Abstract
Contemporary systems for in vitro culture of ovarian follicles do not recapitulate the mechanical heterogeneity in mammalian ovary. Here we report microfluidic generation of biomimetic ovarian microtissue for miniaturized three-dimensional (3D) culture of early secondary preantral follicles by using alginate (harder) and collagen (softer) to fabricate the ovarian cortical and medullary tissues, respectively. This biomimetic configuration greatly facilitates follicle development to antral stage. Moreover, it enables in vitro ovulation of cumulus-oocyte complex (COC) from the antral follicles in the absence of luteinizing hormone (LH) and epidermal growth factor (EGF) that are well accepted to be responsible for ovulation in contemporary literature. These data reveal the crucial role of mechanical heterogeneity in the mammalian ovary in regulating follicle development and ovulation. The biomimetic ovarian microtissue and the microfluidic technology developed in this study are valuable for improving in vitro culture of follicles to preserve fertility and for understanding the mechanism of follicle development and ovulation to facilitate the search of cures to infertility due to ovarian disorders.
Keywords: microfluidic, microtissue, follicle, mechanobiology, ovulation, alginate
1. Introduction
The ovarian follicle consisting of a centrally located oocyte, an inner layer of granulosa cells, and an outer layer of theca cells is the fundamental functional tissue unit of mammalian ovary. Therefore, in vitro culture of ovarian follicles to obtain oocytes has been proposed as an attractive strategy for restoring infertility of women who are born with ovarian disorder and for preserving fertility of women who may want to delay child bearing or lose gonadal function due to aggressive medical treatment (e.g., chemotherapy) or exposure to environmental/occupational biohazards [1–3].
Both two and three-dimensional approaches have been developed for in vitro culture of ovarian follicles [4, 5]. For two-dimensional (2D) culture on the surface inside culture dishes, both the theca and granulosa cells easily detach from the follicles, spread out, and attach on the surface of culture dishes, leading to a diffused morphology that is non-physiological [6]. Moreover, endogenous paracrine and autocrine factors produced by granulosa and theca cells are easily diluted into the bulk culture medium, which can negatively affect follicle development [7]. On the other hand, three-dimensional (3D) approaches involving the use of homogeneous hanging drop or hydrogel encapsulation (in millimeter sized alginate hydrogel) can better preserve the native 3D follicular architecture during in vitro culture [8–12]. Although these approaches have been successfully used for in vitro culture of follicles from inbred mouse model and contribute significantly to the understanding of follicle biology [8–12], they are far away from being used as an assisted reproductive technology (ART) to restore infertility or preserve fertility for humans. This is because most of the culture systems developed using inbred mice have been shown not to be directly applicable to even outbred mouse and primate models [6, 13], not to mention humans. Moreover, both the 2D and homogeneous 3D (alginate hydrogel or liquid hanging drop) microenvironment used to culture follicles are non-physiological because it does not recapitulate the heterogeneous nature of the extracellular matrix (ECM) in the ovary with the ECM of medulla (inner region) being much softer than that in the cortex (outer region) [14–16]. The ECM is believed to not only provide a 3D network to support the ovarian tissue architecture but also regulate (together with soluble endocrine, paracrine, and autocrine factors) cell-ECM and cell-cell interactions that are important for follicle development [7, 12, 14, 15].
To overcome these challenges, we employed outbred deer mice as the model to study the effect of culturing microenvironment on follicle development and ovulation with particular emphasis on mechanical heterogeneity that has been barely explored in the field of assisted reproduction. Deer mice are indigenous rodents in North America and believed to be more suitable than inbred mice for research aimed for medical applications due to their outbred nature as with humans [6, 13]. Moreover, we engineered the in vitro culturing microenvironment of follicles by mimicking their native 3D milieu including the mechanical heterogeneity in ECM of medulla versus cortex and its 3D distribution. To create the mechanical heterogeneity native in ovary, we employed non-planar microfluidic flow focusing devices to encapsulate early secondary preantral follicles in ~350 µm microcapsules composed of an alginate (harder) shell and collagen (softer) core. With the biomimetic ovarian microtissue, we investigated the role of mechanical heterogeneity in follicle development from the early secondary to antral stage. Because luteinizing hormone (LH) and epidermal growth factor (EGF) are conventionally believed to trigger and facilitate ovulation [17–22], we further investigate their effect on ovulation of the antral follicles obtained by in vitro culturing the biomimetic 3D ovarian microtissue.
2. Experimental
2.1. Animals and materials
Peromyscus maniculatus bairdii (BW stock) deer mice were purchased from the Peromyscus Genetic Stock Center at the University of South Carolina, Columbia, SC and were maintained on a 16-8 h light-dark cycle. All procedures for animal use were approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University and every effort was made to minimize animal suffering. A total of 15 animals were used in this study to ensure that follicles from at least 3 animals were used for each experimental condition except the 2D culture condition for which 2 animals were used. L-15 Leibovitz-glutamax and α-MEM-glutamax medium were purchased from Invitrogen. Fetal bovine serum (FBS) from Hyclone was purchased from Fisher Scientific. Sodium alginate was purchased from Sigma and purified by washing in chloroform with charcoal, dialyzing against deionized water, and freeze-drying. Unless specifically noted otherwise, all other chemicals were purchased from Sigma.
