Abstract
Objective
To assess for the presence of cognitive impairment and depression in aging former NFL players, and identify neuroimaging correlates of these dysfunctions.
Design
Comparison of aging NFL players with cognitive impairment and depression to those without these dysfunctions and with matched healthy controls
Setting
Research center in the North Texas region of the United States.
Patients
We performed a cross-sectional study of retired professional football players with and without a history of concussion recruited from the North Texas region, along with age-, education-, and IQ-matched controls. We studied thirty-four retired NFL players (mean age 62) neurologically and neuropsychologically. A subset of 26 also underwent detailed neuroimaging; imaging data in this subset were compared to imaging data acquired in 26 healthy matched controls.
Main Outcome Measures
Neuropsychological measures, clinical diagnoses of depression, neuroimaging measures of white matter pathology, and a measure of cerebral blood flow (CBF).
Results
Of the 34 participants, 20 were cognitively normal, 4 were diagnosed with a fixed cognitive deficit, 8 with Mild Cognitive Impairment, and 2 with dementia; 8 were diagnosed with depression. Of the subgroup in which neuroimaging data were acquired, cognitively impaired (CI) participants showed greatest deficits on tests of naming, word finding, and visual/verbal episodic memory. We found significant differences in white matter abnormalities in CI players and depressed players compared to their respective controls. Regional blood flow differences in the CI group (left temporal pole, inferior parietal lobule, superior temporal gyrus) corresponded to regions associated with impaired neurocognitive performance (problems with memory, naming and word finding).
Conclusions
Cognitive deficits and depression appear to be more common in aging NFL players compared to controls. These deficits are correlated with white matter abnormalities and changes in regional CBF.
Keywords: concussion, memory, depression, cognition, NFL, white matter, DTI, ASL, aging
INTRODUCTION
There has been considerable interest in the neurobehavioral changes, both in terms of cognitive and mood related, that occur in retired National Football League (NFL) players, but despite increased media attention, little has been done to systematically examine later-life functioning in retired professional athletes. Neurocognitive assessments in athletes and non-athletes in the short-term following concussion have demonstrated deficits in working memory, attention, and processing speed.1–3 There is wide variation in the persistence of these defects, with the majority of individuals showing good recovery within days, weeks or months following injury and a small minority showing persistent deficits.4–7
A University of Michigan self-report symptom survey of retired NFL players noted that for men between the ages of 30–49, 0.1% of U.S. men reported memory problems, compared with 1.9% of retired players.8 Over age 56, this number increased to 1.2% in the general population, but to 6.1% among retired players. Among aging, retired NFL players, 11% have reported having depression with a correlation between history of recurrent concussions and a lifetime history of depression.9 Traumatic brain injury has been noted as a potential risk factor for neurodegenerative diseases including Alzheimer’s Disease (AD).10 An association between recurrent concussion and Mild Cognitive Impairment (MCI) and reported memory impairments has also been shown in retired NFL players, with a fivefold increase in MCI diagnosis and a threefold greater prevalence of reported memory problems compared with retirees without a history of concussion. Furthermore, an earlier onset of AD was found in these players compared with the general population.11 Unfortunately, these statistics were based upon responses to a questionnaire about subjective memory difficulties, without further detail, corresponding examination, or neurocognitive testing to assess for impairments or establish formal diagnoses. Another syndrome, Chronic Traumatic Encephalopathy (CTE), is emerging as a putative clinical diagnosis. CTE is described as occurring in individuals who have experienced previous brain injuries and is behaviorally manifested by changes in personality/mood (depression, apathy, suicidality), cognition, and movement. At this point, CTE remains a pathological diagnosis, as clinical diagnostic criteria have not yet been established.12
Examination of autopsy specimens from retired players who committed suicide was demonstrated most recently by McKee and colleagues13 and Omalu et al. (2011).14 CTE is characterized by extensive tau-immunoreactive neurofibrillary tangles and marked accumulation of tau-immunoreactive astrocytes, without the accumulation of beta amyloid that is characteristic of AD. Further evaluation has shown that in a subset of cases, a TDP-43 proteinopathy throughout the frontal and temporal lobe cortical regions and diencephalon is found, with several subjects in the initial sample (n=3) also developing a progressive motor neuron disease (e.g., like ALS) exemplified by progressive weakness and TDP-43 inclusions in the spinal cord.15 These findings suggest that examination is warranted for UMN and LMN findings in these players as they age. In addition, a history of more severe traumatic brain injury has also been linked to the development of AD later in life.16
Neuroimaging techniques can help clarify the brain changes that underpin neurobehavioral dysfunction. For example, Diffusion Tensor Imaging (DTI)17 has shown damage to white matter tracts in boxers with post-concussive injury.18 Magnetic Resonance Spectroscopy (MRS), which measures brain metabolites, was performed in 40 athletes post-concussion, showing alterations of brain metabolites.19 When those data were compared with neuropsychological measures, MRS was found to be sensitive to cognitive impairments.20 However, the optimal use of neuroimaging in assessing brain status in this setting is in combination with neurocognitive measures, so that the findings from each set of investigative techniques can inform the interpretation of the other.21,22
We assessed neurobehavioral, neurological, and neuroimaging markers in a sample of aging former NFL players. To control for the neuroimaging measures used that are sensitive to age (DTI) and history of previous random lesions (ASL), we recruited a matched healthy control group (age-, education- and IQ-matched. We hypothesized that neurobehavioral disorders in former professional (American-style) football players would be present regardless of concussion history and that structural brain abnormalities would be associated with observed neurobehavioral deficits.
METHODS
SUBJECTS AND DIAGNOSTIC CRITERIA
Our cross-sectional sample of participants was recruited after presentations of the study at a local gathering of retired NFL players living in the North Texas region, a meeting of the NFL Players Association local chapter, local advertising, and word of mouth amongst retired NFL players. In an attempt to obtain a reasonable cross-section of retired athletes, we included all 34 retired NFL athletes who inquired about the study, regardless of whether they reported cognitive symptoms or not. Each player received a complete neurologic and neuropsychologic evaluation. From this information, we made clinical assessments of the athletes’ cognitive status to identify them as normal or cognitively impaired, and if impaired, to determine their diagnosis. Participants ranged in age from 41 to 79, with a mean of 61.8 (95% CI, 57.8–65.7). Their NFL/professional football experience ranged from 2 to 15 years, with a mean of 9.7 years (95% CI, 8.4–10.9). Twenty-eight were active businessmen and six were retired. Twenty-three were Caucasian and 11 were African American. Mean education level was 16.3 years (95% CI, 16.0–16.5). Three were left-handed. Eighteen played offense and 16 played defense. Twenty-nine players exercised regularly (at least 3x/week). Subjects had undergone on average 4.5 surgeries with general anesthesia over their lifespan.
