Abstract
Opioid abuse has been postulated as a cofactor in the immunopathogenesis of human immunodeficiency virus (HIV) infection and AIDS. We and others have recently demonstrated that opioid enhances HIV infection of human macrophages through modulation of β-chemokines and the CCR5 receptor and that this effect is reversed by naltrexone, a tertiary opioid antagonist. Tertiary opioid antagonists cannot be used in opioid-dependent patients because they precipitate withdrawal or reversal of analgesia. We determined whether the quaternary opioid antagonist methylnaltrexone (MNTX), now in phase III clinical trials for opioid-induced constipation, reverses the opioid-mediated enhancement of HIV infection of macrophages at clinically relevant doses. MNTX completely abrogated morphine-induced HIV Bal strain infection of macrophages. MNTX also inhibited the R5 strain (ADA) envelope-pseudotyped HIV replication induced by morphine. Furthermore, MNTX abolished morphine-mediated up-regulation of CCR5 receptor expression. The ability of MNTX to block opioid-induced CCR5 expression and HIV replication at clinically relevant doses may have additional benefit for opioid abusers with HIV infection, or patients with AIDS pain receiving opioids.
Drug abuse by injection remains a significant risk for acquiring human immunodeficiency virus (HIV) infection (Risdahl et al., 1998). In fact, drug abuse represents one of the largest reservoirs of HIV in the United States and contributes to faster spread of the virus (Alcabes and Friedland, 1995). Approximately 30% of patients with AIDS have a history of drug abuse of opioids. Opioids exert profound effects on the function of immune system cells (Nair et al., 1997; Kulkarni-Narla et al., 2001; McCarthy et al., 2001) and are implicated as cofactors in the immunopathogenesis of HIV (Donahoe and Falek, 1988; Alcabes and Friedland, 1995; Risdahl et al., 1998; McCarthy et al., 2001). Studies using cells in vitro have demonstrated that morphine activates and enhances HIV replication in human immune cells (Peterson et al., 1990, 1994; Chao et al., 1995). We have recently shown that opioids, including morphine and methadone, enhance HIV infection of human immune cells (Guo et al., 2002; Li et al., 2002). Morphine enhances HIV infection of macrophages through modulation of β-chemokine production and expression of the HIV primary coreceptor CCR5. This opioid-mediated effect can be reversed by the opioid receptor antagonist naltrexone, a tertiary opioid antagonist (Guo et al., 2002; Li et al., 2002). Because tertiary opioid antagonists cannot be used in opioid-dependent patients without precipitating withdrawal, we wished to determine whether the quaternary peripheral opioid antagonist methylnaltrexone (Progenics Pharmaceuticals, Tarrytown, NY), now in phase III clinical trials for opioid-induced constipation (Yuan et al., 2000a), antagonizes opioid-induced CCR5 expression and HIV infection of macrophages.
Materials and Methods
Monocyte Isolation and Culture
Informed consent was obtained from study participants after our Institutional Research Board approved this study. Peripheral blood was obtained from healthy adult donors without known history of drug abuse. Heparinized blood samples were identified as HIV antibody negative by anonymous testing with the enzyme-linked immunosorbent assay method (Coulter Immunology, Hialeah, FL). Monocytes were purified according to a previously described technique (Hassan et al., 1986). In brief, heparinized blood was separated by centrifugation over lymphocyte separation medium (Organon Teknika, Durham, NC) at 400 to 500g for 45 min. The mononuclear cell layer was collected and incubated with Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) in a 2% gelatin-coated flask for 45 min at 37°C, followed by removal of the nonadherent cells with DMEM. Adherent monocytes were detached with 10 mM EDTA. After the initial purification, greater than 97% of the cells were monocytes, as determined by nonspecific esterase staining and flow cytometry analysis using monoclonal antibody against CD14, the marker specific for monocytes and macrophages. Freshly isolated monocytes were plated in 48-well culture plates at a density of 5 × 105 cells/well in DMEM containing 10% fetal calf serum. Monocyte-derived macrophages (MDMs) were 7-day-cultured monocytes in vitro. Monocyte and MDM viability was monitored by trypan blue exclusion and maintenance of cell adherence.
