Abstract
Uncertainty is a component of many gambling games and may play a role in incentive motivation and cue attraction. Uncertainty can increase the attractiveness for predictors of reward in the Pavlovian procedure of autoshaping, visible as enhanced sign-tracking (or approach and nibbles) by rats of a metal lever whose sudden appearance acts as a conditioned stimulus (CS+) to predict sucrose pellets as an unconditioned stimulus (UCS). Here we examined how reward uncertainty might enhance incentive salience as sign-tracking both in intensity and by broadening the range of attractive CS+s. We also examined whether initially-induced uncertainty enhancements of CS+ attraction can endure beyond uncertainty itself, and persist even when Pavlovian prediction becomes 100% certain. Our results show that uncertainty can broaden incentive salience attribution to make CS cues attractive that would otherwise not be (either because they are too distal from reward or too risky to normally attract sign-tracking). In addition, uncertainty enhancement of CS+ incentive salience, once induced by initial exposure, persisted even when Pavlovian CS-UCS correlations later rose toward 100% certainty in prediction. Persistence suggests an enduring incentive motivation enhancement potentially relevant to gambling, which in some ways resembles incentive-sensitization. Higher motivation to uncertain CS+s leads to more potent attraction to these cues when they predict the delivery of uncertain rewards. In humans, those cues might possibly include the sights and sounds associated with gambling, which contribute a major component of the play immersion experienced by problematic gamblers.
Keywords: Reward uncertainty, Incentive motivation, Incentive salience, Sign-tracking, Gambling
1. Introduction
Gambling addiction has become an increasing burden on American society, and possibly worsened by the widespread introduction of video and online gambling [1]. One of the key features that makes games so fun to play and gambling so potentially addictive is uncertainty [2]. In gambling, uncertainty is conveyed through low reliability between the lights and sounds (cues) associated with playing and the winning outcome that they predict. Typically, cues that predict reward will attract attention and therefore are more likely to induce cue-directed behaviors, and the attraction in gambling can outweigh any appraisal that overall odds are against the player. For this reason, cue lights and sounds can become powerfully motivating to a gambler. For example, college students expressed increased craving to gamble when presented with visual cues or imagery associated with gambling [3].
Autoshaping is a Pavlovian conditioning phenomenon that captures the incentive salience attraction attributed to predictive cues even in animals. Incentive salience attribution transforms such cues into motivational magnets. In autoshaping, a metal lever emerges from a wall for several seconds, serving as a conditioned stimulus (CS) to predict an immediately subsequent presentation of a rewarding unconditioned stimulus (UCS) such as palatable food. Although no instrumental action is required to earn the UCS presentation, rats do not passively sit and wait for the food [4,5]. Instead, some individuals become sign-trackers as the CS-UCS association is learned, approaching and vigorously sniffing, nibbling, biting, grabbing and consequently pressing the CS+ lever that predicts reward. Other individuals become goal-trackers, equally triggered by CS+ presentation, but directing their approach, sniffs and nibbles to the physical location or goal dish where the sucrose reward actually appears. The goal dish is minimally predictive of UCS (being present both during UCS delivery and absence) but maximally proximal to UCS (close in space and time to reward receipt), whereas the CS+ lever is maximally predictive as a discrete event highly correlated with UCS delivery, but is more distal to the physical UCS pellet. Finally, some other individuals show comparable amounts of both behavior types, and are considered to be intermediates [6-8]. Motivational attraction to the Pavlovian cues reflects the amount of incentive salience attributed to that cue, and attraction to the discrete predictive CS+ in particular has been suggested to reflect increased susceptibility to addictive behavior and disorders of impulse control [6,9,10].
Traditionally in learning theory, the incentive value of a CS varies with its associative correlation (predictive value) to UCS prediction [11,12]. Thus a 100% certain CS predictor has maximum predictive strength [13], and should have highest incentive value. However, there is evidence that the incentive and predictive components of a learned reward predictor can be dissociated [14]. For example, pharmacological/physiological manipulations of mesolimbic dopamine levels in the nucleus accumbens alter the incentive, but not the predictive, value of a CS in a reversible fashion [8,15-17]. Furthermore, prediction certainty of a Pavlovian CS may in some circumstances detach from incentive value. A CS that predicts UCS with 100% correlation is highly certain, whereas a different CS that predicts reward with a probability of only 50% would be highly uncertain. There is evidence to suggest that under similar conditions of reward uncertainty, rats as well as humans, sometimes tend to more often approach and work harder for rewards whose delivery is uncertain (i.e. impossible to predict) rather than for rewards obtained with certainty [4,18-24]. Regarding sign-tracking specifically, we showed in a previous autoshaping study that an uncertain CS+ (i.e., 50% of trials rewarded) that predicts a varying and uncertain magnitude of UCS reward (i.e., UCS was either 1, 2 or 3 sucrose pellets on a random basis) could attract more approaches and nibbles than a CS+ that predicted reward with 100% certainty [25]. In other words, there was greater incentive salience attribution to the uncertain CS+, which might or might not be rewarded on any given trial (and if so, with uncertain magnitude of reward) than to the more certain CS that always predicted reward. This indicates that uncertain cues for rewards, although less predictive than cues for certain rewards, can possess greater incentive salience – a result in accordance with the findings that the predictive and the incentive components of reward rely on two distinct processes. At a neuronal level, there is evidence to suggest that reward uncertainty can enhance extracellular dopamine levels in nucleus accumbens [26], and sign-tracking also involves a greater dopaminergic response [5,6,9]. Human pathological gambling also has been related to striatal dopamine [27], and compulsive gambling severity is associated with larger dopamine responses [28].
The above suggests that reward uncertainty is a source of incentive motivation, and that uncertainty motivation can be studied in animals by means of sign-tracking. The present study investigates whether the motivating power of uncertainty can actually extend incentive value to cues that are normally not attractive. In addition, we also aimed to assess whether CS+ incentive enhancement by uncertainty could persist beyond the termination of uncertain conditions, and appear subsequently even if reward prediction became 100% certain. Persistence could be relevant to long-term motivational effects that might contribute to compulsive gambling, which is known to depend (at least, in part) on dysregulation of the dopaminergic control of motivated behavior [29].
2. Experiment 1
This experiment aimed to determine (a) whether uncertain rewards can persistently hold a rats' interest despite a reduction in uncertainty and (b) whether reward uncertainty would assign greater incentive value (sign-tracking) to cues normally not preferred. For example, previous studies have shown that pigeons will choose to consistently sign-track when the CS is close to the location of the UCS delivery or goal, but goal-track when the CS is further away from that location [30], suggesting that distal cues normally do not acquire much if any incentive salience. In addition, previous pilot studies in the lab had shown an apparent spontaneous preference for the more sheltered (less exposed) of two equally proximal cues. As previously [25], we used a combination of a 50% probability and variable magnitude in order to induce uncertainty, but here we also incorporated location uncertainty: rats did not know which lever – among three possible – would be presented on a given trial.
