Summary
Signalling C‐type lectin receptors (CLRs) are crucial in shaping the immune response to fungal pathogens, but comparably little is known about the role of these receptors in bacterial, viral and parasitic infections. CLRs have many diverse functions depending on the signalling motifs in their cytoplasmic domains, and can induce endocytic, phagocytic, antimicrobial, pro‐inflammatory or anti‐inflammatory responses which are either protective or not during an infection. Understanding the role of CLRs in shaping anti‐microbial immunity offers great potential for the future development of therapeutics for disease intervention. In this review we will focus on the recognition of bacterial, viral and parasitic pathogens by CLRs, and how these receptors influence the outcome of infection. We will also provide a brief update on the role of CLRs in antifungal immunity.
Introduction
The innate immune system provides the first line of defence against microbial attack, and is induced by recognition of microbial components, known as pathogen‐associated molecular patterns (PAMPs) or microbial‐associated molecular patterns (MAMPs), by pattern recognition receptors (PRRs). PAMPs are highly conserved and generally unique to microbes (Akira et al., 2006). Fungal PAMPs consist primarily of cell wall carbohydrate structures, while bacterial PAMPS range from lipoproteins, lipopolysaccharide (LPS), flagellin and peptidoglycan to bacterial nucleic acid structures. Viruses on the other hand are mainly recognized through their nucleic acids, such as double (dsRNA) or single stranded‐RNA (ssRNA) and viral DNA, although surface envelope glycoproteins can also be recognized. Although the PAMPs are not as well characterized, parasites, particularly helminths such as Schistosoma mansoni and Trichuris muris, or protozoa, such as Leishmania infantum and Plasmodium berghei, are also recognized by mammalian PRRs (McGuinness et al., 2003; Broz and Monack, 2013; Drummond and Brown, 2013).
PRR recognition of a PAMP can lead to the activation of intracellular signalling pathways that elicit innate responses against pathogens and direct the development of adaptive immunity. Also important to mention is the recognition of damage‐associated molecular patterns (DAMPs) by PRRs. Molecules released by stressed cells or cells undergoing necrosis can act as danger signals and promote inflammatory responses (see for example Yamasaki et al., 2008). PRRs of relevance here are the signalling trans‐membrane C‐type lectin receptors (CLRs), which are widely recognized to play an essential role in antifungal immunity (see Hardison and Brown, 2012 for a recent review). Less well recognized is their role in immunity to other microorganisms. This review will therefore focus on the role of signalling CLRs in immunity to bacteria, viruses, helminths and protozoa, and will only briefly discuss the most recent advances in our understanding of their role in antifungal immunity. Furthermore, we have included certain important CLRs in which the signalling pathway is unclear, particularly those from Group VI. The structures of selected CLRs that will be discussed are represented in Fig. 1.
Figure 1.
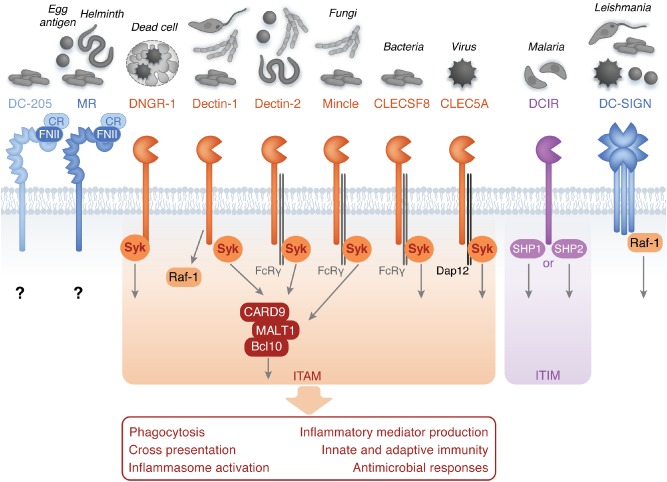
Recognition of microorganisms by signalling CLRs. Cartoon representation of the C‐type lectin receptors discussed in the text. Also shown is the microbes they recognize, the major intracellular signalling pathways utilized by these receptors, and the responses they induce. ITAM indicates receptors utilizing immunoreceptor tyrosine‐based activation motifs; ITIM indicates receptors utilizing immunoreceptor tyrosine‐based inhibitory motifs. CR (cysteine‐rich domain), FNII (fibronectin domain).
C‐type lectin receptors: general overview
CLRs comprise a large family of receptors which bind carbohydrates, through one or more carbohydrate recognition domains (CRDs), or which possess structurally similar C‐type lectin‐like domains (CTLDs) which do not necessarily recognize carbohydrate ligands. CLRs are divided into 17 groups based on features including phylogeny and structure (Zelensky and Gready, 2005). Discussed in this review are CLRs from groups II (calcium‐dependent lectins with single CRDs), group V (calcium‐independent receptors with single CTLDs) and group VI (calcium‐dependent lectins with multiple CRDs) (see Table 1). Based on their signalling potential, CLRs can be further subdivided into (i) activation Syk‐coupled CLRs with immunoreceptor tyrosine‐based activation motif (ITAM) domains, (ii) inhibitory CLRs with immunoreceptor tyrosine‐based inhibition motif (ITIM) domains or (iii) CLRs without clear ITAM or ITIM domains such as MR, DEC‐205 and DC‐SIGN (Zelensky and Gready, 2005; Sancho and Reis e Sousa, 2012).
Table 1.
