Abstract
Human organic anion transporter 3 (hOAT3) belongs to a family of organic anion transporters that play critical roles in the body disposition of numerous clinically important drugs. In the kidney, hOAT3 functions through a tertiary transport mechanism involving two other membrane proteins Na/K ATPase and Na/dicarboxylate cotransporter. In the current study, we established COS-7 cells stably expressing hOAT3 and examined the regulation of hOAT3 by protein kinase C (PKC) and angiotensin II. Both PKC activation and angiotensin II inhibited hOAT3 transport activity. Angiontensin II induced inhibition of hOAT3 activity could be prevented by treating hOAT3-expressing cells with staurosporine, a general inhibitor for PKC, and with Gö6976 (5,6,7,13-Tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]py rrolo[3,4-c]carbazole-12-propanenitrile), a PKCα-specific inhibitor. Examination of hOAT3 expression and transport kinetics revealed that angiotensin II induced inhibition of hOAT3 activity mainly resulted from a decreased cell surface expression kinetically reflected as a decreased Vmax without significant change in Km. Such angiotensin II induced decrease in cell surface expression of hOAT3 was caused by an increase in hOAT3 endocytosis. However, angiotensin II induced endocytosis of Na/K ATPase did not occur under such condition. We concluded that angiotensin II inhibited hOAT3 activity through activation of PKCα, which led to an acceleration of hOAT3 endocytosis.
Keywords: drug transporter, Regulation, Angiotensin II, Protein Kinase C, Endocytosis, COS-7 Cells
1. Introduction
The organic anion transporter (OAT) family mediates the body disposition of a diverse array of environmental toxins, and clinically important drugs, including anti-HIV therapeutics, anti-tumor drugs, antibiotics, anti-hypertensives, and anti-inflammatories (Anzai et al., 2006; El-Sheikh et al., 2008; Srimaroeng et al., 2008; You, 2004). Therefore, understanding the regulation of these transporters has profound clinical significance.
Ten OATs (OAT1-10) have been cloned. These OATs are expressed in distinct tissues and cell membranes (Bahn et al., 2008; Cha et al., 2000; Kusuhara et al., 1999; Schnabolk et al., 2006; Sekine et al., 1998; Sekine et al., 1997; Shin et al., 2007; Sweet et al., 1997; Yokoyama et al., 2008; Youngblood and Sweet, 2004). In the kidney, OAT1 and OAT3 utilize a tertiary transport mechanism to move organic anions across the basolateralmembrane into the proximal tubule cells for subsequent exit across the apical membrane into the urine for elimination (Anzai et al., 2006; El-Sheikh et al., 2008; Srimaroeng et al., 2008; You, 2004) . Through this tertiary transport mechanism, Na+/K+-ATPase maintains an inwardly directed (blood-to-cell) Na+ gradient. The Na+ gradient then drives a sodium dicarboxylate cotransporter, sustaining an outwardly directed dicarboxylate gradient that is utilized by a dicarboxylate/organic anion exchanger, namely OAT, to move the organic anion substrate into the cell. This cascade of events indirectly links organic anion transport to metabolic energy and the Na+ gradient, allowing the entry of a negatively charged substrate against both its chemical concentration gradient and the electrical potential of the cell. Generation of OAT1 and OAT3 null mice confirmed an essential role for these transporters in the renal transport (Eraly et al., 2006; Sweet et al., 2002).
As the major effector of the rennin-angiotensin system, angiotensin II is involved in the regulation of blood pressure, vascular tone and salt and water homeostasis (Matsusaka and Ichikawa, 1997). In addition to its effects in cardiovascular system, angiotensin II also affects cell proliferation and apoptosis (Yamada et al., 1996). Angiotensin II binds to two major high-affinity receptors designated the angiotensin AT1 and the angiotensin AT2 receptors, which belong to the G protein coupled receptor (GPCR) family (Kambayashi et al., 1993; Murphy et al., 1991) . Activation of angiotensin II AT1 subsequently activates a series of protein kinases including PKC (Schmitz and Berk, 1997). We have recently shown that angiotensin II inhibits OAT1 activity through activation of PKC (Li et al., 2009). In the current study, we investigated the effect of angiotensin II on OAT3 and the mechanisms underlying such regulation.
2. Materials and methods
2.1 Materials
[3H] estrone sulfate was purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA, USA). NHS-SS-biotin and streptavidin-agarose beads were purchased from Pierce Chemical (Rockford, IL, USA). Gö6976 (5,6,7,13-Tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]py rrolo[3,4-c]carbazole-12-propanenitrile) was purchased from LC Laboratories (Woburn, MA, USA). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Generation of COS-7 cells stably expressing human OAT3 (hOAT3)
Parental COS-7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 %fetal bovine serum, penicillin/streptomycin (100 U/ml), and glucose (100 mg/ml) in a 5 % CO2 atmosphere at 37°C. Cells were seeded at 5 × 105/well of 6-well cluster plate 24 h before transfection. cDNA plasmid encoding hOAT3-tagged with myc at its carboxyl terminus was transfected into COS-7 cells using Lipofectamine 2000 reagent, following the manufacturer's instruction. Epitope myc was tagged to hOAT3 to facilitate the immunodetection of hOAT3. After 7 to 8 days of selection in medium containing 0.5 mg/ml geneticin (G418; Invitrogen,Carlsbad, CA, USA), resistant colonies were replated into 96 wells for cloning, expansion, and analyzing positive clones. Cells stably expressing hOAT3 were maintained in DMEM medium supplemented with 0.2 mg/ml G418, 10 % fetal bovine serum, penicillin/streptomycin (100 U/ml), and glucose (100 mg/ml).