2.1. Materials and methods
2.1.1. Animals and housing conditions
Female Sprague-Dawley rats (N = 24; 110-150 days old) were bred and reared by the research group from animals purchased from Harlan. Animals were weaned at 21 days of age and housed (cage: 25 × 45 × 20 in.) in groups of 2-3 animals (by gender) with possible litter effects controlled for during group assignment. Shortly prior to the start of the experiment, rats were food restricted (15-20 g of Purina lab chow per rat per day) until reaching approximately 90% of free-feeding bodyweight. Rats were housed under a reverse 12:12 h light-dark cycle (light on at 9 pm) at about 21°C. They had ad libitum access to tap water for the duration of the experiment. All experimental procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
2.1.2. Apparatus
Autoshaping chambers (30 cm × 24 cm × 21 cm) contained four levers (4.5 cm × 2 cm) and a recessed sucrose pellet dish (3 cm × 2 cm × 1 cm). The dish was located in the center of the front wall near to the floor of the chamber. It contained an infrared beam and sensor that recorded an entry each time the beam was broken. Two levers were located on the same (front) wall of the box that contained the sucrose delivery dish or CS goal: one on each side of the dish, and therefore both levers were proximal to the goal dish. Two additional levers were on the opposite or back wall, positioned to mirror the front wall levers, but more distal to the CS goal being separated by 30 cm of open space. Further, one of the two proximal levers, and one of the two distal levers, were positioned near the side door that when open exposed the external room, and through which an experimenter's hand retrieved the rat at the end of trials. The other proximal and other distal levers were further inside the chamber away from the door, and therefore more sheltered from the outside and the hand of the experimenter. Thereafter, these four levers will be referred to as proximal sheltered lever (PS), proximal exposed lever (PE), distal sheltered (DS) and distal exposed (DE) levers. The distal exposed lever was designated as the control lever and remained extended (but never illuminated) throughout every training session and responses on this lever were recorded but bore no consequence. Only the three remaining CS levers (PS, PE, DS) had a light at the base that was turned on with their presentation and an auditory tone (2.9 kHz) was also produced in order to increase the chance of detecting the lever. Lever presses and nose entries into the goal dish were recorded using MedPC® software and Med Associates® hardware, and all behaviors were video recorded through a camera positioned below the transparent floor. Boxes were placed in cabinets whose doors were shut during the training sessions in order to ensure reduced ambient light and noise. Red LED house lights were mounted to the ceiling and floor of the cabinets and turned on during the training sessions.
2.1.3. Groups and experimental conditions
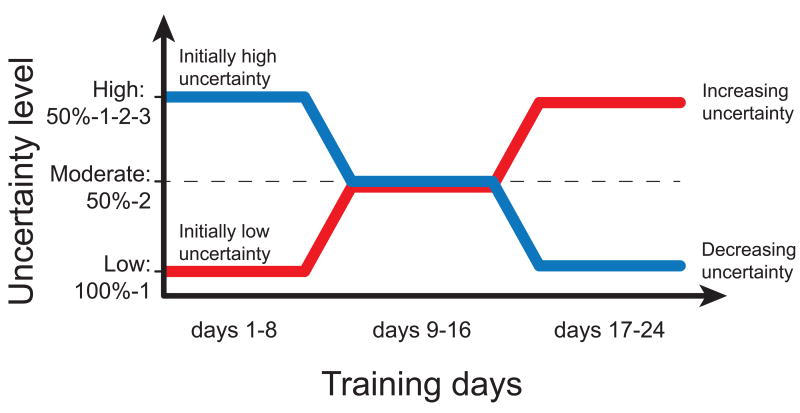
Rats were randomly divided in two groups of eight individuals. In the first group, referred to as the initially high uncertainty group, the rats were initially subjected to high levels of uncertainty (lever location, reward probability, and reward magnitude) for the first 8 days, followed by decreasing levels of uncertainty every 8 days for a total of 24 days. In the second group, referred to as the initially low uncertainty group, the rats began with low levels of uncertainty (lever location only), and were then subjected to increasing levels of uncertainty every 8 days for a total of 24 days. As shown in Figure 1, the three training blocks defining uncertainty level were performed as follows:
Fig. 1. Summary of the groups and experimental conditions for experiment 1.
In the initially high uncertainty group, the rats were tested with decreasing levels of uncertainty (high, moderate, then low), while the rats in the initially low uncertainty group were tested with increasing levels of uncertainty (low, moderate, then high). These conditions changed in a stepwise fashion every 8 days, for a total of 24 days of training.
High Uncertainty Block (50%-1-2-3): location, probability, and magnitude uncertainty (eight sessions). Lever presentation was random and followed by no reward on 50% of trials. On the other 50% of trials when a reward was delivered, it consisted of 1, 2 or 3 sucrose pellets with an equal probability (16.7%).
Moderate Uncertainty Block (50%-2): location and probability uncertainty (eight sessions). On the 50% of trials when random lever presentation was followed by a reward, the reward was always two sucrose pellets – no pellets were delivered in the other 50% of trials.
Low Uncertainty Block (100%-1): location uncertainty (eight sessions). On every trial, rats were presented with one of three levers (two front levers and one back lever) on a random basis for 8 seconds. Immediately after lever retraction, a single sucrose pellet was delivered into the dish.
An additional unpaired control group (N = 8) was run to examine the effects of pseudo-conditioning. These animals received 8 consecutive days of training under high uncertainty conditions (location, probability, and magnitude uncertainty: 50%-1-2-3) but received the CS unpaired, rather than paired, with respect to the UCS.
2.1.4. Procedure
All the rats were handled three times and familiarized with 45-mg sucrose pellets in their home cage the day before the experiment began. They were then trained to receive sucrose pellets from the dish by delivering 25 sucrose pellets in succession (ITI 30-90 s) in the absence of lever presentation.
The rats of each group were subjected to three training blocks (see above), each block consisting of 8 consecutive daily sessions of 40 trials (ITI 30-90 s), where animals received 38-42 sucrose pellets during each session. As mentioned above, the initially high uncertainty group of rats received blocks of decreasing uncertainty starting with high uncertainty, then moderate uncertainty, and ending with the low uncertainty block (where 100% of trials were rewarded with a single pellet). The initially low uncertainty group of rats received blocks of increasing uncertainty starting with low uncertainty (100% of trials were rewarded), then moderate uncertainty, and ending with the high uncertainty block. So doing, all the rats were exposed to the three reward uncertainty levels, where the order of exposure was the only difference between groups. At the conclusion of each block the rats were given a 2-4 day break in testing. Food restriction was continued throughout these periods. Rats were returned to their home cage at the end of each session.
A trial started with the presentation of an illuminated lever (together with the tone turned on) and ended with the retraction of the lever (and the tone turned off) 8 s later. The location of the lever presented on any given trial (PS, PE or DS) was randomly selected with equal probability (1/3) of occurrence for each lever.
2.1.5. Behavioral video scoring and data collection
The number of lever presses and magazine entries was computer-recorded during every CS lever occurrence for each of the 24 sessions of the experiment. In addition, training days 1, 8, and 24 were video recorded for complementary behavior analysis. This analysis was performed for the behaviors mentioned below by manual counting or measure using frame-by-frame or ½ speed analysis of the video recordings on each of the three video scoring days (1, 8, 24). All of these behaviors were scored for the 8-second duration of every third occurrence of a specific lever CS+ – i.e. presentations 1–3–6–9–12. This allowed us to obtain a regular, systematic sampling throughout the key sessions in the different groups. In addition, a portion of these behaviors (all except nibble, slow bite, slow dive, speed and latency) were also scored during the 8 seconds prior to every third CS+ presentation of each type, using the slot in the wall from which the lever is extended as the target for lever directed behaviors, in order to establish a baseline level of responding for comparison to CS+.
Look: head movement towards the lever or magazine without approaching it.
Approach: body (other than head) movement towards the lever or magazine (does not require contact with either the lever or magazine).
Lever/Magazine contact: the lever or magazine was approached to within a distance < 1 cm.
Sniff: small amplitude, short duration exploratory movement of the lever or magazine with the nose, making little or limited contact.
Nibble: small amplitude, short duration exploratory movement of the lever or magazine with the mouth, making contact.
Slow bite (lever only): orally grasping the lever between their mandibles.
Slow dive (magazine only): insertion of the nose and mouth into the food cup, as would normally occur when retrieving a sucrose pellet.
Distance from lever/magazine: distance of the rat's nose from the lever or magazine just before (last video frame) the lever emerges from the wall. (The same behavioral measure is used to note the distance from the lever and the magazine.)
Time latency before lever/magazine contact: time elapsed before reaching the lever or magazine once the lever became available.
Speed before lever/magazine contact: ratio of the distance from lever/magazine to the time latency before contact.
