Summary
Human herpesvirus 8 (HHV8) is an important opportunistic infection of HIV/AIDS. However, very little is known about antibody seropositivities to HHV8 lytic and latent antigens among HIV-infected patients in China. Therefore, a cross-sectional study was conducted to explore HHV8 serostatus among 316 HIV-infected patients in a rural area of central China. The antibody seropositivity to HHV8 ORF65 (lytic) and LANA (latent) antigens was 12.7% and 10.4%, respectively. Patients who were naïve to antiretroviral therapy (ART) were more likely to be seropositive for antibodies to ORF65 (OR: 3.79; 95% CI: 1.71–8.42) and LANA (OR: 3.77; 95% CI: 1.55–9.14) than patients receiving ART. Patients having CD4+ cell counts less than 200 cells/mm3 were more likely to be seropositive for LANA antibody (OR: 3.53; 95% CI: 1.44–8.64) and to have lower LANA antibody titer (p = 0.007). They were also more likely to be seropositive for ORF65 antibody (OR: 2.12; 95% CI: 0.94–4.78) and to have a lower ORF65 antibody titer (p = 0.065), though the difference was marginally significant. No associations between other viral coinfections studied and antibody seropositivity to either latent or lytic HHV8 antigens were identified. Study findings suggest that antibody responses to both lytic and latent HHV8 antigens among HIV patients in China were fairly high and were associated with immunodeficiency status and ART.
Keywords: Antibody seropositivity, HHV8, HIV, LANA, ORF65
1. Introduction
Human herpesvirus 8 (HHV8), also known as Kaposi’s sarcoma-associated herpesvirus (KSHV), has been linked to a number of clinical conditions, notably Kaposi’s sarcoma (KS) (1), multicentric Castleman’s disease (MCD) (2), and primary effusion lymphoma (PEL) (3,4). A higher incidence of HHV8 infection has been observed among HIV-infected patients. In fact, many epidemiological studies have suggested that there were concurrent epidemics of HHV8 and HIV commencing in the early 1980s (5,6). Due to HIV infection and the development of AIDS, individuals were more susceptible to opportunistic infections, including HHV8. Furthermore, progressive immunologic deterioration including CD4+ T-cell depletion and CD8+ T-cell dysfunction, the hallmarks of an untreated HIV infection, lead to impairment of immune control of HHV8 replication and therefore ultimately carcinogenesis, and possibly KS. Fortunately, the introduction of highly active antiretroviral therapy (HAART) in the past two decades has effectively led to a sharp decrease in the incidence of opportunistic infections and KS in HIV-infected patients in developed countries where such therapies were widely available (7–9).
HAART has been widely utilized throughout China since 2003 and has significantly reduced the mortality rate among HIV-infected patients despite the obstacle of drug resistance (10–12). As HIV-infected patients live longer via HAART, their chances for opportunistic infections, including HHV8 infection, might be enhanced as well. Given potentially shared transmission routes between HHV8 and other pathogens as well as the wide spectrum of pathogenic coinfections such as hepatitis B virus (HBV), hepatitis C virus (HCV), herpes simplex virus (HSV), and Epstein-Barr virus (EBV) among HIV-infected patients in China (13), their impact on HHV8 antibody response among HIV-infected patients should be ascertained.
HHV8 viral antigens are broadly categorized into two groups: lytic antigens (e.g. ORF65) and latent antigens (e.g. latent nuclear antigen or LANA) (14). Tests for antibodies to both lytic and latent HHV8 antigens can be used not only to identify HHV8 infection but also to understand their interactions with the host, e.g. the association between antibodies and HHV8 lytic and latent antigens and development of KS (15,16). Most previous studies on the seroprevalence of HHV8 infection report seropositivity of antibodies to any of these antigens without differentiating specific antibody responses to lytic and latent antigens. This could hamper thorough understanding of the HHV8 epidemic and host immune responses to HHV8 infection. As HIV-infected patients live longer when undergoing HARRT, they have a greater likelihood of developing KS. Identification of HHV8 serostatus and cofactors for KS development is paramount given the widespread use of HARRT.
Therefore, the current study specifically examined antibody seropositivities to lytic and latent HHV8 antigens among a sample of previously reported patients infected with HIV (17). Knowledge gained from this study should help to better understand host immune responses to HHV8 infection in the context of HIV infection and viral coinfections.