Reagents
Fluorescein-conjugated antibody against CCR5 was obtained from BD PharMingen (San Diego, CA). Fluorescein-conjugated isotype-matched IgG2b control was purchased from BD PharMingen. Morphine sulfate was obtained from Elkins-Sinn, Inc. (Cherry Hill, NJ). Methylnaltrexone was obtained from Mallinkrodt (St. Louis, MO).
HIV Strain
The macrophage-tropic R5 strain (Bal) was obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health, Bethesda, MD).
Preparation of Pseudotyped HIV
Recombinant luciferase-encoding HIV virions were pseudotyped with the envelopes (Env) from macrophage-tropic (ADA) or amphotropic murine leukemia virus (MLV). Human embryonic kidney cell line (293T) was cotransfected with the plasmids encoding either ADA Env or MLV Env and the plasmid-containing, luciferase-encoding NL4-3 HIV backbone (pNL-Luc-E−R−). Supernatants were collected as virus stock 48 h later. The plasmids encoding HIV, ADA, or MLV Env were generously provided by John Moore (Aaron Diamond AIDS Research Center, New York, NY), and the plasmid with luciferase-encoding NL4 –3 HIV backbone was provided by Ned Landau (Aaron Diamond AIDS Research Center). All virus stocks were assayed for p24 antigen and stored at −70°C as cell-free virus after filtration through a filter with a pore size of 0.22 μm.
Reverse Transcriptase Assay
HIV reverse transcriptase activity was determined based on the technique of Willey et al. (1988) with modifications (Guo et al., 2002; Li et al., 2002). In brief, 10 μl of collected culture supernatants was added to a cocktail containing poly(A), oligo(dT) (Amersham Biosciences Inc., Piscataway, NJ), MgCl2 and [32P]dTTP (Amersham Biosciences Inc.) and incubated for 20 h at 37°C. Then, 30 μl of the cocktail was spotted onto DE81 paper, dried, and washed five times with 2× saline-sodium citrate buffer and once with 95% ethanol. The filter paper was then air-dried. Radioactivity was counted in a liquid scintillation counter (PerkinElmer Life Sciences, Boston, MA).
Flow Cytometry
To determine whether morphine and/or MNTX affect the expression of CCR5 on MDMs, cells were incubated with or without MNTX (10−8 M) for 30 min before the addition of morphine (10−10 M) for 24 h. The cells were then removed from the culture plate and resuspended in 100 μl of phosphate-buffered saline (PBS). After incubation with 20 μl of fluorescein isothiocyanate-conjugated antibody against CCR5 for 45 min at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. Fluorescein isothiocyanate-conjugated control IgG2b was isotype-matched for the antibody against CCR5. Fluorescence was analyzed on an EPICS-elite flow cytometer (Beckman Coulter, Inc., Hialeah, FL).
Pseudotyped Reporter Virus Entry Assay
Seven-day-cultured MDMs in 48-well plates (5 × 105 cells/well) were incubated with MNTX for 30 min before the addition of morphine (10−10 M). The cells were infected with pseudotyped HIV in the presence of polybrene (4 μg/ml) as described previously. At 72 h postinfection, the cells were lysed in 150 μl of 1× reporter lysis buffer (Promega, Madison, WI). The lysate (50 μl) was mixed with an equal volume of luciferase substrate (Promega), and luciferase activity was then determined in a Trilux microbeta luminometer (PerkinElmer Wallac, Turku, Finland).
MNTX or Morphine Treatment and HIV Infection
Seven-day-cultured MDM (5 × 105 cells/well in 48-well plates) were incubated with or without MNTX (10−8 M) and/or morphine (10−10 M) before infection with the HIV Bal strain. In the case of combination treatment of cells with morphine and MNTX, MNTX was added to the MDM cultures 30 min before the addition of morphine for 24 h. The cells were then infected with cell-free HIV for 2 h at 37°C in the presence or absence of MNTX and/or morphine. The cells were then washed three times with DMEM to remove unabsorbed virus, and fresh medium containing MNTX and/or morphine was added to cell cultures. The final wash was tested for viral reverse transcriptase activity and shown to be free of residual inocula. Untreated cells served as controls. The cells were treated with or without morphine every 4 days postinfection. Culture supernatants were collected for assay at day 8 postinfection.
