HDACi MC1568; (4) SIRT1 activators resveratrol and SRT1702; and (5) SIRT1 small interfering RNA (siRNA) knockdown on MMP-9 expression and activity.
Materials and Methods
Sample Collection
Fetal membranes were collected from women with uncomplicated pregnancies at term (gestational ages of the samples ranged from 37 to 40 weeks gestation) undergoing elective cesarean section. The Research Ethics Committee of Mercy Health approved this study. Written informed consent was obtained from participating women.
Primary Amnion Cells Studies
Primary amnion epithelial cultures were used to investigate the effects of activation and inhibition of HDACs on MMP-9 expression and activity. The TSA and resveratrol were purchased from Sigma-Aldrich (Saint Louis, Missouri). The SAHA, MS-275, MC1568, and SRT1720 were purchased from Life Research (Scoresby, Victoria, Australia). Stock solutions of TSA, SAHA, MS-275, and MC1568 were prepared in PBS. Stock solution of SRT1720 and resveratrol were prepared in dimethyl sulfoxide (DMSO). Cells were prepared as we have previously described.21 Cells plated at 1 × 106 cells/well were incubated in a humidified atmosphere of 8% O2 and 5% CO2 at 37°C. Primary amnion cells at 80% to 90% confluence were incubated in the absence or presence of 1 ng/mL IL-1β20,22 with and without 0.3 μmol/L TSA, 5 μmol/L SAHA, 2.5 μmol/L MS-275, 2.5 μmol/L MC1568, 10 μmol/L SRT1720, or 50 μmol/L resveratrol (in Dulbecco’s modified eagle medium [DMEM]/F-12 containing 2% heat-inactivated fetal calf serum). For resveratrol and SRT1720, the basal and IL-1β-treated cells contained DMSO at a final concentration of 0.1%. After 20 hours incubation, medium was collected, and assessment of pro-MMP-2 and pro-MMP-9 was performed by gelatin zymography as previously described. Cells were also collected, and gene expression was analyzed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) as detailed subsequently. Each experiment was performed on amnion from 6 patients. For these experiments, the data are presented as fold change relative to basal.
For the siRNA studies, cells at approximately 50% confluence were transfected using SilenceMag reagent according to manufacturer's guidelines (Oz Biosciences, Marseille, France) and as we have previously described for amnion.20,22 Cells were transfected with 100 nmol/L SIRT1 or NS siRNA (Ambion, Austin, Texas) in DMEM/F-12 for 48 hours. The medium was then replaced with DMEM/F-12 with or without 1 ng/mL IL-1β, and the cells were incubated at 37°C for an additional 20 hours. After 20 hours incubation, medium was collected, and assessment of MMP-9 was performed by gelatin zymography as described subsequently. Cells were collected, and gene expression was analyzed by qRT-PCR as detailed subsequently. Experiments were performed from amnion obtained from 6 patients. For the siRNA experiments, the data are presented as fold change relative to the NS siRNA-transfected cells.
Gelatin Zymography
Secreted pro-MMP-2 and pro-MMP-9 expression in conditioned media was analyzed by gelatin zymography as we have previously described20,21,23 on conditioned media collected from primary amnion cells. Proteolytic activity was visualized as clear zones of lysis on a blue background of undigested gelatin. Gels were scanned using the Chemidoc XRS system (Bio-Rad Laboratories, Hercules, California), inverted, and densitometry performed using Quantity One image analysis software (Bio-Rad Laboratories).
RNA Extraction and qRT-PCR Analysis
RNA extraction, complementary DNA (cDNA) synthesis, and qRT-PCR were performed as we have previously published.19–21 We used predesigned and validated primers for SIRT1, MMP-2, MMP-9, and GAPDH (QuantiTect primer assays, Qiagen, Germantown, Maryland). Average gene CT values were normalized to the average GAPDH CT values of the same cDNA sample. The specificity of the product was assessed from melting curve analysis. The RNA without reverse transcriptase during cDNA synthesis as well as PCR reactions using water instead of template showed no amplification. Relative quantification was determined using the comparative CT method.
Statistical Analysis
Statistical analyses were performed using a commercially available statistical software package (Statgraphics Plus version 3.1; Statistical Graphics Corp, Rockville, Maryland). Statistical analysis was performed using a 1-way analysis of variance (using Tukey honestly significant difference correction to discriminate among the means); homogeneity of data was assessed by Bartlett test, and when significant, data were logarithmically transformed before further analysis. Statistical significance was ascribed to P value <.05. Data were expressed as mean ± standard error of the mean.
