Abstract
Objective
To determine whether risk of endometrial cancer for women without a germline mutation in a DNA mismatch repair (MMR) gene depends on family history of endometrial or colorectal cancer.
Methods
We retrospectively followed a cohort of 79,166 women who were recruited to the Colon Cancer Family Registry, after exclusion of women who were relatives of a carrier of a MMR gene mutation. The Kaplan-Meier failure method was used to estimate the cumulative risk of endometrial cancer. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for association between family history of endometrial or colorectal cancer and risk of endometrial cancer.
Results
A total of 628 endometrial cancer cases were observed, with mean age at diagnosis of 54.4 (standard deviation 15.7) years. The cumulative risk of endometrial cancer to age 70 years was estimated to be 0.94% (95% CI 0.83–1.05) for women with no family history of endometrial cancer, and 3.80% (95% CI 2.75–4.98) for women with at least one first- or second-degree relative with endometrial cancer. Compared with women without family history, we found an increased risk of endometrial cancer for women with at least one first-or second-degree relative with endometrial cancer (HR 3.66, 95% CI 2.63–5.08), and for women with one first-degree relative with colorectal cancer diagnosed at age <50 years (HR 1.48, 95% CI 1.15–1.91).
Conclusion
An increased risk of endometrial cancer is associated with a family history of endometrial cancer or early-onset colorectal cancer for women without a MMR gene mutation; indicating for potential underlying genetic and environmental factors shared by colorectal and endometrial cancers other than caused by MMR gene mutations.
INTRODUCTION
Endometrial cancer is the most common gynaecological cancer and the fourth most common cancer in women in the United States [1]. It is estimated that 49,560 women will be newly diagnosed with and 8,190 will die of endometrial cancer in the United States in 2013 [2]. Endometrial cancer is diagnosed in women at a median age of 62 years with the highest incidence in post-menopausal women aged 55 to 74 years. The overall 5-year relative survival is estimated to be 81.5% but varies by stage at diagnosis, 95.3% for localized cancer and 16.9% for distant metastases. Hence, the early diagnosis of endometrial cancer is important to reduce cancer-related morbidity and mortality [2].
Several personal and lifestyle factors have been identified to be associated with an increased risk of the disease, including increasing age, obesity, use of estrogen only, early menarche, late menopause, nulliparity, polycystic ovarian syndrome, metabolic syndrome, anti-estrogen use, tamoxifen, diabetes, alcohol drinking, and red meat consumption. In contrast, cigarette smoking, use of estrogen and progesterone, intrauterine device, aspirin, increased age at last birth, physical activity, and consumption of dietary fiber, and fruit and vegetables are associated with a decreased risk of endometrial cancer (Supplementary Table 1). Apart from these lifestyle factors, one major genetic predisposition to endometrial cancer is a germline mutation in one of the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2 or EPCAM. Women who carry a MMR gene mutation are at substantially elevated risk of colorectal, endometrial, and several other cancers (Lynch syndrome) [3, 4].
There has been inconsistency on reporting a positive association between family history of endometrial cancer and risk of endometrial cancer [5–14], or between family history of colorectal cancer and risk of endometrial cancer [8, 11–14], or between family history of endometrial cancer and risk of colorectal cancer [6, 11, 15, 16]. All of these studies except two [12, 14] did not exclude Lynch syndrome families in which colorectal and endometrial cancers occurred in multiple family members. Further, these studies were limited as they only considered for first-degree relatives [5–13, 15, 16], or only included women aged <55 years [8, 10] or women aged 55 years and above [5, 9]. The aim of this study was to investigate whether risk of endometrial cancer for women without a MMR gene mutation depend on a family history of endometrial cancer or colorectal cancer.
MATERIALS AND METHODS
Study Cohort
This study comprised women from families who were recruited into the Colon Cancer Family Registry between 1997 and 2007 via people with a newly diagnosed colorectal cancer through population cancer registries (case-probands) in Australia (Victoria), Canada (Ontario), and the USA (Washington, California, Arizona, Minnesota, Colorado, New Hampshire, and North Carolina), or via people without any personal history of cancer (control-probands) through electoral rolls (Victoria, Australia), Medicare and Driver’s License files (Fred Hutchinson Cancer Research Centre, Seattle, USA), and telephone subscribers lists (Cancer Care Ontario, Canada) [17]. Written informed consent was obtained from all study participants, and the study protocol was approved by the institutional human ethics committee at each study center.
Data Collection
At recruitment, baseline information on demographics, personal characteristics, personal and family history of cancer, cancer screening history, hysterectomy, and other surgeries were obtained via questionnaires from all participants. Participants were given follow-up questionnaires at approximately every 5 years after baseline to update this information. The baseline and follow-up questionnaires are available at http://coloncfr.org. Reported cancer diagnoses and ages at which these occurred were confirmed, where possible, using pathology reports, medical records, cancer registry reports, and/or death certificates. Blood samples and permission to access tumor tissue were requested from all participants.