2.2. Isolation of early secondary preantral follicles
Early secondary preantral follicles (100–130 µm) were isolated from ovaries of female deer mice of 12 to 16-week old using a mechanical method reported by us previously [6]. Briefly, the ovaries were placed in 2 ml Leibovitz L-15 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin at 37 °C in 5% CO2 air. Preantral follicles were obtained by using two 30 G needles to mechanically break the extracellular matrix between follicles in the ovarian tissue.
2.3. Fabrication of non-planar microfluidic device
To fabricate polydimethyl siloxane (PDMS, Dow Corning) microfluidic device, silicon master with patterned microfluidic channels was prepared using a multilayer (3-step UV exposure) SU-8 fabrication technique [23]. Briefly, photosensitive epoxy (SU-8 2025, MicroChem) was spun coated onto a 4-inch silicon wafer. The thickness of the first SU-8 coating was 60 µm. The wafers were then soft-baked at 95 °C for 9 min and exposed to UV light through the first shadow mask printed with the core channel. After a post-exposure baking at 90 °C for 7 min, an additional layer (50 µm) of SU-8 photoresist was spun coated, soft baked, exposed with a different shadow mask to pattern shell channel. The third layer for oil channel was similarly patterned. All three exposures were aligned using an EVG620 automated mask aligner. The SU-8 pattern on the substrate was developed in SU-8 developer (MicroChem) for 10 min, rinsed with isopropyl alcohol, and dried using nitrogen gas. PDMS pre-polymer was then poured on the silicon substrate and cured at 65 °C for 3 h to form PDMS slab. Thereafter, the PDMS slab embedded with microchannels (half-depth) was lifted off. Two PDMS slabs with the same channel design were then plasma-treated for 30 s using Harrick PDC-32 G plasma cleaner at 18 W and 27 Pa, wetted with methanol (to prevented instant bonding), and aligned and bonded together under microscope to produce the final microfluidic device. Assembled devices were kept on hotplate at 80 °C for ~ 10 min to evaporate residual methanol and further kept at 65 °C for 2 days to make it sufficiently hydrophobic for experiments.
2.4. Rheological characterization of hydrogel materials for making ovarian microtissues
Rheological measurements were carried out using a TA instrument AR-1000N rheometer. For alginate and collagen (type I, BD Biosciences) hydrogels, 40 mm parallel plate and plate-cone geometries were used, respectively. Stress sweeps at a constant frequency of 1 Hz were first performed to obtain the linear viscoelastic region for collecting subsequent data. Frequency sweeps were performed in the linear viscoelastic regime to determine values of the storage (G′) and loss (G″) modulus. The data at 1 Hz are reported in Fig. 1 for comparison. More rheology data showing the shearing rate (or frequency) dependence of the moduli of the various materials are given in Supplementary Fig. S1. The 2% and 0.5% alginate hydrogels were prepared and gelled on mold using calcium infused mineral oil for 30 min, followed by washing with mannitol solution and transferring onto the rheometer plate. Collagen gels were prepared directly on the rheometer, for which 0.5% collagen solution was placed on the rheometer plate at 4 °C and gelled by raising the rheometer temperature to 37 °C for 30 min. To determining the time dependent mechanical properties, the samples were prepared in the same way and further incubated in the basal culture medium of follicles at 37 °C in 5% CO2 incubator till measurement at the desired times.
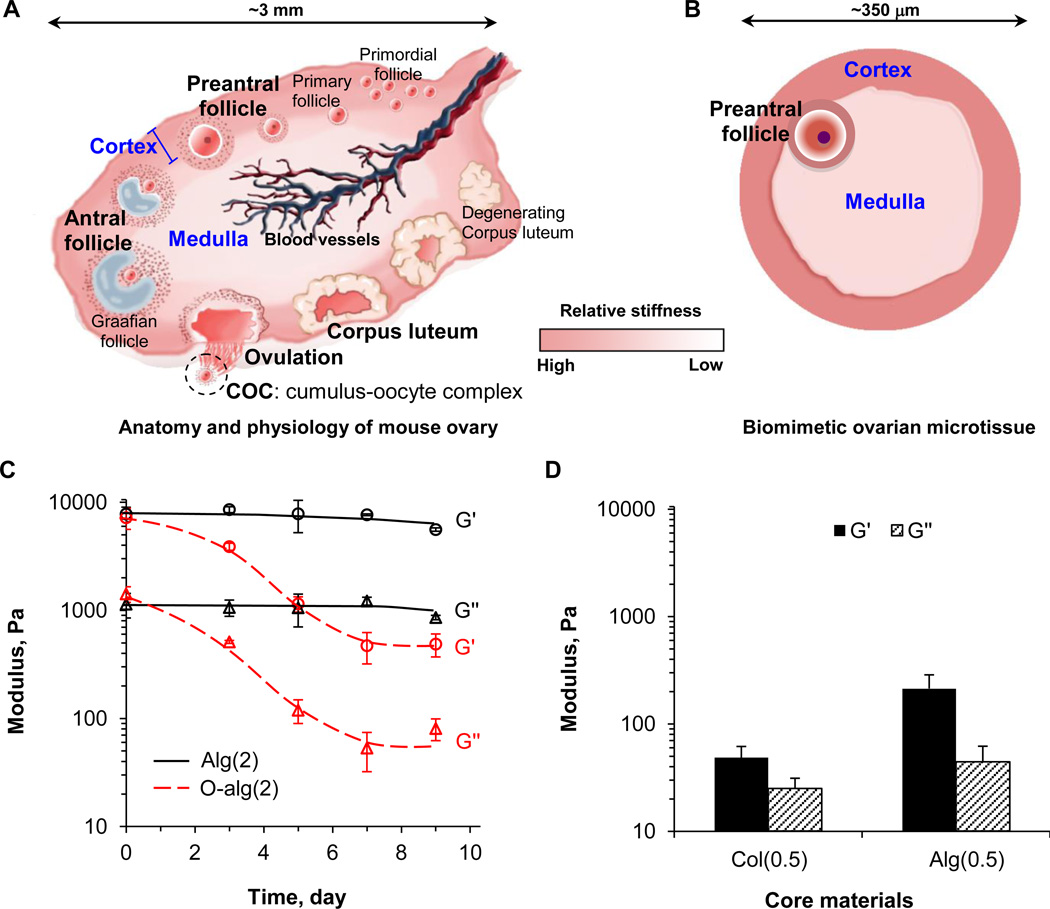
Fig. 1.