Results
Effect of General Class I and Class II HDACi TSA and SAHA on IL-1β-Induced MMP-9 Expression and Activity
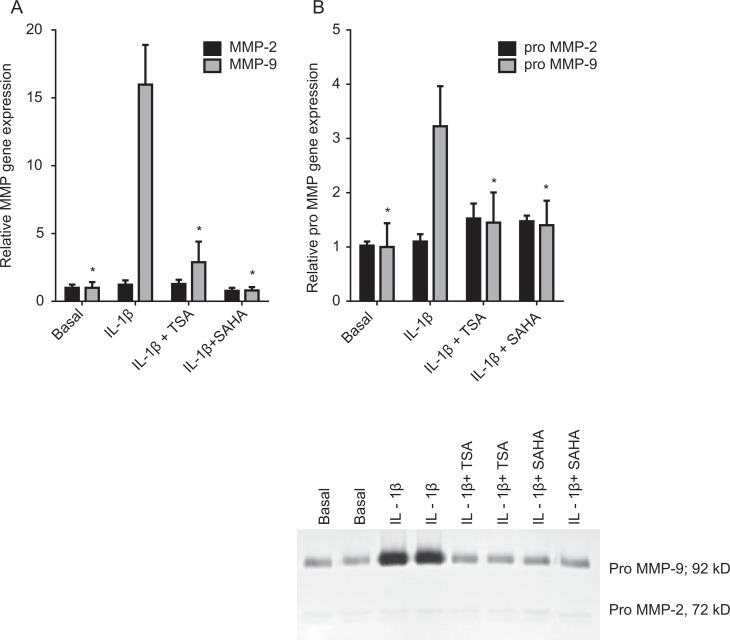
Primary amnion cells were incubated in the absence or presence of 1 ng/mL IL-1β with and without 0.3 μmol/L TSA or 5 μmol/L SAHA for 20 hours. The effect of the TSA and SAHA on the induction of MMP-9 expression and activity is demonstrated in Figure 1. The MMP-9 gene expression was analyzed by qRT-PCR. Gelatin substrate gels were used to determine the effect of treatment on the release of pro-MMP-9 enzyme activity. The IL-1β significantly increased MMP-9 gene expression (Figure 1A) and enzyme activity (Figure 1B) and cotreatment with TSA and SAHA significantly attenuated this increase. There was no effect of IL-1β or inhibitors on MMP-2 messenger RNA (mRNA) or pro-MMP-2 expression (Figure 1A and B).
Figure 1.
Effect of TSA and SAHA on MMP-2 and MMP-9 expression. Primary human amnion cells were incubated with 1 ng/mL IL-1β in the absence or presence of 0.3 μmol/L TSA and 5 μmol/L SAHA for 20 hours (n = 6 patients). A, The MMP-2 and MMP-9 gene expression was normalized to GAPDH mRNA expression, and the fold change was calculated relative to basal. Each bar represents the mean ± SEM. *P < .05 versus IL-1β-treated cells (1-way ANOVA). B, The incubation medium was assayed for pro-MMP-2 and MMP-9 expression by gelatin zymography. Pro-MMP-2 and MMP-9 levels were confirmed by densitometry, and the fold change was calculated to basal. Data are displayed as mean ± SEM (1-way ANOVA). *P < .05 versus IL-1β-treated cells (1-way ANOVA). A representative zymography of 1 patient (performed in duplicate) is also shown. ANOVA indicates analysis of variance; IL, interleukin; MMP, matrix metalloproteinase; SAHA, suberoylanilide hydroxamic acid; SEM, standard error of the mean; TSA, trichostatin A.