Molecular Characterization
All population-based case-probands’ colorectal cancer tumors were characterized for MMR-deficiency by microsatellite instability (MSI) using a ten-marker panel and/or by immunohistochemistry (IHC) for the four MMR proteins.[18] Tumors were classified as MMR-deficient if they were MSI-high (≥30% or more of the markers show instability) and/or showed loss of expression of one or more of the MMR proteins by IHC; and MMR-proficient if they were microsatellite stable (no unstable markers) or MSI-low (<30% unstable markers) and/or showed normal expression of all four MMR proteins by IHC. Probands with CRC that demonstrated MMR-deficiency underwent germline mutation testing.
Mutation testing for the MLH1, MSH2 and MSH6 genes was performed by Sanger sequencing or denaturing high performance liquid chromatography (dHPLC), followed by confirmatory DNA sequencing. Large duplication and deletion mutations including those involving EPCAM, which lead to MSH2 methylation, were detected by Multiplex Ligation Dependent Probe Amplification (MLPA) according to the manufacturer’s instructions (MRC Holland, Amsterdam, The Netherlands) [17, 19, 20]. PMS2 mutation testing involved a modified protocol from Senter et al. [21] where exons 1–5, 9 and 11–15 were amplified in three long range PCRs followed by nested exon specific PCR/sequencing while the remaining exons (6, 7, 8 and 10) were amplified and sequenced direct from genomic DNA. Large-scale deletions in PMS2 were detected using the P008-A1 MLPA kit (MRC Holland, Amsterdam, The Netherlands). The relatives of probands with pathogenic MMR germline mutation [3], who provided a blood sample, underwent testing for the specific mutation identified in the proband.
A fluorescent allele-specific PCR assay was used to detect the somatic T>A mutation at nucleotide 1799 in exon 15 of the BRAF gene (BRAF V600E) as has been previously described [22, 23]. Methylation of the MLH1 gene promoter was measured in all MSI-high and MSI-low cases with sufficient tumor DNA and a random sample of microsatellite stable cases. MLH1 methylation was measured using the MethyLight MLH1-M2Methylight reaction using an ALU control reaction to normalize for bisulfite-converted input DNA,[24] with the modifications described in Poynter et al.[25] We classified samples with a proportion of methylated reference (PMR) greater than or equal to 10 as positive for MLH1 methylation. An ALU control reaction cycle threshold [C(t)] value of <25 was used to retain the largest sample size possible for the analysis while minimizing the potential for false negatives.
In this study, we included first- and second-degree female relatives and female spouses of case-probands and control-probands. We excluded all female relatives of case-probands who were known to have a MMR gene mutation (confirmed Lynch syndrome), and all female relatives of case-probands with a colorectal cancer that had MLH1/PMS2 loss with no evidence of MLH1 methylation and/or BRAF V600E mutation or had MSH2/MSH6 loss or solitary loss of PMS2 or MSH6 or MSI-high, for which no MMR germline mutation had been identified (suspected Lynch syndrome). This analysis was therefore of women unlikely to be a carrier of a MMR gene mutation because: they were relatives of case-probands with no evidence of MMR-deficiency; or they were identified as being a relative of an unaffected woman (i.e., control-proband). We estimate that the probability of being a carrier of a MMR gene mutation in this cohort to be less than 1 in 3,000 (being the estimated prevalence of MMR gene mutations in the general population [26]).
Statistical Analysis
History of endometrial cancer in the first- and/or second-degree relative(s) and history of colorectal cancer in the first- and/or second-degree relative(s) was considered as the main exposures, while diagnosis of endometrial cancer was the main outcome. Observation time for woman began at birth and ended at: first diagnosis of cancer; hysterectomy; last contact; or death, whichever occurred earliest.
Kaplan-Meier failure method was used to estimate cumulative risks of endometrial cancer to age 50 and 70 years. For 95% confidence intervals (CIs) of the cumulative risks, we used the 2.5th and 97.5th percentiles from 10,000 bootstrap samples, using the family as the resampling unit to allow for clustering within families.
Cox proportional hazard regression was conducted to estimate risk of endometrial cancer associated with family history of endometrial cancer after adjusting for total number of first- and second-degree relatives with colorectal cancer (0, 1, ≥2), and with family history of colorectal cancer after adjusting for total number of first- and second-degree relatives with endometrial cancer (0, 1, ≥2). The hazard ratios (HRs) and robust estimates of corresponding 95% CIs were calculated by taking into account clustering by family membership to allow for correlation of risk between relatives from the same family [27, 28]. To account for stratified sampling based on family history, we gave each woman a weight equal to the reciprocal of the family sampling fractions used by each study center. All reported statistical tests were two-sided and p<0.05 was considered nominally statistically significant. All the analysis was performed using Stata 12.1 [29].
RESULTS
A total of 83,475 women from 14,475 families with information available on family history of cancer, personal history of cancer and age at diagnosis, were identified from population-based resources of the Colon Cancer Family Registry. We excluded 2,057 women from 203 confirmed Lynch syndrome families and 2,252 women from 271 suspected Lynch syndrome families; resulting 79,166 women from 14,001 families included in the analyses. They included 33,495 first-degree relatives, 25,640 second-degree relatives and 5,387 spouses of 9,943 case-probands; and 12,536 first-degree relatives, 2,104 second-degree relatives and 4 spouses of 4058 control-probands. We observed that 4,146 (5.2%) women had at least one first- or second-degree relative diagnosed with endometrial cancer and 61,262 (77.4%) had at least one first- or second-degree relative diagnosed with colorectal cancer (Table 1). Of these, 628 (0.8%) women were diagnosed with endometrial cancer at mean age of 54.4 (standard deviation 15.7) years. Overall, the cumulative risk of endometrial cancer to age 70 years was estimated to be 1.07% (95% CI 0.96–1.19) (Table 2).