2.3 Transport measurement
Cells plated in 48-well plates were treated with each reagent at 37 °C for certain time periods as indicated. For each well, uptake solution was added. The uptake solution consisted of phosphate-buffered saline/Ca2+/Mg2+ (137 mM NaCl, 2.7 mMKCl, 4.3 mM Na2HPO41.4 mM KH2PO41 mM CaCl2, and 1 mM MgCl2, pH 7.4) and [3H] estrone sulfate. At the times indicated, the uptake was stopped by aspirating off the uptake solution and rapidly washing the well with ice-cold PBS. The cells were then solubilized in 0.2 N NaOH, neutralized in 0.2 N HCl, and aliquotted for liquid scintillation counting. The uptake count was standardized by the amount of protein in each well. Values are means ±S.D. (n = 3).
2.4 Cell surface biotinylation
Cell surface expression levels of hOAT3 were examined using the membrane-impermeant biotinylation reagent NHS-SS-biotin (Pierce Chemical, Rockford, IL, USA). The cells were seeded onto six-well plates at 8 × 105 cells per well. After 24 h, the medium was removed and the cells were washed twice with 3 ml of ice-cold PBS, pH 8.0. The plates were kept on ice, and all solutions were kept ice-cold for the rest of the procedure. Each well of cells was incubated with 1 ml of freshly made NHS-SS-biotin (0.5 mg/ml in PBS, pH 8.0) in two successive 20 min incubations on ice with very gentle shaking. Biotinylation was quenched by first briefly washing each well with 3 ml of 100 mM glycine and followed by incubation with 100 mM glycine on ice for 20 min. The cells were then dissolved on ice for 40 min in 400 μl of lysis buffer [10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1 % SDS, 1 % Triton X-100, and protease inhibitors (200 μg/ml phenylmethylsulfonyl fluoride and 3 μg/ml leupeptin), pH 7.4]. The unlysed cells were removed by centrifugation at 16,000 X g at 4 °C. Streptavidin-agarose beads (50 μl; Pierce Chemical, Rockford, IL, USA) were then added to the supernatant to isolate cell membrane protein. hOAT3 was detected in the pool of surface proteins by polyacrylamide gel electrophoresis and immunoblotting using an anti-myc antibody (1:500) (Mount Sinai Medical Center, New York, NY, USA). Myc was tagged at the carboxyl terminus of hOAT3 for its immunodetection.
2.5 Internalization assay
We followed the procedure described previously by our laboratory (Zhang et al., 2008a). hOAT3-expressing cells underwent biotinylationwith 0.5 mg/ml sulfo-NHS-SS-biotin as described above. Following biotinylation, one set of cells was washed with PBS and kept at 4 °C to determine the total initial surface hOAT3 and stripping efficiency. To initiate internalization, cells in the duplicate plate were washed repeatedly with pre-warmed (37 °C) PBS containing either 1 μM of angiotensin I I or PBS only and incubated with the same solutions for 10 min at 37 °C. Residual cell surface biotin was stripped by incubating cells three times for 20 min with freshly prepared 50 mM MesNa in NT buffer (150 mM NaCl, 1 mM EDTA, 0.2 % bovine serum albumin, 20 mM Tris, pH 8.6). Stripping efficiency was determined for each experiment on biotinylated cells kept in parallel at 4 °C. Cells were lysed in lysis buffer with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Biotinylated proteins were separated from non-biotinylated proteins by streptavidin-agarose resin (Thermo Scientific, Waltham, MA, USA) similarly as we described above. Samples were then eluted from the beads by adding sample buffer and resolved by SDS-PAGE and analyzed by western blotting with anti-myc antibody. Relative hOAT3 internalized was calculated as % of the total initial cell surface hOAT3 pool.
2.6 Electrophoresis and western blotting
Protein samples (100 μg) were resolved on 7.5 % SDS-PAGEmini-gels and electroblotted onto polyvinylidene difluoride membranes. The blots were blocked for 1 h with 5 % nonfat dry milk in PBS-0.05 %Tween, and incubated overnight at 4 °C with anti-myc antibody (1:500). The membranes were washed and then incubated with goat anti-mouse IgG (Thermo Scientific, Waltham, MA, USA) conjugated to horseradish peroxidase (1: 5,000), and signals were detected using a SuperSignal West Dura extended duration substrate kit (Thermo Scientific, Waltham, MA , USA). Images were captured by Fluorchem ®8800 system (Alpha Innotech, San Leandro, CA, USA). Density of bands was analyzed by Quantity One software (Bio-Rad, Hercules, CA, USA).
