Abstract
OBJECTIVES
In high-tuberculosis (TB)-endemic countries, comorbidity of pulmonary TB in hospitalised patients with non-communicable diseases is well documented. In this study, we evaluated the use of the Xpert® MTB/RIF assay for the detection of concomitant pulmonary TB in patients admitted to the University Teaching Hospital, Lusaka, Zambia, with a primary obstetric or gynaecological condition.
METHODS
The Study population were inpatients admitted with a primary obstetric or gynaecological problem who had a concomitant cough and were able to expectorate a sputum sample. Sputum samples from 94 patients were analysed for the presence of Mycobacterium tuberculosis (M.tb) by standard smear microscopy, MGIT culture, MGIT drug-susceptibility testing (DST) and the Xpert® MTB/RIF assay. The sensitivity and specificity of the Xpert® MTB/RIF assay were evaluated against the culture gold standard.
RESULTS
Twenty-six of 94 (27.7%) patients had culture-confirmed pulmonary TB. The Xpert® MTB/RIF assay had a sensitivity of 80.8% [95% CI: 60.0–92.7%]) compared against MGIT culture. The Xpert® MTB/RIF assay was more sensitive than sputum smear microscopy (21/26 (80.8%) vs. 13/26 (50.0%), P = 0.02) and detected an additional eight culture-confirmed cases. Culture DST analysis identified two monoresistant M.tb strains: one resistant to rifampicin (rifampicin sensitive by the Xpert® MTB/RIF assay) and one to ethambutol. HIV infection was linked with a 3-fold increase in risk of TB, accounting for 87.5% (21/24) of TB cases. 50% of cases presented as comorbidities with other communicable diseases (CDs) and non-communicable diseases (NCDs).
CONCLUSIONS
As an alternative to sputum microscopy, the Xpert® MTB/RIF assay provides a sensitive, specific and rapid method for the diagnosis of pulmonary TB in obstetric or gynaecological inpatients. Pulmonary TB is an important cause of concomitant comorbidity to the obstetric or gynaecological condition necessitating admission. TB and HIV comorbidities with other communicable and non-communicable diseases were also common. More proactive screening for TB comorbidity is required in obstetric and gynaecological wards.
Keywords: tuberculosis, obstetric, gynaecology, maternal, women, Xpert® MTB/RIF assay, sensitivity, specificity, multidrug-resistant TB, MDR-TB, sub-Saharan Africa
Introduction
WHO estimates that tuberculosis (TB) killed 500 000 women in 2011, of which 200 000 cases were associated with HIV co-infection (WHO 2012). In sub-Saharan Africa (SSA), TB causes 15–34% of non-obstetric maternal deaths (Ahmed et al. 1999; Khan et al. 2001; Menendez et al. 2008; Black et al. 2009). African women of child-bearing age carry the highest HIV/TB codisease burden (WHO 2011a,2011b). Maternal and childhood TB are epidemiologically linked (Batra et al. 2012), and there is a close association between maternal TB and post-partum infant morbidity and mortality in children born to both HIV-infected (Cotton et al. 2008; Gupta et al. 2011) and HIV-uninfected women (Tripathy 2003; Lin & Chen 2010). In HIV-infected women, multivariate analysis has shown that maternal TB infection was independently linked with increased risk of mother-to-child transmission of HIV (Gupta et al. 2011). The risk of TB is 24-fold higher in HIV-infected infants (Hesseling et al. 2009) who are at increased risk of TB-associated mortality (Pillay et al. 2001). Pregnancy is an immunosuppressed state and a risk factor for developing active TB (McNerney et al. 2012).
Tuberculosis comorbidity with other communicable diseases and non-communicable disorders is well described, and there are calls for more proactive screening for TB at all points of care (Bates et al. 2012). TB comorbidity in women requiring obstetric or gynaecological inpatient care in high TB and HIV endemic countries may be easily overlooked because the focus of the admitting physician is on the primary obstetric or gynaecological reason necessitating admission. In addition, non-specific symptoms of active TB such as fatigue or night sweats are commonly associated with pregnancy, during and after childbirth, or in chronic gynaecological infectious or non-communicable diseases (Chang et al. 2012). Furthermore, the low sensitivity of sputum smear microscopy and the operational constraints of mycobacterial culture mean that not all TB cases are identified (McNerney et al. 2012). Several new technologies for the rapid diagnosis of TB are now commercially available such as the Xpert® MTB/RIF assay (Lawn et al. 2013). In this study, we evaluated the use of the Xpert® MTB/RIF assay for the detection of concomitant pulmonary TB in patients admitted to the University Teaching Hospital, Lusaka, Zambia, with a primary obstetric or gynaecological disorder.
