Abstract
Cytokines and neuropeptides are modulators of neuroimmunoregulation in the central nervous system (CNS). The interaction of these modulators may have important implications in CNS diseases. We investigated whether interleukin-1β (IL-1β) modulates the expression of neurokinin-1 receptor (NK-1R), the primary receptor for substance P (SP), a potent neuropeptide in the CNS. IL-1β upregulated NK-1R expression in human astroglioma cells (U87 MG) and primary rat astrocytes at both mRNA and protein levels. IL-1β treatment of U87 MG cells and primary rat astrocytes led to an increase in cytosolic Ca2+ in response to SP stimulation, indicating that IL-1β-induced NK-1R is functional. CP-96,345, a specific non-peptide NK-1R antagonist, inhibited SP-induced rise of [Ca2+]i in the astroglioma cells. Investigation of the mechanism responsible for IL-1β action revealed that IL-1β has the ability of activating nuclear factor-κb (NF-κB). Caffeic acid phenethyl ester (CAPE), a specific inhibitor of NF-κB activation, not only abrogated IL-1β-induced NF-κB promoter activation, but also blocked IL-1β-mediated induction of NK-1R gene expression. These findings provide additional evidence that there is a biological interaction between IL-1β and the neuropeptide SP in the CNS, which may have important implications in the inflammatory diseases in the CNS.
Keywords: substance P, neurokinin-1 receptor, interleukin-1β, U87 MG, astrocytes
INTRODUCTION
There is a unique bidirectional communication between the central nervous system (CNS) and the immune system (Benveniste, 1992; Hopkins and Rothwell, 1995; De Simoni and Imeri, 1998; Maier et al., 1998). Cytokines have a dynamic role in this communication (Benveniste, 1992; Hopkins and Rothwell, 1995; De Simoni and Imeri, 1998; Maier et al., 1998). Interleukin-1β (IL-1β), a key inflammatory cytokine, has a key role in the induction of complex immune response to antigens, malignant cells, inflammatory stimuli, and tissue injury (Dinarello, 1988; Fibbe et al., 1989). IL-1β is produced by a wide variety of cell types, including glia and neuronal cells (Ma et al., 2002). The human CNS contains receptors for IL-1, which is important in mediating the acute-phase response (Breder et al., 1988). Increased IL-1β in the CNS is involved in the induction of fever, sickness behavior, and neuroendocrine signaling (Dantzer et al., 1991; Rothwell, 1991; Rothwell and Hopkins, 1995).
Substance P (SP), a neuropeptide in the tachykinin family, modulates neuroimmunoregulation (Ho et al., 2002). SP is involved in immune responses and inflammation within the central and peripheral nervous systems (Ho et al., 2002). SP functions on target cells through its receptor, neurokinin-1 receptor (NK-1R). Increased numbers of SP receptors are expressed in blood vessels at sites of peripheral inflammatory lesions (Mantyh et al., 1988). SP binding sites are highly expressed by glia in vivo after neuronal injury. Initially described as a peptide of neuronal origin, SP is also identified in non-neuronal cell types, including human immune cells (Ho et al., 1997; Lai et al., 1998, 2002). SP activates nuclear factor-κB (NF-κB), a key transcription factor involved in the control of cytokine expression (Lieb et al., 1997; Marriott et al., 2000). SP stimulates the immune cells to produce inflammatory cytokines IL-1, IL-6, IL-12, and tumor necrosis factor-α (TNF-α) and chemokines (Guo et al., 2002; Kanda and Watanabe, 2002). Since the SP-NK-1R pathway and IL-1β are involved in the CNS inflammatory diseases, we investigated whether IL-1β regulates NK-1R expression in astrocytes.
MATERIALS AND METHODS
Cells
Human astroglioma cells (U87-MG) were obtained from the American Type Tissue Culture (ATCC; Manassas, VA) and maintained in DMEM with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat inactive (HI)-fetal bovine serum (HI-FBS).
Primary rat astrocyte cultures were prepared from the forebrains of 1-day-old Sprague-Dawley rats and cultured in100-mm petri dishes in serum-containing medium for 24 h as previously described (Grinspan and Franceschini, 1995). The cultures were then washed once with Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+; DMEM with 10% FCS was added. The cell medium was replaced every other day. Neurons do not survive these conditions. After 1 week, the astrocytes formed a confluent layer at the bottom of the petri dishes and small, round process-bearing cells, most likely oligodendrocytes, were found on top of the astrocytes. To remove the oligodendrocytes, we employed antibody-dependent complement-mediated cell killing (Grinspan et al., 1990; Zelenaia et al., 2000). Cultures were washed with HBSS with calcium and magnesium, incubated with rabbit complement (1:10 in defined medium; Cedarlane, Ontario, Canada), and both A2B5 hybridoma supernatant (final concentration: 1:50) and anti-galactocerebroside hybridoma supernatant (R-mAb, at a final concentration of 1:50) for 45 min at 37°C. This procedure removes almost all oligodendrocyte progenitors and mature oligodendrocytes. After incubation, the cultures were washed several times with HBSS with calcium and magnesium; DMEM with 10% FCS was then added. The purity of these cultures was determined using the A2B5 antibody to oligodendrocyte precursors and anti-GalC antibody to detect mature oligodendrocytes (Grinspan and Franceschini, 1995) by performing immunofluorescence on sister cultures plated on coverslips and treated identically to the 100-mm petri dishes. Virtually all the oligodendrocyte lineage cells were removed by the complement-mediated cell killing procedure. The antibody (ED1) was used to detect microglia. Cell counts of ED1+ cells and DAPI labeled-nuclei (Hara et al., 1999) demonstrated the presence of 98% of astrocytes in the culture.