Effect of Class I-Specific HDACi MS-275 and Class II-Specific HDACi MC1568 on IL-1β-Induced MMP-9 Expression and Activity
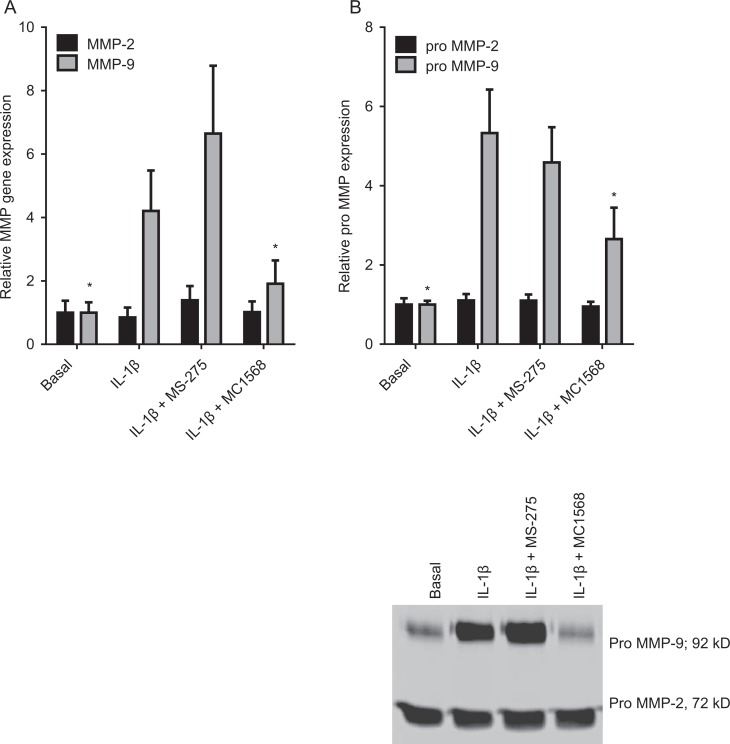
The TSA and SAHA are general inhibitor of class I and class II HDACs. Thus, in order to determine whether both class I and class II HDACs regulate MMP-9, we used the class I-specific HDACi MS-275 and class II-specific HDACi MC1568. For these studies, primary amnion cells were incubated in the absence or presence of 1 ng/mL IL-1β with and without 2.5 μmol/L MS-275 or 2.5 μmol/L MC1568 for 20 hours. As depicted in Figure 2, treatment of primary amnion cells with MC1568 significantly decreased IL-1β-induced MMP-9 gene expression (Figure 2A) and pro-MMP-9 activity (Figure 2B). On the other hand, there was no effect of the class I-specific HDACi MS-275 on MMP-9 gene expression (Figure 2A) and pro-MMP-9 activity (Figure 2B). Of note, there was also no effect of higher concentrations of MS-275 (5 and 10 μmol/L) on MMP-9 (data not shown). There was no effect of IL-1β or inhibitors on MMP-2 mRNA and pro-MMP-2 expression (Figure 2A and B).
Figure 2.
Effect of MS-275 and MC1568 on MMP-2 and MMP-9 expression. Primary human amnion cells were incubated with 1 ng/mL IL-1β in the absence or presence of 2.5 μmol/L MS-275 and 2.5 μmol/L MS1568 for 20 hours (n = 6 patients). A, The MMP-2 and MMP-9 gene expression was normalized to GAPDH mRNA expression, and the fold change was calculated relative to basal. Each bar represents the mean ± SEM. *P < .05 versus IL-1β-treated cells (1-way ANOVA). B, The incubation medium was assayed for pro-MMP-2 and MMP-9 expression by gelatin zymography. Pro-MMP-2 and MMP-9 levels were confirmed by densitometry, and the fold change was calculated to basal. Data are displayed as mean ± SEM (1-way ANOVA). *P < .05 versus IL-1β-treated cells (1-way ANOVA). A representative zymography of 1 patient (performed in duplicate) is also shown. ANOVA indicates analysis of variance; IL, interleukin; MMP, matrix metalloproteinase; SEM, standard error of the mean.
Effect of Class III HDAC Activators on IL-1β-Induced MMP-9 Expression and Activity
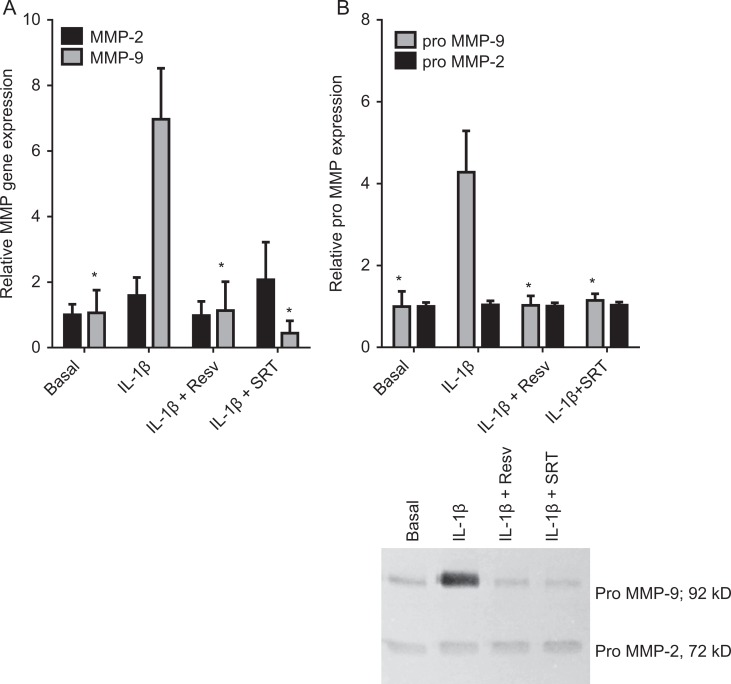
The next aim was to determine the effect of activators of the class III HDAC SIRT1. For these studies, we used resveratrol and SRT1720. We have previously demonstrated the specificity of these compounds for activating SIRT1 expression in fetal membranes and primary amnion cells.19 We found that incubation with resveratrol or SRT1720 significantly decreased MMP-9 mRNA expression (Figure 3A) and activity (Figure 3B) induced by IL-1β. There was no effect of IL-1β or inhibitors on MMP-2 mRNA or secreted pro-MMP-2 expression (Figure 3A and B).