Materials and methods
Ethics approval
This study was approved by the Biomedical Research Ethics Review Committee of the University of Zambia, School of Medicine, Lusaka. All study participants gave written informed consent, and the study was conducted in full accordance with all the ethics committee's guidelines.
Design and aims
This was a descriptive, prospective study to evaluate the use of the Xpert® MTB/RIF assay for the detection of concomitant or undiagnosed pulmonary TB and drug-resistant TB in patients admitted primarily for an obstetric or gynaecological disorder to the University Teaching Hospital, Lusaka, Zambia.
Patient recruitment
Patients admitted during the previous 24 h to the obstetrics or gynaecology wards were eligible for recruitment. Admission criteria were patients who had a cough and could produce a sputum sample unaided (without induction). Patients already on TB treatment were excluded. Informed consent was obtained from all the study participants. Clinical details of all the participants including the diagnosis that necessitated hospital admission were recorded.
Definitions
Smear positive indicates presence of acid-fast bacilli (AFBs) in at least one sputum specimen on sputum smear microscopy. Smear negative means absence of AFBs in sputum specimens on sputum smear microscopy. Culture positive refers to a positive MGIT culture with a positive TBcID confirmatory test result and negative blood agar test. Culture negative denotes a negative MGIT culture or positive MGIT culture with a negative TBcID confirmation test result.
Sample collection and laboratory processing
Patients with a cough, having given their consent, were requested to provide up to three sputum samples (spot-morning-spot). This was supervised by dedicated clinical staff in the wards. Sample decontamination, fluorescent smear microscopy, MGIT culture and phenotypic drug-susceptibility testing were performed as described previously (O'Grady et al. 2012).
Xpert© MTB/RIF assay
0.5 ml of concentrated decontaminated sputum was added to a 15-ml Falcon tube in a 1:3 ratio with the sample reagent (0.5 ml of sputum sample to 1.5 ml of sample reagent), and the resulting mixture added to the Xpert® MTB/RIF assay cartridge and then run in the machine in accordance with the manufacturers guidelines (Lawn et al. 2013). ‘Error’ results were repeated.
Data analysis
Two dedicated laboratory personnel blinded to all clinical recruitment, and sample labelling data processed all samples. Clinical and laboratory data were compiled in a database using Epidata software. Analysis was undertaken using SPSS version 18 (IBM, Armonk, NY, USA). The sensitivity, specificity and predictive values of Xpert® MTB/RIF assay were calculated with 95% confidence intervals (CI) and compared with smear microscopy using Pearson chi-squared test. Risk factors were evaluated by multivariate binary logistic regression.
Results
Study group
Ninety-eight inpatients consented to take part in the study and provided sputum for microbiological TB analysis. Four patients were excluded from the analysis (two specimens misplaced, and two cultures were contaminated), and so, the results from a total of 94 patients were analysed (Figure 1). 67.0% (63/94) of recruited women were pregnant or <6 weeks post-natal when recruited, and 33.0% (31/94) were not pregnant or post-natal. Median age was 28 years (IQR: 24–32). HIV status was available for 84 patients, and HIV prevalence was 73.8%. 11 patients had a history of TB treatment, 10 of whom were HIV positive and one was of unknown HIV status (Table 1). The primary admission diagnosis given by the attending physician was recorded. Suspicion of TB alone was noted for 25.5% (24/94) of patients, with an additional 30.9% (29/94) having an admission diagnosis of pneumonia and suspected TB. The remaining 41 patients presented with a range of admission diagnoses including 29.8% (28/94) with other communicable disease conditions and 13.8% (13/94) with non-communicable conditions (11 with clearly defined NCDs, the majority of which were cancer) (Table 2).