Reagents
Recombinant human and rat IL-1β were purchased from R&D Systems (Minneapolis, MN); rabbit anti-human IL-1β neutralizing antibody (IL-1βAb) from Sigma (St. Louis, MO); caffeic acid phenethyl ester (CAPE) from Calbiochem-Novabiochem (San Diego, CA); and SP from Sigma (St. Louis, MO). A stock solution of SP (10−3 M) was stored at −80°C as frozen aliquots in fast performance liquid chromatography (FPLC) grade water. The SP antagonist (CP-96,345) was generously provided by Pfizer Diagnostics. Stock solution of CP-96,345 (2 × 10−3 M in FPLC grade water) was stored at −80°C.
RNA Extraction and Reverse Transcription PCR
Total cellular RNA was isolated from U87 MG cells or rat astrocytes, using Tri-Reagent (Molecular Research Center, Cincinnati, OH). In brief, total RNA was extracted by single-step guanidium thiocyanate-phenol-chloroform extraction. After centrifugation at 13, 000g for 15 min at 4°C, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were washed once in 75% ethanol and resuspended in 30 μl of RNase-free water. Total cellular RNA (1 μg) was subjected to reverse transcription using the reverse transcription system (Promega, Madison, WI) with specific primers (anti-sense) for NK-1R genes for 1 h at 42°C. The reaction was terminated by incubating the reaction mixture at 99°C for 5 min and then kept at 4°C. The resulting cDNA served as a template for reverse transcription-polymerase chain reaction (RT-PCR) amplification. PCR amplification was performed with one-tenth of the cDNA for 45 cycles, using AmpliTaq Polymerase (Perkin-Elmer, Branchburgh, NJ) in a GeneAmp PCR System 2400 (Perkin-Elmer-Cetus, Norwalk, CT). The NK-1R primer pair (sense: 5′-AGGACAGTGACGAACTATTT-TCTGG-3′ and anti-sense: 5′-CTGCTGGATAAACTTCT-TCAGGTAG-3′) corresponds to +190 and +831 on NK-1R mRNA sequences, with a predicted amplification size of 640 bp, based on the published cDNA sequence for human NK-1R (Rameshwar and Gascon, 1995; Ho et al., 1997; Lai et al., 1998). The cycle condition was designed as follows: 95°C 9 min, followed by 45 cycles of 94°C 30 s, 50°C 30 s, 72°C 30 s, and elongation at 72°C for 7 min. After PCR amplification, the samples were electrophoresed in a 3% NuSieve 3:1 agarose gel (FMC Bio-products, Rockland, ME).
Immunoblot Assay of NK-1R
U87 MG cells (105 cells/well) in 24-well plate were cultured in the presence or absence of IL-1β (4 ng/ml) for 3 h at 37°C. The cells were then washed twice in ice-cold phosphate-buffered saline (PBS) and lysed with lysis buffer (Promega, Madison, WI). The protein concentration was determined by the DC protein assay kit (Bio-Rad, Hercules, CA). Immunoblot analysis of NK-1R protein was performed with Bio-Dot SF apparatus as described by the manufacturer (Bio-Rad). Briefly, total protein (5 μg) was extracted from U87 MG cells incubated with or without IL-1β, and applied onto a nitrocellulose (NC) membrane. The membrane was then blocked with PBS containing 5% nonfat dry milk (NFDM) for 1 h at room temperature, and incubated with a rabbit polyclonal anti-NK-1R antibody (1: 4,000) (Santa Cruz Biotechnology) at 4°C overnight. After three washes with PBS, the NC membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit Ig G for 1 h. Bound antibody was visualized by developing the membrane using SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce, Rock-ford, IL); the results were recorded on a film (Eastman Kodak, Rochester, NY).
Flow Cytometry
To determine whether IL-1β-induced NK-1R affects SP binding on U87 MG cells, these cells were incubated with or without IL-1β (4 ng/ml) for 3 h. The cells were then removed from the culture plate and resuspended in 100 μl of PBS. After incubation with FITC-conjugated SP (1:1,000; Molecular Probes, Eugene, OR) for 45 min at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. Fluorescence was analyzed on an EPICS-elite flow cytometer (Beckman Coulter Electronics, Hialeah, FL).
NF-κB Promoter Activation Assay
The plasmid containing the NF-κB promoter linked to a luciferase gene (pNF-κB-luc) was generated by Dr. D. Petrak (Petrak et al., 1994). Two copies of the mouse κ light chain enhancer (Pierce et al., 1988) were cloned into the pBLCAT3 vector (Luckow and Schutz, 1987). The construct was then modified by replacing the CAT reporter with the luciferase gene obtained from pGEM-luc (Petrak et al., 1994). Plasmid DNA was prepared by Miniprep techniques according to the manufacturer’s instructions (Wizard Plus Minipreps, Promega, Madison, MI) and then used in the transfection experiments.