Concussion history was obtained retrospectively from participants and informants, and classified using the AAN Practice Parameter guidelines for grading concussion (1997).23 Players reported a lifetime history of concussions ranging from just a few seconds of confusion to more severe, with loss of consciousness for several hours. All but two of the 34 players had sustained at least one concussion (range = 1–13) with an average of 4.0 concussions over their lifespan (mean numbers = 2.0 Grade 1, 0.5 Grade 2, and 1.6 Grade 3 concussions per participant). Twenty-six players completed the neuroimaging studies; eight were claustrophobic and did not undergo MRI imaging.
We also screened 85 healthy control participants from normal aging studies at the Center for BrainHealth at the University of Texas at Dallas. Control participants with known concussions were excluded, as were those with a history of playing college or professional football, reported cognitive complaints, or neurologic or psychiatric disorder. Healthy control participants were matched on age as well as estimated IQ and educational background to the retired NFL players who participated in the neuroimaging studies.
Subjects in the control group ranged in age from 41–79 with a mean of 60.1 years (95% CI, 54.6–64.1) and 16.2 mean years of education (95% CI, 16.0–16.7). Two were left-handed, 24 were Caucasian, and two were African American. Five were retired, and 21 were active businessmen. For the 26 control participants, we gathered full clinical, neuropsychological and neuroimaging data. All participants gave written informed consent in accordance with the Institutional Review Board of the University of Texas Southwestern Medical Center and University of Texas at Dallas.
NEUROCOGNITIVE TESTS
The neuropsychological measures included the following (organized by domain of cognitive function): a) General Intelligence: The Wechsler Adult Scale of Intelligence (WASI),24 with estimated IQ calculated from Vocabulary and Matrix Reasoning subtests, b) Attention and Cognitive Flexibility: Trail Making Test parts A and B,25 and the Digit Span subtest from the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV),26 c) Processing Speed: Coding subtest of the WAIS-IV, d) Language: Category Fluency,27 Controlled Oral Word Association Test (COWAT),28,29 and Boston Naming Test (BNT),30 e) Visuospatial Skills: Rey-Osterrieth Complex Figure Test (Copy and Memory components),31 f) Episodic and Semantic Memory: California Verbal Learning Test-II (CVLT-II),32 and the Semantic Object Retrieval Test (SORT).33 The latter test results comprised two components, one score for semantic memory retrieval and another for name production (word finding), and g) Mood: Beck Depression Inventory-II (BDI-II).34
All testing was performed by a trained neuropsychological technician or neuropsychologist. Clinical diagnoses were rendered by the cognitive neurologist (J.H.) following a review of each participant’s history, neurobehavioral status examination, and neurologic examination. Next, neuropsychological scores for each subject were interpreted by two neuropsychologists, blind to identity, history and other data, to determine the presence, level and pattern of impairment, and a consensus was reached in terms of the presence and severity of cognitive deficit.
One-way between-participants ANOVAs were conducted to evaluate differences on all cognitive measures between all three groups: 1) controls and 2) NFL players with and 3) NFL players without cognitive impairment. Significant findings were followed by a Tukey Honest Significant Difference (HSD) post-hoc test. Pearson correlations assessed the relationship between neuropsychological test results with concussion history and years played in the NFL. Fourteen comparisons were made between cognitive measures (Table 1) with number of concussions and number of years played. To protect against inflated Type 1 error and adjust for multiple comparisons, Bonferroni correction was used with the p-value set at p < ·004.
Table 1.
Scores with 95% confidence intervals on all Cognitive Measures by NFL Cognitively Impaired and Intact Players and Control Group
| Healthy CTRL N = 26 |
NFL Not Impaired N = 12 |
NFL CI N = 10 |
F value |
p value |
|
|---|---|---|---|---|---|
| Age | 60.1 (55.6–64.6) | 55.42 (47.2–63.4) | 66.6 (61.6–71.6) | 2.86 | 0.07 |
| Education | 16.2 (15.3–17.1) | 16.6 (16.0–17.2) | 16.1 (15.6–16.6) | 0.29 | 0.75 |
| Attn, Cognitive Flexibility | |||||
| Trails A T-score | 49.0 (45.8–52.1) | 50.2 (44.2–56.2) | 52.0 (47.8–56.2) | 0.55 | 0.58 |
| Trails B T-score | 54.1 (50.6–57.5) | 51.9 (44.9–58.9) | 46.8 (40.5–53.1) | 2.23 | 0.12 |
| WAIS-IV Digit Span ss | 11.0 (9.6–12.3)d | 9.3 (7.2–11.5) | 10.3 (8.4–12.2) | 1.08 | 0.35 |
| Processing Speed | |||||
| WAIS-IV Coding ss | 11.1 (10.0–12.0)e | 10.5 (9.3–11.8) | 10.5 (9.2–11.8) | 0.41 | 0.67 |
| Language | |||||
| Categ. Fluency T-score | 49.5 (45.5–53.5)c | 49.5 (42.5–56.5) | 42.3 (37.0–47.6) | 2.23 | 0.12 |
| COWAT T-score | 50.2 (46.2–54.1)c | 48.1 (44.1–52.1) | 48.3 (39.7–56.9) | 0.26 | 0.77 |
| BNT T-score | 53.4 (48–57.9)e | 48.6 (42.3–54.9) | 36.2 (29.8–42.6) | 10.72 | <0.001*a,b |
| SORT Naming | 15.5 (15.3–15.8) | 14.6 (13.6–15.5) | 12.6 (10.3–14.9) | 11.01 | <0.001*a,b |
| Visuospatial Skills | |||||
| Rey-O Copy T-score | 50.6 (46.0–55.1)d | 46.3 (38.9–53.8) | 48.7 (42.2–55.1) | 0.65 | 0.53 |
| Rey-O Delay T-score | 58.3 (52.3–64.2)d | 56.6 (48.1–65.0) | 35.5 (28.5–42.5) | 11.91 | <0.001*a,b |
|
Episodic and Semantic Memory |
|||||
| CVLT T-score | 60.2 (57.2–63.2) | 52.1 (46.9–57.3) | 39.6 (32.5–46.7) | 23.24 | <0.001*a,b |
| SORT Total | 30.2 (29.6–30.8) | 29.6 (28.9–30.0) | 27.9 (26.6–29.2) | 5.55 | 0.007 |
CTRL= Controls, CI = Cognitively Impaired, ss=scaled score, CVLT=California Verbal Learning Test, SORT=Semantic Object Retrieval Test;
= significant difference Healthy CTRL and NFL CI,
= significant difference between NFL Players without Cognitive Impairment and NFL CI,
=missing data for one person,
=missing data for 4 people,
=missing data for 5 people. Significance value set at
p <0.004.