Figure 1.
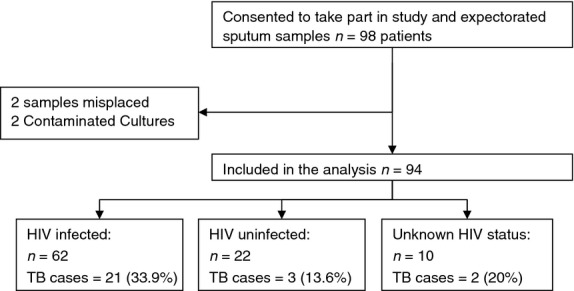
Recruitment flow diagram detailing TB and HIV status.
Table 1.
Study group descriptives for all patients and those with culture-confirmed TB
| Within all patients (n = 94) |
Within culture-confirmed TB cases (n = 26) |
|||
|---|---|---|---|---|
| Proportion | 95% CI | Proportion | 95% CI | |
| Age in years (IQR) | 28 (24–33) | NA | 29 (26–33) | NA |
| HIV status* | ||||
| HIV infected | 62/84 (73.8%) | [63.5–73.8%] | 21/24 (87.5%) | [69.0–95.7%] |
| HIV uninfected | 22/84 (26.2%) | [18.0–36.5%] | 3/24 (13.3%) | [4.3–31.0%] |
| Pregnancy status | ||||
| Pregnant or <6 w post-natal | 63/94 (67.1%) | [57.0–75.7%] | 20/26 (76.9%) | [58.0–89.0%] |
| Not pregnant or post-natal | 31/94 (33.0%) | [24.3–43.0%] | 6/26 (23.1%) | [11.0–42.1%] |
| Treatment history† | ||||
| Previously treated | 11/86 (12.8%) | [7.3–21.5%] | 2/22 (9.1%) | [2.5–27.8%] |
| Not previously treated | 75/86 (87.2%) | [78.5–92.7%] | 20/22 (90.1%) | [72.1–97.5%] |
| Admission diagnosis | ||||
| Pneumonia | 29/94 (30.9%) | [22.4–40.8%] | 8/26 (30.8%) | [16.5–50.0%] |
| Suspected PTB | 24/94 (25.5%) | [17.8–35.2%] | 12/26 (42.3%) | [28.8–64.5%] |
| PID | 11/94 (11.7%) | [6.6–19.8%] | 2/26 (7.7%) | [2.1–24.1%] |
| Cancer | 8/94 (8.5%) | [4.4–15.9%] | 0/26 (0%) | [0–12.9%] |
| Sepsis | 6/94 (6.4%) | [3.0–13.2%] | 2/26 (7.7%) | [2.1–24.1%] |
| Malaria | 4/94 (4.3%) | [1.7–10.4%] | 1/26 (3.8%) | [0.7–19.0%] |
| EPTB | 4/94 (4.3%) | [1.7–10.4%] | 1/26 (3.8%) | [0.7–19.0%] |
| CCF | 3/94 (3.2%) | [1.1–9.0%] | 0/26 (0%) | [0–12.9%] |
| UTI | 2/94 (2.1%) | [0.6–7.4%] | 0/26 (0%) | [0–12.9%] |
| Pre-eclampsia | 1/94 (1.1%) | [0.2–5.8%] | 0/26 (0%) | [0–12.9%] |
| Puerperal psychosis | 1/94 (1.1%) | [0.2–5.8%] | 1/26 (3.8%) | [0.7–19.0%] |
| Vulval Filariasis | 1/94 (1.1%) | [0.2–5.8%] | 0/26 (0%) | [0–12.9%] |
IQR, interquartile range; PID, pelvic inflammatory disease; EPTB, extrapulmonary tuberculosis; CCF, congestive cardiac failure; UTI, urinary tract infection.
HIV status was available for 84 of 94 cases.
Treatment history was unavailable for 8 cases.
Table 2.