U87 MG cells were cultured in a 24-well tissue culture plate at a density of 105 cells/well. The cells were transfected with the pNF-κB-luc plasmid using Fugene 6 Transfection Reagent (Fugene 6) (Roche Molecular Biochemicals, Indianapolis, IN) in a ratio of Fugene 6:plasmid 6:1 (6 μl:1 μg). At 24 h after transient transfection, the cells were treated either with IL-1β (4 ng/ml) for 12 h or in the absence of IL-1β. When IL-1β and CAPE were used in the same experiment, CAPE was incubated for 2 h before the addition of IL-1β. At the termination of the experiments, the cells were harvested, washed twice with PBS, and centrifuged at 3,300g for 3 min at room temperature. The cell pellets were lysed by 1× Reporter Lysis Buffer (Promega, Madison, WI) and underwent a cycle of freezing and thawing in dry ice. Cell-free lysates were obtained by centrifugation at 10,000g for 30 s at room temperature. The effects of IL-1β on the activation of the NF-κB promoter in these transient transfected cells were determined by the NF-κB promoter-directed luciferase activity. Luciferase activity in cell lysates (25 μl/sample) was quantified with a Luciferase assay system (Promega) and a Luminometer. The results were presented as relative light units (RLU).
Measurement of [Ca2+]i
U87 MG cells (3 × 105 cells) or rat astrocytes (5 × 105 cells) were plated on glass coverslips (Fisher Scientific, Pittsburgh, PA) sized to fit a homeothermic perfusion chamber platform of an inverted Nikon microscope. The cells were loaded with 2.5 μM fura-2 acetoxymethylester (Molecular Probes) and 0.2 mg/ml pluronic F-127 (Molecular Probes) in 2 ml HBSS that was supplemented with 1% FBS and 1.25 mM CaCl2 for 30 min at 37°C. The cells were superfused with HBSS supplemented with 1% FBS and 1.25 mM CaCl2 at 37°C containing SP (10−7 M) at a flow rate of 1.5 ml/min and excitation was performed at 334 and 380 nm with two narrow bandpass filters. The emitted fluorescence was filtered (520 nm), captured with a Hamamatso CCD video camera (512 × 480-pixel resolution), digitized (256 gray levels), and analyzed with SimplePCI (Version 3.7.9) software (Cranberry, PA). The Ca2+ was calculated by comparing the ratio of fluorescence at each pixel to an in vitro 2-point calibration curve. The Ca2+ concentration presented is obtained by averaging the values of all pixels over a cell body. The data points were collected at intervals of 4 s.
RESULTS
IL-1β Upregulates NK-1R Expression
IL-1β, when added to U87 MG cells, induced NK-1R expression in a dose-dependent fashion (Fig. 1A). IL-1β also stimulated NK-1R gene expression in primary rat astrocytes (Fig. 1B). When U87 MG cells were treated with IL-1β (4 ng/ml) at 0, 1, 3, 6, 9, and 24 h, we observed an increase of NK-1R mRNA during the incubation (from 1 to 9 h after IL-1β treatment) (Fig. 2). NK-1R expression returned to the basal level at 24 h post-treatment (Fig. 2). The stimulatory effect of IL-1β on NK-1R expression was abrogated by the antibody to IL-1β (Fig. 3). In addition, IL-1β enhanced NK-1R protein expression in U87 MG cells as demonstrated by immunoblot assay (Fig. 4A). NK-1R expression on the cell membrane of U87 MG was also increased by IL-1β treatment, as shown by the flow cytometry assay (Fig. 4B).
Fig. 1.
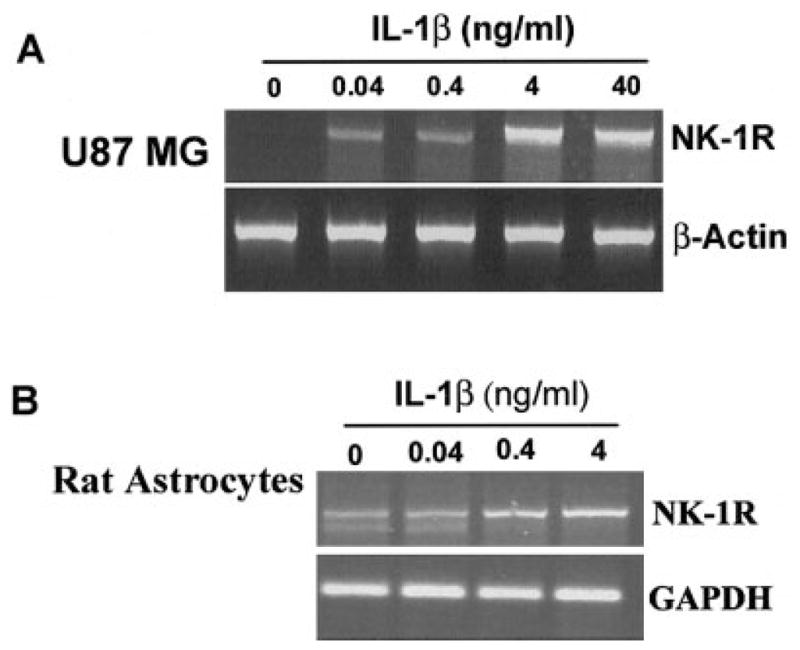
Effect of interleukin-1β (IL-1β) on neurokinin-1 receptor (NK-1R) expression in U87 MG cells (A) and primary rat astrocytes (B). Cells were incubated with IL-1β at the indicated concentrations for 3 h, and total RNA was isolated and subjected to reverse transcription-polymerase chain reaction (RT-PCR) analysis. PCR was performed for NK-1R, as well as for β-actin for U87 MG cells or GAPDH (primary rat astrocytes), as a control to ensure that RNA amounts were equal. The data shown are representative of three experiments.