Figure 3.
Effect of resveratrol and SRT1720 on MMP-2 and MMP-9 expression. Primary human amnion cells were incubated with 1 ng/mL IL-1β in the absence or presence of 50 μmol/L resveratrol (Resv) and 10 μmol/L SRT1720 (SRT) for 20 hours (n = 6 patients). A, The MMP-2 and MMP-9 gene expression was normalized to GAPDH mRNA expression, and the fold change was calculated relative to basal. Each bar represents the mean ± SEM. *P < .05 versus IL-1β-treated cells (1-way ANOVA). B, The incubation medium was assayed for pro-MMP-2 and MMP-9 expression by gelatin zymography. Pro-MMP-2 and MMP-9 levels were confirmed by densitometry, and the fold change was calculated to basal. Data are displayed as mean ± SEM (1-way ANOVA). *P < .05 versus IL-1β-treated cells (1-way ANOVA). A representative zymography of 1 patient is also shown. ANOVA indicates analysis of variance; IL, interleukin; MMP, matrix metalloproteinase; SEM, standard error of the mean.
Effect of SIRT1 siRNA Knockdown on IL-1β-Induced MMP-9 Expression and Activity
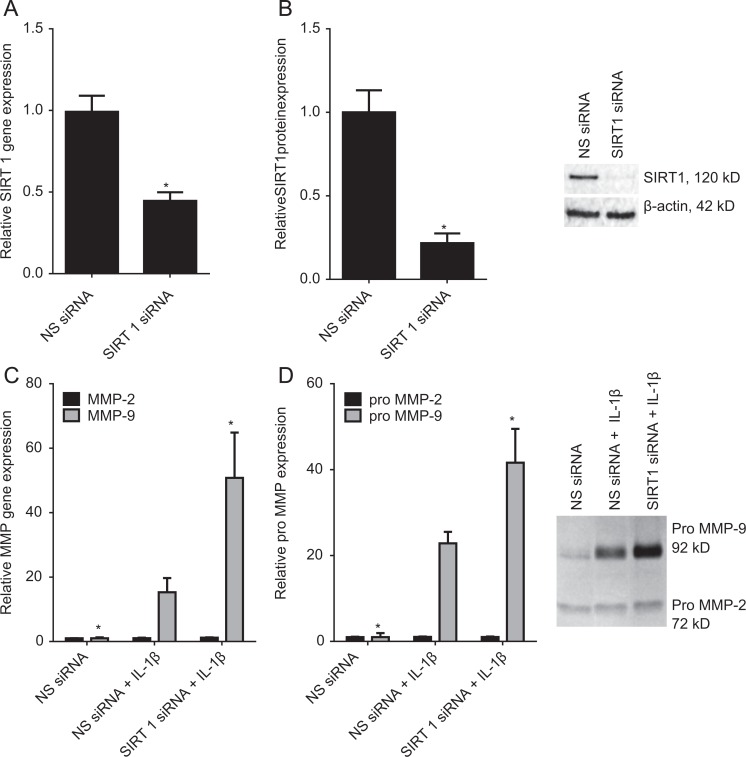
We next confirmed the results obtained in Figure 3 using siRNA against SIRT1. The efficacy of transfection was analyzed by qRT-PCR and Western blotting and the data presented in Figure 4A and B. Compared to NS siRNA-transfected cells, transfection with SIRT1 siRNA resulted in a significant decrease in SIRT1 mRNA (Figure 4A) and protein expression (Figure 4B). The MMP-9 mRNA expression and activity in response to SIRT1 siRNA knockdown is also depicted in Figure 4. As expected, in amnion cells transfected with NS siRNA, IL-1β treatment significantly increased MMP-9 gene expression (Figure 4C) and activity (Figure 4D). However, in cells where SIRT1 was knocked down, this increase in gene expression and enzyme activity was significantly augmented. There was no effect of SIRT1 knockdown or SIRT1 inhibitors on MMP-2 mRNA or secreted pro-MMP-2 expression (Figure 4C and D).
Figure 4.