Table 1.
Baseline characteristics of women with and without a diagnosis of endometrial cancer (Total = 79,166)
| Variables | Women with endometrial cancer (N=628) |
Women without endometrial cancer (N=78,538) |
|---|---|---|
| Age (years)* | ||
| Mean (SD) | 54.4 (15.7) | 55.0 (22.6) |
| Median (range) | 55 (16–95) | 55 (1–100) |
| Country | ||
| Canada | 277 (44.1) | 30,842 (39.3) |
| Australia | 82 (13.1) | 14,926 (19.0) |
| United States | 269 (42.8) | 32,770 (41.7) |
| Race | ||
| Caucasian | 129 (20.5) | 12,402 (15.8) |
| Others** | 9 (1.5) | 1,023 (1.3) |
| Missing | 490 (78.0) | 65,113 (82.9) |
| Educational status | ||
| Grade 10 or below | 41 (6.53) | 4,218 (5.37) |
| Grade 11–12 | 32 (5.10) | 2,563 (3.26) |
| Vocational and Technical School | 17 (2.71) | 1,057 (1.35) |
| University degree | 39 (6.21) | 4,404 (5.61) |
| Missing | 499 (79.5) | 66,296 (84.4) |
| Family History of endometrial cancer | ||
| No family history | 516 (82.2) | 74504 (94.9) |
| 1 FDR or SDR | 87 (13.8) | 3709 (4.7) |
| ≥2 FDR or SDR | 25 (4.0) | 325 (0.4) |
| Family History of colorectal cancer | ||
| No family history | 120 (19.1) | 17,784 (22.6) |
| 1 FDR or SDR | 334 (53.2) | 42,598 (54.2) |
| ≥2 FDR or SDR | 174 (27.7) | 18,156 (23.1) |
| Cigarette smoking | ||
| Never | 79 (12.6%) | 7,973 (10.2%) |
| Ever | 59 (9.4%) | 5,111 (6.5%) |
| Missing | 490 (78.0) | 65,454 (83.3) |
| Age at menarche (years) | ||
| Mean (SD) | 12.6 (2.49) | 12.9 (1.57) |
| <12 | 30 (4.8) | 1,428 (1.8) |
| ≥12 to <15 | 63 (10.0) | 5,951 (7.6) |
| ≥15 | 7 (1.1) | 1,217 (1.6) |
| Missing | 528 (84.1) | 69,942 (89.0) |
| Menopausal status | ||
| Premenopausal | 2 (0.3) | 155 (0.2) |
| Postmenopausal | 87 (13.9) | 4,254 (5.4) |
| Missing | 539 (85.8) | 74,129 (94.4) |
| Age at menopause (years) | ||
| Mean (SD) | 41.2 (10.3) | 38.2 (10.4) |
| ≤50 | 75 (11.9) | 3162 (4.0) |
| >50 to ≤55 | 6 (1.0) | 706 (0.9) |
| >55 | 4 (0.6) | 147 (0.2) |
| Missing | 543 (86.5) | 74,523 (94.9) |
| Hormone replacement therapy users | ||
| Never | 86 (13.7) | 9,480 (12.1) |
| Ever | 43 (6.8) | 2,292 (2.9) |
| Missing | 499 (79.5) | 66,766 (85.0) |
| Estrogen only users | ||
| Never | 6 (1.0) | 689 (0.9) |
| Ever | 36 (5.7) | 1,482 (1.9) |
| Missing | 586 (93.3) | 76,367 (97.2) |
| Progesterone users | ||
| Never | 30 (4.8) | 1,166 (1.5) |
| Ever | 11 (1.7) | 974 (1.2) |
| Missing | 587 (93.5) | 76,398 (97.3) |
| Anti-estrogen use status | ||
| Never | 87 (13.9) | 7,740 (9.9) |
| Ever | 5 (0.8) | 233 (0.3) |
| Missing | 536 (85.3) | 70,565 (89.8) |
| Pregnancy | ||
| Never | 10 (1.6) | 2,142 (2.7) |
| Ever | 130 (20.7) | 11,362 (14.5) |
| Missing | 488 (77.7) | 65,034 (82.8) |
| Body mass index (kg/m2) at age 20 | ||
| Mean (SD) | 22.3 (4.65) | 21.8(5.10) |
| <25 | 80 (12.7) | 7,228 (9.2) |
| ≥25 to <30 | 13 (2.1) | 754 (1.0) |
| ≥30 | 4 (0.6) | 302 (0.4) |
| Missing | 531 (84.6) | 70,254 (89.4) |
| Recent body mass index (kg/m2) | ||
| Mean (SD) | 28.5 (6.6) | 25.7 (5.70) |
| <25 | 32 (5.1) | 4,128 (5.2) |
| ≥25 to <30 | 26 (4.1) | 2,011 (2.6) |
| ≥30 | 31 (5.0) | 1,402 (1.8) |
| Missing | 539 (85.8) | 70,997 (90.4) |
| Diabetes (both Type I and Type II) | ||
| Never | 85 (13.5) | 8,273 (10.5) |
| Ever | 16 (2.6) | 496 (0.6) |
| Missing | 527 (83.9) | 69,769 (88.8) |
Age at diagnosis for women with endometrial cancer and last known age for women without endometrial cancer.
