Abstract
Background. It is urgent to find alternative agents due to increasing failure rate of Helicobacter pylori (H. pylori) eradication. The study surveyed the long-term effect of silver nanoparticles (AgNP) on H. pylori based on Mongolian gerbil's model. Materials and Methods. Fifty gerbils were randomly allocated to six groups (A–F). Group (Gr) A: the gerbils were fed with broth; Gr B and D: the gerbils were fed with AgNP/clay complex (0.1% of weight); Gr C and E: the gerbils were fed with AgNP/clay complex(1% of weight); and Gr D, E, and F: the gerbils were inoculated with H. pylori. At the 20th experimental week, the gerbils were sacrificed. Histology was evaluated according to the classification of the Sydney system. P < 0.05 was considered to be statistically significant. Results. The AgNP/clay has more obvious inhibitory effect on H. pylori in vitro. There was a trend of higher concentrations of AgNP with stronger inhibitory effect on H. pylori growth (P = 0.071). There were no significant differences of inflammation among groups D, E, and F (P = 0.688).Conclusion. AgNP/clay would be a potential and safe agent for inhibiting H. pylori. It should be helpful for eradication of H. pylori infection.
1. Introduction
Helicobacter pylori (H. pylori) infection is an important factor of many gastrointestinal diseases such as chronic gastritis, peptic ulcer, and gastric cancer. Eradication of H. pylori is the most important strategy for treatment of these diseases. However, the failure rate of currently used first-line therapies has increased up to 30% [1–4]. Therefore, there is an urgent need to find alternative agents to improve the efficacy.
The potential side effects of bismuth decrease the compliance of second-line eradication therapies. For the conventional antimicrobial treatments, some metals including silver in large quantities are applied to control skin infection [5], so previous studies applied silver to be the alternative treatment for gastrointestinal symptoms and infections (including H. pylori) [6]. Besides this, silver compounds have been used as antiulcer agents [7]. Because H. pylori is a major peptic-ulcer cause, it is logical that the antiulcer activity of silver might be related to its effects on H. pylori.
Currently, metal-based nanopreparations have become more and more important in their applications in various fields including antimicrobial abilities [8–14]. The synthesis of silver nanoparticles (AgNP) has aroused more interest because of the broad applications including wound dressings and medical devices [10, 11, 13, 15, 16]. The possible mechanism of action of metallic agents is the inhibition of H. pylori urease [17–19]. However, previous studies were mostly in vivo studies, so we need an ideal animal model to survey the long-term effects of AgNP exposure on human physiology and H. pylori. Watanabe et al. [20] demonstrated that H. pylori infection could induce well-differentiated adenocarcinoma based on a Mongolian gerbil's model. The Mongolian gerbils provide a suitable experimental animal model, so the aim of our study was to survey the long-term effect of AgNP on H. pylori based on a Mongolian gerbil's model.
2. Materials and Methods
Experiments were performed according to the experimental guidelines of the Ethics Committee of Kaohsiung Medical University Laboratory Animal Center.
2.1. Animals and Housing
Eight-week-old gerbils with body weight of 30–40 gm were purchased from the Kaohsiung Medical University Experimental Animals Center, Kaohsiung, Taiwan. In usual time, 4 to 5 gerbils per cage were housed and maintained under standard laboratory conditions (room temperature, 23°C~26°C; relative humidity, 55%~65%; 12/12-hour light/dark cycle) with free access to a commercial rodent diet and tap water.
2.2. Synthesis of AgNP/Clay Nanohybrid
The AgNP/clay complex was prepared via the reduction of silver ions in water according to the procedures reported previously [21]. In a typical experimental procedure, the lucentite SWN clay slurry (30 g, 1 wt% in water; DEUCHEM Co., Taiwan) was prepared by swelling in deionized water at 80°C and the AgNO3 solution (3.4 g, 1.0 wt% in water; J.T. Baker, USA) was then added to this slurry. The reducing agent was added to the AgNO3/clay solution and the mixture was vigorously stirred and heated at 80°C for 3 h. During the process, a color change was observed from yellowish to deep red, indicating the reduction of Ag+ to Ag0. The UV absorption at 420 cm−1 was observed using a UV-mini 1240 spectroscope. The Ag particle size on clay was measured with a field emission scanning electron microscope (FE-SEM, Zeiss EM 902A) at 80 kV. The d spacing was analyzed using a Shimadzu SD-D1 X-ray diffractometer with a Cu target (γ = 1.5405 Å) at a generator voltage of 35 kV, a generator current of 30 mA, and a scanning rate of 2°/min. The inorganic fraction was determined by decomposing the composites at temperatures up to 900°C. The concentration of dissolved Ag+ in solution was determined with inductively coupled plasma mass spectrometry (ICP-MS) provided in National Sun Yat-Sen University and National Tsing Hua University of Taiwan. The supernatant of a 0.1 wt% AgNP/clay sample in solution was collected after centrifugation at 16,000 ×g for 30 min. ICP-MS analysis showed the Ag+ concentration to be in a range of 139.33 ± 16.04 ppb. After adding 3% HNO3 to the supernatant to convert the free Ag0 to Ag+, the concentration increased to 155.33 ± 34.53 ppb. [15]
2.3. Dose Escalation of AgNP
Part I: the optimal concentration of the antibacterial activity of AgNP/clay: AgNP/clay (0.06%, 0.08%, 0.1%, 0.2%, and 0.3% of weight) was added into the Brucella broth containing bacteria 1 × 103 CFU/mL and then they were incubated at 37°C. The incubation time was 12 hours. Then 100 μL of these cultured broths were spread on CDC Anaerobe 5% Sheep Blood Agar (BD, USA) and incubated at 37°C with 5% O2 conditions. The colony numbers were counted after 48 hours of incubation. Part II: the time course of the antibacterial activity of AgNP/clay: AgNP/clay (0.01%, 0.05%, and 0.1% of weight) was added into the Brucella broth containing bacteria 1 × 103 CFU/mL and then they were incubated at 37°C. The incubation time was 0, 0.5, 1, 2, 4, 12, and 24 hours, irrespectively. Then 100 μL of these cultured broths was spread on CDC Anaerobe 5% Sheep Blood Agar (BD, USA) and incubated at 37°C with 5% O2 conditions. The colony numbers were counted after 48 hours of incubation.