Effect of SIRT1 siRNA on MMP-2 and MMP-9 expression. A and B, Primary amnion cells were transfected with 100 nmol/L SIRT1 or NS siRNA for 96 hours (n = 6 patients). C and D, Primary amnion cells were transfected with 100 nmol/L SIRT1 or NS siRNA and after 72 hours, cells were incubated in the absence or presence of 1 ng/mL IL-1β for a further 20 hours (n = 6 patients). A and C, The MMP-2 and MMP-9 gene expression was normalized to GAPDH mRNA expression, and the fold change was calculated relative to NS siRNA-transfected cells. Each bar represents the mean ± SEM. *P < .05 versus NS siRNA + IL-1β (1-way ANOVA). B, Representative Western blot demonstrating efficacy of transfection. D, The incubation medium was assayed for pro-MMP-2 and MMP-9 expression by gelatin zymography. Pro-MMP-2 and MMP-9 levels were confirmed by densitometry, and the fold change was calculated to basal. Data are displayed as mean ± SEM (1-way ANOVA). *P < .05 versus NS siRNA + IL-1β (1-way ANOVA). A representative zymography of 1 patient is also shown. ANOVA indicates analysis of variance; IL, interleukin; MMP, matrix metalloproteinase; SEM, standard error of the mean; siRNA, small interfering RNA.
Discussion
This is the first study to report that classes I to III HDACs regulate MMP-9 expression in human fetal membranes. We found that the addition of either of the 2 chemically distinct inhibitors of class I and II HDAC—TSA and SAHA—to amnion primary cell cultures blocks IL-1β-induced pro-MMP-9 expression in the conditioned culture medium. Gene expression studies showed that the expression of MMP-9 gene expression induced by IL-1β was also repressed by these HDACi. The regulation of MMP-9 expression was confirmed using the class II-specific HDACi MC1568. Interestingly, however, we found no effect of the class I-specific HDACi MS-275 on MMP-9 gene expression and expression of pro-MMP-9. On the other hand, knockdown of the class III HDAC SIRT1 was associated with increased transcription and activation of IL-1β-induced MMP-9. Conversely, the 2 activators of SIRT1, resveratrol and SRT1720, decreased IL-1β-induced MMP-9 gene expression and secreted pro-MMP-9 expression.
Matrix metalloproteinase 9is important for the processes that result in successful labor and delivery; however, aberrant and uncontrolled MMP-9 activity has been implicated in the degradation of matrix components of the amnion and chorion leading to preterm birth.24–31 The MMP-9 attacks the collagen fibers in the fetal membranes, thereby dissociating the layers that make up the fetal membranes and thickening the spongy layer that separates the amnion from the chorion.32 Altogether these structural changes lead to a significantly thinner and weaker region in the fetal membranes. There is also ample evidence demonstrating a role for infection or inflammation role in fetal membrane production of MMP-9.33,34 For example, MMP-9 levels are increased by in vitro exposure of fetal membranes to bacterial products35 and in the amniotic fluid of women with intrauterine infections.27,35 Thus, understanding MMP-9 regulation is crucial to define strategies to prevent pathological rupture of fetal membranes, particularly in the context of preterm birth.
Our current data show for the first time that a specific inhibitor of class II HDAC can also function as potent repressors of inflammation-induced MMP-9 gene expression and secretion of pro-MMP-9, a key MMP involved in the degradation of collagen in the fetal membranes. However, interestingly, although the 2 general inhibitors of class I and II HDACs, TSA and SAHA, decreased MMP-9 gene expression and secretion of pro-MMP-9, there was no effect of the class I-specific inhibitor MS-275 on MMP-9 expression. We thus hypothesize that the actions of TSA and SAHA may be attributed to their inhibitory actions on class II HDAC. There are a few studies on gestational tissues, which show a major effect of TSA and SAHA in repressing the production of proinflammatory cytokines,16,36 COX-2, and resultant prostaglandin production.16,18 There have been no studies that have specifically assessed the anti-inflammatory effects of specific class I or II inhibitors of HDAC in human fetal membranes or myometrium. However, the class I-specific HDACi suberic bishydroxamate has been shown to exert potent inhibitory effects on human uterine contractions; similar results were obtained using TSA and SAHA.37 Taken together, these data indicate that class II HDACi exert anti-inflammatory effects in human fetal membranes and myometrium and may thus be useful in achieving a gene expression profile that favors the maintenance of pregnancy.