Results
Effect of MNTX on Morphine-Mediated Enhancement of HIV Infection
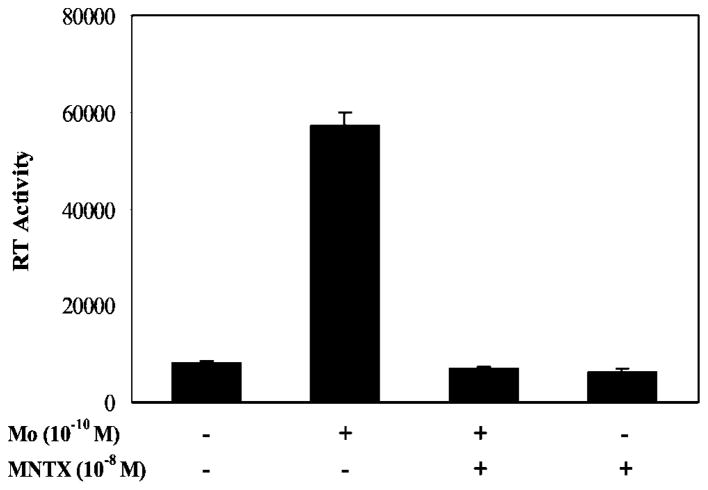
Because we have demonstrated that morphine enhances HIV infection of MDM and naltrexone reverses morphine action (Li et al., 2002), we wished to determine whether MNTX blocks the promoting effect of morphine on HIV replication. We incubated macrophages with or without MNTX (10−8 M) for 30 min and then incubated them with morphine for 24 h. The cells were infected with HIV Bal strain for 2 h, and the supernatants at day 8 postinfection were collected for HIV reverse transcriptase activity assay. Morphine (10−10 M) enhanced HIV infection of macrophages approximately 3-fold, which is consistent with our previous reports (Li et al., 2002); preincubation of MDMs with MNTX completely blocked the morphine effect (Fig. 1).
Fig. 1.
Effect of MNTX on HIV infection of MDMs. MDMs cultured for 7 days were preincubated with or without MNTX and/or morphine (Mo) for 24 h and then infected with HIV Bal strain. HIV reverse transcriptase activity was determined 8 days postinfection. The data shown are mean ± S.E.M. of triplicate cultures, representative of three independent experiments.
Effect of MNTX on Pseudotyped HIV Infection
Because opioids up-regulate the expression of HIV entry receptors such as CCR5 (Miyagi et al., 2000; Guo et al., 2002; Li et al., 2002), we determined whether MNTX blocks morphine-mediated enhancement of HIV infection of MDM through viral entry. We first examined the effect of MNTX on ADA (CCR5-dependent and macrophage-tropic) Env- or MLV (HIV receptor-independent) Env-pseudotyped HIV infection of MDM. The pseudotyped HIV genome that encodes a luciferase reporter gene allows a quantitative measure of the levels of single round infection (Deng et al., 1996). MDMs, incubated with or without morphine and/or MNTX (10−8 M), were infected with luciferase-encoding HIV particles pseudotyped with ADA Env or MLV Env. When infected with ADA Env-pseudotyped virus, a significant 2.8-fold increase of luciferase activity was observed in the morphine-treated MDMs compared with the untreated MDM (Fig. 2). MNTX completely blocked morphine-induced up-regulation of ADA Env-pseudotyped HIV infection (Fig. 2). As expected, morphine did not affect MLV Env-pseudotyped HIV infection of MDMs (Fig. 2). The addition of MNTX alone had no impact on either ADA Env- or MLV Env-pseudotyped HIV infection (Fig. 2). The blocking effect of MNTX on morphine-mediated up-regulation of ADA Env-pseudotyped HIV infection was dose-dependent (Fig. 3). MNTX at the concentrations of 10−10–10−8 M blocked morphine action; MNTX at 10−12 M lost its blocking effect on morphine (Fig. 3).
Fig. 2.

Effect of MNTX on pseudotyped HIV infection of MDMs. MDMs cultured for 7 days were treated with or without MNTX and/or morphine (Mo) for 24 h and then challenged with recombinant luciferase-encoding HIV pseudotyped with either ADA-Env or MLV-Env. Luciferase activity was quantitated in the cell lysates 72 h postinfection. The data are expressed as light units of MNTX and/or morphine-incubated cells relative to that of controls. The data shown are mean ± S.E.M. of triplicate cultures and representative of three experiments.