Univariate and multivariate risk factor analysis for culture-positive TB infection in obstetric and gynaecological inpatients
| Culture-positive TB |
Univariate |
Multivariate* |
||||
|---|---|---|---|---|---|---|
| Risk factor | Proportion | 95% CI | OR [95% CI] | P = | OR [95% CI] | P = |
| HIV status | ||||||
| HIV infected | 33.9% (21/62) | [23.3–46.3%] | 3.244 [0.861–12.22] | 0.082 | 3.244 [0.861–12.22] | 0.082 |
| HIV uninfected | 13.6% (3/19) | [5.5–37.6%] | – | – | – | – |
| Pregnancy status | ||||||
| Pregnant or <6 w post-natal | 31.7% (20/63) | [21.6–44%] | 1.938 [0.687–5.466] | 0.211 | 1.909 [0.649–5.612] | 0.240 |
| Not pregnant or post-natal | 19.4% (6/31) | [9.2–36.3%] | – | – | – | – |
| Treatment history | ||||||
| Previously treated | 18.2% (2/11) | [5.1–47.7%] | 0.611 [0.121–3.074] | 0.550 | 0.471 [0.090–4.469] | 0.373 |
| Not previously treated | 26.7% (20/75) | [18.0–37.6%] | – | – | – | – |
| Age group† | 1.001 [0.944–1.060] | 0.983 | 0.990 [0.925–1.060] | 0.773 | ||
| 15–20 years | 0% (0/7) | [0–35.4%] | – | – | – | – |
| 21–25 years | 27.3% (6/22) | [13.2–48.2%] | – | – | – | – |
| 26–30 years | 33.3% (12/36) | [20.2–49.7%] | – | – | – | – |
| 31–35 years | 42.9% (6/14) | [21.4–67.4%] | – | – | – | – |
| 36–40 years | 25% (2/8) | [7.1–59.1%] | – | – | – | – |
| ≥41 years | 0% (0/7) | [0–35.4%] | – | – | – | – |
Data are n TB positive/n tested (%) [95% CI], odds ratios (ORs) and p values present results of binary logistic regression analysis.
Multivariate analysis was controlled for effect of HIV.
Age was analysed as a continuous variable but is displayed as grouped to illustrate the age distribution of TB cases.
Performance of the Xpert® MTB/RIF assay for detecting pulmonary tuberculosis
Of 26 culture-confirmed TB cases, the Xpert® MTB/RIF assay detected 21 of 26 culture-confirmed cases giving a sensitivity of 80.8% [95% CI: 60.0–92.7%]. With respect to specificity, the assay identified 66 of 68 culture-negative samples, giving a specificity of 97.1% [95% CI: 88.8–99.5%] (Table 3). The Xpert® MTB/RIF assay was significantly more sensitive than microscopy (21/26 (80.8%) vs. 13/26 (50.0%), P = 0.02) and detected an additional eight culture-confirmed cases. Numbers were low, but stratification of Xpert® MTB/RIF assay performance by HIV status found the assay to be 100% sensitive and specific in HIV-negative patients, with possible (but not statistically significant) poorer performance in HIV-infected patients (Table 3). The Xpert® MTB/RIF assay detected no cases of rifampicin resistance, failing to detect one rifampicin-resistant strain identified by culture DST. Two monoresistant Mycobacterium tuberculosis strains were detected in total, one rifampicin and one ethambutol resistant. No MDR-TB cases were identified by culture DST analysis (data not shown). Three samples initially gave an error on the Xpert® MTB/RIF assay. These were repeated and found to be negative.
Table 3.
Xpert® MTB/RIF assay performance compared with MGIT culture and smear microscopy
| Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | |
|---|---|---|---|---|
| Xpert® MTB/RIF assay versus MGIT | ||||
| All patients* | 21/26 (80.8%) [60.0–92.7%]† | 66/68 (97.1%) [88.8–99.5%] | 91.3% [70.5–98.5%] | 93.0% [83.7–97.4%] |
| HIV negative | 3/3 (100%) [31.0–100%] | 19/19 (100%) [79.1–100%] | 100% [31.0–100%] | 100% [79.1–100%] |
| HIV positive | 17/21 (80.1%) [57.4–93.7%]‡ | 39/41 (95.1%) [82.2–99.2%] | 89.5% [65.5–98.2%] | 90.7% [76.9–97.0%] |
| Sputum smear microscopy versus MGIT | ||||
| All patients | 13/26 (50.0%) [30.4–69.6%]† | 66/66 (100%) [93.3–100%] | 100% [71.7–100%] | 84.0% [73.8–90.8%] |
| HIV negative | 2/3 (66.7%) [12.5–98.2%] | 19/19 (100%) [79.1–100%] | 100% [19.8–100%] | 95.0% [73.1–99.7%] |
| HIV positive | 10/21 (47.6%) [26.4–69.7%]‡ | 41/41 (100%) [89.3–100%] | 100% [65.5–100%] | 78.8% (65.5–88.5%] |
Data displayed as number correct/number tested, (%) [95% CI]; PPV, positive predictive value; NPV, negative predictive value.