2.1.6. Statistical analyses
Two-way ANOVAs were computed to determine the Group × Day effects with respect to the number of lever presses and of magazine entries. One-way ANOVAs were used in order to assess the effects of group on the behavioral parameters electronically recorded or extracted from videos. Most comparisons between two data sets were computed using planned comparisons or assessed separately by means of t-tests when appropriate. Repeated measures ANOVAs allowed us to compare sign-tracking and goal-tracking behaviors within a session, and across training trials. To determine whether an animal displayed a tendency towards either sign- or goal-tracking behavior, the probability that the animal would contact the presented CS lever cue after 8 days of training was compared to the probability of contacting either the CS cue or the magazine ((ProbLever/(ProbLever + ProbMag))*100), providing a preference index for the lever CS ranging from 0-100%. An animal was deemed a sign-tracker if its lever preference was > 66%, an intermediate if it was < 66% but > 33%, and a goal-tracker if it was < 33%. Two-tailed tests were used and the null hypothesis was rejected at p < 0.05.
2.2. Results
2.2.1. Uncertainty enhances the incentive salience attributed to cues
In most traditional autoshaping studies that have distinguished individual sign-tracking vs goal-tracking phenotypes, only a single immovable lever position is used as CS+ (often positioned on the same wall as the goal dish). By contrast, here three levers were used, including on the opposite wall, with the CS+ lever able to move location. Based on traditional classification criteria for phenotyping individuals [7] using the goal-proximal lever, after 8 days of training most of our rats were sign-trackers in all groups: the initially low uncertainty group was comprised of 5 sign-trackers and 3 intermediates (no goal-trackers), while the initially high uncertainty group was composed of 8 sign-trackers. However, as described below, when behavior towards a distal lever located on the opposite wall far from the magazine was considered as an independent measure of sign-tracking, uncertainty levels appeared to have a strong impact on whether an individual was a sign-tracker or a goal-tracker. That is, for the initially high uncertainty rats, most individuals remained sign-trackers toward the distal CS+ lever (75%; 6 sign-trackers and 2 goal-trackers). By comparison, for the initially low uncertainty rats, traditional sign-tracker individuals mostly transformed into goal-trackers when a distal lever was used as CS+ (25%; 2 sign-trackers, 1 intermediate and 5 goal-trackers). For this reason, in results below, we do not classify individuals as sign- or goal-trackers. Instead we describe uncertainty effects on all individuals in the group under their current condition.
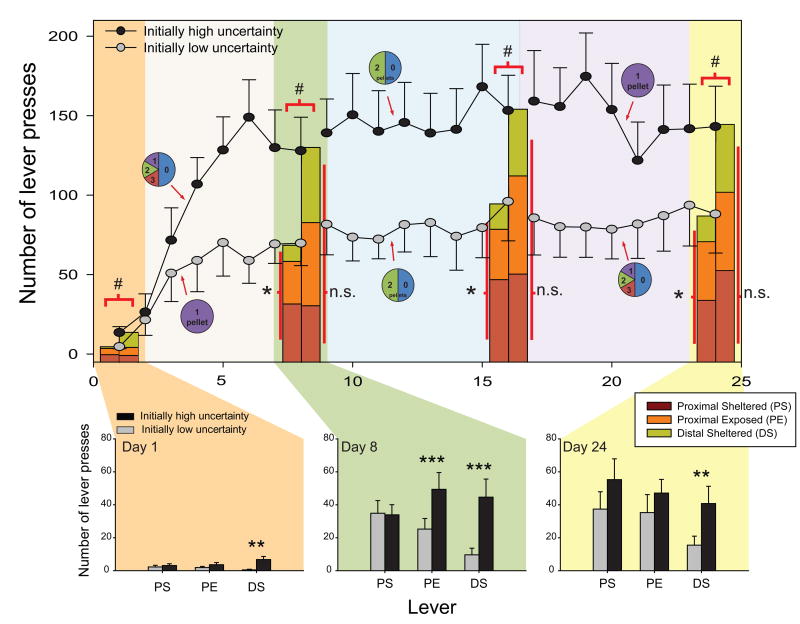
Beginning with the first day of training, rats in the initially high uncertainty group, pressed CS+ levers or showed overall more sign-tracking than rats initially subjected to the low uncertainty conditions (F(1,47) = 9.150, p = 0.004), suggesting an early impact of uncertainty on the attribution of incentive salience to the CS+. However, in the absence of any CS+, initially high uncertainty rats did not differ in their behavior during baseline periods (prior to CS+ lever insertion) from the rats in the initially low uncertainty group (t14's ≥ 0.916, p's ≥ 0.375). Initially high uncertainty conditions also resulted in a greater increase in lever pressing across the following 7 days when compared to initially low uncertainty conditions (Group: F(1,14) = 6.682, p = 0.021; but no Group × Day interaction: F(1,14) = 3.722, p = 0.074). The greater increase of pressing by rats in the initially high uncertainty group occurred despite an overall increase in lever pressing seen in both groups, reflecting a general acquisition of the Pavlovian task and the development of a CS motivational magnet (Day: F(1,14) = 49.438, p = 0.000). As shown in Figure 2 (upper part), this uncertainty-based enhancement resulted on day 8 in an overall greater attraction to all three cues in the form of more lever presses (F(1,138) = 14.948, p = 0.000).
Fig. 2. Number of lever presses in both groups of rats.
Upper part: lever presses for all CS+ levers across varying levels of reward uncertainty. The initially high uncertainty group started with high uncertainty (d1-8) and ended with low uncertainty (d17-24), while the initially low uncertainty group started with low uncertainty (d1-8) and ended with high uncertainty (d17-24). Circles represent reward contingencies in each training block; # indicates significant differences (p < 0.05) in total responding between groups on the last session of a training block; * indicates significant differences (p < 0.05) in responding within group on the last session of a training block; n.s. indicates a lack of significance. Bottom part: lever presses for each lever type (PS, PE, and DS) on sessions 1, 8, and 24. The rats of both groups pressed the PS lever in a similar way throughout training, but the rats of the initially high uncertainty group pressed the PE lever more than those of the initially low uncertainty group at the end of the first training block (on day 8). Interestingly, the distal lever was pressed to a larger extent by the rats of the initially high uncertainty group throughout training. Legends: ** p < 0.01, *** p < 0.001.
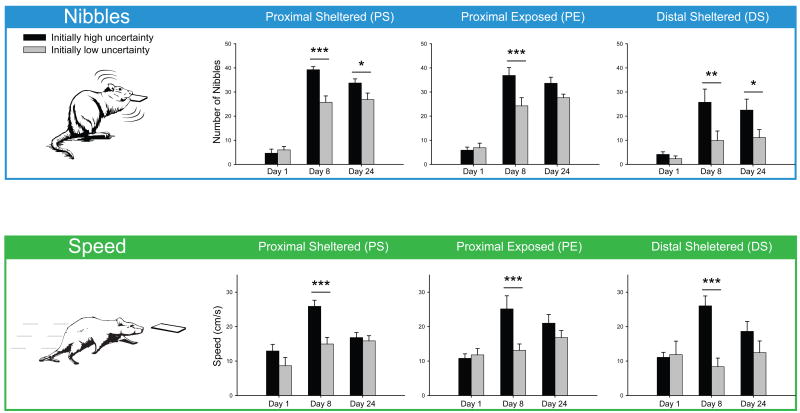
Regarding approach speed and consummatory actions when the CS+ lever was reached, repeated measure ANOVAs indicate that the number of lever-directed nibbles (F(2,6)'s ≥ 22.439, p's ≤ 0.002) and the speed to reaching the lever (F(2,6)'s ≥ 32.851, p's ≤ 0.001) both increased between days 1 and 8 for all CS+ levers, except for the DS lever in animals initially exposed to low uncertainty conditions for which there was no increase in nibbles (F(2,6) = 3.194, p = 0.114) and which even saw a slight decrease with respect to speed (F(2,6) = 5.613, p = 0.042). Figure 3 indicates that exposure to 8 days of high uncertainty conditions transformed CS lever cues into more powerful motivational magnets, causing animals initially exposed to high uncertainty conditions to approach the CS+ levers more rapidly (F(1,42)'s ≥ 13.007, p's ≤ 0.001), and attracting more vigorous interaction in the form of nibbles (F(1,42)'s ≥ 9.514, p's ≤ 0.003). Finally, the number of lever-directed sniffs decreased in both groups between days 1-8 (F(2,6)'s ≥ 5.545, p's ≤ 0.043). The reduction in sniffs coupled with an increase in nibbles suggests a transition towards a more predominantly intense engagement with and greater interest towards the CS+ lever during its presentation.