Fig. 2.
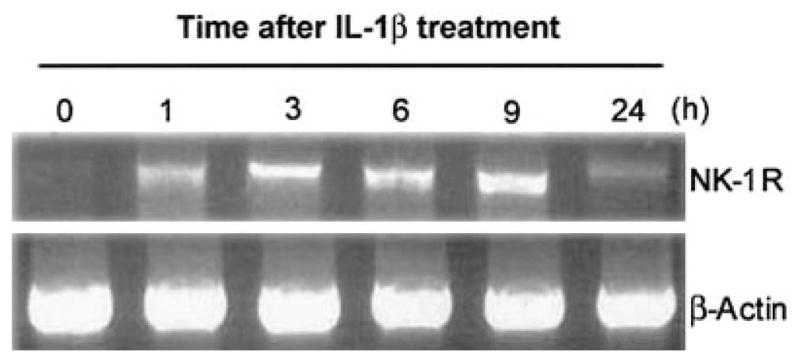
Time course of interleukin-1β (IL-1β) effect on neurokinin-1 receptor (NK-1R) mRNA expression in U87 MG cells. U87 MG cells were incubated with (+) or without (−) IL-1β (4 ng/ml) for the time points post-treatment as indicated. Total RNA was isolated and subjected to reverse transcription-polymerase chain reaction (RT-PCR) analysis. PCR was performed for NK-1R as well as for β-actin as a control to ensure that RNA amounts were equal. The data shown are representative of three experiments.
Fig. 3.
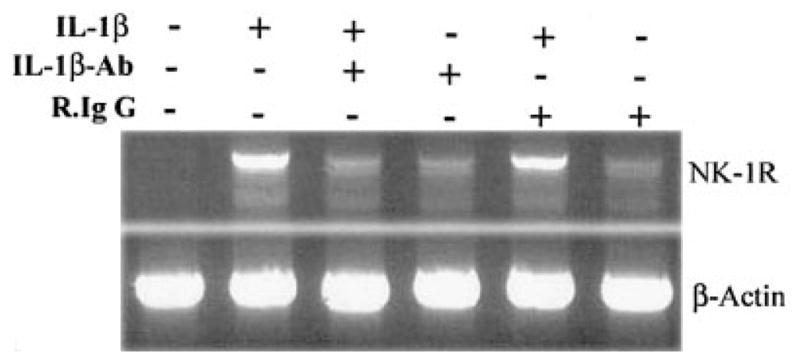
Effect of interleukin-1β (IL-1β) Ab on IL-1β-induced neurokinin-1 receptor (NK-1R) mRNA expression in U87 MG cells. U87 MG cells were incubated with (+) or without (−) IL-1β (4 ng/ml) and/or rabbit anti-human IL-1βAb (50 μg/ml) or normal rabbit IgG (50 μg/ml) for 3 h. Total RNA was isolated and subjected to reverse transcription-polymerase chain reaction (RT-PCR) and electrophoresis for NK-1R mRNA. PCR was performed for NK-1R as well as for β-actin as a control to ensure that RNA amounts were equal. The data shown are representative of two experiments.
Fig. 4.
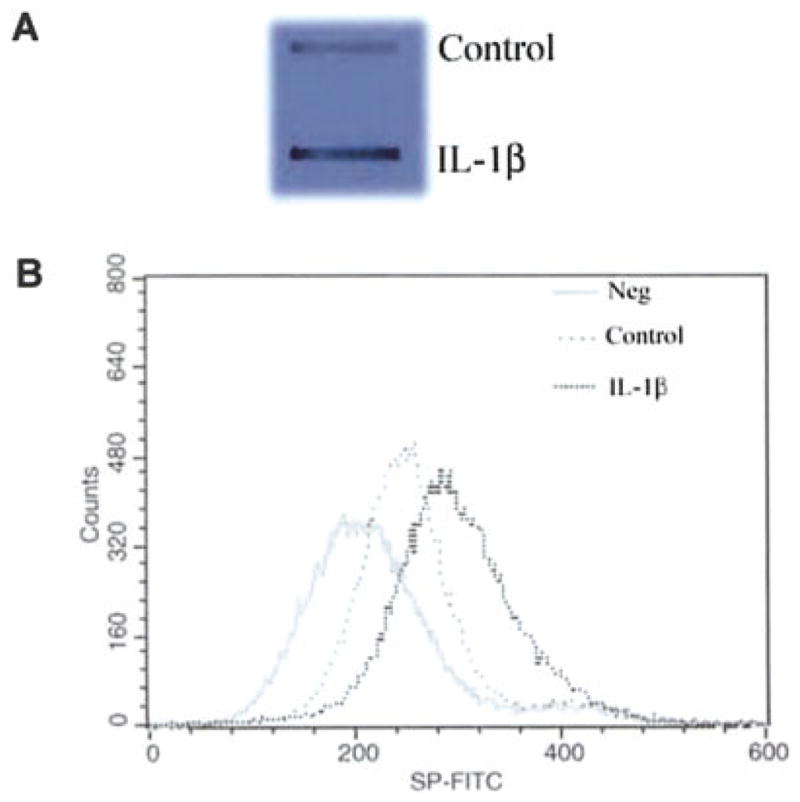
Induction of neurokinin-1 receptor (NK-1R) protein expression by interleukin-1β (IL-1β). A: Effect of IL-1β on NK-1R protein expression in U87 MG cells. U87 MG cells were incubated with or without (control) IL-1β at 4 ng/ml for 3 h. Cell lysates were quantified with a DC protein assay kit. Equal amounts (5 μg) of protein extracted from treated and untreated U87 MG cells were applied onto a nitro-cellulose membrane for immunoblot assay. The results were recorded on the film (1-min exposure). B: U87 MG cells were incubated with or without IL-1β (4 ng/ml) for 3 h. The cells were then removed from the culture plate and resuspended in 100 μl of phosphate-buffered saline (PBS). After incubation with 20 μl of FITC-conjugated substance P (SP) (1:1,000) for 45 min at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. Fluorescence was analyzed on an EPICS-elite flow cytometry. The data shown are representative of three experiments.