Selected CLRs mentioned in this review
| CLR | Ligands | Ligand origin | Selected references | |
|---|---|---|---|---|
| Group II: Calcium‐dependent CRD | Dectin‐2 |
α‐mannans O‐linked mannobiose‐rich glycoprotein |
M. tuberculosis S. mansoni SEA C. albicans Malassezia spp. HDM allergens |
Ritter et al., 2010; Meevissen et al., 2012; Ishikawa et al., 2013; Prasanphanich et al., 2013; Salazar et al., 2013 |
| CLECSF8 | TDM |
M. bovis K. pneumonia |
Miyake et al., 2013; Steichen et al., 2013 | |
| Mincle |
α‐mannose mannitol‐linked glyceroglycolipid mannosyl fatty acids TDM |
M. tuberculosis C. albicans Malassezia spp. |
Ishikawa et al., 2009; Lee et al., 2012; Sancho and Reis e Sousa, 2012; Ishikawa et al., 2013 | |
| DC‐SIGN |
High mannose SlpA |
HIV‐1 Measles Dengue Mycobacterium spp. Influenza A SEA Leishmania spp. Helicobacter pylori Lactobacillus spp. |
Gringhuis et al., 2007; Konstantinov et al., 2008; Geijtenbeek and Gringhuis, 2009; Mesman et al., 2012; Avota et al., 2013; Chen et al., 2013; Harman et al., 2013; Hillaire et al., 2013 | |
| SIGNR3 | High mannose and fucose |
L. infantum SEA |
Powlesland et al., 2006; Meevissen et al., 2012; Lefevre et al., 2013; Prasanphanich et al., 2013 | |
| SIGNR1 | High mannose and fucose | SEA | Galustian et al., 2004; Meevissen et al., 2012; Prasanphanich et al., 2013 | |
| MGL | Lewis X | SEA | Van Vliet et al., 2005; Meevissen et al., 2012; Tundup et al., 2012 | |
| DCIR | unknown | HIV‐1 | Sancho and Reis e Sousa, 2012 | |
| Group V: Calcium‐independent non‐CRD | Dectin‐1 | β‐glucans |
L. infantum C. albicans Mycobacterium spp. |
Hardison and Brown, 2012; Lefevre et al., 2013 |
| CLEC5A | Unknown |
Dengue virus JEV |
Chen et al., 2012; Drummond et al., 2013 | |
| DNGR‐1 (CLEC9A) | F‐actin |
Vaccinia virus Herpes simplex virus |
Iborra et al., 2012; Zelenay et al., 2012 | |
| Group VI: Calcium‐dependent multiple CRD | Mannose Receptor (MR) |
High mannose Omega‐1 ManLam |
SEA Trichuris muris Mesocestoides corti HDM allergens Mycobacterium spp. K. pneumonia S. pneumonia F. tularesis |
Kang et al., 2005; Deschoolmeester et al., 2009; Geijtenbeek and Gringhuis, 2009; Everts et al., 2012; Meevissen et al., 2012; Mishra et al., 2013; Prasanphanich et al., 2013; Salazar et al., 2013 |
| DEC‐205 (CD205) | PLA | Y. pestis | Zhang et al., 2008 |
Relevant references are indicated in text.
HDM, house dust mite; HIV, human immunodeficiency virus; JEV, Japanese encephalitis virus ManLam, mannosylated lipoarabinomannan; PLA, plasminogen activator; SEA, schistosoma egg antigen; SlpA, surface layer A protein; TDM, trehalose‐6,6′‐dimycolate.
Activation of CLRs can induce intracellular signalling pathways in two ways: firstly through indirect signalling, where receptors such as macrophage‐inducible C‐type lectin (Mincle or CLEC4E), Dectin‐2 (CLEC6A) and C‐type lectin domain family 5A (CLEC5A) associate with ITAM containing adaptor molecules such as Fc Receptor γ‐chain (FcRγ) or DAP12.
The second mechanism employed by Dectin‐1 (or CLEC7A) and DNGR‐1 (CLEC9A) involves direct signalling through ITAM‐like motifs located within the cytoplasmic tail of these receptors (Rogers et al., 2005; Geijtenbeek and Gringhuis, 2009). Signalling via both mechanisms involves the recruitment to phosphorylated tyrosine residues of spleen tyrosine kinase (Syk), which in turn co‐ordinates a complex of CARD9, B cell lymphoma 10 (Bcl10) and mucosa‐associated lymphoid tissue lymphoma translocation protein 1 (Malt1). Furthermore, apoptosis‐associated speck‐like protein containing a CARD (ASC) has also been shown to be phosphorylated in a Syk‐ and Jnk‐dependent manner (Hara et al., 2013). Protein kinase C‐δ (PKCδ) is also an essential element in this pathway (Strasser et al., 2012). Signalling from Dectin‐1 and other lectins also involves additional pathways, such as the Syk‐independent activation of Raf‐1 (Gringhuis et al., 2009). These signalling pathways lead to activation of several downstream molecules, including NFκβ and mitogen‐activated protein kinases (MAPK), eventually triggering cellular responses, including phagocytosis, DC maturation, chemotaxis, the respiratory burst, inflammasome activation, and cytokine and other mediator production (Drummond et al., 2011; Strasser et al., 2012). Moreover, these receptors can also drive the development of adaptive immunity, particularly T helper (Th)1 and Th17 CD4+ and CD8+ T cell adaptive responses, although some CLRs are also capable of driving Th2 immunity (Kerrigan and Brown, 2011; Sancho and Reis e Sousa, 2012).
While signalling through ITAM‐bearing receptors results in cell activation, ITIM‐bearing receptors usually mediate inhibitory functions. For example, DCIR contains an ITIM in its cytoplasmic tail which recruits tyrosine phosphatases (SHP‐1 and SHP‐2) following ligand binding to modulate the signalling pathways induced by other PRRs, such as inhibition of TLR8‐ or TLR9‐induced cytokine production (Geijtenbeek and Gringhuis, 2009). However, the function of inhibitory CLRs is not always clear cut as these receptors can also have activation functions, thereby mediating cellular activation. They may either recruit novel substrates to their cytoplasmic domains or inhibit other inhibitory receptors (Redelinghuys and Brown, 2011).