We have previously reported that activators of the class III HDAC SIRT1 can block infection-induced prolabor mediators in human gestational tissues.19 Specifically, the SIRT1 activators resveratrol and SRT1720 significantly decreased LPS-induced cytokine gene expression and release, COX-2 expression, and prostaglandin release from fetal membranes. Furthermore, knockdown of SIRT1 by RNA interference in primary amnion cells diminished the anti-inflammatory effects of resveratrol. In this study, we report that resveratrol and SRT1720 inhibit MMP-9 gene expression and activation induced by IL-1β. Further to this, siRNA knockdown of SIRT1 augments IL-1β-induced MMP-9 expression and activity. These data are also keeping in with our recent studies on SIRT6, another class III HDAC.20 In these studies, we reported that siRNA knockdown of SIRT6 in primary amnion cells was associated with an augmentation of IL-1β-induced prolabor mediators. Collectively, these studies demonstrate that, in contrast to class I and II HDACs, class III HDACs are anti-inflammatory in human gestational tissues.
Histone deacetylation is associated with transcriptional repression38; thus, the inhibition of HDAC activities might be expected to result in increases in gene expression. However, the paradoxical inhibitory effects of HDACi on gene expression observed in this study and others16,18,36,39–42 suggest that other components of the transcriptional response, in addition to histones, are regulated by acetylation. In support, a number of nonhistone proteins have been identified as targets for HDACi including nuclear factor κB (NF-κB).43 There is now compelling evidence implicating the NF-κB signaling pathway in the processes involved in human labor and delivery, both at term and preterm. We, and others, have demonstrated that inhibition of NF-κB activity in ex situ human gestational tissues suppresses the formation of labor-mediating effectors.23,44–46 Likewise, our previous studies have shown the importance of the NF-κB pathway in the regulation of inflammation-induced MMP-9 in human fetal membranes.23 Indeed, there is also now much evidence to show that class I and II HDAC inhibitors (HDACi) inhibit inflammation via acetylation of nonhistone proteins like transcription factors.47 For example, the class I and II HDACi TSA and vorinostat (SAHA) inhibit infection-induced MMP-9 via decreasing NF-κB activity.41 The class III HDACs, especially SIRT1, have also been shown to regulate inflammation through NF-κB.48–51 The SIRT1 physically interacts with the RelA/p65 subunit of NF-κB and inhibits transcription by deacetylating RelA/p65 at lysine 310. Knockout or knockdown of SIRT1 gene leads to increases in inflammation, whereas SIRT1 activation inhibits inflammation.19,49,52–54
Both MMP-2 and MMP-9 (known as gelatinase A and B, respectively) can degrade collagen type IV, elastin, and fibronectin; however, MMP-9 is induced by infection, active labor, and PROM, while MMP-2 protein and activity appear to be constitutively expressed during gestation.24 Our findings that MMP-2 is unchanged with inflammation is consistent with other studies reporting unchanged MMP-2 tissue protein or activity levels in combined fetal membranes with the onset of labor at term and preterm.26 That MMP-2 expression was unchanged in human primary amnion cells with HDACis may indicate a role that is tissue specific; in cancer research, TSA inhibited MMP-2 expression in 3T3 fibroblast cells55 but not in SW620 colon carcinoma cells.56
Some limitations of this study include looking at MMP-9 activation; active MMP-9 was unable to be detected in our samples by gelatin zymography, and the effect of our agents on MMP-9 activity was unable to be performed. Secreted pro-MMPs remain inactive due to the interaction of the unpaired cysteine sulfhydryl group; removal of the propeptide containing the cysteine group by enzyme cleavage; or disruption of the zinc–cysteine interaction can result in activation of the latent enzyme. Additionally, for these studies, it is not possible to determine whether the treatments cause their effects through HDAC inhibition or some other mechanisms. Future experiments examining changes in histone acetylation patterns, specifically histone modification associated with the MMP-9 promoter would answer this. Moreover, as only mRNA and protein expression were measured, it is not known of the HDACi affects MMP-9 transcription directly or through an indirect mechanism (such as alterations in the expression of transcription factor including NF-κB).
The regulation of MMPs is important in understanding the mechanisms of membrane rupture and thus developing potential therapeutics to stop PPROM. This study explores the role of HDACi in MMP-9 gene expression and secretion of pro-MMP-9. In conclusion, our data support a role for class I-III HDACs in the regulation of MMP-9 by IL-1β in human primary amnion cells. Based on the data presented in this study, inhibition of class II HDACs is associated with repression of MMP-9 expression. On the other hand, activation of the class III HDAC SIRT1 is also associated with repression of MMP-9 expression. Aberrant ECM degradation by MMPs in the fetal membranes is a critical event in preterm birth.24–27,33,34 It is, thus, possible that HDACs may be a possible therapeutic target to reduce ECM degradation and risk of preterm birth. Indeed, administration of TSA to pregnant mice late in gestation increased histone acetylation and delayed the initiation of parturition by 24 to 48 hours, suggesting the functional importance of the decline in histone acetylation in the initiation of labor.57
Acknowledgments
The authors gratefully acknowledge the assistance of the Clinical Research Midwives Genevieve Christophers, Renee Grant, Gabrielle Fleming, Debra Jinks, and Rachel Murdoch; and the Obstetrics and Midwifery staff of the Mercy Hospital for Women for their cooperation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Associate Professor Martha Lappas was supported by a Career Development Fellowship from the National Health and Medical Research Council (NHMRC ; grant no. 1047025). Dr Ratana Lim was, in part, supported by an early career researcher grant from the University of Melbourne. Funding for the iMark microplate spectrophotometer and Chemidoc XRS system was, in part, provided by the Medical Research Foundation for Women and Babies.