Fig. 3.
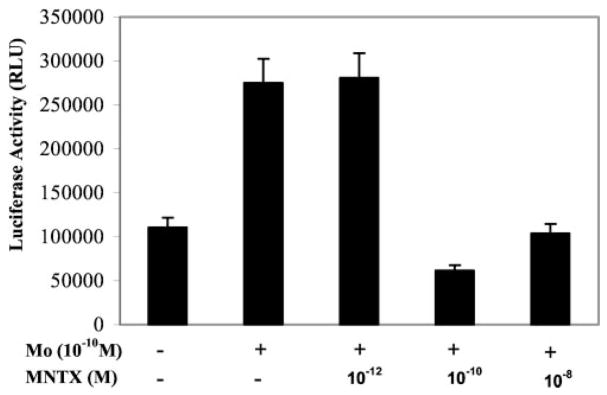
Effect of different concentrations of MNTX on ADA-Env pseudotyped HIV infection of MDMs. MDMs cultured for 7 days were treated with or without MNTX (10−12–10−8 M) and/or morphine (Mo, 10−10 M) for 24 h and then challenged with recombinant luciferase-encoding HIV pseudotyped with ADA-Env. Luciferase activity was quantitated in the cell lysates 72 h postinfection. The data are expressed as relative light units. The data shown are mean ± S.E.M. of triplicate cultures, representative of three experiments.
Effect of MNTX on Morphine-Mediated CCR5 Expression
The CCR5 receptor is a primary coreceptor for HIV R5 strain entry into macrophages (Deng et al., 1996). Morphine has the ability to induce CCR5 expression in human immune cells (Miyagi et al., 2000; Guo et al., 2002). We speculated that MNTX blocks the inducing effect of morphine on CCR5 expression when added to MDM cultures. Pretreatment of MDM with MNTX blocked morphine-induced CCR5 expression; MNTX alone had no effect on CCR5 expression (Table 1).
TABLE 1.
The effect of methylnaltrexone on CCR5 receptor expression in monocyte-derived macrophages
| Treatment | CCR5
|
|
|---|---|---|
| Percentage of Positive Cells | MFI | |
| Control | 15.84 | 35.73 |
| Morphine (10−10 M) | 55.26 | 103.64 |
| Morphine (10−10 M) + MNTX (10−8 M) | 20.29 | 30.55 |
| MNTX (10−8M) | 11.1 | 26.84 |
MFI, mean channel fluorescence intensity.
Discussion
In this communication, we used the in vitro macrophage system to assess the effect of MNTX on morphine-induced CCR5 expression and HIV replication. MNTX completely blocked the morphine-induced replication of the HIV Bal strain in macrophages. This effect occurred at the HIV entry step, because MNTX abrogated morphine-mediated enhancement of ADA Env-pseudotyped HIV infection and had no effect on MLV Env-pseudotyped HIV infection. These data are consistent with our previous report that naltrexone blocked morphine action on HIV infection of macrophages (Li et al., 2002). We also assessed the effect of MNTX on morphine-mediated CCR5 expression in macrophages (Table 1).
MNTX is a quaternary derivative of the pure narcotic antagonist naltrexone. Antagonists such as naloxone, naltrexone, and nalmefene, as tertiary compounds, are fairly lipid-soluble and cross the blood-brain barrier easily. Addition of the methyl group to naltrexone at the amine in the ring forms a compound, N-methylnaltrexone, with greater polarity and lower lipid solubility. Although we have not directly measured intracellular concentrations, MNTX does not cross the blood-brain barrier. The lack of penetration into the central nervous system permits discrimination between the analgesic and constipating effects of opioids (Yuan et al., 1996). In a recent study, 33 patients with advanced medical illness (four with AIDS) experiencing opioid-induced constipation were treated with subcutaneous methylnaltrexone (1, 5, 12.5, and 20 mg). Of patients who received doses of greater than or equal to 5 mg, 60% laxated within 4 h compared with 8% at lower doses (p = <0.0001; Fisher’s exact test). Importantly, no patients experienced withdrawal, and there was no increase in analgesic use, supporting the inability of the drug to cross the blood-brain barrier (Thomas et al., 2003). Also, because of its polarity, MNTX discriminates between cell membrane effects and intracellular effects. The fact that MNTX attenuates opioid-mediated enhancement of CCR5 receptor expression (Table 1) strongly suggests that the mechanism is initiated at the cell membrane, which is consistent with our observations (Fig. 2) in the experiments using pseudotyped HIV.