2.7 Data analysis
Each experiment was repeated a minimum of three times. The statistical analysis given was from multiple experiments. Statistical analysiswas performed using Student's paired t-tests. A P value < 0.05 was considered significant. Kinetics studies of hOAT3 (Km, Vmax) was analyzed by GraphPad Prism® 5 (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1 Characterization of hOAT3 in COS-7 cells
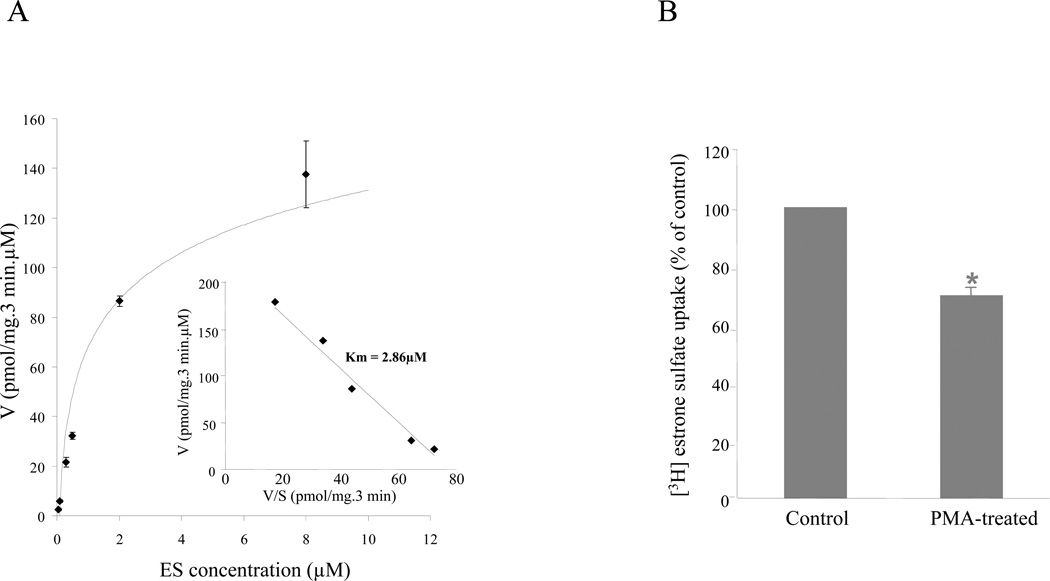
To study the mechanisms underlying regulation of hOAT3-mediated drug transport, we established COS-7 cells stably expressing hOAT3. The hOAT3-mediated transport of estrone sulfate across the cell membrane was saturable (Fig. 1A). Based on Eadie-Hofstee plot analysis (Fig. 1A, inset), the Km value for estrone sulfate was 2.86 μM and Vmax was 221.63 pmol/mg/3 min. Such transport characteristic is consistent with that of hOAT3 in other systems (Cha et al., 2001), suggesting that COS-7 cells are suitable model for studying hOAT3. We further showed that treatment of hOAT3-expressing cells with phorbol 12-myristate 13-acetate (PMA), a PKC activator, at 1 μM for 30 min, resulted in a significant decrease in hOAT3-mediated estrone sulfate transport (Fig. 1B). Therefore, the regulation of hOAT3 by PKC is similar to that of hOAT1 (Zhang et al., 2008a) and hOAT4 (Zhou et al., 2007) previously demonstrated by our laboratory.
Figure 1. Characterization of hOAT3 in COS-7 cells.
A. Kinetic analysis of hOAT3-mediated estrone sulfate uptake. Kinetic characteristics were determined at substrate concentration ranging from 0.05 to 20 μM (3-min uptake). The data represent uptake into hOAT3-expressing cells minus uptake into mock cells. Values are mean ± S.D. (n = 3). Inset: Transport kinetic values were calculated using the Eadie–Hofstee transformation. B. Activation of PKC by PMA inhibits hOAT3 activity. COS-7 cells stably expressing hOAT3 were incubated for 30 min with or without 1 μM PMA added directly to the culture media. After washing the cells, 3-min uptake of [3H] estrone sulfate (0.1 μM) was measured. Uptake activity was expressed as a percentage of the uptake measured in cells without treatment with PMA. Values are mean ± S.D. (n = 3). *P < 0.05, significantly different from untreated cells.
3.2 Effects of angiotensin II on hOAT3 activity
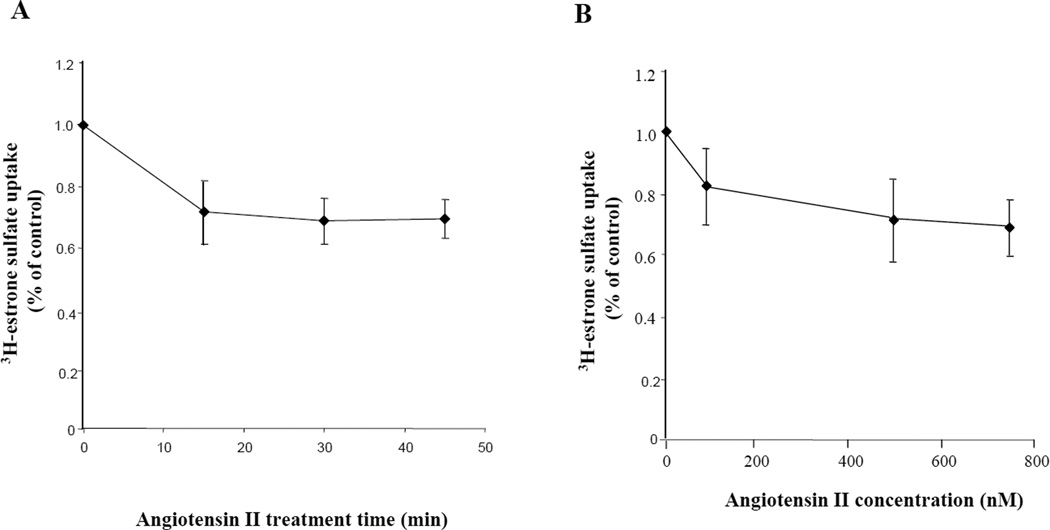
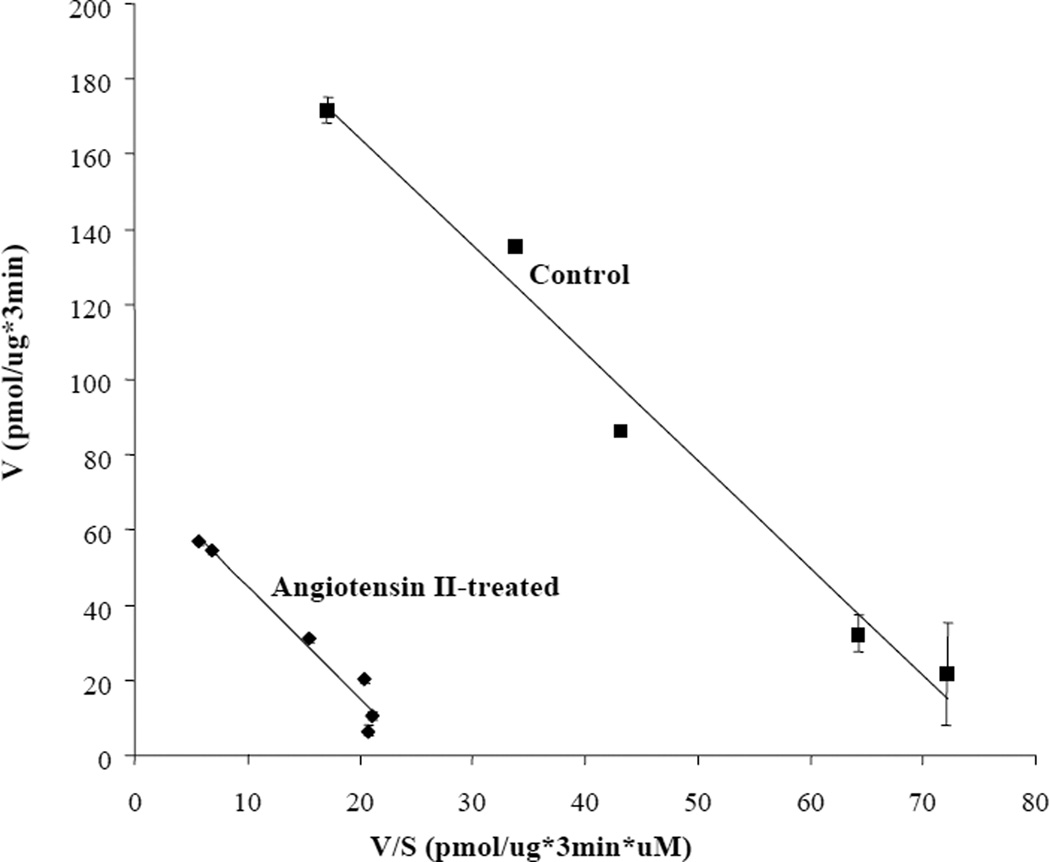