Fig. 3. Number of lever-directed nibbles and speed before reaching the lever in both groups on sessions 1, 8, and 24.
Nibbles: during acquisition (d1-8), performance increased, except in the initially low uncertainty group for the distal lever. Between days 8-24, nibbling slightly decreased in the initially high uncertainty group and slightly increased in the initially low uncertainty group, but a significant group difference was maintained for the PS and distal levers on day 24. Speed: during acquisition (d1-8), speed increased, except in the initially low uncertainty group for the PE and distal levers. Between days 8-24, speed slightly decreased in the initially high uncertainty group (but strongly with respect to the PS lever, p = 0.001) and slightly increased in the initially low uncertainty group. No group difference was shown on day 24. Legends: * p < 0.05, ** p < 0.01, *** p < 0.001.
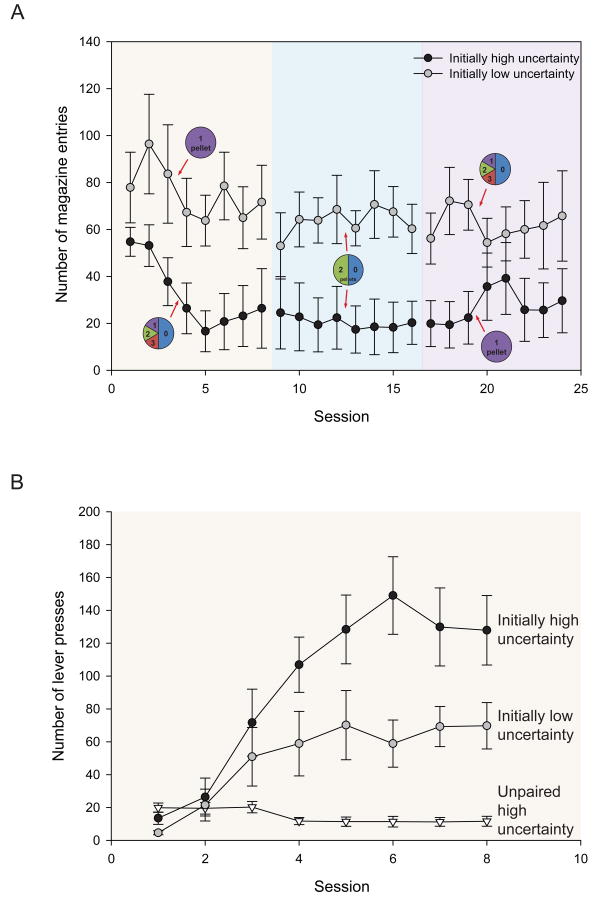
In contrast, the animals initially exposed to low uncertainty conditions focused more of their attention on the CS dish (Fig. 4A), exhibiting a larger number of head intromissions (Group: F(1,14) = 5.287, p = 0.037; but no effect of Day and no Group × Day interaction: F(1,14)'s ≤ 1.683, p's ≥ 0.215), where UCS pellets were delivered reliably after each cue encounter. A video analysis comparison between training days 1 and 8 indicated that these animals reached the magazine faster and faster during presentation of the CS+s (F(2,6)'s ≥ 12.638, p's ≤ 0.007), presumably because they were more attracted by the CS dish relative to CS+ levers, whereas animals exposed initially to high uncertainty conditions took increasingly more time to reach the CS dish during the lever presentation across training days (F(2,6)'s ≥ 75.734, p's < 0.001), by contrast to their faster approach to CS+ levers. In addition, responses on the control lever increased moderately across the first 8 days of training but showed no difference between the two groups (Group: F(1,14) = 0.289, p = 0.599; Day: F(7,98) = 2.592, p = 0.017; but no Group × Day interaction: F(7,98) = 1.263, p = 0.277; mean = 12.25 and 14.64 responses for low and high uncertainty respectively).
Fig. 4. Number of magazine entries in both groups of rats and the impact of unpaired high uncertainty on lever pressing.
A: Magazine entries during the presentation of all CS+ levers across varying levels of reward uncertainty. The initially high uncertainty group started with high uncertainty (d1-8) and ended with low uncertainty (d17-24), while the initially low uncertainty group started with low uncertainty (d1-8) and ended with high uncertainty (d17-24). Circles represent reward contingencies in each training block. B: Lever presses for all CS+ levers across varying levels of reward uncertainty, including a group of animals exposed to high uncertainty conditions, but where CS+ presentations were unpaired from UCS delivery.
In contrast to animals in both the initially high and low uncertainty groups, animals undergoing pseudo-conditioning, consisting of unpaired (yet highly uncertain) CS+ and UCS presentations, showed a decrease in lever pressing across 8 sessions (F(1,7) = 5.947, p = 0.000; Fig 4B), which was significantly lower than that of either of the paired groups (F(1,14)'s ≥ 8.484, p's ≤ 0.011). There was also no effect of the CS presentation on the number of magazine entries, which remained elevated – 600-900 per session – across the 8 days (F(1,7) = 2.871, p = 0.134). These results suggest that uncertainty alone does not produce enhanced attribution of incentive salience if the cue and reward are unpaired.
2.2.2. Uncertainty broadens the scope of incentive salience attribution: recruitment of distal cues (days 1-8)
Initially low uncertainty conditions meant that animals ignored the distal cue (DS lever on opposite wall, approximately 30 cm from dish) in favor of the cues located more proximally to the goal dish (on same wall as dish, 4 cm away). On the first day of training, animals exposed to low uncertainty conditions only came in contact with the DS lever on half (58%) of all cue presentations, and came to mostly ignore it, coming in contact on only 1/3 (36%) of all presentations after 8 days of training (F(1,7) = 4.314, p = 0.076). In contrast, initially high uncertainty conditions recruited the DS lever from the very first day and had animals running to contact it on more than 75% of all cue presentations, increasing to more than 80% with 8 days of training. The impact of high uncertainty on higher recruitment of distal cues could be seen after only one day of training, at which point the number of presses of the distal lever, was already significantly higher in the group of initially high uncertainty compared with the group of initially low uncertainty (t14 = 3.279, p = 0.005; Fig. 2, bottom part), but equally high between the two groups for the proximal (PS and PE) levers (PS: t14 = 0.592, p = 0.563; PE: t14 = 1.163, p = 0.264). Video analysis indicated that rats exposed to only one session of high uncertainty also more often came in contact with the distal lever (t14 = 2.203, p = 0.045). Yet this did not reflect any initial individual differences prior to learning, since there was no difference in the amount of lever contact during the first five CS+ presentations (t8 = 1.768, p = 0.115), but rather the level of attraction grew across the dozen trials of the first training session (last five CSs: t8 = 2.449, p = 0.040). In other words, one session of 40 trials under low or high uncertainty conditions was sufficient to induce differential responding, at least for the most distal CS+.