Design and materials of biomimetic ovarian microtissue. (A) A schematic illustration of mouse ovary that consists of two mechanically distinct tissue layers: the more rigid cortex and the softer medulla. (B) A schematic illustration of the in vitro engineered biomimetic ovarian microtissue. (C) The storage (G′, representing elastic effect) and loss (G″, representing viscous effect) moduli of materials for making the microtissue shell. Two different harder hydrogels, 2% alginate (Alg(2)) and 2% alginate with oxidization (O-alg(2)), were used for making the shell (cortex). The moduli of O-alg(2) decrease by >16 times after ~ 7 days incubation in culture medium at 37 °C. (D) The two moduli of materials for making the microtissue core. Two different softer hydrogels, 0.5% alginate (Alg(0.5)) and 0.5% collagen (Col(0.5)), were used for making the core (medulla).
2.5. Encapsulation of early secondary preantral follicles to produce ovarian microtissues
The fluid entering the core microchannel via I-1 (Fig. 2) was 0.5% collagen or sodium alginate (non-oxidized by default) solution. The dispatching fluid was the same as the core fluid. The fluid entering the shell microchannel via I-2 was 2% sodium alginate or a mixture of 1% sodium alginate and 1% sodium alginate with oxidization using a protocol reported previously [24, 25]. For convenience, the mixture is called oxidized alginate (O-alg) in this work. To increase the viscosity in the core solution, which was found to be necessary for formation of core-shell structure, 1% sodium carboxymethyl cellulose was included. For the extraction channel, 1% sodium carboxymethyl cellulose solution was used which was necessary for forming stable interface between oil emulsion and the aqueous phase. All the solutions were sterile and buffered with 10 mM HEPES to maintain pH at 7.2 before use. Further, osmolality of all the solutions were maintained at 300 mOsm by the addition of d-mannitol. To make mineral oil infused with calcium chloride for flowing in the oil channel, stable emulsion of mineral oil and 0.7 g/ml calcium chloride solution (volume ratio: 3 to 1 with the addition of 1.2% SPAN 80) was prepared by sonication for 1 min using a Branson 450 digital sonifier. Water in the emulsion was then removed by rotatory evaporation for ~ 2 min at 55 °C. All solutions (except collagen that was kept at 4°C) were injected into the microfluidic device using syringe pump at room temperature to generate microcapsules in oil phase and then extract them into aqueous phase. Flow rates for core, dispatching, shell, oil, and aqueous extracting fluids were 50 µl/hr, 30 µl/hr, 120 µl/hr, 2 ml/hr, and 4 ml/hr, respectively. Outlets of the device were connected to a 50 ml centrifuge tube containing M2 medium (Millipore) to collect microtissues at room temperature.
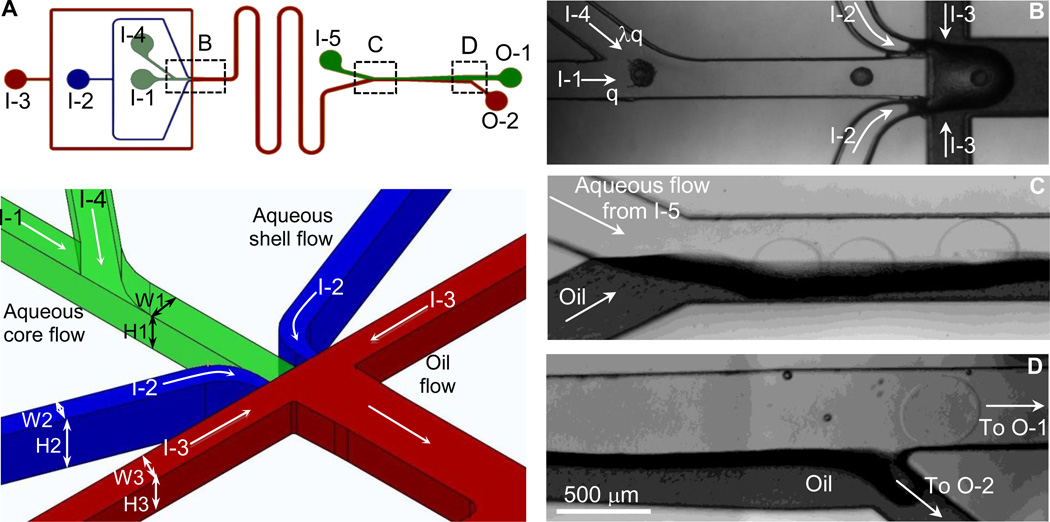
Fig. 2.