We next examined whether treatment with angiotensin II could affect hOAT3 transport activity. Since the hOAT3 expression vector for the current study does not contain the promoter region of hOAT3, the long-term regulation at the transcriptional level cannot be investigated. We only focused on the short-term regulation of the transporter (within a time frame of 1 h). Angiotensin II induced a time- and concentration-dependent inhibition of estrone sulfate uptake (Fig. 2). To further examine the mechanism of angiotensin II-induced inhibition of hOAT3 activity, we determined [3H] estrone sulfate uptake at different substrate concentrations. An Eadie-Hofstee analysis of the derived data (Fig. 3) showed that pretreatment with angiotensin II resulted in a decreased Vmax (221.6 ± 11.6 pmol·μg−1·3 min−1 with untreated cells and 74.9± 4.9 pmol·μg−1·3 min−1 in the presence of angiotensin II) with no significant change in the affinity (Km) for estrone sulfate (2.86 ± 0.23 μM with untreated cells and 2.98 ± 0.30 μM in the presence of angiotensin II).
Figure 2. Effect of angiotensin II on hOAT3 activity.
hOAT3-expressing cells were treated with angiotensin II at various periods of time (A), or at various concentrations (B), followed by [3H] estrone sulfate uptake (3 min, 0.1 μM). Uptake activity was expressed as a percentage of the uptake measured in untreated cells. The results represent data from 3 experiments. The uptake values in mock cells (parental COS-7 cells) were subtracted. Values are means ± S.D. (n = 3).
Figure 3. Effect of angiotensin II on the kinetics of hOAT3-mediated estrone sulfate transport.
COS-7 cells expressing hOAT3 were pretreated with or without angiotensin II (1 μM) for 30 min, and initial uptake (3 min) of [3H] estrone sulfate was measured at 0.05–20 μM estrone sulfate. The data represent uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are means ± S.D. (n = 3). V, velocity; S, substrate concentration.
3.3 Effect of angiotensin II on hOAT3 expression
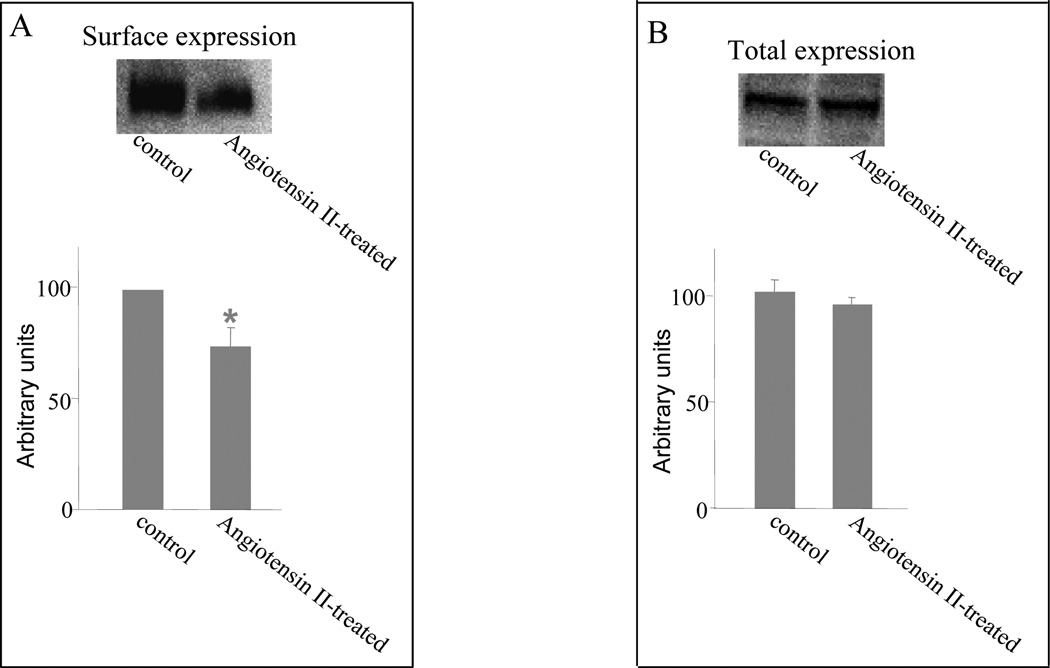
A decreased Vmax could be affected by either a reduced number of the transporter at the cell surface or a reduced transporter turnover rate. To differentiate between these possibilities, we determined transporter expression both at the cell surface and in the total cell lysates. We showed that angiotensin II treatment resulted in a reduced cell surface expression of hOAT3 (Fig 4A) without affecting the total cell expression of the transporter (Fig. 4B).