2.4. H. pylori Inoculation
The gerbils were randomly allocated to six groups according to a randomized number (A–F): group A: the gerbils were fed with broth only; group B: the gerbils were fed with AgNP/clay complex (0.1% of weight) in the 8th to 20th week; group C: the gerbils were fed with AgNP/clay complex (1% of weight) in the 8th to 20th week; group D: the gerbils were inoculated with H. pylori [CagA (+)/VacA (+)] during the 1st to 4th week and then they were fed with AgNP/clay complex (0.1% of weight) in the 8th to 20th week; group E: the gerbils were inoculated with H. pylori [CagA (+)/VacA (+)] during the 1st to 4th week and then they were fed with AgNP/clay complex (1% of weight) in the 8th to 20th week; group F: the gerbils were inoculated with H. pylori [CagA (+)/VacA (+)] during the 1st to 4th week. At the end of the 20th experimental week, the animals were fasted for 24 hours before being sacrificed (Figure 1).
Figure 1.
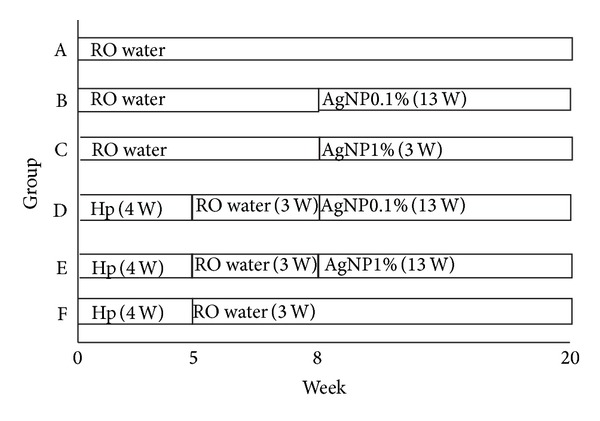
The timing of AgNP/clay and H. pylori given. Group (Gr) A: the gerbils were fed with broth only. Gr B and D: the gerbils were fed with AgNP/clay (0.1% of weight) in the 8th to 20th week. Gr C and E: the gerbils were fed with AgNP/clay (1% of weight) in the 8th to 20th week. Gr D, E, and F: the gerbils were inoculated with H. pylori [CagA (+)/VacA (+)] in the 1st to 4th week. At the end of the 20th experimental week, the animals were fasted for 24 hours before being sacrificed.
2.5. Histological Evaluation of the Gastric Mucosa in Gerbils
Samples of the gastric mucosa were excised from each gerbil stomach for the assessment of the presence of H. pylori and gastric inflammation using Giemsa and hematoxylin-eosin (HE) staining for histological examination, respectively. The samples were fixed in 10% buffered formalin and embedded in paraffin [II-34]. The paraffin sections were cut at a thickness of 5 mm and stained. Two experienced pathologists, blinded to the treatment given, performed histological examinations. Histological features of mucosal inflammation and intestinal metaplasia were evaluated for each specimen under a light microscope according to the classification of the Sydney system. The degree of inflammatory cell infiltration and the area of intestinal metaplasia were scored as follows: 0, normal; 1, mild; 2, moderate; 3, marked.
2.6. Statistical Analyses
We analyzed the collected data using the statistical software package SPSS. Kruskal-Wallis test was used for comparing histological change of mucosa. P < 0.05 was considered to be statistically significant.
3. Results
The inhibitory effects of different materials on H. pylori were surveyed. The results revealed that AgNP/clay has more obvious inhibitory effect on H. pylori. This inhibitory effect became more obvious when the concentration of AgNP/clay was more than 0.08% of weight. However, the clay did not show any inhibitory effect in any concentration. The AgNP had mild inhibitory effect only at high concentration (0.3% of weight) (Figure 2).
Figure 2.