2. Materials and Methods
2.1. Study sample
As previously described (17), study participants were patients confirmed to be infected with HIV who had been registered with the National HIV/AIDS Information System and who were participating in an ongoing HIV cohort study that was established in 2006 in the City of Yuncheng, Shanxi Province in Central China. This site is where the HIV epidemic was first reported in 1996 and HIV was predominantly transmitted through plasma/blood donation or transfusion. Free antiretroviral treatment (ART) has been available for HIV-infected patients since 2003 in the area studied.
Venous blood was collected by trained nurses using disposable sterile needles and tubes and then transferred to a local laboratory within 4 h of collection. Serum samples were stored at −80°C for HHV8, HSV-1 and HSV-2, HBV, HCV, and EBV testing. Specimens were coded by unique identification numbers and were analyzed without knowledge of the individual identity of the study participant. This study was approved by the Institutional Review Board of Fudan University, China. All study participants provided written informed consent.
Data on participants’ sociodemographic characteristics, HIV transmission mode, and receipt of HAART were obtained from the National HIV/AIDS Information System using a standard questionnaire form.
2.2. HBV, HCV, HSV-1, HSV-2, and EBV testing
HBV surface antigen (HBsAg) and anti-HCV IgG antibody were tested using an enzyme-linked immunosorbent assay (ELISA) (Wantai Biological Pharmacy Enterprise Co., Beijing, China). IgG antibodies to HSV-1 and HSV-2 were detected by type-specific ELISA (HerpeSelect 1 ELISA IgG Kit and HerpeSelect 2 ELISA IgG Kit, Focus Technologies, CA, USA). Anti-EBV nucleic antigen (EBNA) IgG antibody was tested for using ELISA (Euroimmun, Lübeck, Germany). All tests were performed by two independent technicians according to the manufacturers’ standard protocols. Duplicate negative, positive, and blank controls were always used.
2.3. HHV8 testing
An immunofluorescence assay (IFA) was performed to detect the presence of lytic or latent antigen-specific antibodies, as previously reported (18). Briefly, Spodoptera frugiperda clone 9 cells infected with baculovirus expressing ORF65 antigen (lytic antigen) or ORF73 (latent nucleic antigen, LANA) were harvested, fixed, and spotted individually on separate slides for further sample testing. All serum samples were then tested at 1:40 dilution. Sera from KS patients who previously tested seropositive and healthy individuals who previously tested seronegative served as controls. Both lytic and latent antibody titers were further determined with IFA using serially diluted samples ranging from 1:40 to 1:10,240. Each slide was read independently by two experienced laboratory workers. Serostatus was categorized as antibody seropositivity for lytic antigen (ORF65), latent antigen (LANA), and ANY and BOTH lytic and latent antigens as previously reported (19).
2.4. CD4+ and CD8 T+ cell counts and HIV RNA quantification
The absolute number of CD4+ and CD8+ T lymphocytes in peripheral blood was estimated using a fluorescence-activated cell analyzer with monoclonal antibodies (BD FACSCount System, BD Biosciences, San Jose, CA, USA). Plasma HIV viral loads were quantified using the Amplicor HIV-1 RNA Monitor Test v1.5 (Roche Diagnostics Alameda, CA, USA).
2.5. Statistical analysis
Original questionnaires and laboratory testing results were entered and managed in EpiData3.0, and then the database was transferred to an SPSS database for further management and analysis. Seroprevalence of both lytic and latent antibodies was calculated. Pearson’s Chi-squared tests were performed to evaluate differences in seroprevalence between subgroups. Nonparametric tests (Mann-Whitney U tests) were used to assess the difference in geometric mean titers (GMTs) of antibodies between different groups. Concordance between the latent and lytic serology assay was assessed using the kappa statistic. Univariate logistic regression analysis was first done and then followed by multivariate analysis to explore associations with seropositivity for both lytic and latent antibodies. Odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated and used to determine whether a variable was associated with antibodies against LANA, ORF65, ANY, or BOTH. All statistical analyses were performed using SPSS software 15.0 (SPSS, Chicago, Illinois, USA) and GraphPad Prism 5.0 (GraphPad, La, Jolla, CA, USA). A two-sided p-value of 0.05 or less was considered statistically significant.
3. Results
3.1. Sociodemographic characteristics and seroprevalence of viral coinfections
A total of 316 HIV-infected patients were included in this study. Their sociodemographic characteristics are shown in Table 1. Of the sample, 53.8% were males and 46.2% were females with a mean age of 42.03 years (S.D. = 10.25). About 99.1% of the participants belonged to the Han ethnic group. Males were more likely to drink alcohol (p < 0.001) and smoke (p < 0.001) than females. No gender differences existed for other demographic characteristics (Table 1).
Table 1.