Non-planar microfluidic flow-focusing device for encapsulating early secondary preantral follicle in core-shell microcapsules to produce the biomimetic ovarian microtissue. (A) A schematic view of the microchannel system (top) together with a zoom-in view of the nonplanar design of the flow-focusing junction (bottom) where W1×H1 = 200×200 µm, W2×H2 = 80×300 µm, and W3×H3 = 200×400 µm. (B) Typical image of the boxed region in panel A showing the dispatching and flow-focusing areas. (C) Typical image of the boxed region in panel A showing the entrance of the extraction channel. (D) Typical image of the boxed region in panel A showing the exit of the extraction channel. I-1, I-2, I-3, I-4, and I-5 are the inlets of core, shell, mineral oil emulsion, dispatching, and extraction flows, respectively. O-1 and O-2 are outlets for the aqueous (containing microtissues) and oil emulsion flows, respectively.
2.6. Preparation of ovarian cell-conditioned medium
To prepare ovarian cell-conditioned medium, ovarian cells were first isolated by following a protocol reported previously [6]. Briefly, the ovaries of 12–14 week-old of deer mice were collected and chopped after the removal of adherent tissues such as fat pad. The specimens were incubated initially for 30 minutes in a dissociation medium consisting of a 50:50 (v:v) mixture of 0.25% (v/w) trypsin-EDTA (ethylenediaminetetraacetic acid) and DMEM (Dulbecco’s modified eagle’s medium) supplemented with 750 units/ml type I collagenase and 0.03% (v/v) fetal bovine serum at 37 °C in 5% CO2 air. The dissociated cells were filtered through a 40 µm filter and subsequently centrifuged at 390×g for 4 minutes. The collected cells were further cultured onto a 60 mm culture dish in 5 ml DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. After 20 h of culture in the dish, the DMEM-based medium was removed and the cells were washed once using 1x PBS. A total of 5 ml (non-conditioned) α-MEM–glutamax medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin solution was then added into the dish. The cells were incubated with the non-conditioned medium at 37 °C in 5% CO2 air for two days and the resultant conditioned medium (CM) was collected and the procedure was repeated once to eventually make a total of 10 ml conditioned medium supplemented with 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml selenium, and 100 mIU/mL recombinant human follicle stimulating hormone (FSH) for further use.
2.7. In vitro culture of early secondary preantral follicles encapsulated in microtissues
For 2D culture, the early secondary preantral follicles were placed singly in 10 µl drops of ovarian cell conditioned culture medium overlaid with mineral oil in 60 mm culture dishes [6]. The preantral follicles encapsulated in microtissues were cultured singly in each well with 100 µl of ovarian cell conditioned culture medium in 96-well plate. On the following day, 10 µl and 100 µl of fresh medium were added to each drop and well, respectively. Starting from day 3, half the medium (10 and 100 µl) was replaced with resh medium every other day till day 13. To study the influence of epidermal growth factor (EGF) and pituitary luteinizing hormone (LH) on follicle growth and ovulation, 2.5 IU/ml LH and 5 ng/ml EGF were added in the medium of some microencapsulated follicles once they developed to the antral stage. Ovulated cumulus-oocyte complexes (COCs) were further cultured in the same medium for 18 hours. Afterward, the COCs were incubated in M2 medium containing 200 IU/ml hyaluronidase at 37 °C for up to 3 min to remove cumulus cells and then washed twice in fresh M2 medium to obtain clean oocytes. The nucleus of the obtained oocytes was stained by incubating them with Hoechst 33342 (1 µg/ml) for 5 minutes at 37 °C in 5% CO2 air.