The role of C‐type lectins in microbial recognition and immunity
Bacteria
The role of CLRs in anti‐bacterial immunity has been best characterized for M. tuberculosis (MTB). Multiple CLRs including Dectin‐1, DC‐SIGN, mannose receptor (MR), and Mincle have been implicated in MTB control in vitro, however each of these CLRs appears to be redundant in controlling infection in vivo. For example, Dectin‐1 was shown to be required for the production of IL‐12p40 by splenic DCs in response to mycobacteria in vitro, but was redundant during infection in vivo (Rothfuchs et al., 2007; Court et al., 2010; Marakalala et al., 2010). MR binds to mannose‐capped lipoarabinomannan (ManLAM) on the surface of M. tuberculosis, mediating bacterial phagocytosis and limiting phagosome–lysosome fusion within macrophages, but loss of the MR did not influence antimycobacterial immunity in vivo (Schlesinger et al., 1994; Kang et al., 2005; Court et al., 2010). Mincle mediates recognition of mycobacterial cord factor, trehalose‐6,6′‐dimycolate (TDM), and was shown to be essential for driving immune responses to TDM in vivo, including granuloma formation (Ishikawa et al., 2009). Yet Mincle knockout mice had normal granulomas and did not show obvious defects during live MTB infection (Lee et al., 2012; Heitmann et al., 2013). DC‐SIGN recognizes ManLAM and α‐glucan, preventing DC maturation and the production of IL‐10 (Geijtenbeek et al., 2003; Geurtsen et al., 2009). Although mice deficient in a murine homologue for DC‐SIGN, SIGNR3−/− showed defects in early control of MTB, they mounted an efficient anti‐mycobacterial adaptive immune response with granulomatous lesions comparable to wild‐type controls (Tanne et al., 2009).
Despite the apparent redundancy of these receptors in vivo, the shared CLR downstream signalling pathway involving CARD9 is critical for protection, as CARD9−/− mice present with uncontrolled bacterial replication and exacerbated neutrophilic pulmonary inflammation, which is followed by death (Dorhoi et al., 2010). This suggests either that a combination of CLRs or an unknown CLR is essential for protection against MTB. Recently studies have shown that Mincle can form a receptor complex with CLECSF8 (CLEC4D or MCL) and FcεRIγ, and this heterotrimeric complex is proposed to be the functionally optimal form for these CLRs (Lobato‐Pascual et al., 2013). However, the role of CLECSF8 in live MTB infection has not been defined.
Recently, CLECSF8 was also shown to be important in the resolution of pneumonia caused by Klebsiella pneumoniae (Steichen et al., 2013). CLECSF8−/− mice were more susceptible than wild‐type mice to pneumonic sepsis, with increased bacterial burdens, hyper‐inflammation and severe lung pathology which correlated with a massive accumulation of neutrophils. These results suggest that CLECSF8 plays an important role in resolution of inflammation, and is the first report describing a physiological function for this CLR.
In addition to M. tuberculosis, DC‐SIGN also interacts with a wide range of other bacterial pathogens including M. leprae, Helicobacter pylori and Lactobacillus species (Geijtenbeek and Gringhuis, 2009). Lactobacillus reuteri and L. casei have been shown to bind DC‐SIGN and induce regulatory T‐cells (Smits et al., 2005), while surface (S) layer A protein (SlpA) on the surface of L. acidophilus has been identified as a ligand of this CLR (Konstantinov et al., 2008). The MR also recognizes a number of other mannose‐expressing bacterial species, including M. kansasii, K. pneumonia, Streptococcus pneumoniae and Francisella tularensis. However, the MR seems not to be essential during infection with these pathogens in vivo (Geijtenbeek and Gringhuis, 2009).
Other less well characterized CLRs have also been shown to recognize bacteria. DEC‐205, for example, is a member of the mannose receptor family and binds to plasminogen activator (PLA) on the surface of Yersinia pestis, which mediates bacterial attachment (Lähteenmäki et al., 1998). This CLR was found to promote dissemination of this pathogen, and this had detrimental implications for the host (Zhang et al., 2008).
Viruses
Viruses are abundant, rapidly evolving pathogens which pose a continual challenge to the host immune system. Unlike the predominantly protective responses that CLRs mediate to other pathogens, viral recognition by CLRs tends to favour transmission, infection and inflammation. Recognition of HIV by DC‐SIGN is a well characterized example of the detrimental effect of CLR signalling in response to a virus. Firstly, binding of HIV‐1 to DC‐SIGN not only modulates TLR‐induced IL‐10 production by signalling via Raf‐1, but also impairs T cell proliferation and TLR‐induced dendrite formation of DCs (Gringhuis et al., 2007). Secondly, HIV gp120 facilitates DC‐SIGN‐mediated viral entry into the cells, results in infected CD4+ target cells (Harman et al., 2013) and accelerated DC apoptosis, negatively affecting DC maturation which would normally promote pathogen recognition by the immune system (Chen et al., 2013). Therefore, preventing HIV from binding to mucosal DCs by blocking CLRs could potentially prevent HIV transmission.
DC‐SIGN ligands are not limited to HIV but include a range of other viruses, such as Cytomegalovirus, Dengue virus, Ebola virus, Hepatitis C virus, SARS‐coronavirus, West Nile virus and the Measles virus (Mesman et al., 2012; Avota et al., 2013; Hillaire et al., 2013). More recently, DC‐SIGN was shown to assist in the replication of Influenza A virus, by binding to glycans on haemagglutinin, promoting viral binding to cells and internalization in vitro (Hillaire et al., 2013). However, the contribution of DC‐SIGN was dependent on the extent of the glycosylation of viral haemagglutinin.
Viral exploitation of CLRs can also induce pro‐inflammatory cytokines leading to severe pathology for the host. Previously, MR and CLEC5A have been shown to bind Dengue virus in vitro leading to infection of macrophages and inflammasome activation respectively. Unlike conventional CLRs which are involved in Dengue virus entry into target cells, CLEC5A regulates virus‐induced pro‐inflammatory cytokines and blocking CLEC5A‐mediated signalling attenuates pro‐inflammatory cytokine production by infected macrophages, reducing mortality, and maintaining host immunity, leading to resolution of infection. This indicates that CLEC5A is critical in regulating inflammatory reactions triggered by pathogens. The elevated levels of TNF‐α during infection were associated with DAP12 activation, suggesting that CLEC5A directly interacts with the Dengue virion (Chen et al., 2008). Similarly, Japanese encephalitis virus (JEV) also binds CLEC5A directly, and induces neuro‐inflammation through DAP12 activation in macrophages. Blocking CLEC5A reduced neuronal damage, pro‐inflammatory cytokine secretion, blood‐brain barrier permeability, and cellular infiltration into the central nervous system (CNS) in vivo (Chen et al., 2012). Together these studies suggest that CLR blockage could alleviate tissue damage and increase survival of patients with virus‐induced inflammatory diseases.