Other races included African American, Latino, Hispanic, Mexican American, Mexican, Cuban, Puerto Rican, Japanese, Chinese, Filipino, Malay, Indonesian, Korean, South Asian, Native American, Polynesian, Micronesian, Australian aboriginal, Melanesian, Caribbean Black, Central/South American, Black African, North African, Middle Eastern and others.
SD, standard deviation.
Table 2.
Cumulative risk of endometrial cancer to age 50 and 70 years
| Variables | Percentage cumulative risk (95% confidence interval) to age |
|
|---|---|---|
| 50 years | 70 years | |
| All Women | 0.38 (0.33–0.44) | 1.07 (0.96–1.19) |
| Family history of endometrial cancer | ||
| No family history | 0.32 (0.27–0.37) | 0.94 (0.83–1.05) |
| ≥1 FDR or SDR | 1.62 (1.11–2.18) | 3.80 (2.75–4.98) |
| 1 FDR or SDR | 1.33 (0.87–1.87) | 3.40 (2.32–4.66) |
| ≥2 FDR or SDR | 4.57 (0.36–4.86) | 7.94 (1.04–9.12) |
| Family history of colorectal cancer | ||
| No family history | 0.43 (0.31–0.58) | 1.14 (0.90–1.40) |
| ≥1 FDR or SDR | 0.37 (0.31–0.44) | 1.06 (0.93–1.20) |
| 1 FDR or SDR | 0.37 (0.29–0.44) | 1.02 (0.87–1.18) |
| ≥2 FDR or SDR | 0.38 (0.28–0.51) | 1.17 (0.93–1.43) |
FDR, first-degree relative; SDR, second-degree relative.
Association with family history of endometrial cancer
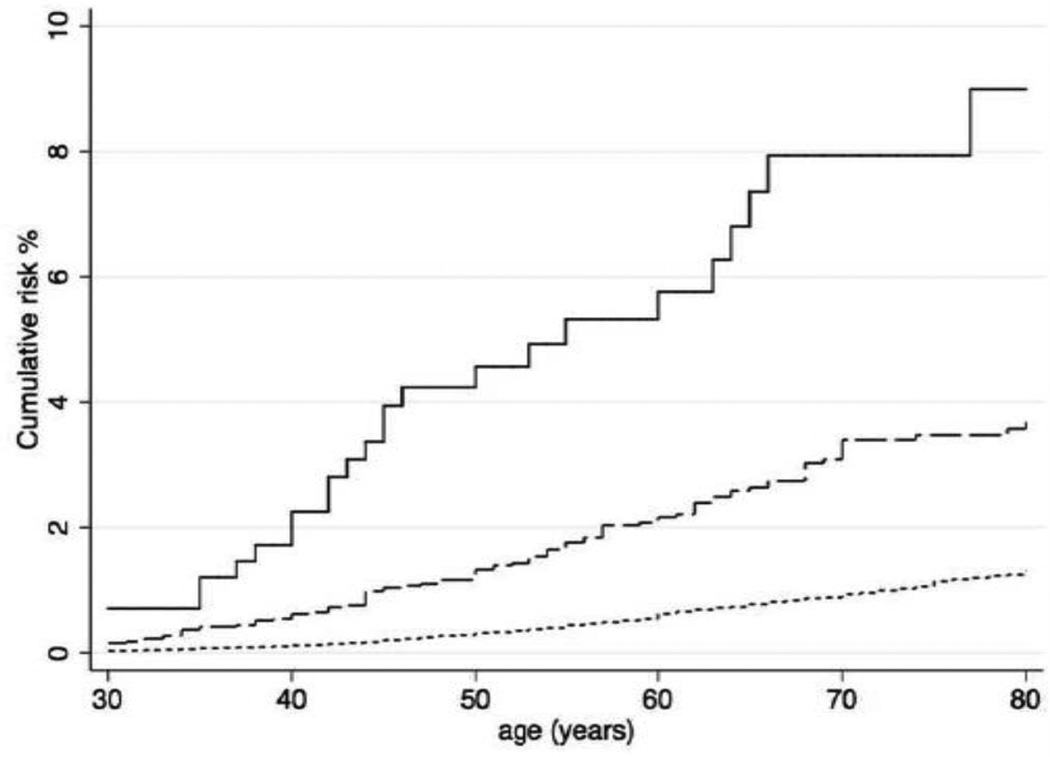
The cumulative risk of endometrial cancer to age 70 years was estimated to be 0.94% (95% CI 0.83–1.05) for women without family history of endometrial cancer, 3.40% (95 CI 2.32–4.66) for women with one first- or second-degree relative with endometrial cancer, and 7.94% (95% CI 1.04–9.12) for women with two or more first- or second-degree relatives with endometrial cancer (Figure 1).
Figure 1.
Cumulative risks of endometrial cancer for women by family history of endometrial cancer. For women without a family history of endometrial cancer (dotted lines), women with one first- or second-degree relative with endometrial cancer (dashed lines), and women with two or more first- or second-degree relatives with endometrial cancer (unbroken lines).