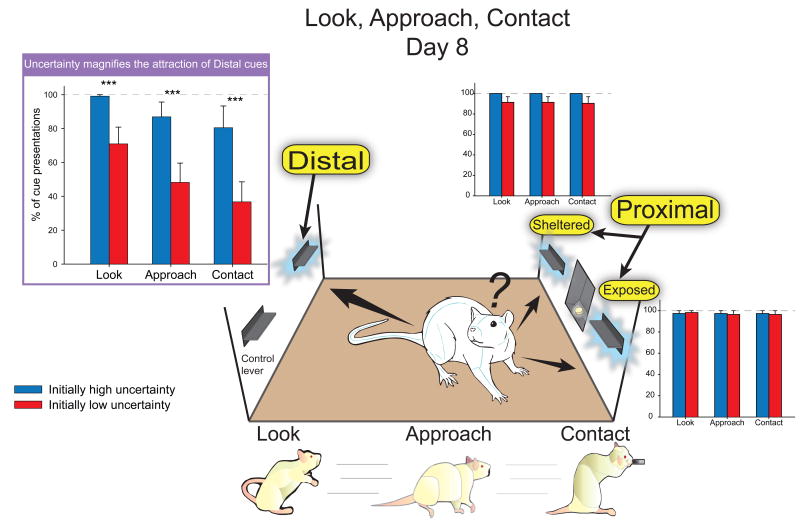
The increased attraction for the distal lever cue seen on the first day of training in the initially high uncertainty group only grew over the course of the following 7 days of high uncertainty exposure, leading to more attention (looks, approaches and contacts) being directed towards the distal lever than in the initially low uncertainty group (look: F(1,42) = 16.991, p = 0.000; approach: F(1,42) = 17.579, p = 0.000; contact: F(1,42) = 15.693, p = 0.000; Fig. 5). Rats exposed to high uncertainty for 8 days displayed an increase in the speed to reach the distal lever (F(1,42) = 18.097, p = 0.000; see Fig. 3) and an increase in the number of distal lever-directed nibbles (F(1,42) = 9.514, p = 0.003), leading to a significantly greater number of presses for the distal lever on day 8 compared to the rats trained under low uncertainty (F(1,42) = 12.785, p = 0.001). In contrast, high uncertainty significantly reduced the attraction (looks, approaches and contacts) directed at the CS goal dish only when the distal lever was presented (look: F(1,42) = 6.64, p = 0.013; approach: F(1,42) = 6.682, p = 0.013; contact: F(1,42) = 6.191, p = 0.017).
Fig. 5. Number of looks, approaches, and contacts directed to the PS, PE, and DS levers on day 8.
Those behaviors were significantly more frequent in the initially high uncertainty group compared with the initially low uncertainty group only relative to the distal lever. It is interesting to note that, though non-significant, rats in both groups had a tendency to decrease the number of behaviors emitted, as they required more effort. On average, looks > approaches > contacts. Legend: *** p < 0.001.
Initial exposure to high uncertainty conditions effectively rendered distal cues as attractive as proximal cues, in contrast to animals initially exposed to low uncertainty conditions that greatly favored proximal cues and tended to mostly ignore the distal cue. When proximal and distal cues were explicitly compared, rats exposed to low uncertainty were naturally more drawn to proximal cues (F(1,23) = 7.135, p = 0.014), whereas exposure to eight days of high uncertainty enhanced the attraction of distal cues to that of proximal cues, making them equally as attractive (F(1,23) = 0.067, p = 0.797). In addition, the differences seen between the two groups after 8 days of training cannot be attributed simply to differences in the distribution of sign-trackers and goal-trackers in each group. Presentations of the PS lever led to predominantly sign-tracking rather than goal-tracking responses in both groups: 62.5% (5 sign-trackers; 3 intermediates) in the initially low uncertainty group versus 100% (8/8 sign-trackers) in the initially high uncertainty group (t14 = 1.040, p = 0.316). In contrast, presentation of the distal lever led to predominantly sign-tracking only in the high uncertainty group (t14 = 2.331, p = 0.035; high uncertainty: 6 sign-trackers; 2 goal-trackers; low uncertainty: 2 sign-trackers; 1 intermediate; 5 goal-trackers).
2.2.3. Uncertainty broadens the scope of incentive salience attribution: recruitment of exposed cues (days 1-8)
Beyond uncertainty recruiting and increasing incentive salience attributed to (distal) cues that are otherwise unattractive, repeated exposure to highly uncertain conditions also appeared to bias the attraction of animals towards ‘riskier’ (or more exposed) cues in comparison to ‘safer’ (or more sheltered) cues. Levers nearer the entry door were closer to where the experimenter's hand enters the chamber at the end of the session (exposed), whereas levers at the inner end were more sheltered and further away from the outer room and approaching human hands. Here we saw a significant interaction between Group (low uncertainty vs high uncertainty) × CS lever (exposed vs sheltered) on day 8, which indicated there was a difference between the groups' preference for either the exposed proximal versus the sheltered proximal lever (Interaction: F(1,14) = 8.877, p = 0.010), while the two groups were not different in total lever pressing on proximal levers overall (F(1,14) = 1.618, p = 0.224). That is, the initially high uncertainty group preferred the exposed cue (t(1,7) = 2.359, p = 0.050), as shown in Figure 2 (bottom part) on day 8, where high uncertainty almost doubled (195% increase) the number of presses for the exposed proximal lever compared to low uncertainty (F(1,42) = 18.737, p = 0.000). By contrast, the initially low uncertainty group did not have a significant preference for either lever, and instead trended toward the sheltered lever with 145% more responding for the sheltered cue (t(1,7) = 1.811, p = 0.113). This suggests that animals being initially exposed to high-level uncertainty in reward are more likely to recruit cues that are both far away from the reward (distal) and nearer to perceived danger (exposed) as targets for incentive salience attribution.
Overall these results suggest that across the first eight sessions, high reward uncertainty (location, probability, and magnitude combined) enhanced the incentive salience of all CS+ cues – whether distal, exposed or sheltered. In contrast, animals exposed to low reward uncertainty (location only) tended to ignore distal cues (and to a lesser extent exposed proximal cues) altogether in favor of directing their attention towards the proximal sheltered CS+ and the CS goal dish. These uncertainty effects transformed a broader spectrum of cues into motivational magnets, raised the attraction intensity of all motivational magnets, and support the hypothesis of a motivational enhancement effect of uncertainty in reward.
2.2.4. The enduring effects of uncertainty: incentive persistence (days 9-24)
In a second round of training, beginning on day 9, uncertainty conditions began to reverse. All rats were shifted to moderate uncertainty (location and probability, not magnitude) for 8 consecutive days, regardless of their initial condition. Effectively this was a reduction in uncertainty for the initially high uncertainty rats, and an increase in uncertainty for the initially low uncertainty rats. In moderate uncertainty conditions, half of all cue presentations were followed by the delivery of two sugar pellets, while the other half went unrewarded. Finally, during a third block of training, conditions were fully reversed (relative to the first training block). The initially high uncertainty group was finally exposed to 8 days of low uncertainty. Conversely, the initially low uncertainty group was exposed to high uncertainty. The goal of this full reversal manipulation was to determine how enduring was the enhancement of incentive salience induced by an early exposure to high uncertainty when uncertainty conditions subsequently reversed gradually but completely. For this reason, the two training blocks (between days 9-16 and between days 17-24) will not be discussed separately; we are only interested in the evolution of performance between days 8 and 24 – i.e., before and after rats have experienced a reversal in the training conditions.
On day 24, rats initially exposed to high uncertainty still showed a greater attraction to lever cues (F(1,47) = 5.054, p = 0.029) – that is, after 8 days of low uncertainty training – than rats initially exposed to low uncertainty, which had now been exposed to 8 days of high uncertainty. There was also no decrement in the total number of lever presses for the initially high uncertainty group – now under low uncertainty – as a result of exposure to decreasingly uncertain conditions from day 8 and day 24 on any of the three levers (F(1,7)'s ≤ 0.25, p's ≥ 0.632). If anything, responses on the sheltered lever almost showed a significant increase (F(1,7) = 5.184, p = 0.057). Similarly, there was no increase in the number of lever presses on any of the three levers for the initially low uncertainty group – now under high uncertainty – as a result of exposure to increasingly uncertain conditions from day 8 to day 24 (F(1,7)'s ≤ 1.548, p's ≥ 0.254). The initially high uncertainty group did however show a decrease in the speed to reach all three levers (F(1,23) = 14.895, p = 0.001) as a result of the change in conditions, suggesting a slight reduction in motivation. Whereas exposure to high uncertainty produced a just significant increase in the number of sniffs directed at the levers (F(1,23) = 4.264, p = 0.050), suggesting a possible ramping up in motivation.
