Background
Adherence to guidelines during public health emergencies is a national priority. Throughout the 2009 H1N1 influenza pandemic, (1) the Centers for Disease Control and Prevention (CDC) issued guidelines recommending antiviral prescribing only to selected patients at high risk for complications, including patients younger than 2 years and patients 65 years or older and not for prophylaxis.(2) The extent to which antivirals were prescribed and how these practices differed from previous years is unknown
Methods and Findings
We used data from the National Disease and Therapeutic Index (NDTI), a nationally representative survey of visits to ambulatory physicians produced by IMS Health, Plymouth Meeting Pennsylvania. The survey includes approximately 4800 sampled physicians each calendar quarter who provide information about each clinical encounter 2 consecutive workdays. Physicians are selected by random stratified sampling by specialty and geographic region from the master lists of the American Medical Association and the American Osteopathic Association. Data for each visit include patient diagnoses based on International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9-CM) and medications prescribed during these visits. The complex sampling frame allows extrapolation to national estimates for office visits and associated prescriptions. The NDTI has been used to examine patterns of medication prescribing (3,4) with results that are consistent with the federally-conducted National Ambulatory Medical Care Survey (5).
Using the NDTI, we estimated the number of monthly visits where influenza was diagnosed from October 2006–March 2010 by identifying all visits with an ICD-9-CM code for influenza (ICD-9-CM code 487). This period included 3 winter influenza seasons (2006–2007, 2007–20008, 2008–2009), as well as the 2009 H1N1 pandemic beginning in April 2009. Our estimates rely on a sample of 3122 influenza visits included in NDTI. To evaluate trends in antiviral prescribing for influenza, we also examined the proportion of these visits where an antiviral (oseltamivir phosphate, zanamivir, rimantadine hydrochloride) was prescribed for influenza, as well as the number of visits where an antiviral was prescribed for any diagnosis.
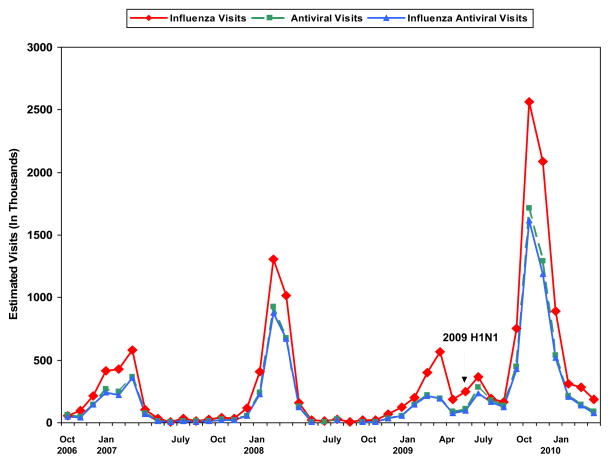
During the last 4 years, peak influenza visits and antiviral use occurred during January through March (Figure). During 2009, in association with 2009 H1N1 pandemic, and unlike previous years, influenza visits increased during May and June and subsequently surged to a peak of 2.6 million (95% CI 2.2–3.0 million) during October. Antiviral drug uses were reported during 1.7 million of these visits (95% CI 1.4–2.0 million), of which 99% noted oseltamivir and 94% included an influenza diagnosis. The percentage of influenza visits where an antiviral was prescribed varied annually, but did not differ during the H1N1 period (58%) compared with previous years (59%, Figure). During the H1N1 period, antivrirals were prescribed for 47% of patients younger than 2 years and 68% of patients 65 years and older.
Figure.
Estimated number of U.S. monthly visits (in thousands) where a diagnosis of influenza was reported (influenza visits), visits where an influenza antiviral was prescribed for any diagnosis (antiviral visits) and influenza visits where an antiviral was prescribed (influenza antiviral visits) from October 2006–March 2010. Source: IMS HEALTH National Disease and Therapeutic Index.
Discussion
Using a nationally representative sample, we document a large surge of influenza visits and antiviral prescribing attributable to 2009 H1N1 influenza during October-December 2009. This pattern of ambulatory visits is similar to that from CDC surveillance (6) suggesting the validity of our findings), but adds previously undescribed information on antiviral prescribing. Although reported antiviral use increased due to the increase in influenza diagnoses, there was no change in the percentage of ambulatory patients diagnosed with influenza who were prescribed antivirals. Antivirals appear to have been underused for patients at high risk for complications based on age (for example <2 and ≥65), populations for whom CDC guidelines recommended treatment. In contrast,, only a very small percentage of visits where antivirals were prescribed (6%) were not associated with an influenza diagnosis, suggesting that prescribing for prophylaxis was likely limited.
A previous study showed that CDC recommendations (via the Health Alert Network) are effective in rapidly influencing antiviral prescribing patterns for influenza (7). During 2009, the CDC used similar communication methods.5 The message that only certain high-risk patients warrant therapy may have aided physicians in judiciously prescribing antivirals, however, we found evidence of underuse among young children and older adults which may have led to preventable complications.
Our findings are subject to limitations. We were unable to evaluate the extent of antiviral prescribing for patients diagnosed with influenza and co-existing high-risk conditions such as asthma. This dataset has limited capture of emergency department visits, hospitalizations and telephone encounters; settings where antiviral prescribing may have differed.
Despite a large surge in influenza visits, we found no change in the overall propensity to prescribe in ambulatory settings during the H1N1 epidemic compared with previous years.. Nonetheless, antivirals appear to be underused for patients in high-risk age groups, suggesting opportunities to improve the translation of public health guidelines into clinical practice.
Acknowledgments
Grant Support: By grant T32HD044331 from the National Institute of Child Health and Human Development (Dr. Hersh) and training grant K24HL086703 from the National Heart, Lung, and Blood Institute (Dr. Stafford).
Footnotes
Disclaimer: The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the National Disease and Therapeutic Index (2006 to 2010), IMS Health. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health or any of its affiliated or subsidiary entities.
Potential Conflicts of Interest: Dr. Stafford: Expert testimony: Mylan Pharmaceuticals. Grants/grants pending (money to institution): Toyo-Shinyaku, Procter & Gamble, Partnership for Prevention, Stanford University, American Heart Association, Wako Inc., GlaxoSmithKline. Grants/grants pending: University of Chicago. Honoraria: American Drug Utilization Review Society, American College of Preventive Medicine, Partnership for Prevention. Payment for manuscript preparation: Massachusetts Medical Society. Travel/accommodations/expenses covered or reimbursed: Partnership for Prevention, National Committee for Quality Assurance, American College of Preventive Medicine, American Heart Association, University of Chicago, IMS Health, Drug Information Association, Bayer. Dr. Hersh: Grants received (money to institution): National Institutes of Health. Consultancy: Colorado Guidelines Committee.
Contributor Information
Adam L. Hersh, University of Utah, Salt Lake City, UT 84108.
Randall S. Stafford, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA 94305.
References
- 1.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009 Jun 18;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Updated interim recommendations for the use of antiviral medications inthe treatment and prevention of influenza for the 2009–2010 season. [Accessed 6/02/10]; http://www.cdc.gov/H1N1flu/recommendations.htm.
- 3. [Accessed 5/31/10];FluView. http://www.cdc.gov/flu/weekly/index.htm.
- 4.Hersh AL, Maselli JH, Cabana MD. Changes in prescribing of antiviral medications for influenza associated with new treatment guidelines. Am J Public Health. 2009 Oct;99( Suppl 2):S362–364. doi: 10.2105/AJPH.2009.171462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health Alert Network. [Accessed 6/01/10]; http://www2a.cdc.gov/han/Index.asp.