3. Results and discussion
3.1. Design and materials of biomimetic ovarian microtissue
As illustrated schematically in Fig. 1A, all primordial and primary follicles stay within the rigid cortex. Once some of them are activated to grow at the beginning of each estrous cycle, they move from the cortex towards the medulla where profuse blood supply is available to provide the necessary nutrients and supplements for them to develop into antral follicles, followed by ovulation to release cumulus-oocyte complex (COC) [1, 16, 26]. To mimic the mechanical heterogeneity in mammalian ovary in vitro, we propose to produce ovarian microtissue by encapsulating early secondary preantral follicles in microcapsules consisting of a softer, biodegradable collagen (0.5%) hydrogel core and a harder, slowly degradable alginate (2%) hydrogel shell (Fig. 1B). This miniaturized 3D culture ensures effective transport of oxygen and nutrients to all cells in the fast growing follicles [7, 23, 27]. Alginate was used because of its excellent biocompatibility and mild gelation condition using divalent cations such as Ca2+ that are not harmful to living cells [23, 28–30]. As shown in Fig. 1C-D, the mechanical properties including both storage (G′, representing elastic effect) and loss (G″, representing viscous effect) modulus of 2% alginate (Alg(2)) for shell/cortex are much higher than that of 0.5% collagen (Col(0.5)) for core/medulla. For comparison, we also used 2% oxidized alginate (O-alg(2)) that degrades fast (Fig. 1C) as the shell materials and 0.5% alginate (Alg(0.5)) with higher modulus than 0.5% collagen (Fig. 1D) as the core materials to understand how the change in mechanical cue in the ovarian microtissue could affect the development and ovulation of the encapsulated early secondary preantral follicles.
3.2. Microfluidic fabrication of biomimetic ovarian microtissue
The ovarian microtissues were produced using a non-planar microfluidic flow-focusing device by injecting collagen (or alginate) core solution with follicles, alginate shell solution, and mineral oil infused with aqueous calcium chloride solution from the I-1, I-2, and I-3 inlets into the device, respectively (Fig. 2A). At the flow-focusing junction, the core and shell solutions were pinched into droplets by the oil emulsion flow as a result of the Rayleigh-Plateau instability [31, 32]. Sodium alginate solution in the shell was crosslinked (i.e., hardened) to form calcium alginate hydrogel by aqueous calcium chloride solution infused in the mineral oil-calcium chloride emulsion during traveling in the serpentine channel before entering the extraction channel [23, 33]. The aqueous core flow is arranged in the center of alginate shell flow both horizontally and vertically, to ensure that the core flow can be encompassed by the shell flow (Fig. 2A-B and Video S1). However, in some cases, two or more follicles could be so close in the core channel immediately before flow focusing that they would be encapsulated in one microtissue (Fig. S2). This is undesired because the core of the microtissue is too small to accommodate more than one fully-grown follicle. To overcome this problem, we designed a dispatching channel shortly upstream of flow focusing to increase the distance between neighboring follicles by injecting the same solution as the core flow through I-4 inlet (Fig. 2A-B). For the Stokes laminar flow in microchannels, microparticles (or follicles) can be taken to flow along the flow streamlines [34–36]. As a result of mass conservation, the ratio of the distance between two follicles before dispatching to that after dispatching can be calculated as follows:
| (1) |
Where and are the distances between follicles before and after dispatching, respectively, q is the flow rate of the follicle-loaded core solution, and λ is the ratio of the dispatching to core flow rate. The effectiveness of this dispatching channel (λ=3) in separating two adjacent microparticles is demonstrated in Video S2 using 125 µm polystyrene beads. With dispatching at a much lower λ (0.6) to minimize the effect of the dispatching flow on droplet formation, we were able to encapsulate one follicle in each microcapsule or microtissue with high efficiency (> 97%).
To minimize the stay of the hardened (by crosslinking alginate in the shell) microtissues in oil emulsion and collect them in aqueous culture medium, we further incorporated an extraction channel downstream of the serpentine channel in the microfluidic device to efficiently extract microtissues from the oil emulsion into an isotonic aqueous phase. Since the Reynolds Number for our flow conditions is much less than one (~ 0.1), the movement of microparticles is dictated by viscous force and the microtissues would travel with oil emulsion if undisturbed [34–36]. Therefore, we used an expansion design for the extraction channel that is narrow (400 µm) at the beginning part (Fig. 2C) and wide (800 µm) at the end (Fig. 2D). Because the diameter of the microtissue is ~350 µm that is only slightly smaller than the width (400 µm) of the extraction channel at the entrance, the hardened microtissue is forced to penetrate through the oil-water interface and touch the aqueous extracting solution once it enters the extraction channel at the entrance where the channel is occupied partially by oil (half) and the aqueous extracting solution (half) (Fig. 2C). In other words, the microtissue is partially in oil and partially in the aqueous extracting solution at the entrance of the extraction channel. Because the interfacial tension between the microtissue (hydrophilic) and oil (hydrophobic) is high while it is nearly zero between the microtissue and aqueous extracting solution (hydrophilic), the microtissue should experience an unbalanced hydrophobic force as a result of the difference in interfacial tension on its surface. Consequently, the microtissue is pushed away from the oil into aqueous extracting solution further downstream in the extraction channel as the width increases (Fig. 2C-D and Videos S3–S4).
The expansion design at the exit should make it more convenient for collecting the microtissue by allowing them going straight into a 500 µm aqueous channel to exit from O-1 while the oil flow turns into a side channel to exit from O-2 (Fig. 2D and Video S4). Thereafter, the microtissues were collected and cultured for up to 13 days (the typical time length of an estrous cycle of deer mice) to monitor their development and ovulation in vitro.