Figure 4. Effect of angiotensin II on cell surface and total cell expression of hOAT3.
A. Western blot analysis of cell surface expression of hOAT3 in cells treated with or without angiotensin II (1 μM, 30 min). Top: COS-7 cells stably expressing hOAT3 were biotinylated, and the labeled cell surface proteins were precipitated with streptavidin beads and separated by SDS-PAGE, followed by western blotting with anti-myc antibody (1:500). Bottom: the intensity of the transporter expression from the experiment shown at top and other experiments was quantified. *P < 0.05, significantly different from untreated cells. B. Western blot analysis of total cell expression of hOAT3 in cells treated with or without angiotensin II (1 μM, 30 min). Top: total cell expression of hOAT3 in cells treated with or without angiotensin II (1 μM, 30 min). Cells were lysed, and their proteins were separated by SDS-PAGE, followed by Western blotting with anti-myc antibody (1:500). Bottom: the intensity of the transporter expression from the experiment shown at top and other experiments was quantified. There is no statistically difference between control and angiotensin II treated.
3.4 Angiotensin II regulation of hOAT3 activity through PKCα
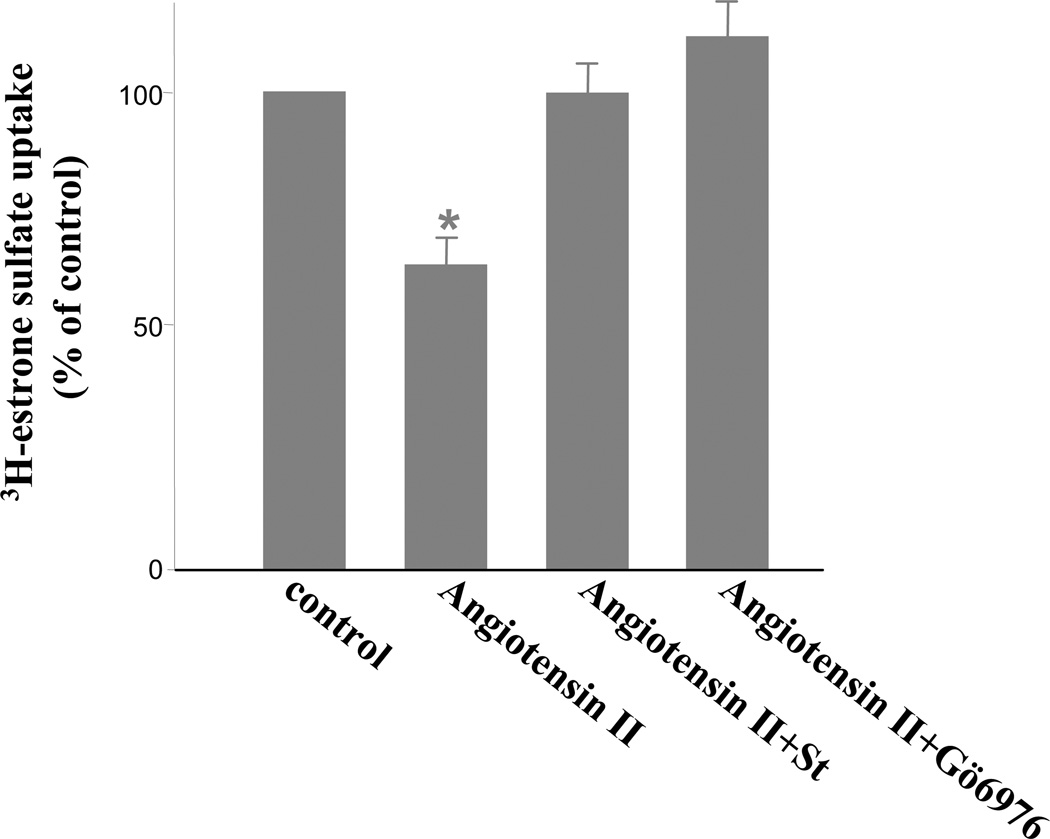
Angiotensin II was shown to exert its effects through PKC. We have recently shown that angiotensin II inhibited OAT1 activity through activation of PKCα (Li et al., 2009). To determine whether angiotensin II regulates hOAT3 function through a similar mechanism, we treated hOAT3-expressing COS-7 cells with angiotensin II in the presence of staurosporine, a general PKC inhibitor as well as Gö6976, a PKCα-specific inhibitor. As shown in Fig. 5, both staurosporine and Gö6976 efficiently reversed the inhibitory effect of angiotensin II on hOAT3 activity.
Figure 5. Effect of PKC inhibitors staurosporine (St) and Gö6976 on angiotensin II induced inhibition of hOAT3 activity.
hOAT3-expressing cells were pretreated with staurosporine (St) or Gö6976 (St, 2 μM, Gö6976, 1 μM, 20 min) followed by incubation with angiotensin II (1 μM, 30 min) in the presence or absence of staurosporine or Gö6976. The uptake of [3H] estrone sulfate (3 min, 0.1 μM) was then performed. The results represent data from three experiments. The uptake values in mock cells (parental COS-7 cells) were subtracted. Values are mean ± S.D. (n = 3). *P < 0.05, significantly different from control cells.