Note: 4 patients with diagnoses of depression were not included.
NEUROLOGICAL ASSESSMENT
A neurological history (including questionnaire for post-concussive symptoms), standardized neurological examination (cranial nerves, motor, sensory, coordination, gait, reflexes), and neurobehavioral examination were performed by a cognitive neurologist (J.H.) for each player. The evaluation also included a clinician-administered survey of cognitive symptoms (attention, language, memory, visuospatial skills, executive/cognitive control functions, psychomotor speed) and post-concussive symptoms (Appendix 1).
The neurobehavioral examination included detailed history of mental status including assessment of attitude, behavior, mood/affect, speech, thought process, perceptions, insight, and judgment, which are described in Appendix 2. Clinical psychiatric diagnoses were made by a behavioral neurologist (J.H.) and psychologist (N.D.) using standard DSM-IV-TR criteria in conjunction with clinical mental status examination data and a self-report of depressive symptoms (Beck Depression Inventory-II; BDI).
After blind review and ratings of the neuropsychological information by a board-certified clinical neuropsychologist (M.C.), the neuropsychological data and interpretations were evaluated in conjunction with the neurological evaluation and structural MRI scan to obtain a clinical neurological diagnosis by consensus.
Criteria for neurological diagnosis consisted of DSM-IV criteria for dementia, McKhann et al. (1984)35 criteria for Alzheimer’s disease, and Peterson et al. (1999)36 for Mild Cognitive Impairment (MCI). A diagnosis of ‘fixed cognitive deficit’ was determined using criteria of reported cognitive symptoms following a neurological event (e.g., cerebrovascular accident, traumatic brain injury) that are reported to be static and not progressive (ICD-10 guidelines) and having a corresponding deficit on neurocognitive testing. Neurological examination was also used to assess for amyotrophic lateral sclerosis (ALS), as this has been noted to be more common among retired players in some reports.37
NEUROIMAGING MEASURES
The imaging data were acquired on a 3 Tesla MRI instrument (Philips, Andover, MA).
Fluid Attenuated Inversion Recovery (FLAIR)
FLAIR images were acquired in an oblique axial plane (TI/TR/TE = 2800 ms/11000 ms/150ms) using a field of view (FOV) = 230 × 230 mm and a reconstructed resolution = 0.45 × 0.45 mm2. We acquired 24 - 5 mm thick slices with a 1 mm inter-slice gap. Lesions were defined as exhibiting FLAIR signal intensity greater than 2 sd above the mean. The lesions were then edited manually to remove spurious voxels due to fat signal, motion, edge effect or coil sensitivity inhomogeneity. Total lesion volume was calculated and lesions were manually divided into two groups, deep or periventricular white matter lesions.
Hemosiderin Scan
Imaging parameters for hemosiderin scans that assess for previous bleeding were: Gradient Echo (GE), voxel size = 0.44 × 0.44 × 4 mm3, gap = 1 mm, TR = 753 ms, flip angle 18°, acquisition number = 8, scan duration = 124 s.
Diffusion Tensor Imaging
DTI data were acquired with an acquisition matrix of 128 × 128, field of view of 224 × 224 mm, slice thickness of 2 mm and inter-slice gap of 1 mm. images were acquired from 30 gradient directions (b = 1000 s/mm2) along with one, B0 image. Preprocessing included correction for motion and eddy current distortions followed by skull stripping38 using FSL (www.fmrib.ox.ac.uk/fsl/).39 Tensors were estimated and FA maps created using MedINRIA (www-sop.inria.fr/asclepios/software/MedINRIA/). The FA data were analyzed with tract based spatial statistics (TBSS)40 in FSL, using a mean FA skeleton threshold of 0.2. Voxel-wise analysis of the skeletonized data was performed using the “randomise” tool41 in FSL with threshold-free cluster enhancement42 and correction for multiple comparisons using family-wise error rate. All of the DTI comparisons included age as a covariate.
Arterial Spin Labeling
To estimate cerebral blood flow,pseudo-continuous Arterial Spin Labeling (pCASL) and phase contrast MRI were performed.43 From the phase-contrast data, the total flux in the four feeding arteries (left/right internal carotid arteries, left/right vertebral arteries) was calculated, providing an estimate of the blood flow to the entire brain. The whole brain volume was estimated from MPRAGE data, from which the average blood flow per unit brain mass was calculated in units of ml/100 g per minute. Next, a brain mask was applied to the pCASL difference images and the whole-brain averaged pCASL signal (in units of MR signal) was calculated. Comparing these two averaged values, the conversion constant between pCASL MR signal and the physiologic unit was obtained and used to calibrate the pCASL signal for individual voxels, yielding CBF maps. Imaging parameters for pCASL scan were: Gradient Echo, voxel size = 2.5 × 2.5 × 5 mm3, TR = 4 s, labeling duration/delay 1.6/1.5 s, 30 averages, labeling RF interval 1 ms, RF duration 0.5 ms, flip angle 18°.
RESULTS
NEUROCOGNITIVE STATUS
Diagnoses
Of the 34 participants, 20 were cognitively normal (59%), 4 were diagnosed with a fixed cognitive deficit (12%), 8 with Mild Cognitive Impairment (23%), and 2 with dementia (6%). Of those with dementia, one had vascular dementia (history of diabetes and stroke) and the other had regions of cystic change on MRI that had the appearance of lesions remotely associated with traumatic brain injury consistent with clinical history. No participants demonstrated signs suggestive of Amyotrophic Lateral Sclerosis. For subsequent analyses, those diagnosed with a fixed deficit, Mild Cognitive Impairment, or dementia were grouped as ‘cognitively impaired’ in order to maximize sample sizes for statistical comparisons.
Eight of the 34 participants (24%) were diagnosed with depression, six of whom had not been previously diagnosed or treated. Three of the eight with depression had concurrent cognitive deficits that were not felt to be attributable solely to depression. Thus, these three were included in both their cognitive diagnostic group (and as cognitively impaired) and depression analyses.