In addition, the distal lever remained a more powerful motivational magnet for the group initially exposed to high uncertainty, than for the group initially exposed to low uncertainty but now highly uncertain. In other words, the enhancement induced by initial high uncertainty was persistent, and initial exposure proved enduringly more potent than later exposure at enhancing incentive salience. The distal lever elicited more looks and approaches (F(1,42)'s ≥ 7.554, p's ≤ 0.009), more nibbles (F(1,42) = 4.885, p = 0.032), and more lever presses (F(1,42) = 7.329, p = 0.01) from the initially high uncertainty group than from the initially low uncertainty group on day 24 – i.e., when the uncertainty conditions were reversed. In contrast, the initially low uncertainty group showed no increase in attraction towards the distal cue in the form of either look, approach, or contact (F(1,7)'s ≤ 0.839, p's ≥ 0.390), or lever presses (F(1,7) = 1.548, p = 0.254).
Approaches and presses on the proximal levers did not differ (F(1,42)'s ≤ 2.588, p's ≥ 0.115) – as was already the case with the sheltered proximal lever on day 8, although the number of nibbles directed at the sheltered proximal lever was larger in the initially high uncertainty group (F(1,42) = 23.103, p = 0.000). The lack of observed difference seen between the two groups on the proximal levers may be due to a ceiling effect, whereby both groups were approaching and contacting both proximal levers on >95% of trials.
As one final comparison, we compared each group after they were respectively exposed to 8 consecutive days of high uncertainty – i.e. day 8 for the group of initially high uncertainty and day 24 for the group of initially low uncertainty. The initially high uncertainty group again showed dramatically higher motivation than the initially low uncertainty group (F(1,42)'s ≥ 6.189, p's ≤ 0.017) – i.e. 2.3 times more nibbles on and 2.1 times faster to approach the distal lever when both were tested under high uncertainty conditions. In addition, the distal lever cue generated far more attraction when animals were exposed to high uncertainty early on (look: F(1,42) = 58.295, p = 0.000; approach: F(1,42) = 19.131, p = 0.000; contact: F(1,42) = 9.995, p = 0.003; presses: F(1,42) = 4.868, p = 0.033). This evidence lends support to the idea that early exposure to uncertain conditions enduringly enhances the incentive value of discrete predictive cues (particularly distal ones) in a manner that is long lasting and persists even when reward uncertainty is returned back down to low levels.
3. Experiment 2
Experiment 1 suggested that the impact of uncertainty could be particularly felt on distal cues, where a distal lever was more attractive when rats received early exposure to highly uncertain rewards. However it could be argued that location uncertainty for a shifting position of lever CS+ regarding where the cue would be presented next might be responsible for the added attraction garnered by the distal cue. Experiment 2 further examines this relationship between reward uncertainty and the incentive value of a distal lever without the possible confounds of presenting a shifting location.
3.1. Materials and methods
Female Sprague-Dawley rats (N = 24; age: 66-211 days old) were housed in the same conditions as in Experiment 1.
3.1.1. Apparatus
The apparatus used was identical to that presented in Experiment 1 but the front proximal exposed lever acted merely as a control stimulus, while the proximal sheltered lever was never extended. Only a single back right (distal sheltered, DS) lever or back left (distal exposed, DE) lever was presented to rats as CS+ (counterbalanced assignment across rats).
3.1.2. Groups
Rats were randomly separated into two groups of 12 individuals. Each group was characterized in terms of a probability (100% or 50%) and a magnitude (1 or 1-3 pellets) of reward delivery per trial. In the certain group (100%-1), rats received one sucrose pellet for each presentation of a lever, that is, a total of 40 pellets per session. In the uncertain group (50%-1-2-3), rats received, on average, either no pellet with a 50% probability, or 1, 2 or 3 pellets with an equal probability (16.7% for each reward amount) for each lever presentation – i.e. a total of 38-42 pellets per session. Thus those rats were exposed to a combined probability/magnitude reward uncertainty.
3.1.3. Procedure
All the rats were exposed to a daily session of 40 trials for five consecutive days. They were trained with only one distal lever (either DS or DE counterbalanced) paired with either reward certainty (100%-1 group) or reward uncertainty (50%-1-2-3 group).
3.1.4. Statistical analyses
Behavior was analyzed on the basis of computer-recorded information (number of lever presses and of magazine entries), and video recordings on the first and last training sessions (days 1 and 5) were used to determine the number of looks, approaches, and contacts directed to the lever and to the magazine. The statistical tests used here were identical to those used in Experiment 1.
3.2. Results
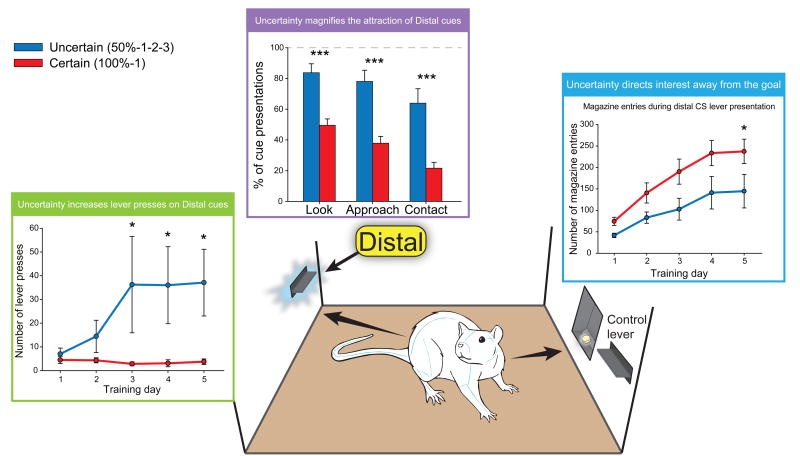
After only three sessions of training under either certain (100%-1) or uncertain (50%-1-2-3) conditions, the uncertain group showed a far greater attraction towards the distal cue in approach and CS+ lever pressing (F(1,110) = 5.798, p = 0.018), which persisted through to the fifth day of training (day 5: F(1,110) = 5.946, p = 0.016). Uncertain rats were 3 times more likely (64% vs. 21.7%) to sign-track and approach and contact the distal lever than the certain rats (look: t(22) = 4.790, p = 0.000; approach: t(22) = 4.734, p = 0.000; contact: t(22) = 4.182, p = 0.000), whereas the 100%-1 certain rats were more likely to goal-track and approach the sucrose dish (look, approach, contact: t(22)'s ≥ 2.479, p's ≤ 0.021). The difference developed gradually over 5 days of training, and did not exist on the first day (F(1,110) = 0.033, p = 0.855; Fig. 6). A comparison of the first and last training days indicated a significant increase in lever pressing within the 50%-1-2-3 uncertain group (F(2, 10) = 4.576, p = 0.039). On average, rats under uncertain conditions pressed the CS+ lever 37 times on day 5 compared to less than 4 times for rats in the certain group, and 25% of the uncertain individuals (3/12) pressed the lever more than 100 times during the last session. By contrast, 100%-1 certain rats showed a significant decrease in the number of lever presses across days 1-5 (F(2,10) = 7.296, p = 0.011). There was however no difference between either group in their attraction for the control lever (F(2,10) = 2.081, p = 0.163; Certain = 17.15 responses, Uncertain = 26.28 responses).
Fig. 6. Uncertainty draws attention towards distal cues and away from the goal.
While both groups were initially similar, the 50%-1-2-3 rats came to press a distal lever more than the 100%-1 rats after only two training days. Accordingly, the 50%-1-2-3 rats looked, approached, and contacted the distal lever more than the 100%-1 rats. However, magazine-directed behaviors (goal-tracking) gradually increased in both groups over the five training sessions, but the increase was significantly higher in the 100%-1 rats than in the 50%-1-2-3 rats. Legends: * p < 0.05, *** p < 0.001.
When examined separately, the animals in the certain group (100%-1) showed a 4.6 fold preference for the sheltered distal (DS) lever over the more exposed distal lever (DE), while this preference for the DS over the DE was only 1.8 for the animals in the uncertain group (50%-1-2-3). At first glance, this might suggest that the certain rats tend to avoid the exposed lever more than the uncertain rats. However, there was no effect of lever type and no Group × Lever type interaction on day 5 (F(1,10)'s ≤ 0.642, p's ≥ 0.442).