In contrast to these detrimental roles, CLRs can also induce protective responses. This is exemplified by DNGR‐1 (CLEC9A) in the control of both vaccinia (Iborra et al., 2012) and Herpes Simplex (Zelenay et al., 2012) viruses. DNGR‐1 is expressed by a subset of DC's and detects dead cells, promoting antigen cross‐presentation to CD8+ T cells. Although DNGR‐1‐deficient DCs are activated following interaction with virus‐infected cells, they are no longer capable of cross‐presenting antigens. This results in weaker CD8+ T‐cell responses, delayed lesion resolution and a higher viral load, suggesting that tissue damage sensing by DNGR‐1 is a key component in anti‐viral immunity.
Another example of protective responses mediated by CLRs is the role of DCIR during infection with Chikungunya virus (Long et al., 2013). DCIR−/− mice developed more severe inflammatory disease with a skewed cytokine response both in vivo and in vitro. Thus the inhibitory functions of this receptor play an important role in suppressing pathological inflammatory responses induced by this pathogen (Long et al., 2013).
Helminths
Helminth parasites drive host CD4+ Th cells toward Th2 and anti‐inflammatory responses, and induce alternative activation of macrophages. Helminth glycans are thought to play a critical role in driving these responses, and given the large number of glycan moieties they possess; it is likely that many CLRs are involved in immunity to these pathogens. Soluble egg antigen (SEA) of Schistosoma mansoni cercariae was the first parasite‐specific ligand for DC‐SIGN described (Meyer et al., 2005). Subsequently, glycoproteins from SEAs of several schistosome species (S. mansoni, S. hematobium, S. japonicum) have been described as ligands for DC‐SIGN (van Die et al., 2003).
Other signalling CLRs have been shown to recognize SEA or S. mansoni glycans, although not all of the actual ligands have been identified. Among these, Dectin‐2 recognizes an unknown ligand in SEA; SIGNR1, SIGNR3 and MR all recognize Lewis x and high‐mannose N‐glycans; and macrophage galactose lectin (MGL) recognizes Lewis x, LDN and LDN‐F in SEA (Meevissen et al., 2012; Prasanphanich et al., 2013). Furthermore, MR recognizes Omega‐1 in SEA and conditions DCs for Th2 priming (Everts et al., 2012). Dectin‐2, in particular, was the first Syk‐coupled CLR to be associated with helminth infections, and plays a role in regulating helminth immune responses by indirectly reducing Th2‐mediated pathology. Here, Dectin‐2 was found to induce active IL‐1β secretion by activating the Nlrp3 inflammasome in response to S. mansoni SEA (Ritter et al., 2010).
Other CLRs have also been implicated in anti‐helminth immunity but their role in vivo is less clear. For example, murine SIGNR1 (a homologue of DC‐SIGN) binds SEA in vitro, but SIGNR1−/− mice mount a normal response during S. mansoni infection. Both MGL and DC‐SIGN are able to recognize SEA antigens, but the importance of these interactions in vivo is still unknown (Meevissen et al., 2012; Tundup et al., 2012). Similarly, MR was shown to bind Trichuris muris excretory/secretory proteins but did not affect parasite clearance, as MR−/− mice cleared the infection normally (Deschoolmeester et al., 2009).
Notably, a recent publication has shown a role for MR against Mesocestoides corti, a tape worm that releases glycan antigens within the CNS causing neurocysticercosis (Mishra et al., 2013). In vivo, MR−/− mice have increased survival, with accumulation of regulatory granulocytic myeloid cells and reduced T cell numbers. Therefore, the pathogenesis of neurocysticercosis appears to be directly attributable to the immune response against the parasite induced by MR. Although these in vivo results should be interpreted with caution due to the fact that a microRNA (miR‐511‐1) is encoded within the MR gene and co‐regulated with MR, possibly influencing the observed phenotype (Tserel et al., 2011).
In the context of Th2 immunity, CLRs also play a key role in promoting allergic responses. Of particular importance are MR, DC‐SIGN and Dectin‐2 (Salazar et al., 2013). While, MR and DC‐SIGN both recognize Dermatophagoides pteronyssinus group 1 antigen (Der p 1) from house dust mite (HDM), Dectin‐2 recognizes both HDM (D. farinae and D. pteronyssinus) and mold (Aspergillus fumigatus) extracts. Dectin‐2 was found to trigger the generation of cysteinyl leucotrienes (cys‐LT), which mediated pulmonary inflammation. In absence of LTC4 synthase (a critical enzyme in cys‐LT generation) or the cys‐LT receptor, pulmonary inflammation was reduced (Barret et al., 2009).
Protozoa
Several CLRs have been implicated in the recognition of Leishmania species, but their role is only starting to be understood. DC‐SIGN and L‐SIGN (a close homologue of DC‐SIGN which also recognizes high‐mannose glycans) molecule have both been shown to recognize Leishmania, but the receptors differ in their ability to interact with these organisms, depending on the species and the stage of parasite maturation (Caparros et al., 2005). Dectin‐1 and MR have been shown to be crucial for the ‘killing’ response against L. infantum, by inducing ROS in macrophages and triggering Syk‐coupled secretion of IL‐1β (Lefevre et al., 2013). SIGNR3, on the other hand, has been lined with parasite survival both in vivo and in vitro, by inhibiting the LTB4/IL‐1β axis (Lefevre et al., 2013). As LTB4 is known to play a crucial role in the mechanisms responsible for killing Leishmania, specifically through the activation of IL‐1β; it has been suggested that elevated LTB4 benefits the host while reduced LTB4 benefits the pathogen. These studies highlight the divergent but essential roles of CLRs in Leishmania pathogenesis.