References
- 1. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawn JE, Osrin D, Adler A, Cousens S. Four million neonatal deaths: counting and attribution of cause of death. Paediatr Perinat Epidemiol. 2008;22(5):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. [DOI] [PubMed] [Google Scholar]
- 4. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. [DOI] [PubMed] [Google Scholar]
- 5. Goldenberg RL, Culhane JF, Iams JD, Romero R. Preterm birth 1—epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malak TM, Bell SC. Structural characteristics of term human fetal membranes—a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynaecol. 1994;101(5):375–386. [DOI] [PubMed] [Google Scholar]
- 7. El Khwad M, Stetzer B, Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72(3):720–726. [DOI] [PubMed] [Google Scholar]
- 8. McLaren J, Taylor DJ, Bell SC. Increased incidence of apoptosis in non-labour-affected cytotrophoblast cells in term fetal membranes overlying the cervix. Hum Reprod. 1999;14(11):2895–2900. [DOI] [PubMed] [Google Scholar]
- 9. McParland PC, Taylor DJ, Bell SC. Mapping of zones of altered morphology and chorionic connective tissue cellular phenotype in human fetal membranes (amniochorion and decidua) overlying the lower uterine pole and cervix before labor at term. Am J Obstetr Gynecol. 2003;189(5):1481–1488. [DOI] [PubMed] [Google Scholar]
- 10. Reti NG, Lappas M, Riley C, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol. 2007;196(5):484 e1–484 e10. [DOI] [PubMed] [Google Scholar]
- 11. McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14(1):237–241. [DOI] [PubMed] [Google Scholar]
- 12. Malak T. M., Mulholland G, Bell S. C. Morphometric Characteristics of the Decidua, Cytotrophoblast, and Connective Tissue of the Prelabor Ruptured Fetal Membranes. Annals of the New York Academy of Sciences. 1994; 734: 430–432. [DOI] [PubMed] [Google Scholar]
- 13. Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11-12):1037–1051. [DOI] [PubMed] [Google Scholar]
- 14. Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, et al. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- 15. Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF., III Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol. 1996;174(4):1371–1376. [DOI] [PubMed] [Google Scholar]
- 16. Lindström TM, Mohan AR, Johnson MR, Bennett PR. Histone deacetylase inhibitors exert time-dependent effects on nuclear factor-κB but consistently suppress the expression of proinflammatory genes in human myometrial cells. Mol Pharmacol. 2008;74(1):109–121. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell MD, Ponnampalam A, Rice GE. Epigenetic regulation of cytokine production in human amnion and villous placenta. Mediators Inflamm. 2012;2012:159709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitchell MD. Unique suppression of prostaglandin H synthase-2 expression by inhibition of histone deacetylation, specifically in human amnion but not adjacent choriodecidua. Mol Biol Cell. 2006;17(1):549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84(1):167–178. [DOI] [PubMed] [Google Scholar]
- 20. Lim R, Barker G, Lappas M. SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod. 2013;88(1):17. [DOI] [PubMed] [Google Scholar]
- 21. Lim R, Barker G, Wall CA, Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol Hum Reprod. 2013;19(7):451–462. [DOI] [PubMed] [Google Scholar]
- 22. Lim R, Barker G, Riley C, Lappas M. Apelin is decreased with human preterm and term labor and regulates prolabor mediators in human primary amnion cells. Reprod Sci. 2013;20(8):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88(4):1723–1729. [DOI] [PubMed] [Google Scholar]
- 24. Fortunato SJ, Menon R, Lombardi SJ. Collagenolytic enzymes (gelatinases) and their inhibitors in human amniochorionic membrane. Am J Obstet Gynecol. 1997;177(4):731–741. [DOI] [PubMed] [Google Scholar]
- 25. Yonemoto H, Young CB, Ross JT, Guilbert LL, Fairclough RJ, Olson DM. Changes in matrix metalloproteinase (MMP)-2 and MMP-9 in the fetal amnion and chorion during gestation and at term and preterm labor. Placenta. 2006;27(6-7):669–677. [DOI] [PubMed] [Google Scholar]
- 26. Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87(3):1353–1361. [DOI] [PubMed] [Google Scholar]
- 27. Maymon E, Romero R, Pacora P, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183(4):887–894. [DOI] [PubMed] [Google Scholar]