NF-κB Is Involved in IL-1β-Induced NK-1R Expression
Since NF-κB is a key transcriptional factor whose binding sites are also found on NK-1R genes (Simeonidis et al., 2003), we hypothesized that NF-κB activation is involved in the IL-1β-mediated enhancing effect on NK-1R expression. To test this hypothesis, we first examined whether IL-1β has the ability to activate NF-κB promoter. We transfected U87 MG cells with a plasmid containing the NF-κB promoter (pNF-κB-luc); we then incubated the cells with or without IL-1β and/or CAPE, a potent and specific NF-κB inhibitor. IL-1β activated NF-κB promoter-directed luciferase activity, while CAPE completely blocked the stimulatory effect of IL-1β on NF-κB promoter activation in U87 MG cells (Fig. 5A). To determine whether NF-κB plays a direct role in upregulation of NK-1R by IL-1β, we investigated whether CAPE, when added to the U87 MG and rat astrocyte cultures, had the capacity of blocking IL-1β action. Pre-incubation of U87 MG and primary rat astrocytes with CAPE blocked IL-1β-induced NK-1R expression (Fig. 5B, C).
Fig. 5.
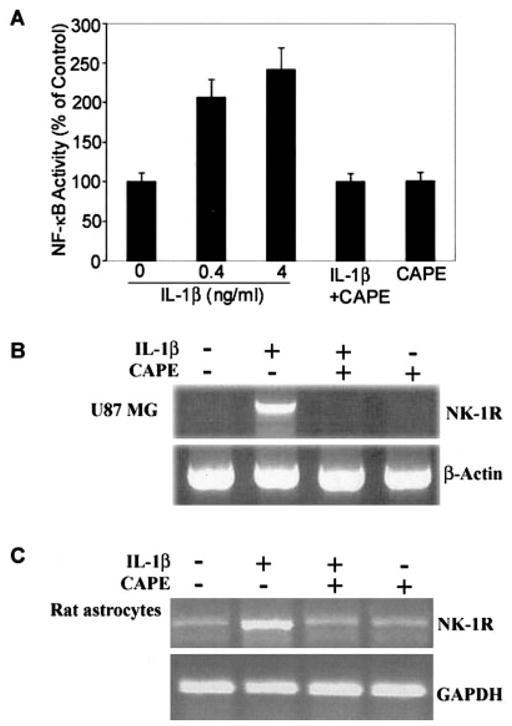
Effect of nuclear factor-κB (NF-κB) inhibitor caffeic acid phenethyl ester (CAPE) on interleukin-1β (IL-1β)-induced activation of the NF-κB promoter (A) and neurokinin-1 receptor (NK-1R) mRNA expression in U87 MG cells (B) and primary rat astrocytes (C). A: U87-MG cells were transfected with the plasmid containing the NF-κB promoter (pNF-κB-luc) and incubated with or without IL-1β and/or CAPE for 12 h. Data presented as means ± SD of triplicate cultures, a representative of three independent experiments. B, C: U87 MG cells and primary rat astrocytes were pre-incubated with or without CAPE (25 μg/ml) for 2 h, the cells were then treated with IL-1β (4 ng/ml) for 3 h. Total cellular RNA extracted was subjected to reverse transcription-polymerase chain reaction (RT-PCR) for NK-1R mRNA expression. PCR was performed for NK-1R as well as for β-actin U87 MG cells or GAPDH for primary rat astrocytes as a control to ensure that RNA amounts were equal. The data shown are representative of three experiments.