Socio-demographic characteristics of study participants
| Item | Male (170) n (%) | Female (146) n (%) | Total (316) n (%) |
|---|---|---|---|
| Ethnicity (p = 0.252) | |||
| Han | 167 (98.2) | 146 (100.0) | 313 (99.1) |
| Other | 3 (1.8) | 0 (0) | 3 (0.9) |
| Age group (p = 0.530) | |||
| 0–18 | 5 (2.9) | 6 (4.1) | 11 (3.5) |
| 19–49 | 123 (72.4) | 111 (76.0) | 234 (74.1) |
| 50+ | 42 (24.7) | 29 (19.9) | 71 (22.5) |
| Marital status (p = 0.346) | |||
| Married | 156 (91.8) | 136 (93.2) | 292 (92.4) |
| Single | 7 (4.1) | 8 (5.5) | 15 (4.7) |
| Divorced/widowed | 7 (4.1) | 2 (1.4) | 9 (2.8) |
| Education (p < 0.001) | |||
| Illiterate | 10 (5.9) | 17 (11.6) | 27 (8.5) |
| Primary school | 52 (30.6) | 67 (45.9) | 119 (37.7) |
| Middle school | 95 (55.9) | 59 (40.4) | 154 (48.7) |
| High school or higher | 13 (7.6) | 3 (2.1) | 16 (5.1) |
| Farmer (p = 0.409) | |||
| Yes | 154 (90.6) | 136 (93.2) | 290 (91.8) |
| No | 16 (9.4) | 10 (6.8) | 26 (8.2) |
| Monthly income (RMB, p = 0.779) | |||
| < 1,000 | 97 (57.1) | 82 (56.2) | 179 (55.6) |
| 1,001–2,000 | 35 (20.6) | 36 (24.7) | 71 (22.5) |
| 2,001–3,000 | 12 (7.1) | 10 (6.8) | 22 (7.0) |
| > 3,000 | 26 (15.3) | 18 (12.3) | 44 (13.9) |
| Alcohol Consumption (p < 0.001) | |||
| Yes | 26 (15.3) | 3 (2.1) | 29 (9.2) |
| No | 144 (84.7) | 143 (97.9) | 287 (90.8) |
| Smoking (p < 0.001) | |||
| Yes | 109 (64.1) | 5 (3.4) | 114 (36.4) |
| No | 61 (35.9) | 141 (96.6) | 202 (63.6) |
| HBsAg (p = 0.786) | |||
| Positive | 13 (7.6) | 10 (6.8) | 23 (7.3) |
| Negative | 157 (92.4) | 136 (93.2) | 293 (92.7) |
| HCV (p < 0.001) | |||
| Positive | 142 (83.5) | 93 (63.7) | 235 (74.4) |
| Negative | 28 (16.5) | 53 (39.3) | 81 (25.6) |
| EBV (p = 0.096) | |||
| Positive | 161 (94.7) | 131 (89.7) | 292 (92.4) |
| Negative | 9 (5.3) | 15 (10.3) | 24 (7.6) |
| HSV-1 (p = 0.430) | |||
| Positive | 130 (76.5) | 106 (72.6) | 236 (74.7) |
| Negative | 40 (23.5) | 40 (27.4) | 80 (25.3) |
| HSV-2 (p = 0.012) | |||
| Positive | 26 (15.3) | 39 (26.7) | 65 (20.6) |
| Negative | 144 (84.7) | 107 (73.3) | 251 (79.4) |
Among the participants, 23 (7.3%) were seropositive for HBsAg, 235 (74.4%) for HCV, 236 (74.4%) for HSV-1, 65 (20.6%) for HSV-2, and 292 (92.4%) for EBV. Males had a higher prevalence of HCV infection but lower prevalence of HSV-2 infection than females (Table 1).
3.2. Prevalence and correlates of HHV8 lytic and latent antibody seropositivity
The serostatus of lytic and latent antibodies were determined separately. Antibody seropositivity was 12.7% for lytic antigen (ORF65) and 10.4% for latent antigen (LANA). The two serology assays showed a moderate concordance (Kappa = 0.582). Two separate multiple logistic regression analyses were performed to explore independent correlates with ORF65 and LANA antibody seropositivity by adjusting for gender and age group. As shown in Table 2, both ORF65 and LANA antibody seropositivity were significantly associated with ART and CD4+ cell counts. Participants who were ART-naïve were more likely to be positive for ORF65 antibody (OR: 3.79; 95% CI: 1.71–8.42) and LANA antibody (OR: 3.77; 95% CI: 1.55–9.14) than those receiving ART. Patients having CD4+ cell counts less than 200 cells/mm3 were more likely to be seropositive for LANA antibody (OR: 3.53; 95% CI: 1.44–8.64) and to have a lower LANA antibody titer (p = 0.007). They were also more likely to be seropositive for ORF65 antibody (OR: 2.12; 95% CI: 0.94–4.78) and to have a lower ORF65 antibody titer (p = 0.065) although these associations were marginally significant. No associations between other examined viral coinfections and antibody seropositivity to either latent or lytic HHV8 antigen were identified.