Demographic variables
Independent t-tests were performed to compare demographic variables between 26 NFL players and their 26 matched controls. There were no significant differences between all NFL players and controls in terms of age [t (50) =0.21, p = 0.84], education [t (50) = −0.41, p = 0.68] or estimated FSIQ [t (50) = 0.49, p = 0.63]. One-way between-participants ANOVAs (two-tailed) were conducted to evaluate differences in demographic variables among controls (N = 26), NFL players without cognitive impairment or depression (N = 12), and NFL players with cognitive impairment (N = 10). There were no significant differences across groups for education [F (2, 45) = 0.29, p = 0.75], estimated FSIQ [F (2, 45) = 0.13, p = 0.88], or age [F (2, 45) = 2.86, p = .07]. In examining differences between cognitively impaired versus non-impaired players, there was no significant difference in number of concussions [t (34) = −0.86, p=0.39], or years played in the NFL [t (34) = −1.93, p=0.06].
There were no differences between the impaired vs. nonimpaired NFL players’ groups for vascular risk factors (hypertension, diabetes, high cholesterol, etc.) or alcohol use. None of the players reported steroid use while playing and there was no current drug abuse in either group. Seven of the NFL players complained of headaches and one complained of dizziness; however, there were no significant differences between impaired vs. nonimpaired players on these symptoms.
Neuropsychological measures
Using age adjusted scores, one-way ANOVAs revealed significant differences on tests of naming (Boston Naming Test), word finding (Semantic Object Retrieval Test naming) and visual (Rey Osterrieth Figure delayed recall) and verbal (California Verbal Learning Test) episodic memory (Table 1). There were no significant correlations between neuropsychological measures and concussions or years played in the NFL.
NEUROIMAGING
Fluid Attenuated Inversion Recovery (FLAIR)
Total and deep white matter lesion volumes were significantly different between NFL players with cognitive deficits (N = 10) and age-matched controls (N = 20). The periventricular white matter lesion volume alone was not significantly different between the two groups (Table 2).
Table 2.
The results from the FLAIR images are shown below, including the volume of all white matter lesions (ml), deep subcortical white matter lesions and subependymal periventricular white matter lesions.
| Age | White Matter Lesions |
Deep White Matter Lesions |
White Matter Lesions around Ventricles |
|
|---|---|---|---|---|
| NFL | 66.6 | 8.13 | 1.01 | 7.13 |
| Control | 65.15 | 2.38 | 0.22 | 2.23 |
| p-value | 0.6 | 0.04 | 0.02 | 0.06 |
There were no difference in white matter lesion volumes between cognitively impaired players and those who were not impaired or between symptomatic players (cognitive impairment and/or depression) and those who were not impaired (p > .05).
Hemosiderin Scan
Of all of the subjects, only three normal controls and 3 NFL players (two with no impairments and one with MCI and depression) had small foci of hemosiderin deposition. The one impaired subject with MCI and mild depression demonstrated a focus of hemosiderin deposition in the pons that had an appearance most compatible with a cavernous angioma). None of the foci of likely hemosiderin deposition in any of the participants were collocated with foci of elevated FLAIR signal that would suggest gliosis. There was no encephalomalacia in the vicinity of any of these foci, either. The subject with the most numerous foci (four; two on each side) demonstrated no cognitive abnormalities.
Diffusion Tensor Imaging
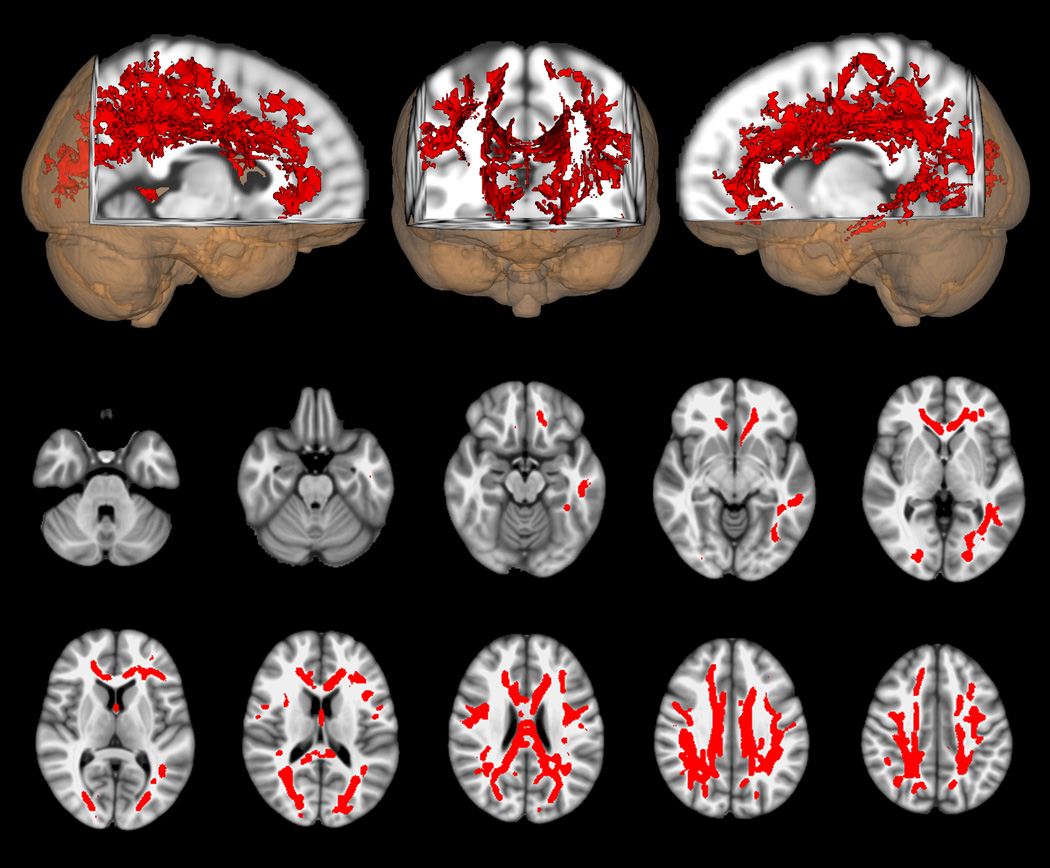
The primary DTI analysis compared FA between the group of retired athletes with impairments of cognition and/or mood (symptomatic athletes, N=14) and their age, education and IQ matched controls (N=14). This comparison demonstrated widely distributed reductions of FA in frontal and parietal regions bilaterally as well as along the corpus callosum and in the left temporal lobe (Figure 1). The reverse contrast between these same groups yielded no significant voxels where FA was higher in the symptomatic athletes than in their matched controls. The comparison of the asymptomatic retired athletes who had neither cognitive impairment nor depression (N=12) with their matched controls likewise showed no voxels in which FA differed. (We performed a secondary analysis to see if the finding of reduced FA in the symptomatic athletes persisted if this group was compared to the asymptomatic athletes [Supplementary Figure 1] instead of their matched controls. This comparison had similar results to the one between symptomatic athletes and their matched normal controls.)