As training progressed, although the overall number of dish entries increased for both groups over 5 days (Day: F(4,88) = 2.454, p = 0.052; Group × Day: F(4,88) = 2.938, p = 0.025), the mean number of goal dish entries was greater in the 100%-1 group, but became significantly different from the 50%-1-2-3 group only on day 5 (F(1,22) = 5.575, p = 0.027). Interestingly, on day 5, all 100%-1 certain rats had become strong goal-trackers (12/12). In contrast, goal-trackers represented only 58% (7/12) of the 50%-1-2-3 uncertain group, whereas 25% (3/12) of animals in this group were strong sign-trackers and 17% (2/12) displayed mixed behavior. This indicates that reward uncertainty tends to make rats more attracted to the lever CS+ than they are under reward certainty, even relative to a distal cue.
4. General discussion
Our study first showed that initial exposure to high levels of uncertainty in CS-UCS reward prediction (in location, probability, and magnitude) leads to a greater and more persistent attraction towards discrete predictive cues than a lower level of uncertainty associated with the more absolute certainty of a 100% reliable prediction. First, the enhanced attraction and sign-tracking response to CS+ levers seen after initial exposure to uncertainty endured even when CS-UCS prediction switched to purely 100% certain (although there were signs to suggest a mild decrease in motivation). This suggests that the behavioral consequences of an initial exposure to CS+ reward high uncertainty are relatively long-lasting. Second, we showed that reward uncertainty expands the stimulus domain of incentive candidates to include discrete CS+s that normally would be non-preferred (either because they were too exposed to potentially threatening association with outside humans or because they were too distant physically from UCS reward to be optimal as incentives). Uncertainty recruited those non-preferred exposed and/or distal cues and imbued them with higher levels of incentive salience, so that they too became potent motivational magnets able to elicit rapid approach and intense nibbles, and draw the rat away from the location of the UCS reward itself (goal dish).
4.1. Incentive salience attribution to distal cues
The proximity of the CS+ cue to the goal dish site of reward retrieval seemed to be of importance to the attribution of incentive salience. In general, the opposite-wall distal levers (30 cm away) were interacted with up to 2.5 times less than the proximal same-wall levers (4 cm away). This is likely due to the increased distance of the distal levers from the sucrose dish. It indicates that the proximal levers ordinarily tend to receive more incentive salience than the distal levers (see also [30]), but also that the spatial envelope of incentive salience attribution can be expanded by uncertainty to include discrete CS+ targets further away. This may tend to push an uncertainty-influenced gambler to be drawn in by a greater myriad of cues that surround the gambling experience. The results of our study also indicate that having several different cues appear from different locations (Experiment 1) makes a given distal CS+ lever more ‘wanted’ than when it is presented alone as sole CS+ (Experiment 2), and that the overall attraction of a distal cue is greater when cues are uncertain (Experiment 1 sign-trackers: high uncertainty (75%) vs. low uncertainty (25%)). This suggests that the occurrence of many uncertain cues tend to recruit each other and have a cumulative effect, just as the lights, sounds, and a game's outcome may have in gambling situations.
4.2. ‘Risky’ and ‘safe’ levers
Though rats were never in any danger during autoshaping, they may still have perceived threat or affordances for anxiety, especially related to intrusions from the larger outside world of the laboratory, as might be engendered in an elevated plus maze [31]. The door on one wall of the chamber opened to allow the human researcher to reach in and remove the animal (an occurrence which rats often retreat from), and exposed the animal to the bright lights of the testing room, and a potentially unsafe area (ledge) far above the floor. As such, rats may have associated the exposed side of the chamber with anxiety, relative to the more sheltered and safer recessed side of the chamber. This would make the levers on the exposed side of the cage relatively unattractive compared to levers on the sheltered side of the chamber, and may explain why after 8 days of training the animals under initially certain conditions showed a bias (145% increase in lever presses) in their lever pressing responses towards the proximal sheltered lever over the proximal exposed lever. However the most apparent effect was that initial high uncertainty enhanced the attractiveness of the proximal exposed lever in Experiment 1 (156% more lever presses than for the sheltered lever), and reduced the moderate incentive advantage of the proximal sheltered lever, suggesting that high levels of uncertainty may tend to make ‘riskier’ cues more attractive. Experiment 2 failed to highlight this effect using exposed and sheltered distal levers, perhaps because the overall attractiveness of a distal lever remains modest when compared with that of a proximal lever. As a result, the responses to exposed and sheltered distal levers may appear less contrasted. In other words, uncertainty seems to produce a relative increase in the value of ‘riskier’ cues to the point that animals are willing to put themselves into perceived danger to interact with them.
Overall, the recruitment of non-preferred exposed/distal cues suggests a possible slant towards riskier behavior, or at least towards riskier CSs. These results bring additional support to the idea that reward uncertainty is a source of motivation in animals and humans, which may help account for the powerful attraction seen in gambling [19,20,23-25].
4.3. Initial learning is powerfully persistent
The increased attraction (seen as sign-tracking responses) bestowed upon a CS+ lever cue by uncertainty was largely influenced by the first experiences with the CS-UCS relationship. Rats exposed to initially high uncertainty still demonstrated higher sign-tracking than rats exposed to initially low uncertainty even after their conditions were reversed. This result seems consistent with evidence that early exposure to an unpredictable environment helps lead to subsequent susceptibility to gambling [32] and that early gambling behaviors are predictive of later gambling behavior [33]. Animals who experience for the first time the sucrose pellets under highly uncertain conditions of prediction might develop a persistently greater interest in the CS+ than animals that are initially exposed to the sucrose pellets with a more certain delivery. It also might be relevant to note that rats reared in isolation develop a stronger propensity to sign-track [34] and to prefer uncertain, suboptimal rewards in a dual-choice test [35] compared with individuals reared in socially enriched environments.
The effects of initial exposure to uncertainty persisted even when conditions changed to more certain prediction in a manner that resembles the enduring pattern of incentive sensitization. The process of incentive sensitization denotes the fact that repeated drug consumption induces persistent neuroadaptations of the dopaminergic systems that progressively sensitize an individual to the drug as well as to Pavlovian incentive cues associated with drug taking [36]. The result is specifically higher ‘wanting’ triggered by drug-associated CS+s. In our experiments, no drugs were used but it seems interesting to note similarities in behavioral patterns resulting from repeated drug consumption and repeated exposure to early uncertainty.
According to incentive-sensitization theory, mesolimbic dopamine elevation helps transform ‘cold’ addiction-related stimuli into more ‘wanted’ incentives. The suggestion we raise here is that Pavlovian uncertainty might recruit brain mesolimbic systems to enduringly become hyper-reactive to cues in ways that resemble sensitization of the brain reward systems seen in drug addiction (more below) [37-39]. This may also interact with other routes to produce mesolimbic sensitization. For example, stress-induced behavioral sensitization has been shown to increase vulnerability to acquisition of amphetamine self-administration [40]. If uncertainty caused or coincided with a stress response, this might induce a long-lasting sensitization increasing the incentive salience of the CS+ levers. Consistent with this suggestion, it has been shown that in autoshaping, sign-trackers release more dopamine, and possess higher corticosterone levels, than goal-trackers [41,42], and microinjections of corticotropin-releasing factor – a stress-related neurotransmitter – into the shell region of the nucleus accumbens increase cue-triggered motivation for sucrose reward in a similar way to amphetamine [43].
4.4. Uncertainty and dopamine
Evidence suggests that presentation of a CS+ cue elicits greater release of mesolimbic dopamine and recruits subcortical brain regions to a larger extent in sign-trackers, rather than goal-trackers who preferentially engage the goal dish [5,6,44]. Thus sign-tracking is a reliable way of indexing incentive motivation (or ‘wanting’), a process influenced by dopamine levels in the nucleus accumbens [9,45-47].