CARD9 has recently been linked to cerebral malaria (CM), where its expression was upregulated in a mouse model induced by Plasmodium Berghei (Hafalla et al., 2012). However, CARD9−/− mice were not protected from infection, suggesting that CM develops independently of CARD9 despite its upregulation during disease. In contrast, striking protection against CM was observed when DNGR‐1+ DCs were depleted. Protection was associated with reduced numbers of CD8+ cells, reduced parasite burdens in the brain and reduced IFN‐γ levels (Piva et al., 2012). More recently a study using DCIR−/− mice also demonstrated significant survival compared with wild‐type controls. Protection was associated with reduced CD8+ cells and reduced brain inflammation, highlighting the activation functions of some inhibitory receptors (Redelinghuys and Brown, 2011; Maglinao et al., 2013). Although the specific mechanism underlying this protection is unknown, it is clear that CLRs play a critical role in CM.
Fungi
Innate and adaptive immune responses to fungi are primarily mediated by CLRs, with Dectin‐1 being the best characterized in the context of fungal infections (Hardison and Brown, 2012). For fungal infections innate and adaptive immune responses are primarily regulated by CLRs. Due to the rapid increase of advances made in this field we will discuss some of the recent studies which have dissect CLR‐mediated mechanisms involved in antifungal immunity. Dectin‐1 recognizes β‐glucan and induces multiple cellular functions through its cytoplasmic signalling domain, and is essential for protective immune response to Candida albicans and other fungi in mice and humans. Recently we have discovered that the requirement for Dectin‐1 in the control of C. albicans is strain specific, as different C. albicans strains have variations in the composition and nature of their cell walls which only become apparent during infection in vivo (Marakalala et al., 2013). Dectin‐1‐mediated protection to C. albicans infections has also recently been attributed to the production of type I IFN by renal infiltrating DCs, a response which required Syk, CARD9 and IRF5 (del Fresno et al., 2013). Other signalling CLRs which play important roles include Dectin‐2, Mincle, DC‐SIGN and the MR (Hardison and Brown, 2012).
The influence of the fungal microbiota on immune regulation is another recent area of progress. The mammalian intestinal microbiota was found to include a myriad of fungal species, with over 100 known and 100 novel fungal species being identified (Iliev et al., 2012). Mice lacking Dectin‐1 were shown to have increased susceptibility to dextran sodium sulfate (DSS) induced colitis when specific fungal species were present in their gastrointestinal tract. Moreover, a polymorphism of Dectin‐1 was identified which associated with patients presenting with severe ulcerative colitis (Iliev et al., 2012). Similarly, SIGNR3 recognition of fungi was shown to influence immune regulation in the gut, as SIGNR3−/− mice exhibit an exacerbated DSS‐induced colitis compared with wild‐type controls (Eriksson et al., 2013). Together, these studies highlight the importance of fungal recognition by CLRs and the role this plays in maintaining intestinal immune homeostasis and control of disease.
As we have already discussed, CLRs are important in initiating innate immunity and link pathogen recognition to the development of adaptive immunity. More recently, the concept of innate immune memory or ‘trained immunity’ has emerged and challenged conventional paradigms of T and B cell‐mediated adaptive memory. Essentially, trained immunity is induced after a primary infection or vaccination, confers protection independently of T or B cells, mediated by innate immune cells such as NK cells and monocytes/macrophages, and increases resistance to infection by the same or other pathogens (Netea et al., 2011; Netea, 2013). Trained immunity can be distinguished from immune priming due to the fact that after recovery from infection, innate immune responses do not return to the steady‐state level. This is due to the epigenetic reprogramming of innate immune cells rather than the short‐lived change of state seen in immune priming. Dissecting the mechanisms involved in trained immunity provides an exciting new approach to protection against infection. A role for CLRs in trained immunity has recently been described, where the reprogramming of monocytes by Dectin‐1/ Raf‐1 signalling prevented infection with C. albicans and other organisms (Quintin et al., 2012). Insights into trained immunity triggered by CLRs could therefore form the basis for novel strategies in immunotherapy and vaccination.
Lastly, it is well established that collaboration between CLRs and TLRs initiates optimal antifungal responses. In fact, the collaborative responses induced by Dectin‐1 and TLR2 was one of the first collaborative PRR responses ever described (Hardison and Brown, 2012). A more recent example of the importance of such collaboration is the recognition of Fonsecaea pedrosoi. This organism, which causes chromoblastomycoses, was shown to be recognized by CLRs, but not TLRs, and this resulted in defective inflammatory responses and susceptibility to infection. Amazingly, exogenous administration of TLR agonists restored protective inflammatory responses and led to clearance of the infection in vivo (Sousa et al., 2011). This approach is now being tested in humans (G.D.B., unpubl. data).
Conclusions
It is well established that CLRs play an important role in recognizing fungi and orchestrate both innate and adaptive immune responses to these pathogens. Recent discoveries have revealed an ever increasing repertoire of pathogens that are also recognized by these receptors, including bacteria, helminths and protozoa. Moreover, we are discovering that CLRs play key roles in autoimmunity, allergy and in maintaining homeostasis. Yet we are only just beginning to understanding the importance of these receptors, and the next few years are likely to yield a wealth of exciting new breakthroughs.
Acknowledgements
This work was supported in part by the Wellcome Trust, Medical Research Council (UK) and the University of Cape Town. J.C. Hoving is a Carnegie Corporation postdoctoral researcher at the University of Cape Town. We apologize of all our colleagues whose important contributions we could not cite due to space constraints. The authors disclose no conflicts of interest.