3.3. Regulation of follicle development and ovulation
Typical micrographs showing an early secondary preantral follicle in the biomimetic ovarian microtissue with a collagen core and alginate (non-oxidized, by default) shell on day 0 and its development into the antral stage are shown in Fig. 3A. The fibrous collagen core enclosed in the alginate shell is clearly visible, which is further confirmed by confocal reflectance microscopy (Fig. S3). The defining feature of an antral follicle is that it contains a cumulus-oocyte complex (COC) inside a fluid-filled antral cavity (also called antrum) as shown in Fig. 3A. An enlarged view of the antral follicle on day 9 for better visualizing the COC and antral cavity together with a schematic illustration of the anatomic features of COC and follicles at the preantral and antral stages is given in Fig. S4. The cumulus cells surrounding the oocyte in the COC are differentiated from granulosa cells.
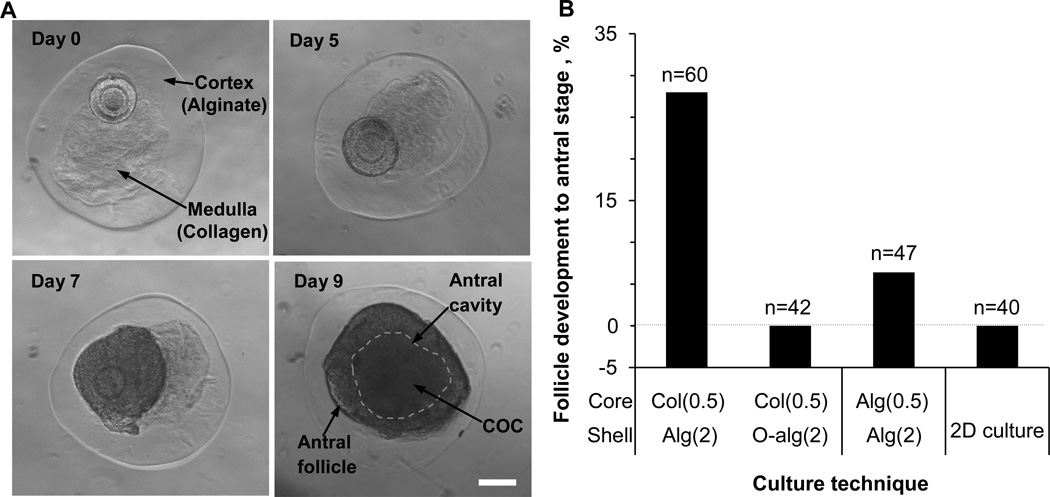
Fig. 3.
In vitro development of early secondary preantral follicles of deer mice under miniaturized 3D culture in microtissue. (A) Typical micrographs showing the development to antral stage over 9 days of an early secondary preantral follicles in the collagen core of biomimetic ovarian microtissue with an alginate (non-oxidized) shell. The defining feature of an antral follicle is that it contains a cumulus-oocyte complex (COC) inside a fluid-filled antral cavity (the area enclosed in dashed line). A schematic illustration of the anatomic features of COC and follicles at various stages together with an enlarged view of the antral follicle on day 9 for better visualizing the COC and antral cavity is shown in Fig. S4. (B) Quantitative data (pooled, the number of early secondary preantral follicles n ≥ 40) showing the effect of the core (0.5% collagen or alginate) and shell (2% oxidized or nozn-oxidized alginate) materials for making the ovarian microtissue on the development of early secondary preantral follicle into antral stage together with that from 2D culture. Col(0.5): 0.5% collagen. Alg(2): 2% alginate. O-alg(2): 2% alginate with oxidization. Alg(0.5): 0.5% alginate. Scale bar: 100 µm.
A total of ~28% (17/60) of the preantral follicles in the biomimetic microtissue could develop to the antral stage, typically on day 9 (Fig. 3B and Table S1). This percentage dropped drastically to 0% (0/42) when oxidized alginate was used to make the shell of the microtissue even though the core was the same (0.5% collagen). The hydrogel shell of oxidized alginate degrades in 5–7 days with much reduced (by >16 times) mechanical strength thereafter compared to the hydrogel shell of alginate without oxidization (Fig. 1C). These data indicate the critical role of mechanical heterogeneity of the cortical and medullary tissue in the ovary in regulating follicle development. This is supported by the low percentage of development to the antral stage (~6%, 3/47) when the shell was the same (2% alginate) and the collagen (0.5%) core was replaced with the harder alginate (0.5%), even though homogeneous hydrogel of alginate up to 1% has been conventionally used for culturing preantral follicles isolated from inbred mice [8–11]. It is worth noting that the low percentage of development with the alginate core could be partially attributed to the loss of potential molecular signals from collagen to the follicle in the microtissue although the signaling between collagen and follicle should be minimal. The latter is because the follicles were observed to grow as a whole rather than getting diffused into the collagen matrix as shown in Fig. 3A. However, further investigation is warranted to investigate the change in mechanical property of the core matrix on follicle development without compromising any potential signaling event in the microtissue although it is beyond the scope of this study. Lastly, it is not surprising that 0% (0/40) of the early secondary preantral follicle developed to the antral stage from 2D culture since it is non-physiological at all and could not maintain the 3D architecture of the follicles during culture (Figs. 3B and S5).