Table 2.
Multivariate analysis of correlates with HHV8 lytic and latent antibody seropositivities among study participants
| Characteristics/Risk factor | LANA
|
ORF65
|
||||
|---|---|---|---|---|---|---|
| Positives/total (%) | OR (95%CI)* | p-value* | Positives/total (%) | OR (95% CI)* | p-value* | |
| Socio-demographics | ||||||
| Gender | ||||||
| Male | 11/170 (6.5) | 1.00 | 18/170 (10.6) | 1.00 | ||
| Female | 22/146 (15.1) | 2.53 (1.12–5.73) | 0.026* | 22/146 (15.1) | 1.38 (0.67–2.85) | 0.385 |
| Age group | ||||||
| 0–18 | 2/11 (18.2) | 1.00 | 3/11 (27.3) | 1.00 | ||
| 19–49 | 24/243 (10.3) | 0.36 (0.06–2.16) | 0.265 | 30/234 (12.8) | 0.33 (0.07–1.60) | 0.172 |
| 50 + | 7/71 (9.9) | 0.38 (0.05–2.74) | 0.340 | 7/71 (9.9) | 0.28 (0.05–1.65) | 0.162 |
| HIV-related factors | ||||||
| ART | ||||||
| Yes | 19/238 (8.0) | 1.00 | 22/238 (9.2) | 1.00 | ||
| No | 14/78 (17.9) | 3.77 (1.55–9.14) | 0.003** | 18/78 (23.1) | 3.79 (1.71–8.42) | 0.001** |
| CD4 (cell/mm3) | ||||||
| > 200 | 15/187 (8.0) | 1.00 | 22/187 (11.8) | 1.00 | ||
| ≤ 200 | 18/129 (14.0) | 3.53 (1.44–8.64) | 0.006** | 18/129 (14.0) | 2.12 (0.94–4.78) | 0.07 |
| CD8 (cell/mm3) | ||||||
| > 400 | 27/251 (10.8) | 1.00 | 32/251 (12.7) | 1.00 | ||
| ≤ 400 | 6/65 (9.2) | 0.73 (0.25–2.14) | 0.571 | 8/65 (12.3) | 0.97 (0.37–2.57) | 0.955 |
| Viral load (copies/mL) | ||||||
| ≤ 400 | 4/25 (16.0) | 1.00 | 6/25 (24.0) | 1.00 | ||
| > 400 | 29/291 (10.0) | 0.47 (0.12–1.86) | 0.287 | 34/291 (11.7) | 0.42 (0.13–1.35) | 0.143 |
| Duration of HIV infection (yr) | ||||||
| ≤ 10 | 14/97 (14.4) | 1.00 | 18/97 (18.6) | 1.00 | ||
| > 10 | 19/219 (8.7) | 0.59 (0.25–1.38) | 0.220 | 22/219 (10.0) | 0.54 (0.25–1.19) | 0.129 |
| Co-infections | ||||||
| HBsAg | ||||||
| Negative | 29/293 (9.9) | 1.00 | 35/293 (11.9) | 1.00 | ||
| Positive | 4/23 (17.4) | 2.36 (0.67–8.47) | 0.182 | 5/23 (21.7) | 2.41 (0.75–7.72) | 0.140 |
| HCV | ||||||
| Negative | 10/81 (12.3) | 1.00 | 13/81 (16.0) | 1.00 | ||
| Positive | 23/235 (9.8) | 0.52 (0.89–1.44) | 0.211 | 27/235 (11.5) | 0.63 (0.25–1.58) | 0.323 |
| EBV | ||||||
| Negative | 2/24 (8.3) | 1.00 | 4/24 (16.7) | 1.00 | ||
| Positive | 31/292 (10.6) | 1.88 (0.36–9.72) | 0.452 | 36/292 (12.3) | 0.93 (0.27–3.25) | 0.909 |
| HSV-1 | ||||||
| Negative | 8/80 (10.0) | 1.00 | 9/80 (11.3) | 1.00 | ||
| Positive | 25/236 (10.6) | 1.30 (0.52–3.24) | 0.574 | 31/236 (13.1) | 1.42 (0.60–3.36) | 0.427 |
| HSV-2 | ||||||
| Negative | 26/251 (10.4) | 1.00 | 32/251 (12.7) | 1.00 | ||
| Positive | 7/65 (10.8) | 1.03 (0.39–2.73) | 0.956 | 8/65 (12.3) | 0.95 (0.38–2.37) | 0.917 |
The odds ratio (OR) and 95% CI were obtained by adjusting for other variables listed in the table.