The actions of opioids on the immune system are complex and involve both cytokine production and the expression of cytokine receptors. Previous studies suggest that κ-agonists and μ-receptor ligands exert different effects. κ-Opioids may attenuate HIV proliferation, whereas μ-opioids may in fact lead to increased expression of the CCR5 receptor in monocytes (Chao et al., 1995, 1998, 2000; Rogers and Peterson, 2003). It is likely that the action of MNTX in this study was mediated through its activity on the μ-opioid receptor. A previous in vitro study showed that MNTX completely antagonized the action of the μ-opioid receptor agonist (Yuan and Foss, 1999). In that study, a 19-fold higher concentration of the compound was required to achieve a similar effect with a κ-opioid receptor agonist trans-3,4-dichloro-N-methyl-N[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide methanesulfonate hydrate (U-50,488H). MNTX did not antagonize the δ-receptor agonist’s activity within the concentration range tested. In addition, effects of [D-Ala2,N-Me-Phe4,Gly5-ol]-en-kephalin and U-50,488H were reversed by naloxone, the non-selective opioid receptor antagonist.
In previous studies of parenteral and oral administration of MNTX, the effect on CCR5 expression and viral replication occurred well within the therapeutic range required to cause laxation. In our study of patients who received methadone maintenance and mean doses of 0.1 mg/kg MNTX intravenously, all patients had immediate laxation without evidence of withdrawal. Mean peak plasma levels of MNTX were 162 ng/ml (range, 33–774 ng/ml) (Yuan et al., 2000a). In a recent study, after repeated i.v. doses of MNTX in volunteers, levels of plasma MNTX were maintained well above the range in which we observed the CCR5 effect (Yuan et al., 2003). In another study of subcutaneous MNTX, volunteers who received 0.1 mg/kg achieved sustained plasma levels of >10 ng/ml (Yuan et al., 2002). Administration of subcutaneous MNTX over a month was not associated with tolerance to gastrointestinal effects (Thomas et al., 2003). Finally, we have observed that oral administration of MNTX attenuates not only constipation but also the systemic effects of opioids (nausea, skin itch, and flushing) at plasma concentrations of 10 ng/ml (25 nM) (Yuan et al., 1997, 2000b), severalfold higher than is necessary to achieve the effect observed on CCR5 and transcript number (Yuan et al., 1998). Although the concentration tolerance and stability of MNTX dosing do not seem to be an issue in reversing opiate-induced constipation, whether the in vitro effect on MDMs we have observed can be extrapolated to a direct therapeutic role remains unproven. Alvimopan, another selective opioid antagonist, is in late stage development for prophylaxis of postoperative ileus and treatment of opioid-induced constipation, but it is not available for parenteral use and purportedly does not enter the systemic circulation (Taguchi et al., 2001).
Because opioid antagonists such as naltrexone cannot be used in opioid-dependent patients without precipitating withdrawal, our data, demonstrating that MNTX reversed the opioid-induced increase in HIV infection of macrophages, suggest a potential therapeutic role for MNTX. Patients with AIDS who take opioids for pain or HIV-infected opioid abusers, who account for approximately 30% of the drug abuse population, could potentially receive MNTX for opioid-induced constipation. Because morphine or methadone at therapeutic doses enhances HIV infection of macrophages through up-regulation of CCR5 receptor expression, MNTX treatment may have an additional benefit for opioid abusers with HIV infection. It has recently been suggested that agents that target viral entry may offer significant advantages over those that interfere with steps in the viral cycle after the cell has been infected (Kilby and Eron, 2003). Further investigations are needed to determine the mechanism(s) responsible for opioid-mediated induction of the CCR5 receptor and to evaluate the impact of MNTX on CCR5 receptor expression and viral load in vivo using HIV-infected opioid-abusing subjects.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DA12815 and DA16022 to W.-Z.H., MH49981, AA13547 to S.D.D., and CA79042 to C.-S.Y.).