Figure 1.
DTI voxel-wise comparison of FA differences between symptomatic athletes with cognitive impairment and/or depression (N = 14) and their matched normal controls (N = 14). Red indicates voxels in which FA is lower in the symptomatic athletes than in controls (p < 0.05, corrected). Axial images are in radiologic orientation with the results thickened for better visibility using the “tbss_fill” script.
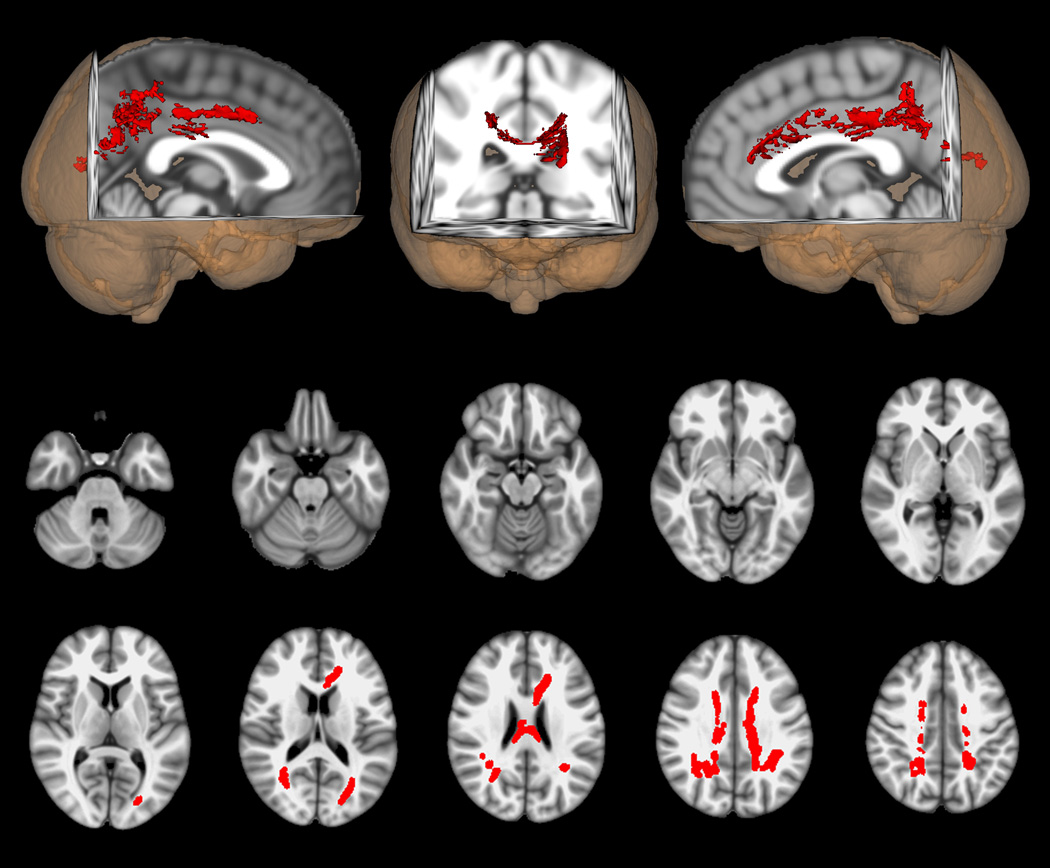
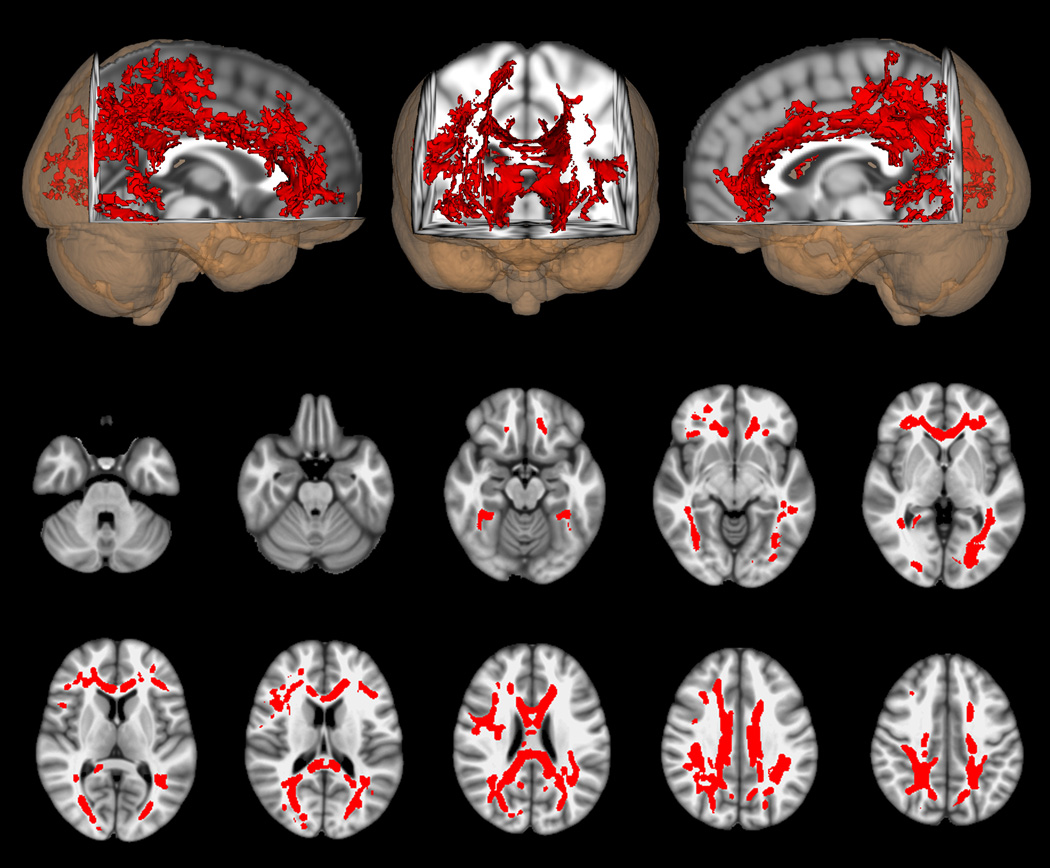
In additional analyses, cognitive impairment and depression were considered separately. Figure 2.a., shows the comparison of FA between the retired athletes with cognitive impairment (N=10) and their matched controls (N=10), while Figure 2.b. shows the same comparison for those with depression (N=6) and their matched controls (N=6). Both comparisons again show widely distributed voxels with lower FA in the athlete groups; however, the changes for those with cognitive impairment is more constricted in topographic extent than was seen in the entire symptomatic athlete group.