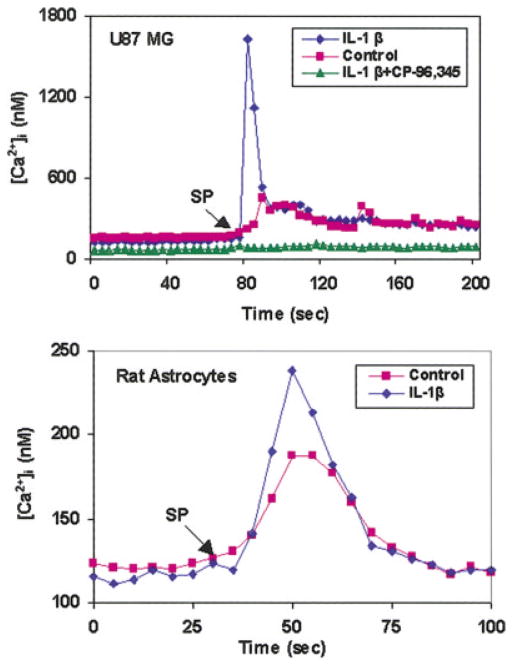
IL-1β-Treated Astrocytes Have Increased [Ca2+]i in Response to SP
To determine IL-1β-induced NK-1R are biologically functional, we examined whether IL-1β-treated U87 MG and rat astrocytes have increased cytosolic [Ca2+]i. The cells were washed once to remove IL-1β and loaded with fura-2 AM for 30 min. Stimulation with SP led to a significant increase of [Ca2+]i in IL-1β-treated U87 MG cells and primary rat astrocytes (Fig. 6). Preincubation of the cells with the SP antagonist (CP-96, 345), completely inhibited the SP-induced rise of [Ca2+]i (Fig. 6).
Fig. 6.
Measurement of cytosolic [Ca2+]i in cultured U87 MG cells and primary rat astrocytes in response to substance P (SP). Cells incubated with or without (control) interleukin-1β (IL-1β) (4 ng/ml) were loaded with 2.5 μM fura-2 AM for 30 min and exposed to SP (10−7 M) and/or (CP-96,345, 10−6 M) and change in [Ca2+]i was recorded from 20–40 cells. The data shown are representative of five experiments.
DISCUSSION
The neuropeptide SP is widely distributed throughout mammalian central and peripheral nervous systems. SP modulates the interaction between the nervous system and immune system. SP and NK-1R have a critical role in immune regulation in infection and inflammation. In mice, NK-1R helps govern mucosal injury caused by Clostridium difficile toxin (Castagliuolo et al., 1998). NK-1R blockade decreases the intestinal interferon-γ (IFN-γ) response in murine salmonellosis, leaving animals more susceptible to infection (Kincy-Cain and Bost, 1996). Mice with NK-1R gene deletion are less susceptible to immune complex-induced pulmonary injury (Bozic et al., 1996) and to IL-1-induced neutrophil migration (Ahluwalia et al., 1998). Also, mice given an NK-1R antagonist have less CNS inflammation when infected with Trypanosoma brucei (Kennedy et al., 1997). In injury to the optic nerve, SP binding is restricted to reactive astrocytes, whereas reactive gliosis in the striatum is not associated with expression of the SP receptor. SP receptors are expressed in restricted glial cell subpopulations derived from the white matter (Beaujouan et al., 1991; Marriott and Wilkin, 1993), as well as in astrocytes from cortical areas (Torrens et al., 1989; Martin et al., 1992). Investigations in rodent astrocytes have shown that SP induces accumulation of inositol phosphate (IP), calcium mobilization, and secretion of IL-1 (Martin et al., 1992), prostaglandin E2 (PGE2), and IL-6 (Palma et al., 1997). SP immunoreactive astrocytes were identified within the glial scars in multiple sclerosis (MS) brain tissue (Kostyk et al., 1989). Our data showing that the astrocytes express NK-1R are consistent with studies reporting that U87 MG and rat astrocytes express functional NK-1R (Martin et al., 1992; Ogo et al., 1996).
NK-1R is stimulated upon activation by proinflammatory cytokines in a wide spectrum of cell types, including cells of the immune system (e.g., T cells and macrophages). It is critical to identify factors that regulate NK-1R expression in the CNS. We hypothesized that IL-1β, an important inflammatory cytokine in the early immune response, may induce NK-1R expression by astrocytes. The human astrocyte line (U87MG), as well as primary rat astrocytes, are suitable for this study, as these cells express NK-1R. Our data differ from the data reported by Johnson and Johnson (1991), who observed that IL-1 downregulated receptors for SP in human astrocytoma cells (UC11) after long-term exposure to the cytokine (6 days). The discrepancy between our findings and those of Johnson and Johnson (1991) may be attributable to different exposure times to IL-1. In our study, the astrocytes were treated with IL-1β for 1 h (mRNA) and 3 h (protein synthesis). This argument is supported by a recent study showing that IL-1β induces NK-1R expression in THP-1 monocytes after 1–3-h treatment with the cytokine (Simeonidis et al., 2003). The notion that NK-1R is inducible by the inflammatory cytokine in astrocytes is also supported by the result reported by Weinstock et al. (2003).