Compared with women without family history of endometrial cancer, we found an increased risk of endometrial cancer for women with one first- or second-degree relative with endometrial cancer (HR 3.20, 95% CI 2.23–4.59) and for women with two or more first- or second-degree relatives with endometrial cancer (HR 8.73, 95% CI 4.25–17.9) after adjusting for family history of colorectal cancer. The strength of association with family history of endometrial cancer was higher if multiple relatives were affected, if the relatives with endometrial cancer were more closely related, and if ages at diagnoses of endometrial cancer in relatives were younger (Table 3). For example, risk of endometrial cancer was increased 14.2-fold (95% CI 5.70–36.3) if a woman had two or more first-degree relatives with endometrial cancer. Supplementary Table 2 shows that strength of association with for specific combinations of family history of endometrial cancer depending on the numbers of relative affected and the degree of relatedness to an affected relative.
Table 3.
Associations between family history and risk of endometrial cancer by the numbers of affected first- and second-degree relatives and age at diagnosis of cancer
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Family history of endometrial cancer* | |||
| No family history of EC | 1.00 | referent | |
| ≥1 FDR or SDR | 3.66 | 2.63–5.08 | <0.001 |
| 1 FDR or SDR | 3.20 | 2.23–4.59 | <0.001 |
| ≥2 FDR or SDR | 8.73 | 4.25–17.9 | <0.001 |
| First-Degree relatives | |||
| ≥1 FDR | 4.99 | 3.50–7.12 | <0.001 |
| 1 FDR | 4.42 | 3.01–6.49 | <0.001 |
| 1 FDR <50 | 6.68 | 4.02–11.1 | <0.001 |
| 1 FDR ≥50 | 3.38 | 2.24–5.09 | <0.001 |
| ≥2 FDR | 14.2 | 5.70–36.3 | <0.001 |
| ≥2 FDR <50 | 140 | 50.4–393 | <0.001 |
| ≥2 FDR ≥50 | 6.51 | 2.05–20.7 | 0.002 |
| Second-Degree relatives | |||
| ≥1 SDR | 1.83 | 1.13–2.98 | 0.02 |
| 1 SDR | 1.83 | 1.12–3.01 | 0.02 |
| 1 SDR <50 | 2.84 | 1.58–5.10 | <0.001 |
| 1 SDR ≥50 | 1.21 | 0.66–2.21 | 0.54 |
| ≥2 SDR | 1.77 | 0.43–7.30 | 0.43 |
| ≥2 SDR <50 | – | – | |
| ≥2 SDR ≥50 | 1.99 | 0.25–15.5 | 0.51 |
| Family history of colorectal cancer** | |||
| No family history | 1.00 | referent | |
| ≥1 FDR or SDR | 0.96 | 0.76–1.21 | 0.76 |
| 1 FDR or SDR | 0.96 | 0.75–1.23 | 0.74 |
| ≥2 FDR or SDR | 0.98 | 0.74–1.29 | 0.87 |
| First-Degree relatives | |||
| ≥1 FDR | 1.17 | 0.96–1.42 | 0.11 |
| 1 FDR | 1.12 | 0.91–1.38 | 0.27 |
| 1 FDR <50 | 1.48 | 1.15–1.91 | 0.002 |
| 1 FDR ≥50 | 1.01 | 0.82–1.24 | 0.92 |
| ≥2 FDR | 1.38 | 1.05–1.82 | 0.02 |
| ≥2 FDR <50 | 2.77 | 1.37–5.58 | 0.004 |
| ≥2 FDR ≥50 | 1.19 | 0.88–1.61 | 0.25 |
| Second-Degree relatives | |||
| ≥1 SDR | 0.92 | 0.76–1.11 | 0.37 |
| 1 SDR | 0.91 | 0.75–1.12 | 0.38 |
| 1 SDR <50 | 1.19 | 0.93–1.52 | 0.17 |
| 1 SDR ≥50 | 0.83 | 0.66–1.04 | 0.11 |
| ≥2 SDR | 0.93 | 0.65–1.31 | 0.67 |
| ≥2 SDR <50 | 1.24 | 0.56–2.75 | 0.60 |
| ≥2 SDR ≥50 | 0.82 | 0.53–1.27 | 0.38 |
FDR, first-degree relative; SDR, second-degree relative; <50, age at diagnosis less than 50 years; ≥50, age at diagnosis at 50 years and above.
All estimates were adjusted for family history of colorectal cancer.
All estimates were adjusted for family history of endometrial cancer.
Hazard ratio could not be estimated due to small numbers.
Association with family history of colorectal cancer
The cumulative risk of endometrial cancer to age 70 years was estimated to be 1.14% (95% CI 0.90–1.40) for women with no family history of colorectal cancer, 1.02% (95% CI 0.87–1.18) for women with one first- or second-degree relative with colorectal cancer, and 1.17% (95% CI 0.93–1.43) for women with two or more first- or second-degree relatives with colorectal cancer (Table 2).