Figure 2.
a. DTI voxel-wise comparison of FA differences between cognitively impaired, athletes (n = 10) and matched normal controls (n = 10). Red indicates voxels in which FA is lower in the cognitively impaired athletes than in controls (p < 0.05, corrected). Axial images are in radiologic orientation with the results thickened for better visibility using the “tbss_fill” script.
b. DTI voxel-wise comparison of FA differences between athletes with depression (n = 6) and matched normal controls (n = 6). Red indicates voxels in which FA is lower in the athletes with depression than in controls (p < 0.05, corrected). Axial images are in radiologic orientation with the results thickened for better visibility using the “tbss_fill” script.
Arterial Spin Labeling
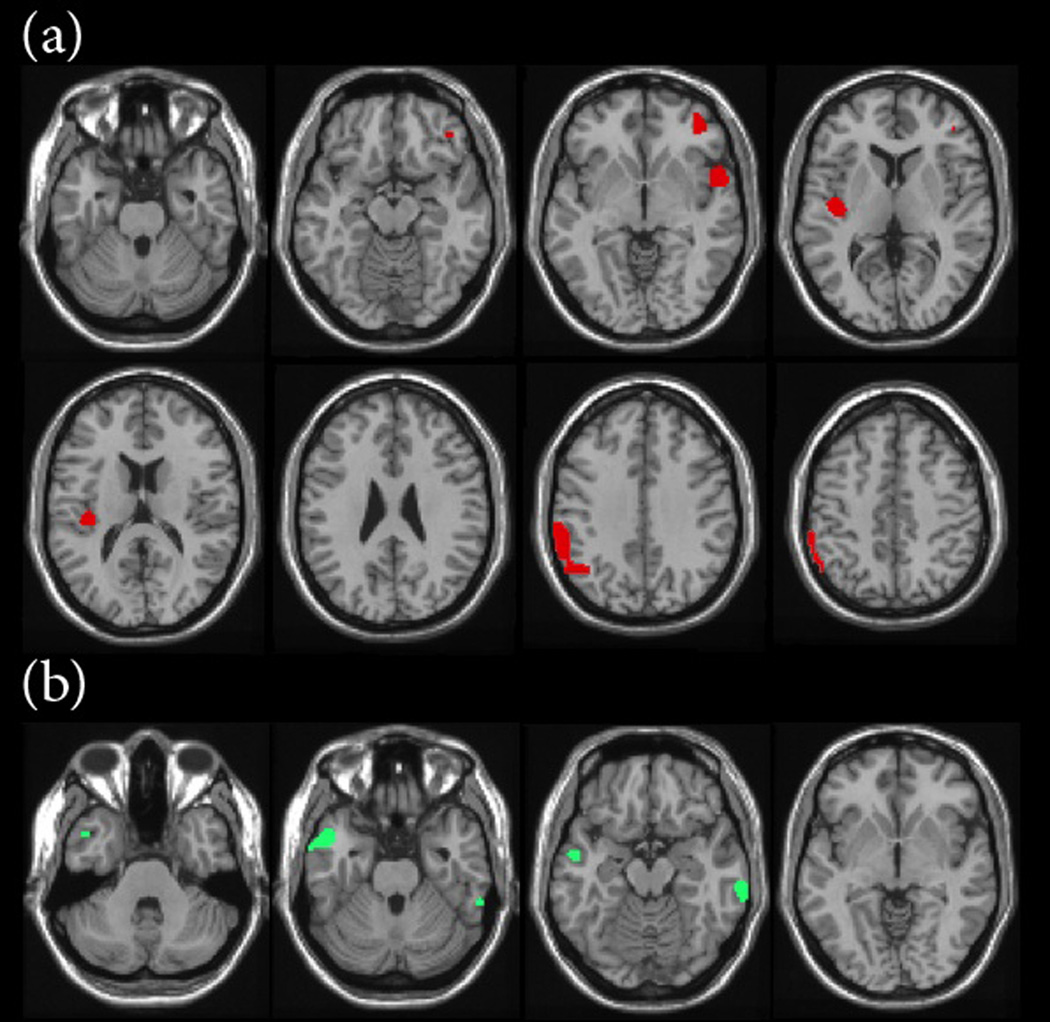
We found regions in the left inferior parietal lobe, posterior superior temporal gyrus, bilateral mid-cingulate gyri, and right middle frontal gyrus that demonstrated significant (p < 0.001) increases in regional blood flow for impaired players compared to all matched controls (Figure 3.a). The impaired players also had significantly less blood flow compared to controls in the left temporal pole and right occipital region (Figure 3.b). Four of the 10 impaired NFL players and 6 controls were not used due to > 4 mm of motion misalignment.
Figure 3.
(a) Increases in estimated regional cerebral blood flow for NFL players with cognitive impairment compared to controls (p < 0.001) and (b) decrease in estimated regional cerebral blood flow for NFL players with cognitive impairment compared to controls (p < 0.001).
COMMENT
In the group of retired NFL players that participated in our study, 41% demonstrated cognitive deficits, including 4 with fixed cognitive deficits (12%), 8 with Mild Cognitive Impairment (23%), and 2 with dementia (6%). Comparing players with cognitive deficits to a) players without cognitive deficits and b) normal controls, the neuropsychological tests that distinguished the groups were measures of naming and word finding, and visual and verbal episodic memory.
In our sample, the number of individuals with dementia was not different than expected in the general population at this age. Previous studies8 have noted a higher incidence of dementia in retired players as they aged. The lower incidence in the present study could be due to a motivated volunteer sample (although bias is typically towards more, not fewer, with impairment) or the higher average IQ of participants. The number of participants with Mild Cognitive Impairment is slightly higher than expected in the general population,44,45 and these individuals will be followed to determine if their clinical progression approximates that of amnestic Mild Cognitive Impairment with a presumed degenerative etiology, or if Mild Cognitive Impairment in individuals with significant concussion history exhibits a different course. There were no neurological abnormalities suggestive of Amyotrophic Lateral Sclerosis detected in any individuals; however, we acknowledge that the sample size of the study is small. None of the retired players met presently available criteria for CTE, while we acknowledge that individuals may now have or develop pathology consistent with CTE.