3.5 Angiotensin II enhanced hOAT3 endocytosis
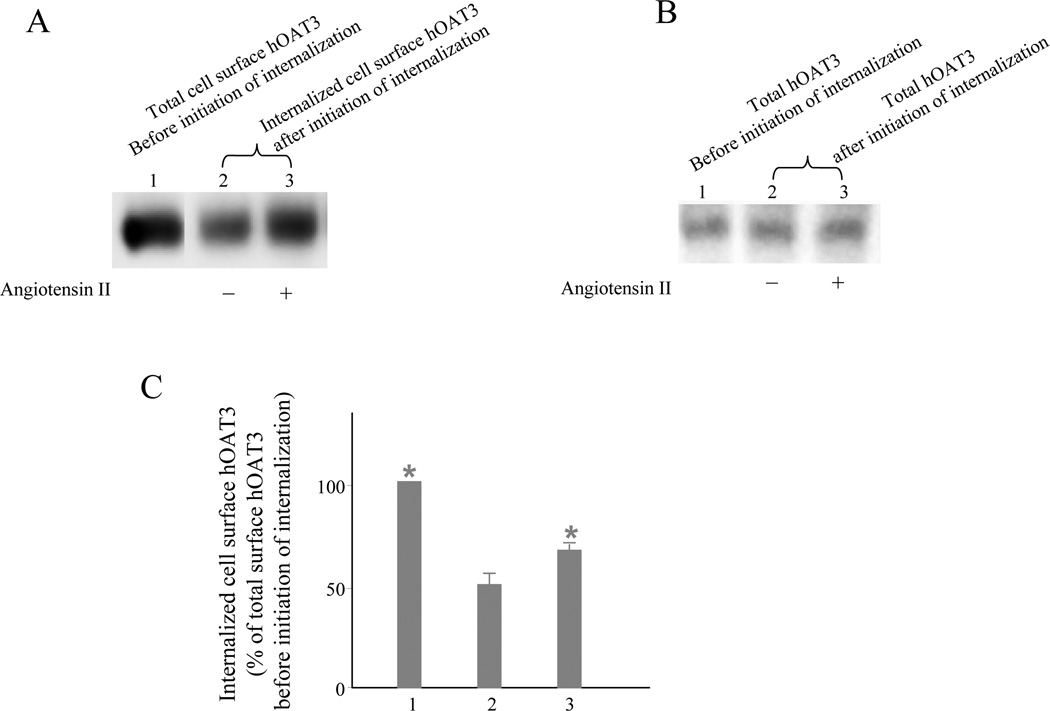
We have recently shown that hOAT1 undergoes constitutive internalization from and recycling back to the cell surface, and that activation of PKC accelerated hOAT1 internalization (Zhang et al., 2008a) . To examine whether the reduced surface expression of hOAT3 after angiotensin II treatment was a result of the enhanced internalization of hOAT3, a biotinylation-based strategy was employed. hOAT3-expressing COS-7 cells were biotinylated with cell-impermeable biotinylation reagent sulfo-NHS-SS-biotin under trafficking-impermissive condition (4 °C). The labeled cells were then incubated on trafficking-permissive condition (37 °C) to allow internalization to occur. At indicated time points after initiation of internalization, biotin from biotinylated proteins remaining on the surface was removed by treatment with MesNa, an impermeant reducing agent that cleaves disulfide bond and liberates biotin from biotinylated proteins at the cell surface. "The amount of protein internalized" was defined as the amount of biotinylated proteins resistant (inaccessible) to MesNa treatment. Incubation of the cells at 37 °C in the absence or presence of angiotensin II before MesNa reversal of surface biotinylation permitted the evaluation of hOAT3 internalization occurring in the absence of angiotensin II (basal internalization) versus angiotensin II-mediated changes in hOAT3 internalization (angiotensin II stimulated internalization). Our result (Fig. 6) showed that 10 min after initiation of internalization, about 50 % of surface-labeled hOAT3 was detectable in the intracellular compartments (Fig. 6A, lane 2). Therefore, hOAT3 undergoes constitutive internalization in COS-7 cells. Our result further demonstrated that the amount of surface-labeled hOAT3 internalized in the presence of angiotensin II was much greater (Fig. 6A, lane 3) than that in the absence of angiotensin II (Fig. 6A, lane 2), suggesting that the reduced surface expression of hOAT3 after angiotensin II treatment shown in Fig. 4 was due to the accelerated hOAT3 internalization into the intracellular compartments.
Figure 6. Biotinylation analysis of angiotensin II modulated hOAT3 internalization.
A. hOAT3 internalization (10 min) was analyzed as described in “materials and methods” section in cell treated with or without angiotensin II (1 μM) followed by Western blotting using anti-myc antibody (1:500). “+”: in the presence of angiotensin II. “−“: in the absence of angiotensin II. B. As a loading control for Fig. 6A, total expression of hOAT3 in cell lysate was measured in parallel to experiments shown in Fig. 6A by Western blotting using anti-myc antibody (1:500). C. Densitometry plot of results from Fig. 6A. Internalized hOAT3 was expressed as % of total initial cell surface hOAT3 pool. Values are mean ± S.D. (n = 3). *P < 0.05, significantly different from the amount of hOAT3 internalization in the absence of angiotensin II.
As mentioned before, hOAT3 functions through a tertiary transport mechanism involving two other membrane proteins Na/K ATPase and Na/dicarboxylate cotransporter. In addition to hOAT3, angiotensin II has also been shown previously to affect Na/K ATPase expression (Fekete et al., 2008). We therefore asked whether angiotensin II induced hOAT3 internalization is accompanied by Na/K ATPase internalization. To address this issue, the internalization of Na/K ATPase was determined in the presence and the absence of angiotensin II. Our result (Fig. 7) showed that, in contrasts to hOAT3, Na/K ATPase underwent neither constitutive nor angiotensin II induced internalization.