Overall, we found no evidence of an association between family history of colorectal cancer and an increased risk of endometrial cancer. When we investigated the association by the degree of relatedness to the affected relative(s), number of affected relative(s), and ages at diagnosis of colorectal cancer, we found an increased risk of endometrial cancer for women with one first-degree relative with colorectal cancer diagnosed at age <50 years (HR 1.48, 95% CI 1.15–1.91), for women with two or more first-degree relatives with colorectal cancer (HR 1.38, 95% CI 1.05–1.82), and for women with two or more first-degree relatives with colorectal cancer diagnosed at age <50 years (HR 2.77, 95% CI 1.37–5.58) (Table 3).
DISCUSSION
In this study, for women presumed to not be a carrier of a germline mutation in a DNA MMR gene we found a family history of at least one first- or second-degree relative with endometrial cancer increased risk of endometrial cancer compared with those without a family history. The magnitude of an increased risk of endometrial cancer was higher if women had multiple affected relatives, closer degree of relatedness to the affected relative, and younger onset of cancer in the affected relatives with endometrial cancer. Further, we found an increased risk of endometrial cancer if at least one first-degree relative is diagnosed with colorectal cancer at age <50 years though we found no evidence of association between family history of colorectal cancer in first- and second-degree relatives and risk of endometrial cancer for overall.
Our estimated cumulative risk of endometrial cancer to age 50 years and 70 years (0.38% and 1.07% respectively) for all women are comparable with the Surveillance, Epidemiology and End Results (SEER) data for the United States general population (0.27% at age 50 and 1.63% at age 70) [2].
To our knowledge, none of the previous studies [5–11, 13] except two [12, 14] excluded women from families with Lynch syndrome and, therefore, the reported associations of endometrial cancer with family history of endometrial or colorectal cancer may be attributable, in part, to underlying MMR gene mutations. Approximately 2% of all endometrial cancer cases and 9% of endometrial cancer cases diagnosed before age 50 years are due to Lynch syndrome [30–33]. Lorenzo Bermejo et al. [12] found that first-degree relatives of colorectal cancer cases had an increased risk of endometrial cancer after excluding families that met Amsterdam-I [34] or Amsterdam-II [35] or modified Amsterdam [36, 37] criteria (OR 1.76, 95% CI 1.12–2.62 for siblings; and OR 1.26, 95% CI 1.05–1.49 for offspring). Cook et al. [14] found that women with at least one first- or second-degree relative with colorectal cancer had an increased risk of endometrial cancer (OR 1.4, 95% CI 1.0–2.2) with high-MSI (a phenotype of loss of MMR function caused either by a somatic or germline mutation or methylation in a MMR gene) after excluding families that met the Amsterdam-II criteria [35].
The finding from our study together with the previous two studies [12, 14] support that an increased risk of endometrial cancer is associated with a family history of endometrial cancer or colorectal cancer for women without a MMR gene mutation. That is, MMR gene mutations explain only a small fraction of the familial risk of endometrial cancer. The remaining familial risk may be explained by other genetic and environmental factors shared between the family members. Recently, Win et al. [38] reported that monoallelic mutations in MUTYH are likely to be associated with an increased risk of both colorectal and endometrial cancers. The shared lifestyle factors within families (e.g., dietary habits, obesity, etc.) and other diseases common within families (e.g., diabetes) may also influence the familial risk of endometrial cancer unexplained by MMR gene mutations.
Several studies reported an association between family history of endometrial cancer and risk of endometrial cancer [5–11, 13]; however, none of these studies adjusted for having a family history of colorectal cancer. Consistent with our finding, Parazzini et al. [7] and Dong and Hemminki [11] found about 2- to 4-fold increased risk of endometrial cancer for women with a first-degree relative with endometrial cancer compared with those without a family history. For women aged <55 years, Gruber and Thompson [8] and Parslov et al. [10] found having at least one first-degree relative with endometrial cancer is a risk factor of endometrial cancer (odds ratio [OR] 2.8, 95% CI 1.9–4.2; and OR 2.1, 95% CI 1.1–3.8 respectively). For women aged 55 years and above, Nelson et al. [5] found an increased risk of endometrial cancer is associated with having a first-degree relative with endometrial cancer (OR 1.85, 95% CI 1.44–2.37). Other studies found weak or marginal evidence of an association between family history of endometrial cancer and risk of endometrial cancer [6, 9, 13].
Previous studies have been inconsistent on reporting an association between family history of colorectal cancer and risk of endometrial cancer [5, 8, 9, 11, 13]. Gruber and Thompson [8] found an increased risk of endometrial cancer (OR 1.8, 95% CI 1.0–3.2) for women aged <55 years with at least one first-degree relative with colorectal cancer in a population-based case-control study. Lucenteforte et al. [13] also found an increased risk of endometrial cancer (OR 1.6, 95% CI 1.0–2.7) for women with at least one first-degree relative with intestinal (mainly colorectal) cancer. In a retrospective cohort study, Dong and Hemminki [11] found an increased risk of endometrial cancer for women having a parent with colon cancer (standardized incidence ratio [SIR] 1.46, 95% CI 1.14–1.84) or for women having a sibling with colon cancer (SIR 2.69, 95% CI 1.28–4.96). In contrast, Nelson et al. [5] and Olsan et al. [9] found no evidence of an association between family history of colon cancer and risk of endometrial cancer for women aged 55–69 years. However, all of these studies did not adjust for having a family history of colorectal cancer and therefore these study estimates might be likely to be biased upward.