Cytokines and neuropeptides that interact with specific receptors on both immune and CNS cells are involved in neuroimunoregulation. The intracellular signaling pathway by which IL-1β enhances NK-1R expression is implicated by IL-1β-mediated activation of NF-κB. NF-κB is an inducible transcription factor present in the CNS (Kaltschmidt et al., 1993, 1995). A putative NF-κB-binding site on the NK-1R promoter was noted by Takahashi et al. (1992). NF-κB plays a role in the upregulation of the NK-1R gene after stimulation of the T cells and macrophages with proinflammatory cytokines such as IL-1β (Simeonidis et al., 2003; Weinstock et al., 2003). These findings have provided a basis for our investigation on the interaction of IL-1β with NK-1R in astrocytes. We showed that IL-1β activates the NF-κB promoter in U87 MG cells (Fig. 5A). To determine further whether IL-1β-mediated NF-κB activation is indeed involved in NK-1R expression, we employed CAPE, a potent and specific inhibitor of NF-κB activation (Natarajan et al., 1996), in IL-1β stimulation experiments. When added to NF-κB-promoter containing plasmid-transfected U87 MG cells, CAPE not only abrogated IL-1β-induced NF-κB promoter activation (Fig. 5A), but also blocked IL-1β-induced NK-1R expression in U87 MG cells (Fig. 5B) and primary rat astrocytes (Fig. 5C). These data indicate that NF-κB activation is a mechanism responsible for IL-1β-mediated upregulation of NK-1R in the astrocytes.
To assess specifically the function of the IL-1β-mediated upregulation of NK-1R, we examined the effect of SP- induced signaling on intracellular calcium levels in U87 MG cells and rat astrocytes. IL-1β-treated cells had increased response to SP stimulation (Fig. 6). Pre-treatment with the specific non-peptide NK-1R antagonist (CP-96,345) abrogated SP-induced [Ca2+]i (Fig. 6). The binding of SP to astroglia cells is specific to NK-1R, since the NK-1R antagonist (CP-96,345) and cold SP blocked SP-FITC binding to IL-1β-primed U87 MG cells (data not shown). In addition, CP-96,345 completely inhibited SP-induced calcium mobilization in U87 MG cells (Fig. 6). We observed that at a concentration of ≤10−9 M, SP did not induce [Ca2+]i in U87 MG cells (data not shown). Under pathological conditions, a high concentration of SP may be required to stimulate astrocytes to produce cytokines. The requirement for a high concentration of SP to activate astrocytes in the brain may prevent spurious induction of an inflammatory response in the CNS.
Although our in vitro observations cannot be extrapolated directly to the in vivo situation, astroglial regulation of NK-1R by IL-1β may have pathophysiological relevance. Recent studies suggest that both IL-1β and SP are genetic factors that affect disease severity of multiple sclerosis (MS) (Mann et al., 2002; Vandenbroeck et al., 2002). NK-1R expression is increased in several pathological conditions, including MS (Kostyk et al., 1989). Thus, the regulation of NK-1R in the CNS has important implications in neuroimmunological disorders and neuroinfectious diseases. Upregulation of NK-1R by IL-1β in astrocytes may constitute a critical mechanism which leads to sustained chronic inflammation. IL-1β-induced NK-1R expression may be one of the mechanisms responsible for the pathogenesis of the inflammatory process related to the neuronal disorders in the CNS. Further understanding of factors that control NK-1R expression in the CNS may lead to the development of a new strategy for treatment of inflammation-related neurological disorders.
Acknowledgments
This investigation was supported by grants from the National Institutes of Health (DA12815 and DA16022 (to W.Z.H.), MH49981 and AA13547 (to S.D.D.), and NS43422 (to J.B.G). This study was also supported by the National Multiple Sclerosis Society, grant PP0895 (to W.Z.H).
Abbreviations used
- SP
substance P
- NK-1R
neurokinin-1 receptor
- IL-1β
interleukin-1β
- TNF-α
tumor necrosis factor-α
- LPS
lipopolysaccharide
- IL-8
interleukin-8
- MIP-1β
macrophage inflammatory protein-1β
- CNS
central nervous system
- CAPE
caffeic acid phenethyl ester
- NF-κB
nuclear factor κB
References
- Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol. 1998;124:1013–1015. doi: 10.1038/sj.bjp.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaujouan JC, Teutsch B, Saffroy M, Petitet F, Torrens Y, Glowinski J. NK-1 receptors are the only class of tachykinin receptors found on mouse cortical astrocytes. Peptides. 1991;12:813–820. doi: 10.1016/0196-9781(91)90139-g. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest. 1998;101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Kelley KW. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res. 1991;557:115–120. doi: 10.1016/0006-8993(91)90123-d. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Imeri L. Cytokine-neurotransmitter interactions in the brain. Biol Signals Recept. 1998;7:33–44. doi: 10.1159/000014526. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Ann NY Acad Sci. 1988;546:122–132. doi: 10.1111/j.1749-6632.1988.tb21627.x. [DOI] [PubMed] [Google Scholar]