The Xpert® MTB/RIF assay gave repeat errors in 3 samples and is excluded from this analysis. Fisher's exact test:
P = 0.02
P = 0.03.
Potential risk factors
TB prevalence within HIV-infected patients was nearly 3-fold higher than that in HIV-uninfected patients (33.9% (21/62) vs. 13.6% (3/19)), with an associated 3-fold increase in risk of TB infection amongst HIV-infected patients (OR 3.244 [95% CI 0.861–12.221]) (P = 0.082) (Table 2). Multivariate binary logistic regression analysis (adjusting for the effect of HIV) demonstrated that the risk of TB was unaffected by patient age (OR 0.990 [0.925–1.060], P = 0.773), previous treatment history (OR 0.471 [0.090–4.469], P = 0.927) or pregnancy (OR 1.909 [0.649–5.612], P = 0.240) (Table 2). 42.3% (11/26) of TB cases were detected in patients with no other reason for admission other than suspected TB (Table 1). 50% (13/26) of TB cases were detected in patients with evidence of comorbidity with other communicable diseases: eight cases of pneumonia, two cases of sepsis (one septic abortion and one case of puerperal sepsis in HIV/AIDS), two cases of pelvic inflammatory disease (likely bacterial infection) and one case of malaria. The two remaining cases included one patient with acute cephalopelvic disproportion with suspected abdominal TB and a patient with puerperal psychosis (Table 1).
Discussion
The key findings of this study are that in inpatients admitted to the obstetrics and gynaecology wards at UTH, Lusaka, Zambia, (i) pulmonary TB is an important incidental cause of concomitant comorbidity to the obstetric or gynaecological condition necessitating admission, (ii) as an alternative to sputum microscopy and culture, the Xpert® MTB/RIF assay provides a sensitive, specific and rapid method for diagnosis of pulmonary TB, and (iii) comorbidity of TB with HIV, other communicable and non-communicable diseases was common. The main limitation of this study was the relatively small number of patients. We have previously shown that TB can be detected in up to 10% of patients capable of producing sputum who are not clinically suspected of having pulmonary TB (Bates et al. 2012). As we only recruited patients with a cough, our data may underestimate the true prevalence of TB amongst obstetric and gynaecological admissions. Low numbers impaired the evaluation of the Xpert® MTB/RIF assay to detect rifampicin resistance.
National TB programmes in high HIV/TB burden countries require data from different patient groups to inform on how best to incorporate the Xpert® MTB/RIF assay into their diagnostic algorithms. In obstetric and gynaecological inpatients, we report a sensitivity of 80.8% (95% CI: 60.0–92.7%) and a specificity of 97.1% (95% CI: 88.8–99.5%), which is comparable with previous findings from the internal medicine wards at our centre (O'Grady et al. 2012), and elsewhere (Chang et al. 2012). With the high throughput of obstetrics and gynaecology wards, the Xpert® MTB/RIF assay could be a useful tool to rapidly screen for TB during the opportunity that presents during the obstetric or gynaecological admission or consultation. It may also help distinguish TB from other co-infections and comorbidities and ensure prompt initiation of TB therapy, reducing the risk of transmission to the baby and other family members. The one case of rifampicin resistance detected by DST was missed by the Xpert® MTB/RIF assay. False-negative rifampicin resistance has been well documented, and larger studies within high MDR-TB prevalence countries are required to evaluate whether the manufacturer has successfully addressed this issue (O'Grady et al. 2012; Lawn et al. 2013).