There is no standard or routine screening test recommended for endometrial cancer [39]. However, approximately 70% of endometrial cancer cases are diagnosed at an early stage because of postmenopausal bleeding [1] and, therefore, women with a family history of endometrial cancer or early-onset colorectal cancer should report any unexpected bleeding or spotting to their physicians for early diagnosis of endometrial cancer by a transvaginal ultrasound scan and an endometrial biopsy [40].
The strengths of our study included: (i) both confirmed and suspected Lynch syndrome families were excluded from the analyses to investigate association of endometrial cancer with family history of endometrial or colorectal cancer; (ii) associations were investigated after adjusting for family history of endometrial or colorectal cancer; (iii) associations were evaluated for in relation with family history of cancer by the number of affected relatives, degree of relatedness to the affected relative(s), and age at diagnosis in the affected relative(s); (iv) we used a very large dataset from population-based resources of the Colon Cancer Family Registry that used standardized and validated protocols uniformly across the study centers to collect data across three countries; and (v) our application of weights to each relative depending on the different sampling strategies would minimize any selection bias due to sampling based on family history.
The limitations of our study included: (i) our estimates are based on family histories of endometrial or colorectal cancer that were self-reported or reported by their relatives and, therefore, likely to be biased due to recall bias; (ii) we had limited data on environmental factors in this cohort and, therefore, were not able to adjust for them as potential confounders in assessing associations between family history of cancer and risk of endometrial cancer; and (iii) we excluded families with confirmed and suspected Lynch syndrome only based on MMR-deficiency status of case-proband’s colorectal cancer tumor, and therefore, there was a small probability that this study cohort may contain unidentified women with a MMR gene mutation given they were not tested for MMR germline mutations.
In summary, we found that women with a family history of endometrial or colorectal cancer had an increased risk of endometrial cancer depending on the number of relatives affected, degree of relatedness, and age at diagnosis in affected relatives. These findings might be clinically useful for early detection of endometrial cancer, and indicative for potential underlying genetic and environmental factors shared by colorectal and endometrial cancers other than caused by MMR gene mutations.
Supplementary Material
Highlights.
It was not known whether endometrial cancer risk for women without a mismatch repair gene mutation depend on family history of endometrial or colorectal cancer.
We found that having a family history of endometrial cancer or early-onset colorectal cancer increased their risk of endometrial cancer.
This might be clinically useful for early detection of endometrial cancer, and indicative for potential underlying genetic and environmental factors.
ACKNOWLEDGEMENTS
The authors thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
FUNDING
This work was supported by grant UM1 CA167551 from the National Cancer Institute, National Institutes of Health and through cooperative agreements with members of the Colon Cancer Family Registry and Principal Investigators. Collaborating centers include Australasian Colorectal Cancer Family Registry, University of Southern California (USC) Consortium, Mayo Clinic Cooperative Family Registry for Colon Cancer Studies, Ontario Registry for Studies of Familial Colorectal Cancer, Seattle Colorectal Cancer Family Registry, and University of Hawaii Colorectal Cancer Family Registry. RB was supported by the Endeavour Postgraduate Scholarship, Australia. AKW was supported by the Picchi Brothers Foundation Cancer Council Victoria Cancer Research Scholarship, Australia. MAJ is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow. JLH is a NHMRC Senior Principal Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
DISCLOSURE
The authors have no conflict of interest to declare with respect to this manuscript.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, et al. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 3.Win AK, Young JP, Lindor NM, Tucker K, Ahnen D, Young GP, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Win AK, Lindor NM, Winship I, Tucker KM, Buchanan DD, Young JP, et al. Risks of Colorectal and Other Cancers After Endometrial Cancer for Women With Lynch Syndrome. J Natl Cancer Inst. 2013;105:274–279. doi: 10.1093/jnci/djs525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CL, Sellers TA, Rich SS, Potter JD, McGovern PG, Kushi LH. Familial clustering of colon, breast, uterine, and ovarian cancers as assessed by family history. Genet Epidemiol. 1993;10:235–244. doi: 10.1002/gepi.1370100404. [DOI] [PubMed] [Google Scholar]
- 6.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic Population-Based Assessment of Cancer Risk in First-Degree Relatives of Cancer Probands. J Natl Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 7.Parazzini F, La Vecchia C, Moroni S, Chatenoud L, Ricci E. Family history and the risk of endometrial cancer. Int J Cancer. 1994;59:460–462. doi: 10.1002/ijc.2910590404. [DOI] [PubMed] [Google Scholar]
- 8.Gruber SB, Thompson WD Cancer and Steroid Hormone Study Group. A population-based study of endometrial cancer and familial risk in younger women. Cancer Epidemiol Biomarkers Prev. 1996;5:411–417. [PubMed] [Google Scholar]