- Fibbe WE, Schaafsma MR, Falkenburg JH, Willemze R. The biological activities of interleukin-1. Blut. 1989;59:147–156. doi: 10.1007/BF00320059. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Franceschini B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor cells. J Neurosci Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Stern JL, Pustilnik SM, Pleasure D. Cerebral white matter contains PDGF-responsive precursors to O2A cells. J Neurosci. 1990;10:1866–1873. doi: 10.1523/JNEUROSCI.10-06-01866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1beta expression in human T lymphocytes. J Neuroimmunol. 2002;131:160–167. doi: 10.1016/s0165-5728(02)00277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A, Niwa M, Iwai T, Nakashima M, Bunai Y, Uematsu T, Yoshimi N, Mori H. Neuronal apoptosis studied by a sequential TUNEL technique: a method for tract-tracing. Brain Res Brain Res Protoc. 1999;4:140–146. doi: 10.1016/s1385-299x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Ho WZ, Evans DL, Douglas SD. Substance P and human immunodeficiency virus infection: psychoneuroimmunology. CNS Spectrom. 2002;7:867–874. doi: 10.1017/s1092852900022483. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I. Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Johnson CL, Johnson CG. Tumor necrosis factor and interleukin-1 down-regulate receptors for substance P in human astrocytoma cells. Brain Res. 1991;564:79–85. doi: 10.1016/0006-8993(91)91354-4. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech Dev. 1993;43:135–147. doi: 10.1016/0925-4773(93)90031-r. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc Natl Acad Sci USA. 1995;92:9618–9622. doi: 10.1073/pnas.92.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. Substance P enhances the production of interferon-induced protein of 10 kDa by human keratinocytes in synergy with interferon-gamma. J Invest Dermatol. 2002;119:1290–1297. doi: 10.1046/j.1523-1747.2002.19626.x. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Rodgers J, Jennings FW, Murray M, Leeman SE, Burke JM. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc Natl Acad Sci USA. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincy-Cain T, Bost KL. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J Immunol. 1996;157:255–264. [PubMed] [Google Scholar]
- Kostyk SK, Kowall NW, Hauser SL. Substance P immunoreactive astrocytes are present in multiple sclerosis plaques. Brain Res. 1989;504:284–288. doi: 10.1016/0006-8993(89)91369-3. [DOI] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Shaheen F, Pleasure DE, Ho WZ. Quantification of substance p mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2002;9:138–143. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Gottschall PE, Chen LT, Wiranowska M, Phelps CP. Role and mechanisms of interleukin-1 in the modulation of neurotoxicity. Neuroimmunomodulation. 2002;10:199–207. doi: 10.1159/000068322. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Mann CL, Davies MB, Stevenson VL, Leary SM, Boggild MD, Ko Ko C, Jones PW, Fryer AA, Strange RC, Thompson AJ, Hawkins CP. Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity. J Neuroimmunol. 2002;129:197–204. doi: 10.1016/s0165-5728(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott DR, Wilkin GP. Substance P receptors on O-2A progenitor cells and type-2 astrocytes in vitro. J Neurochem. 1993;61:826–834. doi: 10.1111/j.1471-4159.1993.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Marriott I, Mason MJ, Elhofy A, Bost KL. Substance P activates NF-kappaB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- Martin FC, Charles AC, Sanderson MJ, Merrill JE. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Res. 1992;599:13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo H, Kuroyanagi N, Inoue A, Nishio H, Hirai Y, Akiyama M, DiMaggio DA, Krause JE, Nakata Y. Human astrocytoma cells (U-87 MG) exhibit a specific substance P binding site with the characteristics of an NK-1 receptor. J Neurochem. 1996;67:1813–1820. doi: 10.1046/j.1471-4159.1996.67051813.x. [DOI] [PubMed] [Google Scholar]
- Palma C, Minghetti L, Astolfi M, Ambrosini E, Silberstein FC, Manzini S, Levi G, Aloisi F. Functional characterization of substance P receptors on cultured human spinal cord astrocytes: synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia. 1997;21:183–193. doi: 10.1002/(sici)1098-1136(199710)21:2<183::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Petrak D, Memon SA, Birrer MJ, Ashwell JD, Zacharchuk CM. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J Immunol. 1994;153:2046–2051. [PubMed] [Google Scholar]
- Pierce JW, Lenardo M, Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci USA. 1988;85:1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood. 1995;86:482–490. [PubMed] [Google Scholar]
- Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system. II. Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D, Pothoulakis C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanaka A, Hara M, Nakanishi S. The primary structure and gene organization of human substance P and neuromedin K receptors. Eur J Biochem. 1992;204:1025–1033. doi: 10.1111/j.1432-1033.1992.tb16724.x. [DOI] [PubMed] [Google Scholar]
- Torrens Y, Daguet De Montety MC, el Etr M, Beaujouan JC, Glowinski J. Tachykinin receptors of the NK1 type (substance P) coupled positively to phospholipase C on cortical astrocytes from the newborn mouse in primary culture. J Neurochem. 1989;52:1913–1918. doi: 10.1111/j.1471-4159.1989.tb07276.x. [DOI] [PubMed] [Google Scholar]
- Vandenbroeck K, Fiten P, Heggarty S, Goris A, Cocco E, Hawkins SA, Graham CA, Marrosu MG, Opdenakker G. Chromosome 7q21–22 and multiple sclerosis: evidence for a genetic susceptibility effect in vicinity to the protachykinin-1 gene. J Neuroimmunol. 2002;125:141–148. doi: 10.1016/s0165-5728(02)00023-1. [DOI] [PubMed] [Google Scholar]
- Weinstock JV, Blum A, Metwali A, Elliott D, Arsenescu R. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J Immunol. 2003;170:5003–5007. doi: 10.4049/jimmunol.170.10.5003. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]