The prevalence of HIV infection within our study population was 73.4%. Whilst our study sample was small and major conclusions of HIV prevalence cannot be drawn, this high HIV burden amongst obstetric and gynaecological inpatients with suspected TB is comparable to that seen amongst females on the adult general internal medicine wards at UTH (Bates et al. 2012; O'Grady et al. 2012). This focus of HIV-associated TB cases highlights the need for broader active case finding in asymptomatic and lower-risk patient groups with community-based studies in Zambia previously showing that as little as 43% of TB cases occur in patients who would be classically defined as TB suspects (Ayles et al. 2009). In our study, there was a trend for HIV infection to be linked with a threefold increase in risk of TB infection compared with a 2-fold increase in risk of TB infection amongst HIV-infected patients on the internal medicine wards (Bates et al. 2012). A large community-based study of antenatal attendants in South Africa also found TB rates to be 3-fold higher amongst HIV-infected women (Gounder et al. 2011). We did not detect any significant decrease in risk of TB infection, with increasing age as shown previously (Bates et al. 2012), but this may likely be due to the younger age profile of our study group: median age of 28 (IQR: 24–33) vs. 35 (IQR: 28–43) (Bates et al. 2012), Mann–Whitney U-test, P < 0.001).
One of the challenges at busy, high-turnover referral hospitals is the focus on addressing the immediate obstetric or gynaecological reason for admission, whilst concomitant comorbidities with chronic communicable diseases like TB can be easily overlooked (Marais et al. 2013). High levels of TB comorbidity with other communicable and non-communicable disease have been previously documented amongst general internal medicine inpatients at the University Teaching Hospital in Lusaka (Bates et al. 2012). We hence sought to assess to what degree TB cases presented as comorbidities on the obstetric and gynaecological wards. We documented five cases of TB comorbidity with infectious diseases including pelvic inflammatory disease (PID), bacterial sepsis and malaria. 11.7% (11/94) of the recruited patients had a clearly defined non-communicable disease (cancer and congestive cardiac failure), and active TB was not detected in these cases. We detected 20 cases of pulmonary TB within women who were either pregnant or <6 weeks post-natal. We did not conduct follow-up to assess the risk of neonatal TB infection in the women recruited, but one South African study detected TB in 16% of neonates born to mothers with suspected or proven TB (Bekker et al. 2012). The majority of neonatal admissions have feeding tubes inserted, which presents an excellent opportunity for TB screening without the need for additional invasive sampling, possibly using the Xpert® MTB/RIF assay, which has been demonstrated to perform well on gastric aspirate at our site (Bates et al. 2013).
Conclusions
The Xpert® MTB/RIF assay provides a sensitive, specific and rapid method for diagnosis of TB in obstetric or gynaecological inpatients. Most of the obstetric admissions and during the study were for deliveries with a short duration of inpatient stay. Similarly, the gynaecology admissions were for miscarriages with a similar short stay. The high TB/HIV co-infection rates in SSA and presence of TB with HIV comorbidities now call for more proactive and rapid screening for pulmonary TB and HIV in obstetric and gynaecological inpatient facilities to provide more optimal and synergistic alignment of health care for communicable and non-communicable disorders.
Acknowledgments
AZ acknowledges support from the European and Developing Countries Clinical Trials Partnership (EDCTP grants REMOX, PANACEA and TB-NEAT), the Netherlands; UK Medical Research Council (MRC); UBS Optimus Foundation, Switzerland; University College London Hospitals, NIHR Biomedical Research Centre; and the UCL Hospitals National Health Service (NHS) Foundation Trust.