- 28. Draper D, McGregor J, Hall J, et al. Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am J Obstet Gynecol. 1995;173(5):1506–1512. [DOI] [PubMed] [Google Scholar]
- 29. Fortunato SJ, Menon R, Bryant C, Lombardi SJ. Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet Gynecol. 2000;182(6):1468–1476. [DOI] [PubMed] [Google Scholar]
- 30. Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184(7):1399–1405; discussion 405-406. [DOI] [PubMed] [Google Scholar]
- 31. Fortunato SJ, Menon R, Lombardi SJ. Support for an infection-induced apoptotic pathway in human fetal membranes. Am J Obstet Gynecol. 2001;184(7):1392–1397; discussion 97-98. [DOI] [PubMed] [Google Scholar]
- 32. Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 33. Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11(7):427–437. [DOI] [PubMed] [Google Scholar]
- 34. Vadillo-Ortega F, Estrada-Gutierrez G. Role of matrix metalloproteinases in preterm labour. BJOG. 2005;112(suppl 1):19–22. [DOI] [PubMed] [Google Scholar]
- 35. Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med. 1999;27(5):362–368. [DOI] [PubMed] [Google Scholar]
- 36. Munro SK, Mitchell MD, Ponnampalam AP. Histone deacetylase inhibition by trichostatin A mitigates LPS induced TNFα and IL-10 production in human placental explants. Placenta. 2013;34(7):567–573. [DOI] [PubMed] [Google Scholar]
- 37. Moynihan AT, Hehir MP, Sharkey AM, Robson SC, Europe-Finner GN, Morrison JJ. Histone deacetylase inhibitors and a functional potent inhibitory effect on human uterine contractility. Am J Obstetr Gynecol. 2008;199(2):167.e1–167.e7. [DOI] [PubMed] [Google Scholar]
- 38. Li YJ, Fu XH, Liu DP, Liang CC. Opening the chromatin for transcription. Int J Biochem Cell Biol. 2004;36(8):1411–1423. [DOI] [PubMed] [Google Scholar]
- 39. Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321(3):892–901. [DOI] [PubMed] [Google Scholar]
- 40. Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35(1):89–96. [DOI] [PubMed] [Google Scholar]
- 41. Choo QY, Ho PC, Tanaka Y, Lin HS. Histone deacetylase inhibitors MS-275 and SAHA induced growth arrest and suppressed lipopolysaccharide-stimulated NF-κB p65 nuclear accumulation in human rheumatoid arthritis synovial fibroblastic E11 cells. Rheumatology (Oxford). 2010;49(8):1447–1460. [DOI] [PubMed] [Google Scholar]
- 42. Venza I, Visalli M, Oteri R, Cucinotta M, Teti D, Venza M. Class II-specific histone deacetylase inhibitors MC1568 and MC1575 suppress IL-8 expression in human melanoma cells. Pigment Cell Melanoma Res. 2013;26(2):193–204. [DOI] [PubMed] [Google Scholar]
- 43. Quivy V, Van Lint C. Regulation at multiple levels of NF-kappa B-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68(6):1221–1229. [DOI] [PubMed] [Google Scholar]
- 44. Lappas M, Permezel M, Georgiou HM, Rice GE. Regulation of phospholipase isozymes by nuclear factor-kappaB in human gestational tissues in vitro. J Clin Endocrinol Metab. 2004;89(5):2365–2372. [DOI] [PubMed] [Google Scholar]
- 45. Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappa B activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal'. Mol Hum Reprod. 2001;7(6):581–586. [DOI] [PubMed] [Google Scholar]
- 46. Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67(2):668–673. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov. 2009;8(12):969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang H, Zhang W, Pan H, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One. 2012;7(9):e46364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292(2): L567–L576. [DOI] [PubMed] [Google Scholar]
- 52. Bishayee A, Waghray A, Barnes K, et al. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res. 2010;27(6):1080–1091. [DOI] [PubMed] [Google Scholar]
- 53. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105(28):9793–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoshizaki T, Schenk S, Imamura T, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(3):E419–E428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ailenberg M, Silverman M. Trichostatin A-histone deacetylase inhibitor with clinical therapeutic potential-is also a selective and potent inhibitor of gelatinase A expression. Biochem Biophys Res Commun. 2002;298(1):110–115. [DOI] [PubMed] [Google Scholar]
- 56. Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60(16):4561–4572. [PubMed] [Google Scholar]
- 57. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100(16):9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]