3.3. Correlates of antibody seropositivity for ANY and BOTH lytic and latent HHV8 antigens
The seropositivity of antibodies to ANY and BOTH lytic and latent HHV8 antigens was 15.8% and 7.3%, respectively. Regression analyses revealed that patients who were ART-naïve (OR: 3.67; 95% CI: 1.75–7.69, p = 0.001) or had low CD4+ cell counts (OR: 2.71; 95% CI: 1.29–5.68, p = 0.008) were more likely to be antibody seropositive for ANY lytic and latent HHV8 antigens (Table 3). They were also more likely to be antibody seropositive for BOTH lytic and latent HHV8 antigens (Table 3).
Table 3.
Multivariate analysis of correlates with ANY and BOTH HHV8 lytic and latent antibody seropositivity among study participants
| Characteristics/Risk factor | ANY
|
BOTH
|
||||
|---|---|---|---|---|---|---|
| Positives/total (%) | OR (95% CI) | p-value | Positives/total (%) | OR (95% CI) | p-value* | |
| Socio-demographics | ||||||
| Gender | ||||||
| Male | 22/170 (12.9) | 1.00 | 7/170 (4.7) | 1.00 | ||
| Female | 28/146 (19.2) | 1.51 (0.78–2.91) | 0.225 | 16/146 (11.0) | 2.83 (1.05–7.62) | 0.039* |
| Age group | ||||||
| 0–18 | 3/11 (27.3) | 1.00 | 2/11 (18.2) | 1.00 | ||
| 19–49 | 38/234 (16.2) | 0.39 (0.08–1.79) | 0.229 | 16/234 (6.8) | 0.26 (0.04–1.69) | 0.185 |
| 50 + | 9/71 (12.7) | 0.32 (0.06–1.75) | 0.191 | 5/71 (7.0) | 0.31 (0.03–2.59) | 0.181 |
| HIV-related factors | ||||||
| ART | ||||||
| Yes | 29/238 (12.2) | 1.00 | 12/238 (5.0) | 1.00 | ||
| No | 21/78 (25.9) | 3.67 (1.75–7.69) | 0.001** | 11/78 (14.1) | 4.36 (1.55–12.27) | 0.005* |
| CD4 (cell/mm3) | ||||||
| > 200 | 25/187 (13.4) | 1.00 | 12/187 (6.4) | 1.00 | ||
| ≤ 200 | 25/129 (19.4) | 2.71 (1.29–5.68) | 0.008** | 11/129 (8.5) | 2.82 (0.98–8.13) | 0.054* |
| CD8 (cell/mm3) | ||||||
| > 400 | 40/251 (15.9) | 1.00 | 19/251 (7.6) | 1.00 | ||
| ≤ 400 | 10/65 (15.4) | 0.87 (0.36–2.08) | 0.748 | 4/65 (6.2) | 0.78 (0.21–2.91) | 0.718 |
| Viral load (copies/mL) | ||||||
| ≤ 400 | 6/25 (24.0) | 1.00 | 4/25 (16.0) | 1.00 | ||
| > 400 | 44/291 (15.1) | 0.51 (0.16–1.61) | 0.252 | 19/291 (6.5) | 0.31 (0.07–1.35) | 0.120 |
| Duration of HIV infection (yr) | ||||||
| ≤ 10 | 20/97 (20.6) | 1.00 | 12/97 (12.4) | 1.00 | ||
| > 10 | 30/219 (13.7) | 0.65 (0.32–1.33) | 0.240 | 11/219 (5.0) | 0.41 (0.15–1.12) | 0.077 |
| Co-infections | ||||||
| HBsAg | ||||||
| Negative | 44/239 (15.0) | 1.00 | 20/293 (6.8) | 1.00 | ||
| Positive | 6/23 (26.1) | 2.42 (0.82–7.12) | 0.108 | 3/23 (13.0) | 2.30 (0.53–9.98) | 0.265 |
| HCV | ||||||
| Negative | 15/81 (18.5) | 1.00 | 8/81 (9.9) | 1.00 | ||
| Positive | 35/235 (14.9) | 0.62 (0.26–1.42) | 0.260 | 15/235 (6.4) | 0.48 (0.14–1.61) | 0.231 |
| EBV | ||||||
| Negative | 4/24 (16.7) | 1.00 | 2/24 (8.3) | 1.00 | ||
| Positive | 46/292 (15.8) | 0.82 (0.24–2.79) | 0.752 | 21/292 (7.2) | 0.74 (0.14–4.11) | 0.736 |
| HSV-1 | ||||||
| Negative | 13/80 (16.3) | 1.00 | 4/80 (5.0) | 1.00 | ||
| Positive | 37/236 (15.7) | 0.87 (0.41–1.84) | 0.716 | 19/236 (8.1) | 2.23 (0.64–7.70) | 0.205 |
| HSV-2 | ||||||
| Negative | 39/251 (15.5) | 1.00 | 19/251 (7.6) | 1.00 | ||
| Positive | 11/65 (16.9) | 1.08 (0.48–2.43) | 0.839 | 4/65 (6.2) | 0.77 (0.25–2.66) | 0.684 |
The odds ratio (OR) and 95% CI were obtained by adjusting for other variables listed in the table.