References
- Ahmed Y, Mwaba P, Chintu C, Grange JM, Ustianowski A, Zumla A. A study of maternal mortality at the University Teaching Hospital, Lusaka, Zambia: the emergence of tuberculosis as a major non-obstetric cause of maternal death. The International Journal of Tuberculosis and Lung Disease. 1999;3:675–680. [PubMed] [Google Scholar]
- Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS ONE. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, O'Grady J, Mwaba P, et al. Evaluation of the burden of unsuspected pulmonary tuberculosis and co-morbidity with non-communicable diseases in sputum producing adult inpatients. PLoS ONE. 2012;7:e40774. doi: 10.1371/journal.pone.0040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, O'Grady J, Maeurer M, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infectious Diseases. 2013;13:36–42. doi: 10.1016/S1473-3099(12)70245-1. doi: 10.1016/S1473-3099(12)70245-1. Epub 2012 Nov 5. [DOI] [PubMed] [Google Scholar]
- Batra S, Ayaz A, Murtaza A, Ahmad S, Hasan R, Pfau R. Childhood Tuberculosis in Household Contacts of Newly Diagnosed TB Patients. PLoS ONE. 2012;7:e40880. doi: 10.1371/journal.pone.0040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker A, Du Preez K, Schaaf HS, Cotton MF, Hesseling AC. High tuberculosis exposure among neonates in a high tuberculosis and human immunodeficiency virus burden setting. The International Journal of Tuberculosis and Lung Disease. 2012;16:1040–1046. doi: 10.5588/ijtld.11.0821. [DOI] [PubMed] [Google Scholar]
- Black V, Brooke S, Chersich MF. Effect of human immunodeficiency virus treatment on maternal mortality at a tertiary center in South Africa: a 5-year audit. Obstetrics and Gynecology. 2009;114:292–299. doi: 10.1097/AOG.0b013e3181af33e6. [DOI] [PubMed] [Google Scholar]
- Chang K, Lu W, Wang J, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. Journal of Infection. 2012;64:580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. The International Journal of Tuberculosis and Lung Disease. 2008;12:225–227. [PubMed] [Google Scholar]
- Gounder CR, Wada NI, Kensler C, et al. Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. Journal of Acquired Immune Deficiency Syndromes. 2011;57:e77–e84. doi: 10.1097/QAI.0b013e31821ac9c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. Journal of Infectious Diseases. 2011;203:358–363. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clinical Infectious Diseases. 2009;48:108–114. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- Khan M, Pillay T, Moodley JM, Connolly CA. Maternal mortality associated with tuberculosis-HIV-1 co-infection in Durban, South Africa. AIDS. 2001;15:1857–1863. doi: 10.1097/00002030-200109280-00016. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chen SF. Increased risk of low birthweight and small for gestational age infants among women with tuberculosis. BJOG. 2010;117:585–590. doi: 10.1111/j.1471-0528.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- McNerney R, Maeurer M, Abubakar I, et al. Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. Journal of Infectious Diseases. 2012;205(Suppl. 2):S147–S158. doi: 10.1093/infdis/jir860. [DOI] [PubMed] [Google Scholar]
- Menendez C, Romagosa C, Ismail MR, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. doi: 10.1371/journal.pmed.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady J, Bates M, Chilukutu L, et al. Evaluation of the Xpert MTB/RIF Assay at a Tertiary Care Referral Hospital in a Setting Where Tuberculosis and HIV Infection Are Highly Endemic. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2012;55:1171–1178. doi: 10.1093/cid/cis631. [DOI] [PubMed] [Google Scholar]
- Pillay T, Adhikari M, Mokili J, et al. Severe, rapidly progressive human immunodeficiency virus type 1 disease in newborns with coinfections. The Pediatric Infectious Disease Journal. 2001;20:404–410. doi: 10.1097/00006454-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Tripathy SN. Tuberculosis and pregnancy. International Journal of Gynaecology and Obstetrics. 2003;80:247–253. doi: 10.1016/s0020-7292(02)00393-4. [DOI] [PubMed] [Google Scholar]
- WHO. Global Tuberculosis Control: WHO Report 2011. Geneva: World Health Organisation; 2011a. [Google Scholar]
- WHO. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access: Progress Report 2011. Geneva: WHO press; 2011b. [Google Scholar]
- WHO. Global Tuberculosis Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. The Lancet infectious diseases. 2013;13:349–361. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infectious Diseases. 2013 doi: 10.1016/S1473-3099(13)70015-X. doi: 10.1016/S1473-3099(13)70015-X. Epub 2013 Mar 22. [DOI] [PubMed] [Google Scholar]