References
- Akira, S. , Uematsu, S. , and Takeuchi, O. (2006) Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- Avota, E. , Koethe, S. , and Schneider‐Schaulies, S. (2013) Membrane dynamics and interactions in measles virus dendritic cell infections. Cell Microbiol 15: 161–169. [DOI] [PubMed] [Google Scholar]
- Barret, N.A. , Maekawa, A. , Rahman, O.M. , Austen, F. , and Kanaoka, Y. (2009) Dectin‐2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol 182: 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz, P. , and Monack, D.M. (2013) Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol 13: 551–565. [DOI] [PubMed] [Google Scholar]
- Caparros, E. , Serrano, D. , Puig‐Kroger, A. , Riol, L. , Lasala, F. , Martinez, I. , et al. (2005) Role of the C‐type lectins DC‐SIGN and L‐SIGN in Leishmania interaction with host phagocytes. Immunobiology 210: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Liu, R. , Wu, M. , Lin, Y. , Chen, S. , Tan, D.T. , et al. (2012) CLEC5A regulates Japanese encephalitis virus‐induced neuroinflammation and lethality. PLoS Pathog 8: e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.T. , Lin, Y.L. , Huang, M.T. , Wu, M.F. , Cheng, S.C. , et al. (2008) CLEC5A is critical for dengue‐virus‐induced lethal disease. Nature 453: 672–676. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Hwang, S. , Chan, V.S.F. , Chung, N.P.Y. , Wang, S. , Li, Z. , et al. (2013) Binding of HIV‐1 gp120 to DC‐SIGN promotes ASK‐1‐dependent activation‐induced apoptosis of human dendritic cells. PLoS Pathog 9: e1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court, N. , Vasseur, V. , Vacher, R. , Frémond, C. , Shebzukhov, Y. , et al. (2010) Partial redundancy of the PRRs, scavenger receptors, and C‐type lectins for the long‐term control of MTB infection. J Immunol 15: 7057–7070. [DOI] [PubMed] [Google Scholar]
- Deschoolmeester, M.L. , Martinez‐Pomares, L. , Gordon, S. , and Else, K.J. (2009) The mannose receptor binds Trichuris muris excretory/secretory proteins but is not essential for protective immunity. Immunology 126: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die, I. , van Vliet, S.J. , Nyame, A.K. , Cummings, R.D. , Bank, C.M. , et al. (2003) The dendritic cell‐specific C‐type lectin DC‐SIGN is a receptor for Schistosoma mansoni egg antigen and recognizes the glycan antigen Lewis x. Glycobiology 13: 471–478. [DOI] [PubMed] [Google Scholar]
- Dorhoi, A. , Desel, C. , Yeremeev, V. , Pradl, L. , Brinkmann, V. , Mollenkopf, H.J. , et al. (2010) The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med 12: 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, R.A. , and Brown, G.D. (2013) Signaling C‐type lectins in antimicrobial immunity. PLoS Pathog 9: e1033417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, R.A. , Saijo, S. , Iwakura, Y. , and Brown, G.D. (2011) The role of Syk/CARD9 coupled C‐type lectins in antifungal immunity. Eur J Immunol 41: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, M. , Johannssen, T. , von Smolinski, D. , Gruber, A.D. , Seeberger, P.H. , and Lepenies, B. (2013) The c‐type lectin receptor SIGNR3 binds to fungi present in commensal microbiota and influences immune regulation in experimental colitis. Front Immunol 4: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts, B. , Hussaarts, L. , Driessen, N.N. , Meevissen, M.H.J. , Schramm, G. , van der Ham, A.J. , et al. (2012) Schistosome‐derived omega‐1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med 209: 1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Fresno, C. , Soulat, D. , Roth, S. , Blazek, K. , Udalova, I. , Sancho, D. , et al. (2013) Interferon‐β production via dectin‐1‐Syk‐IRF5 signalling in dendritic cells in crucial for immunity to C. albicans . Immunity 38: 1176–1186. [DOI] [PubMed] [Google Scholar]
- Galustian, C. , Park, C.G. , Chai, W. , Kiso, M. , Bruening, S.A. , Kang, Y.S. , et al. (2004) High and low affinity carbohydrate ligands revealed for murine SIGN‐R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN‐R3 and langerin. Int Immunol 16: 853–866. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek, T.B.H. , and Gringhuis, S.I. (2009) Signaling through C‐type lectin receptors: shaping immune responses. Nat Rev Immunol 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek, T.B.H. , Van Vliet, S.J. , Koppel, E.A. , Sanchez‐Herandez, M. , Vandenbroucke‐Grauls, C.M. , Appelmelk, B. , and van Kooyk, Y. (2003) Mycobacteria target DC‐SIGN to suppress dendritic cell function. J Exp Med 197: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurtsen, J. , Chedammi, S. , Mesters, J. , Cot, M. , Driessen, N.N. , et al. (2009) Identification of mycobacterial alpha‐glucan as a novel ligand for DC‐SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J Immunol 183: 5221–5231. [DOI] [PubMed] [Google Scholar]
- Gringhuis, S.I. , den Dunnen, J. , Litjens, M. , van het Hof, B. , van Kooyk, Y. , and Geijtenbeek, T.B.H. (2007) C‐type lectin DC‐SIGN modulates toll‐like receptor signalling via Raf‐1 kinase‐dependent acetylation of transcription factor NF‐kappa B. Immunity 26: 605–616. [DOI] [PubMed] [Google Scholar]
- Gringhuis, S.I. , den Dunnen, J. , Litjens, M. , van der Vlist, M. , Wevers, B. , Bruijns, J S.C. , and Geijtenbeek, T.B.H. (2009) Dectin‐1 directs T helper cell differentiation by controlling noncanonical NK‐kappaB activation through Raf‐1 and Syk. Nat Immunol 10: 203–213. [DOI] [PubMed] [Google Scholar]
- Hafalla, J.C. , Burgold, J. , Dorhoi, A. , Gross, O. , Ruland, J. , Kaufmann, S.H.E. , and Matuschchewski, K. (2012) Experimental cerebral malaria develops independently of CARD‐signalling. Infect Immun 80: 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, H. , Tsuchiya, K. , Kawamura, I. , Fang, R. , Hernandez‐Cuellar, E. , Shen, Y. , et al. (2013) Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck‐like aggregates and inflammasome activity. Nat Immunol 14: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison, S.E. , and Brown, G.D. (2012) C‐type lectin receptors orchestrate anti‐fungal immunity. Nat Immunol 13: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman, A.N. , Kim, M. , Nasr, N. , Sandgren, K.J. , and Cameron, P.U. (2013) Tissue dendritic cells as portals for HIV entry. Rev Med Virol 23: 319–333. [DOI] [PubMed] [Google Scholar]