Figure 7. Biotinylation analysis of constitutive and angiotensin II-modulated Na/K-ATPase internalization.
A. Na/K-ATPase internalization (10 min) was analyzed as described in “materials and methods” section in cell treated with or without angiotensin II (1 μM) followed by western blotting using anti-Na/K-ATPase antibody (1:500). “+”: in the presence of angiotensin II. “−“: in the absence of angiotensin II. B. Densitometry plot of results from Fig. 7a. Internalized Na/K-ATPase was expressed as % of total initial cell surface Na/K-ATPase pool. Values are mean ± S.D. (n = 3). *P < 0.05, significantly different from the amount of hOAT3 internalization in the absence of angiotensin II.
4. Discussion
The major finding from the current study is that i) hOAT3 undergoes constitutive internalization, ii) angiotensin II inhibits hOAT3 activity by accelerating hOAT3 internalization through activation of PKCα, and iii) angiotensin II induces hOAT3 internalization without simultaneously inducing Na/K ATPase internalization.
To study the regulation of hOAT3, we established COS-7 cells stably expressing the transporter. Although native systems that endogenously express transporters are great assets to identify the endogenous stimuli controlling the transporter function, heterogonous expression systems are useful for asking mechanistic questions with regard to understanding the relationship between transporter trafficking and regulation. COS-7 cells offer several useful advantages for study of the cloned organic anion transporter. (i) These cells were directly derived from the kidney and have been very useful in understanding other renal transport processes and cellular functions, including organic cation transport (Nagai et al., 2006; Zhang et al., 2002). (ii) This cell line does not express endogenousOATs. Therefore, expression of hOAT3 in COS-7 cells will allow us to dissect the transport characteristics of hOAT3 in a relevant mammalian system without the possibly confounding effects of other organic anion transporters. (iii) They possess endogenousPKC and PKA signaling pathways and provide a good experimentalmodel system for studying the regulatory mechanisms underlying many transport process (Cobb et al., 2002; Zhang et al., 2008b).
Angiotensin II induced a time- and dose-dependent inhibition of uptake of estrone sulfate mediated by hOAT3 (Fig. 2). Our kinetic analysis of the inhibition of hOAT3 activity by angiotensin II (Fig. 3) showed that the reduced transport activity was contributed by a reduced maximum transport velocity (Vmax) without affecting the binding affinity (1/Km) of the transporter for its substrates. We further showed (Fig. 4) that such a reduction in Vmax resulted from a reduced cell surface expression of hOAT3 without affecting its total cell expression. Angiotensin II induced inhibition of hOAT3 activity was prevented by staurosporin, a general PKC inhibitor and by Gö6976, a PKCα-specific inhibitor. Despite that hOAT1 and hOAT3 exhibit distinct substrate specificities, the regulation of hOAT3 by angiotensin II shown in the current study seems similar to the regulation of hOAT1 by angiotensin II shown in our previous study (Li et al., 2009), both of which involve activation of PKCα. However, one important piece of information that was missing from our previous study was the mechanism underlying angiotensin II-induced reduction of the surface expression of the transporter. We addressed this issue in the current study. We showed that hOAT3 underwent constitutive internalization from cell surface to the intracellular compartment (Fig. 6, lane 2), suggesting that a constitutive recycling of the transporter back to the cell surface must occur in order to maintain a steady-state cell surface expression. We further showed that angiotensin II inhibited hOAT3 activity by accelerating hOAT3 internalization (Fig. 6, lane 3).
Several previous reports indicate that many transporters such as dopamine transporter (Loder and Melikian, 2003) are not static at the cell surface; rather they dynamically cycles to and from the plasma membrane under basal conditions. Recently, our lab provided evidence that hOAT1, another member of OAT family, indeed undergoes such constitutive internalization and recycling between cell surface and intracellular compartments (Zhang et al., 2008a). Our demonstration in the current study that hOAT3 also undergoes such dynamic trafficking (Fig. 6) suggests that this characteristic maybe the common feature for all members of OAT family.
It is important to note that the only moderate inhibition of hOAT3 activity (~30 %) by angiotensin II (Fig. 2) is consistent with the dynamic nature of the transporter. At any given time, there is always certain amount of hOAT3 recycling back to the cell surface even with the maximum inhibition by angiotensin II, which explains why we only observed a moderate inhibition of ~30 % by angiotensin II. In vivo, regulation at such a moderate scale may play an important role in providing quick and efficient fine-tuning in body response to environmental changes.
Another interesting finding from the current study was that although Na/K-ATPase is one of the three components (Na/K-ATPase, Na-dicarboxylate cotransporter and OAT) in the tertiary transport mechanism utilized by hOAT3, and although angiotensin II was previously reported to affect Na/K-ATPase expression (Fekete et al., 2008), Na/K-ATPase seems only to participate in hOAT3 function at the cell surface and does not co-traffic with hOAT3 both in the absence and in the presence of angiotensin II (Fig. 7). Na-dicarboxylate cotransporter expresses at the same membrane (basolateral membrane) as that of hOAT3 and Na/K-ATPase. To our knowledge, there has been no report on the effect of angiotensin II on Na-dicarboxylate cotransporter. Whether Na-dicarboxylate cotransporter undergoes constitutive and regulated trafficking will be an interesting topic of future studies.
Angiotensin II levels are elevated in several pathophysiological conditions including bilateral ureteral obstruction (Klahr, 1998; 2000; Klahr and Morrissey, 2002). Our studies suggest that the change in angiotensin II levels may affect the function of OAT3 and then subsequently interfere with the renal elimination of numerous drugs and toxins. In addition to rapid regulation (short-term regulation) as reported in the current study, members of OAT family are also subject to long-term regulation. For example, differential expression of mRNA for both OAT1 and OAT3 were observed during the kidney development and between different sexes (Buist and Klaassen, 2004; Wood et al., 2005).