3.4. Antibody titers by different characteristics
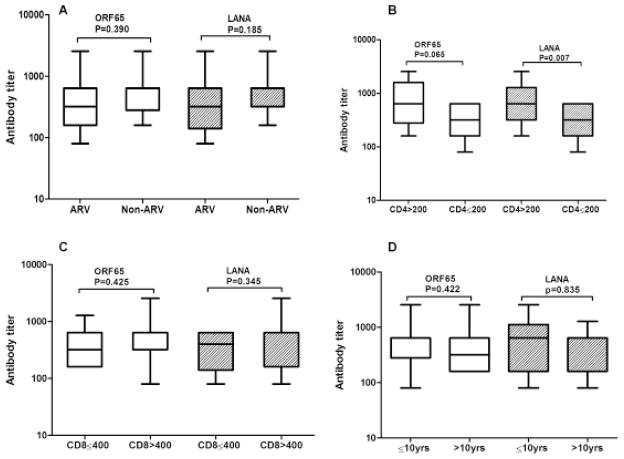
Geometric mean titers (GMT) of antibodies to lytic and latent antigens of HHV8 were compared according to the patient’s ART status, CD4+ count, CD8+ count, and duration of HIV infection. Patients with CD4+ T cell counts less than 200 cell/mm3 had lower antibody titers for latent antigen (p = 0.007) and lytic antigens (p = 0.065) (Figure 1). Antibody titers to lytic and latent antigens of HHV8 did not differ significantly with the patient’s ART status, CD8+ count, or duration of HIV infection (Figure 1).
Figure 1. Box-and-whisker plots of HHV8 lytic and latent antibody titer by HIV-related factors.
(A) Antibody titer for patients receiving ART and not receiving ART. (B) Antibody titer for patients with CD4 ≤ 200 cells/mm3 and CD4 > 200 cells/mm3. (C) Antibody titer for patients with CD8 ≤ 400 cells/mm3 and CD8 > 400 cells/mm3. (D) Antibody titer for patients infected with HIV ≤ 10 yr and those infected with HIV > 10 yr. Titers for different groups were calculated and compared using Mann-Whitney U. “yr” stands for years.
4. Discussion
The present study is the first to extensively examine seroprevalence and epidemiologic characteristics of antibodies to lytic and latent HHV8 antigens among Chinese HIV-infected patients. This study found a seroprevalence of 12.7% for antibodies to lytic antigen (ORF65), 10.4% for antibodies to latent antigen (LANA), 15.8% for antibodies to ANY of the two antigens, and 7.3% for antibodies to BOTH antigens. Previous studies from China often reported only an overall seroprevalence of antibodies to ANY lytic and latent antigens ranging from 16.3% to 43.2% (20–23), and higher than that (15.8%) in the present study. This further suggests that HHV8 infection is unevenly distributed across populations and geographical regions in China (24). Moreover, the present study, by showing individualized antibody responses to the two antigens, facilitates a better understanding of host immune responses to HHV8 infection.
Since the introduction of ART, a dramatic decline in the incidence of HIV/AIDS opportunistic infections has been witnessed worldwide (25,26). Data have shown that ART may also influence HHV8 infection among HIV-infected patients in a number of ways and decrease the risk of AIDS-KS (16,27,28). In the present study, both ART and CD4+ T cell counts were significantly correlated with HHV8 seropositivity, further suggesting the impact of ART on host immune responses to HHV8 infection.