- Heitmann, L. , Schoenen, H. , Ehlers, S. , Lang, R. , and Holscher, C. (2013) Mincle is not essential for controlling M. tuberculosis infection. Immunobiology 218: 506–516. [DOI] [PubMed] [Google Scholar]
- Hillaire, L.B. , Nieuwekoop, N.J. , Boon, A.C.M. , de Mutsert, G. , Vogelzang‐van Trierum, S.E. , et al. (2013) Binding of DC‐SIGN to the Hemagglutinin of Influenza A viruses supports virus replication in DC‐SIGN expressing cells. PLoS ONE 8: e56164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra, S. , Izquierdo, H.M. , Matinez‐Lopez, M. , Blanco‐Menendez, N. , Reis e Sousa, C. , and Sancho, D. (2012) The DC receptor DNGR‐1 mediates cross‐priming of CTLs during vaccinia virus infection in mice. J Clin Invest 122: 1268–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev, I.D. , Funari, V.A. , Taylor, K.D. , Nguyen, Q. , Reyes, C.N. , Strom, S.P. , et al. (2012) Interactions between commensal fungi and the C‐type lectin receptor Dectin‐1 influence colitis. Science 336: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, E. , Ishikawa, T. , Morita, Y.S. , Toyonaga, K. , Yamada, H. , Takeuchi, O. , et al. (2009) Direct recognition of the mycobacterial glycolipid trehalose dimycolate, by C‐type lectin Mincle. J Exp Med 206: 2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, T. , Itoh, F. , Yoshida, S. , Saijo, S. , Matsuzawa, T. , Gonoi, T. , et al. (2013) Identification of distinct ligands for the C‐type lectin receptors mincle and Dectin‐2 in the pathogenic fungus Malassezia. Cell Host Microbe 13: 477–488. [DOI] [PubMed] [Google Scholar]
- Kang, P.B. , Azad, A.K. , Torrelles, J.B. , Kaufman, T.M. , Beharka, A. , et al. (2005) The human macrophage MR directs MTB lipoarabinomannan‐mediated phagosome biogenesis. J Exp Med 202: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan, A.M. , and Brown, G.D. (2011) Syk‐coupled C‐type lectins in immunity. Trends Immunol 32: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov, S.R. , Smidt, H. , de Vos, W.M. , Bruijns, S.C. , Singh, S.K. , et al. (2008) S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA 105: 19474–19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähteenmäki, K. , Virkola, R. , Sarén, A. , Emödy, L. , and Korhonen, T.K. (1998) Expression of plasminogen activator pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect Immun 66: 5755–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W.B. , Kang, J.S. , Yan, J.J. , Lee, M.S. , Jeon, B.Y. , Cho, S.N. , and Kim, Y.J. (2012) Neutrophils promote mycobacterial trehalose dimycolate‐induced lung inflammation via the Mincle pathway. PLoS Pathog 8: e1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre, L. , Lugo‐Villarino, G. , Meunier, E. , Valentin, A. , Olagnier, D. , Authier, H. , et al. (2013) The C‐type lectin receptors Dectin‐1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum . Immunity 38: 1–12. [DOI] [PubMed] [Google Scholar]
- Lobato‐Pascual, A. , Saether, P.C. , Fossum, S. , Dissen, E. , and Daws, M.R. (2013) Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcεRI‐γ. Eur J Immunol 43: 3167–3174. [DOI] [PubMed] [Google Scholar]
- Long, K.M. , Whitmore, A.C. , Ferris, M.T. , Sempowski, G.D. , McGee, C. , Trollinger, B. , et al. (2013) Dendritic cell immunoreceptor regulates Chikungunya virus pathogenesis in mice. J Virol 87: 5697–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness, D.H. , Dehal, P.K. , and Pleass, R.J. (2003) Pattern recognition molecules and innate immunity to parasites. Trends Parasitol 19: 312–319. [DOI] [PubMed] [Google Scholar]
- Maglinao, M. , Klopfleisch, R. , Seeberger, P.H. , and Lepenies, B. (2013) The C‐type lectin receptor DCIR is crucial for the development of experimental cerebral malaria. J Immunol 191: 2551–2559. [DOI] [PubMed] [Google Scholar]
- Marakalala, M.J. , Graham, L.M. , and Brown, G.D. (2010) The role of Syk/CARD9‐coupled C type lectin receptors in immunity to M. tuberculosis infections. Clin Dev Immunol 2010: 567571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakalala, M.J. , Vautier, S. , Potrykus, J. , Walker, L.A. , Shepardson, K.M. , Hopke, A. , et al. (2013) Differential adaptation of C. albicans in vivo modulates immune recognition by Dectin‐1. PLoS Pathog 9: e1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meevissen, M.H.J. , Yazdanbakhsh, M. , and Hokke, C.H. (2012) Schistosoma mansoni egg glycoproteins and C‐type lectins of host immune cells: Molecular partners that shape immune responses. Exp Parasitol 132: 14–21. [DOI] [PubMed] [Google Scholar]
- Mesman, A.W. , de Vries, R.D. , McQuaid, S. , Duprex, W.P. , de Swart, R.L. , and Geijtenbeek, T.B.H. (2012) A prominent role for DC‐SIGN+ dendritic cells in initiation and dissemination of measles virus infection in non‐human primates. PLoS ONE 7: e49573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, S. , van Liempt, E. , Imberty, A. , van Kooyk, Y. , Geyer, H. , Geyer, R. , and Van Die, I. (2005) DC‐SIGN mediates binding of dendritic cells to authentic pseudo‐LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite‐specific ligand of DC‐SIGN. J Biol Chem 280: 37349–37359. [DOI] [PubMed] [Google Scholar]
- Mishra, P.K. , Morris, E.G. , Garcia, J.A. , Cardona, A.E. , and Teale, J.M. (2013) Increased accumulation of regulatory granulocytic myeloid cells in mannose receptor C type 1‐deficient mice correlates with protection in a mouse model of neurocysticercosis. Infect Immun 81: 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, Y. , Toyonaga, K. , Mori, D. , Kakuta, S. , Hoshino, Y. , Oyamada, A. , et al. (2013) C‐type lectin MCL is an FcRgamma‐coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 38: 1050–1062. [DOI] [PubMed] [Google Scholar]