- 9.Olson JE, Sellers TA, Anderson KE, Folsom AR. Does a family history of cancer increase the risk for postmenopausal endometrial carcinoma? A prospective cohort study and a nested case-control family study of older women. Cancer. 1999;85:2444–2449. [PubMed] [Google Scholar]
- 10.Parslov M, Lidegaard Ø, Klintorp S, Pedersen B, Jønsson L, Eriksen PS, et al. Risk factors among young women with endometrial cancer: A Danish case-control study. Am J Obstet Gynecol. 2000;182:23–29. doi: 10.1016/s0002-9378(00)70486-8. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer. 2001;92:144–150. [PubMed] [Google Scholar]
- 12.Lorenzo Bermejo J, Buchner FL, Hemminki K. Familial risk of endometrial cancer after exclusion of families that fulfilled Amsterdam, Japanese or Bethesda criteria for HNPCC. Ann Oncol. 2004;15:598–604. doi: 10.1093/annonc/mdh135. [DOI] [PubMed] [Google Scholar]
- 13.Lucenteforte E, Talamini R, Montella M, Dal Maso L, Pelucchi C, Franceschi S, et al. Family history of cancer and the risk of endometrial cancer. Eur J Cancer Prev. 2009;18:95–99. doi: 10.1097/CEJ.0b013e328305a0c9. [DOI] [PubMed] [Google Scholar]
- 14.Cook LS, Nelson HE, Stidley CA, Dong Y, Round PJ, Amankwah EK, et al. Endometrial cancer and a family history of cancer. Gynecol Oncol. 2013;130:334–339. doi: 10.1016/j.ygyno.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst. 1994;86:1618–1626. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 16.Jang JH, Cotterchio M, Gallinger S, Knight JA, Daftary D. Family history of hormonal cancers and colorectal cancer risk: a case-control study conducted in Ontario. Int J Cancer. 2009;125:918–925. doi: 10.1002/ijc.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 18.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry Versus Microsatellite Instability Testing in Phenotyping Colorectal Tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 19.Southey MC, Jenkins MA, Mead L, Whitty J, Trivett M, Tesoriero AA, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–6532. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- 20.Rumilla K, Schowalter KV, Lindor NM, Thomas BC, Mensink KA, Gallinger S, et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2-associated Lynch syndrome cases. J Mol Diagn. 2011;13:93–99. doi: 10.1016/j.jmoldx.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The Clinical Phenotype of Lynch Syndrome Due to Germ-Line PMS2 Mutations. Gastroenterology. 2008;135:419–428. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchanan DD, Sweet K, Drini M, Jenkins MA, Win AK, English DR, et al. Risk Factors for Colorectal Cancer in Patients with Multiple Serrated Polyps: A Cross-Sectional Case Series from Genetics Clinics. PLoS One. 2010;5:e11636. doi: 10.1371/journal.pone.0011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchanan DD, Win AK, Walsh MD, Walters RJ, Clendenning M, Nagler BN, et al. Family History of Colorectal Cancer in BRAF p.V600E mutated Colorectal Cancer Cases. Cancer Epidemiol Biomarkers Prev. 2013;22:917–926. doi: 10.1158/1055-9965.EPI-12-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–6836. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, Lindor N, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlop MG, Farrington SM, Nicholl I, Aaltonen L, Petersen G, Porteous M, et al. Population carrier frequency of hMSH2 and hMLH1 mutations. Br J Cancer. 2000;83:1643–1645. doi: 10.1054/bjoc.2000.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;3:19–23. [Google Scholar]
- 28.Williams RL. A Note on Robust Variance Estimation for Cluster-Correlated Data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 30.Ollikainen M, Abdel-Rahman WM, Moisio A-L, Lindroos A, Kariola R, Järvelä I, et al. Molecular Analysis of Familial Endometrial Carcinoma: A Manifestation of Hereditary Nonpolyposis Colorectal Cancer or a Separate Syndrome? J Clin Oncol. 2005;23:4609–4616. doi: 10.1200/JCO.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 31.Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. 2003;100:5908–5913. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 33.Berends MJ, Wu Y, Sijmons RH, van der Sluis T, Ek WB, Ligtenberg MJ, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol. 2003;21:4364–4370. doi: 10.1200/JCO.2003.04.094. [DOI] [PubMed] [Google Scholar]
- 34.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 35.Vasen HFA, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 36.Bellacosa A, Genuardi M, Anti M, Viel A, Ponz de Leon M. Hereditary nonpolyposis colorectal cancer: review of clinical, molecular genetics, and counseling aspects. Am J Med Genet. 1996;62:353–364. doi: 10.1002/(SICI)1096-8628(19960424)62:4<353::AID-AJMG7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Benatti P, Sassatelli R, Roncucci L, Pedroni M, Fante R, Di Gregorio C, et al. Tumour spectrum in hereditary non-polyposis colorectal cancer (HNPCC) and in families with "suspected HNPCC". A population-based study in northern Italy. Colorectal Cancer Study Group. Int J Cancer. 1993;54:371–377. doi: 10.1002/ijc.2910540304. [DOI] [PubMed] [Google Scholar]
- 38.Win AK, Cleary SP, Dowty JG, Baron JA, Young JP, Buchanan DD, et al. Cancer risks for monoallelic MUTYH mutation carriers with a family history of colorectal cancer. Int J Cancer. 2011;129:2256–2262. doi: 10.1002/ijc.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Comprehensive Cancer Network. Uterine Neoplasms. National Comprehensive Cancer Network; 2013. NCCN Clinical Practice Guidelines in Oncology Version 1.2013. [Google Scholar]
- 40.Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. BMJ. 2011;343:d3954. doi: 10.1136/bmj.d3954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.