In this study, HIV-infected patients who received ART treatment were less likely to be positive for both lytic (ORF65) and latent (LANA) antibodies, although the antibody titers of patients receiving ART and not receiving ART did not differ significantly. Since LANA is one of the few viral proteins expressed during latency and is one of the most immunogenetic HHV8 antigens (14), detection of antibodies to LANA is primarily used as a marker of an established persistent latent HHV8 infection. The current findings, consistent with results from the Swiss HIV Cohort Study (16), suggest that ART could have a positive effect on HHV8 infection. Nevertheless, whether or not ART can alter the latency of HHV8 infection remains an important scientific question that warrants further study in the near future.
CD4+ and CD8+ T cells play important roles in protection against intracellular pathogens including HHV8. In the present study, HIV-infected patients with CD4+ cell counts less than 200 cell/mm3 were consistently found to have a higher seroprevalence but a lower titer of both lytic (ORF65) and latent (LANA) antibodies. Previous studies also found that HIV-infected patients with CD4+ cell counts less than 200 cell/mm3 had a higher seroprevalence of lytic (ORF65) antibody (15,19). However, they found no association between CD4+ cell counts and seroprevalence of latent (LANA) antibody (15,19). A possible explanation for this discrepancy is that the current study included both patients who were ART-naïve and those receiving ART whereas the two cited studies included either only ART-naïve patients (15) or only patients receiving ART but not both. That said, the CD8+ cell count was not significantly correlated with antibody responses to any of the lytic and latent antigens. This is most likely due to the fact that CD8+ T-cells are mostly involved in cellular responses but not humeral responses.
In addition to HHV8 coinfection, HIV patients are also living with other pathogenic viral coinfections due to shared transmission modes (13). The current study found a high prevalence of coinfections with HBV, HCV, HSV-1, HSV-2, and EBV. However, none were found to be significantly associated with either of the lytic and latent HHV8 antibodies. This finding is consistent with that of a study conducted among a sample of HIV patients in the United States (19) but is inconsistent with that of a study conducted among a sample of Chinese patients with chronic hepatitis B (20). More intensive and extensive research is urgently needed to address such questions.
This study had a couple of limitations. First, the capacity to make valid causal inferences might be limited due to the nature of a cross-sectional study design. Second, none of the study participants had KS. Therefore, the potential relationship between host antibody responses to lytic and latent HHV8 antigens and KS risk has by no means been defined.
In conclusion, HIV patients in Central China had relatively high antibody seropositivity to lytic and latent HHV8 antigens, and this seropositivity was significantly associated with ART status and CD4+ cell counts. More extensive and prospective studies are urgently needed to address controversial findings and to better understand interactions between HHV8 and the host in the context of HIV-induced immunodeficiency being treated by or not being treated by ART.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81072345) to NH and from the United States National Institutes of Health Fogarty International Center (D43 TW001492), NCI (CA75903), and NCRR COBRE (RR15635) to CW.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Burbelo PD, Issa AT, Ching KH, Wyvill KM, Little RF, Iadarola MJ, Kovacs JA, Yarchoan R. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J Infect Dis. 2011;201:1919–1922. doi: 10.1086/652869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 4.Martin JN. The epidemiology of KSHV and its association with malignant disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Chapter 54 Cambridge: Cambridge University Press, Cambridge, London UK; 2007. [Google Scholar]
- 5.Centers for Disease Control. Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men – New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 6.Gottlieb GJ, Ragaz A, Vogel JV, Friedman-Kien A, Rywlin AM, Weiner EA, Ackerman AB. A preliminary communication on extensively disseminated Kaposi’s sarcoma in young homosexual men. Am J Dermatopathol. 1981;3:111–114. doi: 10.1097/00000372-198100320-00002. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: The Swiss HIV Cohort Study. Br J Cancer. 2010;103:416–422. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi S, Maso LD, Rickenbach M, Polesel J, Hirschel B, Cavassini M, Bordoni A, Elzi L, Ess S, Jundt G, Mueller N, Clifford GM. Br J Cancer. 2008;99:800–804. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001;15:629–633. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Dou Z, Yu L, Xu J, Jiao JH, Wang N, Ma Y, Zhao Y, Zhao H, Chen RY. The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47:825–833. doi: 10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao L, Xing H, Shang H, Li J, Zhong P, Kang L, Cheng H, Si X, Jiang S, Li X, Shao Y. The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. J Acquir Immune Defic Syndr. 2010;53 (Suppl 1):S10–S14. doi: 10.1097/QAI.0b013e3181c7d363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, Chen RY. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–251. W-52. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 13.He NCL, Lin HJ, Zhang M, Wei J, Yang JH, Gabrio J, Rui BL, Zhang ZF, Fu ZH, Ding YYZG, Jiang QW, Detels R. Multiple viral coinfections among HIV/AIDS patients in China. Biosci Trends. 2011;5:1–9. doi: 10.5582/bst.2011.v5.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Moore PS, Chang Y. Kaposi’s sarcoma associated herpesvirus. In: Knipe D, Howley P, Griffin D, Lamb R, Martin M, DS, editors. Fields’ virology. 4. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001. pp. 2803–2833. [Google Scholar]
- 15.Goudsmit J, Renwick N, Dukers NH, Coutinho RA, Heisterkamp S, Bakker M, Schulz TF, Cornelissen M, Weverling GJ. Human herpesvirus 8 infections in the Amsterdam Cohort Studies (1984–1997): Analysis of seroconversions to ORF65 and ORF73. Proc Natl Acad Sci U S A. 2000;97:4838–4843. doi: 10.1073/pnas.97.9.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan SG, Hirsch HH, Franceschi S, Steffen I, Amari EB, Mueller NJ, Magkouras I, Biggar RJ, Rickenbach M, Clifford GM Swiss HIV Cohort Study. Kaposi sarcoma herpes virus antibody response and viremia following highly active antiretroviral therapy in the Swiss HIV Cohort study. AIDS. 2010;24:2245–2252. doi: 10.1097/QAD.0b013e32833b7830. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, He N, Ding Y, Crabtree K, Minhas V, Wood C. Prevalence of human herpesvirus 8 and hepatitis C virus in a rural community with a high risk for blood-borne infections in central China. Clin Microbiol Infect. 2011;17:395–401. doi: 10.1111/j.1469-0691.2010.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minhas V, Crosby LN, Crabtree KL, Phiri S, M’soka TJ, Kankasa C, Harrington WJ, Mitchell CD, Wood C. Development of an immunofluorescence assay using recombinant proteins expressed in insect cells to screen and confirm presence of human herpesvirus 8-specific antibodies. Clin Vaccine Immunol. 2008;15:1259–1264. doi: 10.1128/CVI.00487-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guadalupe M, Pollock BH, Westbrook S, et al. Risk factors influencing antibody responses to Kaposi’s sarcoma-associated herpesvirus latent and lytic antigens in patients under antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;56:83–90. doi: 10.1097/QAI.0b013e3181fdc928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Ruan B, Chen Y, Wu N, Hu M, Zhu B. Kaposi’s sarcoma-associated herpesvirus infection in Chinese patients with chronic hepatitis B. J Med Virol. 2011;83:879–883. doi: 10.1002/jmv.22001. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Liu J, Dilimulati, Li L, Ren Z, Wen H, Wang X. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus and risk factors in Xinjiang, China. J Med Virol. 2009;81:1422–1431. doi: 10.1002/jmv.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He F, Wang X, He B, Feng Z, Lu X, Zhang Y, Zhao S, Lin R, Hui Y, Bao Y, Zhang Z, Wen H. Human herpesvirus 8: Serovprevalence and correlates in tumor patients from Xinjiang, China. J Med Virol. 2007;79:161–166. doi: 10.1002/jmv.20730. [DOI] [PubMed] [Google Scholar]
- 23.Mei Q, Ming ZW, Ping YX, Hui JJ, Bin ZY, Hong W, Juan L, Zhe CY, Wei T, Han Y. HHV-8 seroprevalence in blood donors and HIV-positive individuals in Shandong area, China. J Infect. 2007;55:89–90. doi: 10.1016/j.jinf.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Shao X, Chen Y, Minhas V, Wood C, He N. Human herpesvirus 8 seroprevalence, China. Emerg Infect Dis. 2012;18:150–152. doi: 10.3201/eid1801.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Ledergerber B, Tilling K, Weber R, Sendi P, Rickenbach M, Robins JM, Egger M Swiss HIV Cohort Study. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: A prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 26.Montaner JS, Wood E, Kerr T, Lima V, Barrios R, Shannon K, Harrigan R, Hogg R. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S5–S9. doi: 10.1097/QAI.0b013e3181f9c1f0. [DOI] [PubMed] [Google Scholar]
- 27.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, Bordoni A, De Weck D, Franceschi S Swiss HIV Cohort. Cancer risk in the Swiss HIV Cohort Study: Associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 28.Flores R, Goedert JJ. Reconstitution of immune responses against Kaposi sarcoma-associated herpesvirus. AIDS. 2010;24:2279–2281. doi: 10.1097/QAD.0b013e32833c7bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]