The prevalence of depression amongst our retired players (24%) was slightly higher than expected for this age group (approximately 15%).46 These findings underscore the need for screening for depression and cognitive dysfunction in retired athletes.9,46 Interestingly, reports of Chronic Traumatic Encephalopathy in more recently retired athletes have been in individuals who eventually committed suicide.13,47 However, those studies did not examine the relationship between neurobehavioral status or clinical course of symptoms during life; thus, the clinical presentation of depression was not well described. Undetected depression can occur because of the presence of vegetative symptoms of depression (e.g. disturbance of sleep, appetite, volition) without predominant mood indicators (e.g. expressed feelings of hopelessness, crying, etc.) as seen in the former players in this study, resulting in failure to report overt symptoms of sadness or depression per se.
Our findings support a previous study of the relationship of remote concussion and MCI and Alzheimer’s Disease (AD) by Guskiewicz et al. (2005),11 who found an association between recurrent concussion (3 or more) and MCI, but not AD. Tremblay et al (in press)48 compared quantitative voxel-based morphometry, magnetic resonance spectroscopy, neuropsychological testing, and ApoE status in15 former athletes who sustained their last sports concussion >3 decades prior to testing to 15 with no history of traumatic brain injury. Those with concussions showed focal brain volume loss, MRS abnormalities in the medial temporal lobe and prefrontal cortex, and episodic memory verbal fluency decrements, indicating structural, neurochemical and behavioral lesions in these otherwise healthy former athletes. In another study,49 19 healthy former athletes with a history of concussion and 21 without concussion underwent neuropsychological testing, ERP oddball task, and transcranial magnetic stimulation for motor measures. The athletes with a history of concussion demonstrated decreased episodic memory and response inhibition performance, which correlated with delayed ERP and slowed movement speed. These concussion-linked findings in reportedly normal athletes suggest remote concussion may result in brain abnormalities and that these findings may be precursors to those reported here. Unfortunately, none of these studies included neurological and neuropsychological assessment of the clinical diagnostic (e.g., dementia, MCI, etc.) status of the subjects.
In our cohort of retired players we found generally mild difficulties in naming and word finding, and in episodic memory (verbal and visual). The memory impairment we observed is consistent with previous findings of persistent memory impairments following concussions.49,50 We also found word finding difficulties that have not been previously reported in aging cohorts of retired players. Future neurocognitive studies of aging athletes should include these assessments to further explore the potential significance of this finding in terms of cognitive outcomes. A more extensive neuropsychological examination, with additional emphasis on executive function and more detailed analysis of episodic memory and language performances may be useful.
The cognitive dysfunction and depression in the cognitively impaired members of our cohort were associated with disrupted white matter integrity on DTI and with deep white matter lesions on FLAIR. As these differences were not evident in either comparable healthy retired NFL players or matched control participants, it appears that disrupted white matter integrity represents a potentially important biomarker for neurobehavioral impairment. These findings differ from a previous study of post-concussional athletes with depression, in whom quantitative MRI, and functional MRI (fMRI) demonstrated reduced fMRI signal changes without prominent white matter pathology.51 The use of a matched control group without concussions is essential to detect these key differences. Further correlation between white matter pathology and cognitive impairments or depression over time may be of prognostic value among those at risk for future neurobehavioral difficulties.
Altered cerebral blood flow patterns in retired NFL players are concordant with brain regions associated with neuropsychological testing abnormalities. The left inferior parietal lobule and the superior temporal gyrus are thought to play important roles in naming and word finding,52 and the increase in blood flow in the impaired players may reflect compensatory responses to improve performance in dysfunctional regions. The left temporal pole is also associated with both naming and verbal memory,53 and the decreased flow in this region suggests less metabolic activity and associated dysfunction here. Our findings suggest that there is a dynamic process underlying dysfunction in these players as they age, and that their deficits do not simply reflect the static effects of previous damage. In a longitudinal study of white matter hyperintensities in aging individuals, advancing white matter abnormalities were associated with different patterns of cortical blood flow increase and decrease compared to what was evident in a sub-population with static white matter findings. The regions of longitudinally developing cortical blood flow increase were posited to reflect at least transient cortical compensatory efforts to overcome reduced efficacy of interregional neural communications due to white matter deterioration. The decreased blood flow was exhibited in regions that were posited to have reduced function as a consequence of longer- term disconnection.54 We posit that similar mechanisms underlie the regional increases and decreases in cerebral blood flow that we have observed in our subjects with cognitive abnormalities.
In summary, this comprehensive, multimodal investigation suggests that retired NFL players may be more likely to develop cognitive impairments (problems with memory, naming and word finding) or depression as they age compared to the general population. These cognitive impairments correlated with changes in blood flow to specific (left temporal pole, inferior parietal lobule, superior temporal gyrus) brain regions, as well as with white matter abnormalities. NFL players without cognitive impairment or depression did not demonstrate white matter abnormalities compared with controls. Future investigations with larger samples including these types of detailed histories and multimodal neurobehavioral and neuroimaging studies of this population are needed.
Supplementary Material
Supplementary Figure. DTI voxel-wise comparison of FA differences between symptomatic athletes with cognitive impairment and/or depression (N = 14) and asymptomatic athletes with neither cognitive impairment nor depression (N = 12). Red indicates voxels in which FA is lower in the symptomatic athletes than in the asymptomatic athletes. Axial images are in radiologic orientation with the results thickened for better visibility using the “tbss_fill” script.
Acknowledgements
This work was supported by the BrainHealth Institute for Athletes at the Center for BrainHealth, a research center at the University of Texas at Dallas. The authors would like to thank the retired NFL players for their partnership in performing this study. This study was partially funded by NIA grant 5K23AG030006.
Appendix.
The clinical interview included an assessment of the presence and severity of difficulties with focusing attention or concentrating, continually paying attention to complete a job/task, stuttering, expressing or communicating ideas clearly, word finding, naming objects, coming up with the names of people, understanding what is heard or read, reading aloud, handwriting, ability to learn new things, memory for people’s names or faces, memory for facts (grocery lists, phone numbers, appointments, news, daily events), when things happened, things that happened years ago, recognizing objects, volitional movements of hands and feet, sense of direction, calculations, organizing ideas, planning, shifting attention, decision making, judgment, slowed thinking, headaches, dizziness, nausea, vomiting, fainting spells, seizures, sleep disruption, fatigue, blurred vision, double vision, falling, balance problems.