The dramatic increase in sign-tracking and attraction to all cues raises the possibility here that early exposure to high uncertainty conditions may lead to more CS+-related dopamine release in the mesolimbic pathway compared to low uncertainty conditions of perfect prediction. Several studies have highlighted the role of midbrain dopamine in coding reward uncertainty in primates [37,48], healthy humans [49,50], and pathological gamblers [27]. These studies suggest that more dopamine can be released when the event's probability of occurrence is more uncertain (is or is close to 50%) for a two-outcome event than when its probability is less uncertain. More precisely, electrophysiological recordings show sustained activation of midbrain dopamine neurons from the onset of a CS to the expected time of reward delivery provided that the probability of reward is 50% [37]. Conversely, reward uncertainty could also require the activation of additional non-dopaminergic neurons. In this respect, Monosov and Hikosaka [51] found neurons in the primate anterodorsal septal region that are sensitive to uncertain rewards, but not uncertain punishments.
4.5. Conclusion
This study brings additional support to the notion that reward uncertainty can spur cue-triggered incentive motivation in many species, including rats and humans. Our results strongly suggest that uncertainty magnifies the incentive salience attributed to a discrete CS+ for reward, underlying sign-tracking in rats. The pattern of motivation enhancement may implicate a mesolimbic dopamine mechanism. We think that the processes underpinning enhanced motivation of rats may be related to those recruited by the uncertainty about sounds, lights, and game's outcome at casinos, which contributes to the compulsiveness of pathological gambling in humans.
Highlights.
Reward uncertainty elevates the attraction of multiple predictive cues
Reward uncertainty also recruits non-preferred exposed and distal cues
Incentive salience attribution remains persistently enhanced by initial uncertainty
Uncertainty directs motivation towards cues and away from reward itself
Acknowledgments
These studies were made possible by NIH grants (DA015188 and MH63649) to K.C.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dow SchüllN. Addiction by Design: Machine Gambling in Las Vegas. First. Princeton, NJ: Princeton University Press; 2012. [Google Scholar]
- 2.Costikyan G. Uncertainty in Games. MIT Press; 2013. [Google Scholar]
- 3.Ashrafioun L, McCarthy A, Rosenberg H. Assessing the impact of cue exposure on craving to gamble in university students. J Gambl Stud. 2012;28:363–75. doi: 10.1007/s10899-011-9262-0. [DOI] [PubMed] [Google Scholar]
- 4.Boakes RA. Performance on Learning to Associate a Stimulus with Positive Reinforcement. In: Davis H, Hurvitz HMB, editors. Operant Pavlovian Interactions, vol Book Chapter. Hillsdale, N.J: Erlbaum Associates; 1977. pp. 67–97. [Google Scholar]
- 5.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 6.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying Individual Variation in the Propensity to Attribute Incentive Salience to Reward Cues. PLoS ONE. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TE, Flagel SB. Dissociating the Predictive and Incentive Motivational Properties of Reward-Related Cues Through the Study of Individual Differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20:505–35. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 11.Rescorla RA, Wagner AR. A theory of pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 12.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Ward RD, Gallistel CR, Jensen G, Richards VL, Fairhurst S, Balsam PD. Conditioned [corrected] stimulus informativeness governs conditioned stimulus-unconditioned stimulus associability. J Exp Psychol Anim Behav Process. 2012;38:217–32. doi: 10.1037/a0027621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Duuren E, van der Plasse G, Lankelma J, Joosten RNJMA, Feenstra MGP, Pennartz CMA. Single-cell and population coding of expected reward probability in the orbitofrontal cortex of the rat. Journal of Neuroscience. 2009;29:8965–76. doi: 10.1523/JNEUROSCI.0005-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–34. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MJF, Berridge KC. Instant Transformation of Learned Repulsion into Motivational “Wanting”. Current Biology. 2013;23:282–9. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davison MC. Preference for mixed-interval versus fixed-interval schedules. J Exp Anal Behav. 1969;12:247–52. doi: 10.1901/jeab.1969.12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins L, Young DB, Davies K, Pearce JM. The influence of partial reinforcement on serial autoshaping with pigeons. Q J Exp Psychol B. 1983;35:275–90. doi: 10.1080/14640748308400893. [DOI] [PubMed] [Google Scholar]
- 20.Forkman BA. The Effect of Uncertainty on the Food Intake of the Mongolian Gerbil. Behaviour. 1993;124:197–206. [Google Scholar]
- 21.Hurly T, Oseen M. Context-dependent, risk-sensitive foraging preferences in wild rufous hummingbirds. 1999;58:59–66. doi: 10.1006/anbe.1999.1130. [DOI] [PubMed] [Google Scholar]
- 22.Adriani W, Laviola G. Delay aversion but preference for large and rare rewards in two choice tasks: implications for the measurement of self-control parameters. BMC Neurosci. 2006;7:52. doi: 10.1186/1471-2202-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagner JP, Zentall TR. Suboptimal choice behavior by pigeons. Psychonomic Bulletin & Review. 2010;17:412–6. doi: 10.3758/PBR.17.3.412. [DOI] [PubMed] [Google Scholar]
- 24.Molet M, Miller HC, Laude JR, Kirk C, Manning B, Zentall TR. Decision making by humans in a behavioral task: do humans, like pigeons, show suboptimal choice? Learn Behav. 2012;40:439–47. doi: 10.3758/s13420-012-0065-7. [DOI] [PubMed] [Google Scholar]
- 25.Anselme P, Robinson MJF, Berridge KC. Reward uncertainty enhances incentive salience attribution as sign-tracking. Behav Brain Res. 2013;238:53–61. doi: 10.1016/j.bbr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. Journal of Neuroscience. 2012;32:2886–99. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, Gjedde A. Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Res. 2012;204:55–60. doi: 10.1016/j.pscychresns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60:1992–9. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Linnet J. The Iowa Gambling Task and the three fallacies of dopamine in gambling disorder. Front Psychol. 2013;4:709. doi: 10.3389/fpsyg.2013.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva FJ, Silva KM, Pear JJ. Sign- versus goal-tracking: effects of conditioned-stimulus-to-unconditioned-stimulus distance. J Exp Anal Behav. 1992;57:17–31. doi: 10.1901/jeab.1992.57-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haller J, Alicki M. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr Opin Psychiatry. 2012;25:59–64. doi: 10.1097/YCO.0b013e32834de34f. [DOI] [PubMed] [Google Scholar]
- 32.Scherrer JF, Xian H, Kapp JMK, Waterman B, Shah KR, Volberg R, et al. Association between exposure to childhood and lifetime traumatic events and lifetime pathological gambling in a twin cohort. J Nerv Ment Dis. 2007;195:72–8. doi: 10.1097/01.nmd.0000252384.20382.e9. [DOI] [PubMed] [Google Scholar]
- 33.Braverman J, Shaffer HJ. How do gamblers start gambling: identifying behavioural markers for high-risk internet gambling. Eur J Public Health. 2012;22:273–8. doi: 10.1093/eurpub/ckp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–9. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Pattison KF, Laude JR, Zentall TR. Environmental enrichment affects suboptimal, risky, gambling-like choice by pigeons. Animal Behavior. 2013;16:429–34. doi: 10.1007/s10071-012-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 37.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 38.Fiorillo CD. Transient activation of midbrain dopamine neurons by reward risk. Neuroscience. 2011;197:162–71. doi: 10.1016/j.neuroscience.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer BF, Scott-Railton J, Vezina P. Unpredictable saccharin reinforcement enhances locomotor responding to amphetamine. Behav Brain Res. 2012;226:340–4. doi: 10.1016/j.bbr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–6. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- 41.Tomie A, Tirado AD, Yu L, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behav Brain Res. 2004;153:97–105. doi: 10.1016/j.bbr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peciña S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 47.Saunders BT, Yager LM, Robinson TE. Cue-Evoked Cocaine “Craving”: Role of Dopamine in the Accumbens Core. Journal of Neuroscience. 2013;33:13989–4000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lafuente V, Romo R. Dopamine neurons code subjective sensory experience and uncertainty of perceptual decisions. Proc Natl Acad Sci USA. 2011;108:19767–71. doi: 10.1073/pnas.1117636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J Neurophysiol. 2004;92:1144–52. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- 50.Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–90. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Monosov IE, Hikosaka O. Selective and graded coding of reward uncertainty by neurons in the primate anterodorsal septal region. Nat Neurosci. 2013;16:756–62. doi: 10.1038/nn.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]