5. Conclusion
Our current study demonstrated that 1) COS-7 cells are suitable model for charactering hOAT3. 2) hOAT3 undergoes constitutive trafficking between cell surface and intracellular compartment, 3) Angiotensin II inhibits hOAT3 activity by accelerating hOAT3 internalization through activation of PKCα, and 4) Angiotensin II induces hOAT3 internalization without simultaneously inducing Na/K ATPase internalization.
Acknowledgments
This work was supported by grants (to Dr. Guofeng You) from the National Institute of Health (R01-DK 60034 and R01-GM 079123).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J. Pharmacol. Sci. 2006;100:411–426. doi: 10.1254/jphs.crj06006x. [DOI] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13) J. Biol. Chem. 2008;283:16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab. Dispos. 2004;32:620–625. doi: 10.1124/dmd.32.6.620. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol. Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2) Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, Masereeuw R, Russel FG. Mechanisms of renal anionic drug transport. Eur. J. Pharmacol. 2008;585:245–255. doi: 10.1016/j.ejphar.2008.02.085. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J. Biol. Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- Fekete A, Rosta K, Wagner L, Prokai A, Degrell P, Ruzicska E, Vegh E, Toth M, Ronai K, Rusai K, Somogyi A, Tulassay T, Szabo AJ, Ver A. Na+,K+- ATPase is modulated by angiotensin II in diabetic rat kidney--another reason for diabetic nephropathy? J. Physiol. 2008;586:5337–5348. doi: 10.1113/jphysiol.2008.156703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J. Biol. Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- Klahr S. Obstructive nephropathy. Kidney Int. 1998;54:286–300. [PubMed] [Google Scholar]
- Klahr S. In: The Kidney Physiology and Pathophysiology. Seldin DW, Giebisch G, editors. Lippincott Williams & Wilkins Philadelphia; 2000. pp. 2473–2512. [Google Scholar]
- Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J. Biol. Chem. 1999;274:13675–13680. doi: 10.1074/jbc.274.19.13675. [DOI] [PubMed] [Google Scholar]
- Li S, Duan P, You G. Regulation of human organic anion transporter 1 by ANG II: involvement of protein kinase Calpha. Am. J. Physiol. Endocrinol. Metab. 2009;296:E378–E383. doi: 10.1152/ajpendo.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol. Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Annu. Rev. Physiol. 1997;59:395–412. doi: 10.1146/annurev.physiol.59.1.395. [DOI] [PubMed] [Google Scholar]
- Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Nagai K, Takikawa O, Kawakami N, Fukao M, Soma T, Oda A, Nishiya T, Hayashi M, Lu L, Nakano M, Kajita E, Fujita H, Miwa S. Cloning and functional characterization of a novel up-regulator, cartregulin, of carnitine transporter, OCTN2. Arch. Biochem. Biophys. 2006;452:29–37. doi: 10.1016/j.abb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schmitz U, Berk BC. Angiotensin II signal transduction: Stimulation of multiple mitogen-activated protein kinase pathways. Trends in endocrinology and metabolism: TEM. 1997;8:261–266. doi: 10.1016/s1043-2760(97)00101-x. [DOI] [PubMed] [Google Scholar]
- Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20) Am. J. Physiol. Renal Physiol. 2006;291:F314–F321. doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS. Lett. 1998;429:179–182. doi: 10.1016/s0014-5793(98)00585-7. [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J. Biol. Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45:1046–1055. doi: 10.1002/hep.21596. [DOI] [PubMed] [Google Scholar]
- Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica ; the fate of foreign compounds in biological systems. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J. Biol. Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J. Biol. Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- Wood CE, Cousins R, Zhang D, Keller-Wood M. Ontogeny of expression of organic anion transporters 1 and 3 in ovine fetal and neonatal kidney. Exp. Biol. Med. (Maywood) 2005;230:668–673. doi: 10.1177/153537020523000909. [DOI] [PubMed] [Google Scholar]
- Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc. Natl. Acad. Sci. U S A. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Anzai N, Ljubojevic M, Ohtsu N, Sakata T, Miyazaki H, Nonoguchi H, Islam R, Onozato M, Tojo A, Tomita K, Kanai Y, Igarashi T, Sabolic I, Endou H. Functional and immunochemical characterization of a novel organic anion transporter Oat8 (Slc22a9) in rat renal collecting duct. Cell Physiol Biochem. 2008;21:269–278. doi: 10.1159/000129385. [DOI] [PubMed] [Google Scholar]
- You G. The role of organic ion transporters in drug disposition: an update. Curr. Drug Metab. 2004;5:55–62. doi: 10.2174/1389200043489207. [DOI] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am. J. Physiol. Renal Physiol. 2004;287:F236–F244. doi: 10.1152/ajprenal.00012.2004. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter 17 OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J. Biol. Chem. 2008a;283:32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Yang X, Li J, Wu J, Peng WX, Dong XQ, Zhou SF, Yu XQ. Upregulation of rat renal cortical organic anion transporter (OAT1 and OAT3) expression in response to ischemia/reperfusion injury. Am. J. Nephrol. 2008b;28:772–783. doi: 10.1159/000129073. [DOI] [PubMed] [Google Scholar]
- Zhang X, Evans KK, Wright SH. Molecular cloning of rabbit organic cation transporter rbOCT2 and functional comparisons with rbOCT1. Am. J. Physiol. Renal Physiol. 2002;283:F124–F133. doi: 10.1152/ajprenal.00367.2001. [DOI] [PubMed] [Google Scholar]
- Zhou F, Hong M, You G. Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. 2007;293:E57–E61. doi: 10.1152/ajpendo.00696.2006. [DOI] [PubMed] [Google Scholar]