ABBREVIATIONS
- HIV
human immunodeficiency virus
- DMEM
Dulbecco’s modified Eagle’s medium
- MDM
monocyte-derived macrophage
- Env
envelope
- MLV
murine leukemia virus
- PBS
phosphate-buffered saline
- MNTX
methylnaltrexone
References
- Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–1479. doi: 10.1093/clinids/20.6.1467. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Kravitz F, Peterson PK. Kappa-opioid potentiation of tumor necrosis factor-alpha-induced anti-HIV-1 activity in acutely infected human brain cell cultures. Biochem Pharmacol. 1998;56:397–404. doi: 10.1016/s0006-2952(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Portoghese PS, Peterson PK. Upregulation of HIV-1 expression in cocultures of chronically infected promonocytes and human brain cells by dynorphin. Biochem Pharmacol. 1995;50:715–722. doi: 10.1016/0006-2952(95)00176-z. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Gekker G, Lokensgard JR, Heyes MP, Peterson PK. U50,488 protection against HIV-1-related neurotoxicity: involvement of quinolinic acid suppression. Neuropharmacology. 2000;39:150–160. doi: 10.1016/s0028-3908(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Falek A. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. Adv Biochem Psychopharmacol. 1988;44:145–158. [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Kilby JM, Eron JJ. Novel therapies based on mechanisms of HIV-1 cell entry. N Engl J Med. 2003;3(48):2228–2238. doi: 10.1056/NEJMra022812. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Walcheck B, Brown DR. Opioid receptors on bone marrow neutrophils modulate chemotaxis and CD11b/CD18 expression. Eur J Pharmacol. 2001;414:289–294. doi: 10.1016/s0014-2999(01)00727-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes. J Biol Chem. 2000;275:31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mono-nuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83:4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Sharma N, Saleem RM, Sessler DI, Carpenter RL, Seyedsadr M, Kurz A. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345:935–940. doi: 10.1056/NEJMoa010564. [DOI] [PubMed] [Google Scholar]
- Thomas J, Portenoy R, Moehl M, Von Gunten C, Thielemann P, Stambler N, Tran D, Galasso F, Israel R. A phase II randomized dose-finding trial of methylnaltrexone for the relief of opioid-induced constipation in hospice patients (Abstract) Proc Am Soc Clin Oncol. 2003;22:2933. [Google Scholar]
- Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, Capon DJ, Martin MA. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CS, Doshan H, O’Connor M, Maleckar SA, Israel R, Moss J. Methylnaltrexone reduces oral-cecal transit time in humans (Abstract) Dig Dis Week. 2003:A824. [Google Scholar]
- Yuan CS, Foss JF. Gastric effects of methylnaltrexone on mu, kappa and delta opioid agonists induced brainstem unitary responses. Neuropharmacology. 1999;38:425–432. doi: 10.1016/s0028-3908(98)00192-0. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O’Connor M, Karrison T, Osinski J, Roizen MF, Moss J. Effects of enteric-coated methylnaltrexone in preventing opioid-induced delay in oral-cecal transit time. Clin Pharmacol Ther. 2000b;67:398–404. doi: 10.1067/mcp.2000.105037. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O’Connor M, Osinski J, Karrison T, Moss J, Roizen MF. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. J Am Med Assoc. 2000a;283:367–372. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O’Connor M, Osinski J, Roizen MF, Moss J. Efficacy of orally administered methylnaltrexone in decreasing subjective effects after intravenous morphine. Drug Alcohol Depend. 1998;52:161–165. doi: 10.1016/s0376-8716(98)00087-8. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O’Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind, randomized, placebo-controlled trial. Clin Pharmacol Ther. 1996;59:469–475. doi: 10.1016/S0009-9236(96)90117-4. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, Osinski J, Toledano A, Roizen MF, Moss J. The safety and efficacy of oral methylnaltrexone in preventing morphine-induced delay in oral-cecal transit time. Clin Pharmacol Ther. 1997;61:467–475. doi: 10.1016/S0009-9236(97)90197-1. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Wei G, Foss JF, O’Connor M, Karrison T, Osinski J. Effects of subcutaneous methylnaltrexone on morphine-induced peripherally mediated side effects: a double-blind randomized placebo-controlled trial. J Pharmacol Exp Ther. 2002;300:118–123. doi: 10.1124/jpet.300.1.118. [DOI] [PubMed] [Google Scholar]