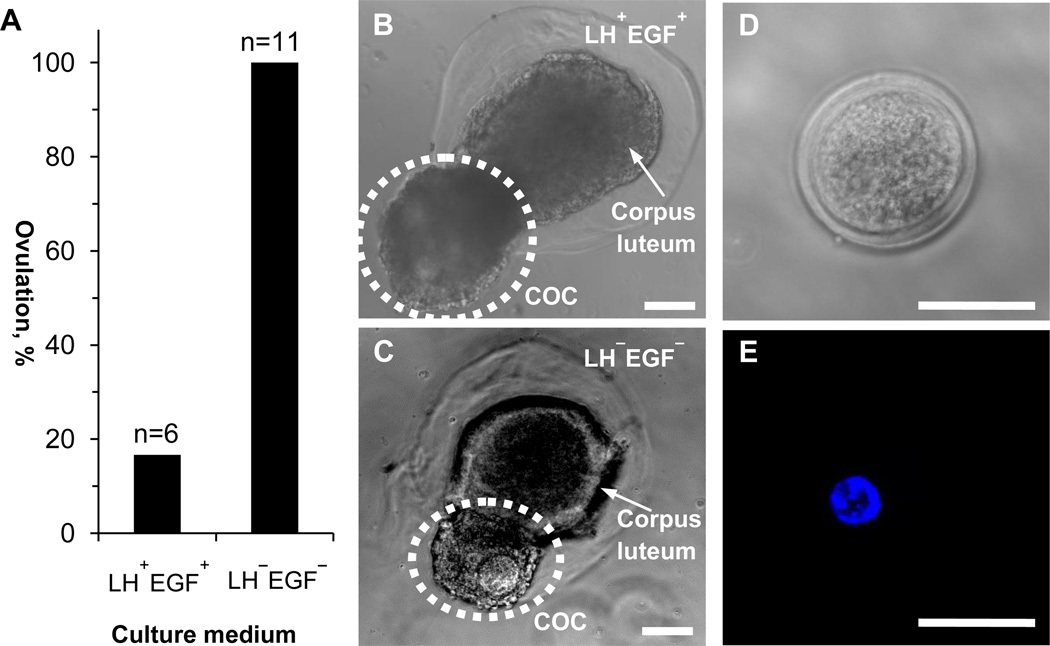
As shown in Fig. 1A, the event following the development of follicles to the antral stage in vivo is ovulation, a delicate reproductive process that results in the release of a cumulus-oocyte complex (COC) from each follicle. Although the exact mechanisms that regulate the ovulation process are still not fully understood, it is well accepted in contemporary literature that ovulation is triggered by the surge of luteinizing hormone (LH) from the pituitary gland. This LH surge activates a cascade of epidermal growth factor (EGF) mediated signaling pathways to induce cumulus cell differentiation (from granulosa cells) and expansion in the follicle, resulting in the escape of the COC out of the follicle [17–22]. Guided by the literature on the critical role of LH and EGF in initiating ovulation, we treated 6 out of the 17 antral follicles obtained by culturing early secondary preantral follicle in the microtissue with a collagen core and alginate shell (Fig. 3B and Table S1) with LH and EGF (LH+EGF+), leaving the remaining 11 antral follicles without LH and EGF treatment (LH−EGF−). In other words, we used less antral follicles for the LH+EGF+ group because we thought that according to the conventional theory, the probability of ovulation from this group should be much higher compared to the LH−EGF− group. In contrast to our anticipation, only 1 out of the 6 antral follicles treated with LH and EGF ovulated while ovulation was observed for all the 11 antral follicles without LH and EGF treatment (Fig. 4A). Typical micrographs of ovulation showing a released COC and a corpus luteum-like tissue complex remaining in the alginate shell are shown in Fig. 4B and C for the LH+EGF+ and LH−EGF− groups, respectively. Typical images showing the morphology and nucleus of the ovulated germinal vesicle (GV) oocytes are given in Fig. 4D and E, respectively. Interestingly, only 1 out of 3 antral follicles obtained from culturing early secondary preantral follicles in microtissue with an alginate (0.5%) core and alginate shell ovulated when they were cultured in the absence of LH and EGF (Table S1). These data suggest that mechanical heterogeneity in the biomimetic ovarian tissue and ovary regulates follicle ovulation and the LH surge might even suppress ovulation of many antral follicles to allow few of them being ovulated during each estrous cycle, as occurs naturally. In this study, we cultured the ovarian microtissue with the commonly used dose of LH and EGF [17–22]. Future research is warranted to determine the critical level of LH and EGF that begins to inhibit ovulation under various mechanical microenvironments.
Fig. 4.
In vitro ovulation of antral follicles obtained by culturing early secondary preantral follicles in the collagen core of biomimetic ovarian microtissues with an alginate shell. (A) Quantitative data (pooled, the number of antral follicles n ≥ 6) showing the effect of luteinizing hormone (LH) and epidermal growth factor (EGF) on ovulation. (B) A typical micrograph of in vitro ovulation showing a cumulus-oocyte complex (COC) was released leaving behind a corpus luteum-like tissue complex in a biomimetic microtissue cultured in the presence of LH and EGF (LH+EGF+). (C) A typical micrograph of in vitro ovulation from a biomimetic microtissue cultured in the absence of LH and EGF (LH−EGF−). (D) A typical phase contrast image showing the oocyte morphology. (E) A typical fluorescence image showing the nucleus of the oocyte in panel D. Scale bars: 100 µm in panels B-C and 50 µm in panels D-E.