- Netea, M.G. (2013) Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest 43: 881–884. [DOI] [PubMed] [Google Scholar]
- Netea, M.G. , Quintin, J. , and van der Meer, J.W.M. (2011) Trained immunity: a memory for innate host defense. Cell Host Microbe 19: 355–361. [DOI] [PubMed] [Google Scholar]
- Piva, L. , Tetlak, P. , Claser, C. , Karjalainen, K. , Renia, L. , and Ruedl, C. (2012) Cutting edge: Clec9A+ dendritic cells mediate the development of experimental cerebral malaria. J Immunol 189: 1128–1132. [DOI] [PubMed] [Google Scholar]
- Powlesland, A.S. , Ward, E.M. , Sadhu, S.K. , Guo, Y. , Taylor, M.E. , and Drickamer, K. (2006) Widely divergent biochemical properties of the complete set of mouse DC‐SIGN‐related proteins. J Biol Chem 281: 20440–20449. [DOI] [PubMed] [Google Scholar]
- Prasanphanich, N.S. , Mickum, M.L. , Heimburg‐Molinaro, J. , and Cummings, R.D. (2013) Glycoconjugates in host‐helminth interactions. Front Immunol 4: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin, J. , Saeed, S. , Martens, J.H.A. , Giamarellos‐Bourboulis, E.J. , Ifrim, D.C. , et al. (2012) C. albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelinghuys, P. , and Brown, G.D. (2011) Inhibitory C‐type lectin receptors in myeloid cells. Immunol Lett 136: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, M. , Gross, O. , Kays, S. , Ruland, J. , Nimmerjahn, F. , Saijo, S. , et al. (2010) Schistosoma mansoni triggers Dectin‐2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA 107: 20459–20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, N.C. , Slack, E.C. , Edwards, A.D. , Nolte, M.A. , Schulz, O. , et al. (2005) Syk‐dependent cytokine induction by Dectin‐1 reveals a novel pattern recognition pathway for C Type lectins. Immunity 22: 507–517. [DOI] [PubMed] [Google Scholar]
- Rothfuchs, A.G. , Bafica, A. , Feng, C.G. , Egen, J.G. , Williams, D.L. , Brown, G.D. , and Sher, A. (2007) Dectin‐1 interaction with MTB leads to enhanced IL‐12p40 production by splenic DCs. J Immunol 15: 3463–3471. [DOI] [PubMed] [Google Scholar]
- Salazar, F. , Sewell, H.F. , Shakib, F. , and Ghaemmaghami, A.M. (2013) The role of lectins in allergic sensitization and allergic disease. J Allergy Clin Immunol 132: 27–36. [DOI] [PubMed] [Google Scholar]
- Sancho, D. , and Reis e Sousa, C. (2012) Signaling by myeloid C‐type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30: 491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger, L.S. , Hull, S.R. , and Kaufman, T.M. (1994) Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of M. tuberculosis to human macrophages. J Immunol 152: 4070–4079. [PubMed] [Google Scholar]
- Smits, H.H. , Engering, A. , van der Kleij, D. , de Jong, E.C. , Schipper, K. , et al. (2005) Selective probiotic bacteria induce IL‐10‐producing regulatory T cells in vitro by modulation dendritic cell function through DC‐SIGN. J Allergy Clin Immunol 115: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Sousa Mda, G. , Reid, D.M. , Schweighoffer, E. , Tybulewicz, V. , Ruland, J. , Langhorne J., et al. (2011) Restoration of pattern recognition receptor co‐stimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 9: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steichen, A.L. , Binstock, B.J. , Mishra, B.B. , and Sharma, J. (2013) C‐type lectin receptor CLEC4D plays a protective role in resolution of Gram‐negative pneumonia. J Leukoc Biol 94: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, D. , Neumann, K. , Bergmann, H. , Marakalala, M.J. , Guler, R. , et al. (2012) Syk Kinase‐coupled C‐type lectin receptors engage protein kinase C‐δ to elicit Card‐9 adaptor‐mediated innate immunity. Immunity 36: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne, A. , Ma, B. , Boudou, F. , Tailleux, L. , Botella, H. , Badell, E. , et al. (2009) A murine DC‐SIGN homologue contributes to early host defense against M. tuberculosis. J Exp Med 206: 2205–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserel, L. , Runnel, T. , Kisand, K. , Pihlap, M. , Bakhoff, L. , and Kolde, R. (2011) MicroRNA expression profiles of human blood monocyte‐derived dendritic cells and macrophages reveal miR‐511 as a putative positive regulator of Toll‐like receptor 4. J Biol Chem 286: 26487–26495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundup, S. , Srivastava, L. , and Harn, D.A. (2012) Polarization of host immune responses by helminth‐expressed glycans. Ann N Y Acad Sci 1253: E1–E13. [DOI] [PubMed] [Google Scholar]
- Van Vliet, S.J. , van Liempt, E. , Saeland, E. , Aarnoudse, C.A. , Appelmelk, B. , and Irimura, T. (2005) Carbohydrate profiling reveals a distinctive role for the C‐type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol 17: 61–669. [DOI] [PubMed] [Google Scholar]
- Yamasaki, S. , Ishikawa, E. , Sakuma, M. , Hara, H. , Ogata, K. , and Saito, T. (2008) Mincle is an ITAM‐coupled activating receptor that senses damaged cells. Nat Immunol 9: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Zelenay, S. , Keller, A.M. , Whitney, P.G. , Schrami, B.U. , Deddouche, S. , et al. (2012) The dendritic cell receptor DNGR‐1 controls endolytic handling of necrotic cell antigens to favor cross‐priming of CTLs in virus‐infected mice. J Clin Invest 122: 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelensky, A.N. , and Gready, J.E. (2005) The C‐type lectin‐like domain superfamily. FEBS J 272: 6179–6217. [DOI] [PubMed] [Google Scholar]
- Zhang, S.S. , Park, C.G. , Zhang, P. , Bartra, S.S. , Plano, G.V. , et al. (2008) Plasminogen activator PLA of Yersinia pestis utilizes murine DEC‐205 as a receptor to promote dissemination. J Biol Chem 283: 31511–31521. [DOI] [PMC free article] [PubMed] [Google Scholar]