The neurobehavioral evaluation included a survey of the presence and severity of symptoms including personality changes (apathy, impulse control, lack of inhibition, emotional lability, etc.), visual and auditory hallucinations, paranoid ideation, delusions, emotional outbursts, uncontrollable feelings or emotions, aggressive behavior, depressed mood, mood swings, lack of self worth, recent stressful life events, desperation concerning the future, inability to make decisions, excessive guilt, suicidal thoughts or actions, lack of drive, inability to initiate things, significant change in energy level, change in weight, change in sleep, change in sex drive, anxiety, excessive worry, panic attacks, pressure to speak, racing thoughts, uncontrollable thoughts, recurring thoughts or ideas, or unusual fears or phobias.
Footnotes
Contributors
JH was principal investigator, obtained funding, designed, directed, and supervised the study, analyzed and interpreted the data, and drafted the first and final drafts of the manuscript. MK helped design the study, analyzed and interpreted the data, and drafted and reviewed the manuscript. KW and HL helped design the study, analyzed and interpreted the neuroimaging data, and reviewed the manuscript. JS analyzed and interpreted the neuroimaging data and reviewed the manuscript. ND, EB, and SM collected data, analyzed and interpreted the data, and reviewed the manuscript. HC coordinated the study, collected data, and reviewed the manuscript. MC helped design the study, supervised, collected and interpreted the neuropsychological data, and drafted and reviewed the manuscript.
Conflicts of interest
The authors report no conflict of interest in the publication of this work, financial or otherwise.
REFERENCES
- 1.McAllister TW, Saykin AJ, Flashman LA, et al. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53(6):1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 2.McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23(10):1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 3.Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22(1):68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- 5.Reddy CC, Collins MW. Sports concussion: management and predictors of outcome. Curr Sports Med Rep. 2009;8(1):10–15. doi: 10.1249/JSR.0b013e31819539ca. [DOI] [PubMed] [Google Scholar]
- 6.Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc. 2010;16(2):262–267. doi: 10.1017/S1355617709991287. [DOI] [PubMed] [Google Scholar]
- 7.Gardner A, Shores EA, Batchelor J. Reduced processing speed in rugby union players reporting three or more previous concussions. Arch Clin Neuropsychol. 2010;25(3):174–181. doi: 10.1093/arclin/acq007. [DOI] [PubMed] [Google Scholar]
- 8.Weir DR, Jackson JS, Sonnega A. National Football League Player Care Foundation Study of Retired NFL Players. Institute for Social Research, University of Michigan; 2009. Sep, [Google Scholar]
- 9.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Medicine & Science in Sports & Exercise. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 10.Alexander MP. Neuropsychiatric correlates of persistent post- concussive syndrome. J Head Trauma Rehabil. 1992;7:60–69. [Google Scholar]
- 11.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–724. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 12.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Me. 2011;30(1):179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]
- 15.McKee AC, Gavett BE, Stern RA, et al. TDP-43 Proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69(9):918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on a partial replication. J Neurol Neurosurg Psychiatry. 2003;74(7):857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10(5):764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 20.Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010;27(1):65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- 21.Ellemberg D, Henry LC, Macciocchi SN, Guskiewicz KM, Broglio SP. Advances in sport concussion assessment: from behavioral to brain imaging measures. J Neurotrauma. 2009;26(12):2365–2382. doi: 10.1089/neu.2009.0906. [DOI] [PubMed] [Google Scholar]
- 22.Slobounov S, Slobounov E, Sebastianelli W, Cao C, Newell K. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery. 2007;61(2):338–344. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- 23.Practice parameter. The management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 25.Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept. Mot Skills. 1958;8:271–276. [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition: Technical and interpretive manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 27.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 28.Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia. University of Victoria: Victoria, BC; 1969. [Google Scholar]
- 29.Benton AL, Hamsher K. Multilingual Aphasia examination. 2nd ed. Iowa City, IA: AJA Associates; 1976. [Google Scholar]
- 30.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea &Febiger; 1983. [Google Scholar]
- 31.Rey A. L’examen psychologique dans les casd’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- 32.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 33.Kraut MA, Cherry B, Pitcock JA, Vestal L, Henderson VW, Hart J. The semantic object retrieval test (SORT) in normal aging and Alzheimer disease. Cognitive and Behavioral Neurology. 2006;19(4):177–184. doi: 10.1097/01.wnn.0000213922.41008.22. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 37.Abel EL. Football increases the risk for Lou Gehrig’s Disease, Amyotrophic Lateral Sclerosis. Perceptual and Motor Skills. 2007;104:1251–1254. doi: 10.2466/pms.104.4.1251-1254. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SM. Overview of fMRI analysis. Br J Radiol. 2004 Spec;77(No 2):S167–S175. doi: 10.1259/bjr/33553595. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxel wise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 43.Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63(3):765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 45.Palmer K, Bäckman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: Occurrence and progression to Alzheimer’s Disease. American Journal of Geriatric Psychiatry. 2008;16(7):603–611. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 46.Schwenk TL, Gorenflo DW, Dopp DR, Hipple E. Depression and pain in retired professional football players. Medicine and Science in Sports and Exercise. 2007;39(4):599–605. doi: 10.1249/mss.0b013e31802fa679. [DOI] [PubMed] [Google Scholar]
- 47.Cantu RC. Chronic traumatic encephalopathy in the National Football League. Neurosurgery. 2007;61:223–225. doi: 10.1227/01.NEU.0000255514.73967.90. [DOI] [PubMed] [Google Scholar]
- 48.Tremblay S, De Beaumont LHenry LC, Boulanger Y, Evans AC, Bourgouin P, Poirier J, Théoret H, Lassonde M. Sports concussions and aging: A neuroimaging investigation. Cerebral Cortex. doi: 10.1093/cercor/bhs102. in press. [DOI] [PubMed] [Google Scholar]
- 49.De Beaumont L, Théoret H, Mongeon D, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132(Pt 3):695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 50.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 51.Chen J-K, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch Gen Psychiatry. 2008;65(1):81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- 52.Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- 53.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 54.Kraut MA, Beason-Held LL, Elkins WD, Resnick SM. The impact of magnetic resonance imaging- detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. Journal of Cerebral Blood Flow & Metabolism. 2008;28:190–197. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. DTI voxel-wise comparison of FA differences between symptomatic athletes with cognitive impairment and/or depression (N = 14) and asymptomatic athletes with neither cognitive impairment nor depression (N = 12). Red indicates voxels in which FA is lower in the symptomatic athletes than in the asymptomatic athletes. Axial images are in radiologic orientation with the results thickened for better visibility using the “tbss_fill” script.