Lastly, it has been reported that disruption of normal physical environment in the ovary may result in ovarian disorders such as premature ovarian failure (POF) and polycystic ovary syndrome (PCOS) [16, 37], which is consistent with the observations in this study. Therefore, our biomimetic ovarian microtissue may be used to study the mechanisms associated with these ovarian disorders to facilitate the search of cures to infertility as a result of these diseases.
4. Conclusions
In summary, we have developed a microfluidic microencapsulation technology that allows us to engineer ovarian microtissue that recapitulates the 3D mechanical, physiological, and anatomical milieu in the ovary. With these biomimetic microtissues, we unraveled the crucial role of mechanical heterogeneity in the ovary in regulating follicle development and ovulation. Our biomimetic ovarian microtissue may be valuable for understanding the mechanisms associated with ovarian disorders and serving as a better in vitro culture system to preserve fertility and restore infertility for women.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH (R01EB012108).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodruff TK. Making eggs: is it now or later? Nat Med. 2008;14(11):1190–1191. doi: 10.1038/nm1108-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodruff TK, Zoloth L, Campo-Engelstein L, Rodriguez S. Oncofertility: ethical, legal, social, and medical perspectives. Preface. Cancer Treat Res. 2010;156:v–vii. [PubMed] [Google Scholar]
- 3.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A, et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119. doi: 10.1186/1477-7827-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2(2):94–104. doi: 10.1530/ror.0.0020094. [DOI] [PubMed] [Google Scholar]
- 6.Choi JK, Agarwal P, He X. In vitro culture of early secondary preantral follicles in hanging drop of ovarian cell-conditioned medium to obtain MII oocytes from outbred deer mice. Tissue Eng Part A. 2013;19(23–24):2626–2637. doi: 10.1089/ten.tea.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25(4):287–299. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brito IR, Lima IM, Xu M, Shea LD, Woodruff TK, Figueiredo JR. Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices. Reprod Fertil Dev. 2013 doi: 10.1071/RD12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise M, Koepsel R, Russell AJ, McGee EA. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3:47. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30(29):5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Woodruff TK, Shea LD. Bioengineering and the ovarian follicle. Cancer Treat Res. 2007;138:75–82. doi: 10.1007/978-0-387-72293-1_6. [DOI] [PubMed] [Google Scholar]
- 13.Choi JK, He X. In vitro maturation of cumulus-oocyte complexes for efficient isolation of oocytes from outbred deer mice. PLoS One. 2013;8(2):e56158. doi: 10.1371/journal.pone.0056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers RJ, van Wezel IL, Irving-Rodgers HF, Lavranos TC, Irvine CM, Krupa M. Roles of extracellular matrix in follicular development. J Reprod Fertil Suppl. 1999;54:343–352. [PubMed] [Google Scholar]
- 15.Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24(4):262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28(1):3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JAS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 18.Fan HY, Liu ZL, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilchrist RB, Mottershead DG, Thompson JGE. Oocyte maturation and ovulation- an orchestral symphony of signaling. Australian Biochemist. 2011;42(1):8–11. [Google Scholar]
- 20.Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23(3):444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y, et al. One-step microfluidic generation of prehatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip. 2013;13(23):4525–4533. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17(5):945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 25.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 26.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Zhao S, Rao W, Snyder J, Choi JK, Wang J, et al. A novel core-shell microcapsule for encapsulation and 3D culture of embryonic stem cells. J Mater Chem B Mater Biol Med. 2013;2013(7):1002–1009. doi: 10.1039/C2TB00058J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, He X. Microencapsulating and banking living cells for cell-based medicine. J Healthc Eng. 2011;2(4):427–446. doi: 10.1260/2040-2295.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 31.Seemann R, Brinkmann M, Pfohl T, Herminghaus S. Droplet based microfluidics. Rep Prog Phys. 2012;75(1):016601. doi: 10.1088/0034-4885/75/1/016601. [DOI] [PubMed] [Google Scholar]
- 32.Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Formation of droplets and bubbles in a microfluidic T-junction - scaling and mechanism of break-up. Lab Chip. 2006;6(3):437–446. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman AS. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944:62–73. doi: 10.1111/j.1749-6632.2001.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 34.Cubaud T, Mason TG. Capillary threads and viscous droplets in square microchannels. Phys Fluids. 2008;20:053302. [Google Scholar]
- 35.Di Carlo D, Edd JF, Humphry KJ, Stone HA, Toner M. Particle segregation and dynamics in confined flows. Phys Rev Lett. 2009;102(9):094503. doi: 10.1103/PhysRevLett.102.094503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baroud CN, Gallaire F, Dangla R. Dynamics of microfluidic droplets. Lab Chip. 2010;10(16):2032–2045. doi: 10.1039/c001191f. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Fan L, Meng Y, Hou Z, Mao YDL, Wang W, et al. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;13(8):527–535. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.