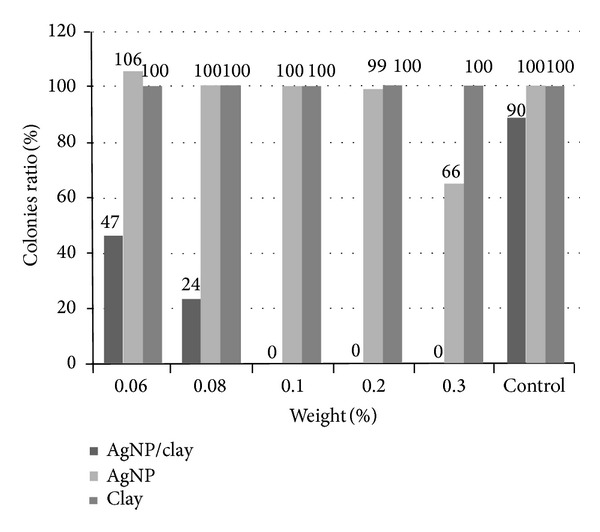
The inhibitory effects of different materials on H. pylori were surveyed. The results revealed that AgNP/clay has more obvious inhibitory effect on H. pylori (concentration more than 0.08% of weight). However, clay did not have the inhibitory effect and the AgNP had mild inhibitory effect only in high concentrations. H. pylori: Helicobacter pylori; AgNP: silver nanoparticles.
We surveyed the reaction time of inhibiting H. pylori in different concentrations of AgNP/clay. We found that the higher the concentration of AgNP/clay, the shorter the reaction time. In concentration of 0.1% weight of AgNP/clay, H. pylori would be completely inhibited since the 12th hour and this effect would persist up to the 24th hour (Figure 3). So the optimal frequency of feeding AgNP/clay might be daily.
Figure 3.
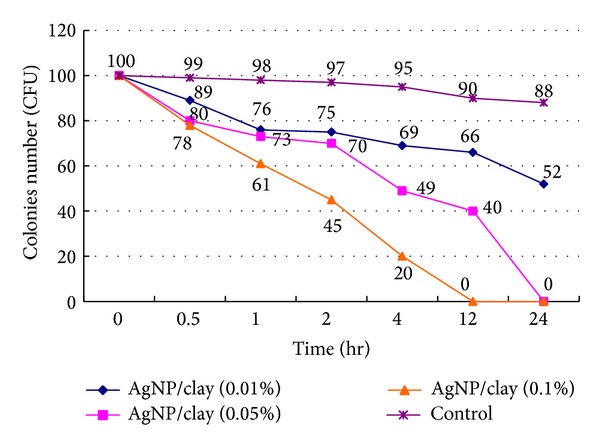
We surveyed the reaction time of inhibiting H. pylori in different concentrations of AgNP/clay. We found that the higher the concentration, the shorter the reaction time. H. pylori could be completely inhibited within 12 hours in a concentration of 0.1% of weight. H. pylori: Helicobacter pylori; AgNP: silver nanoparticles.
Fifty gerbils were used in this study. The numbers of gerbils in each group were 6 (Gr A), 6 (Gr B), 6 (Gr C), 11 (Gr D), 15 (Gr E), and 6 (Gr F), respectively. The study design is shown in Figure 1. In our study, all gerbils were alive till the end of this experiment, and there was no significant difference in the survival rates among the various groups. On the 13th week, the positive rates of H. pylori were all 100% in groups D, E, and F.
We surveyed the densities of H. pylori in groups D, E, and F. We wanted to survey the inhibitory effect of AgNP on H. pylori. The average densities of H. pylori (according to Sydney classification) were 1.45 ± 0.52, 1.27 ± 0.70, and 1.83 ± 0.41 in Gr D, E, and F, respectively. It did not show significant difference between the three groups (P = 0.071); however, we found the trend that higher concentrations of AgNP had stronger effect on inhibiting H. pylori (Figures 4 and 6).
Figure 4.
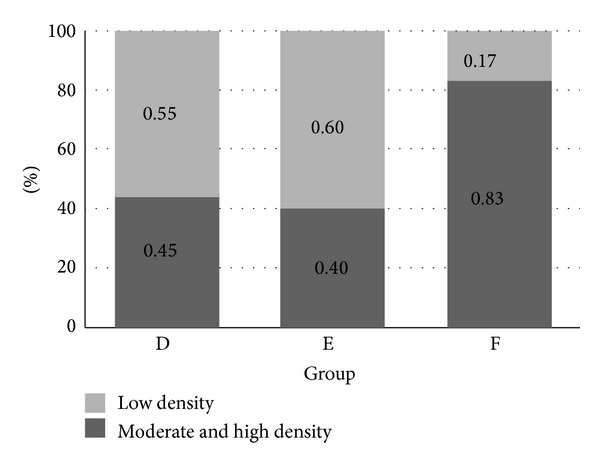
The status of H. pylori densities in groups D, E, and F is shown. Group F had obviously higher proportion of moderate and high H. pylori densities. It disclosed that AgNP/clay had an inhibitory effect on H. pylori. H. pylori: Helicobacter pylori; AgNP: silver nanoparticles.
Figure 6.
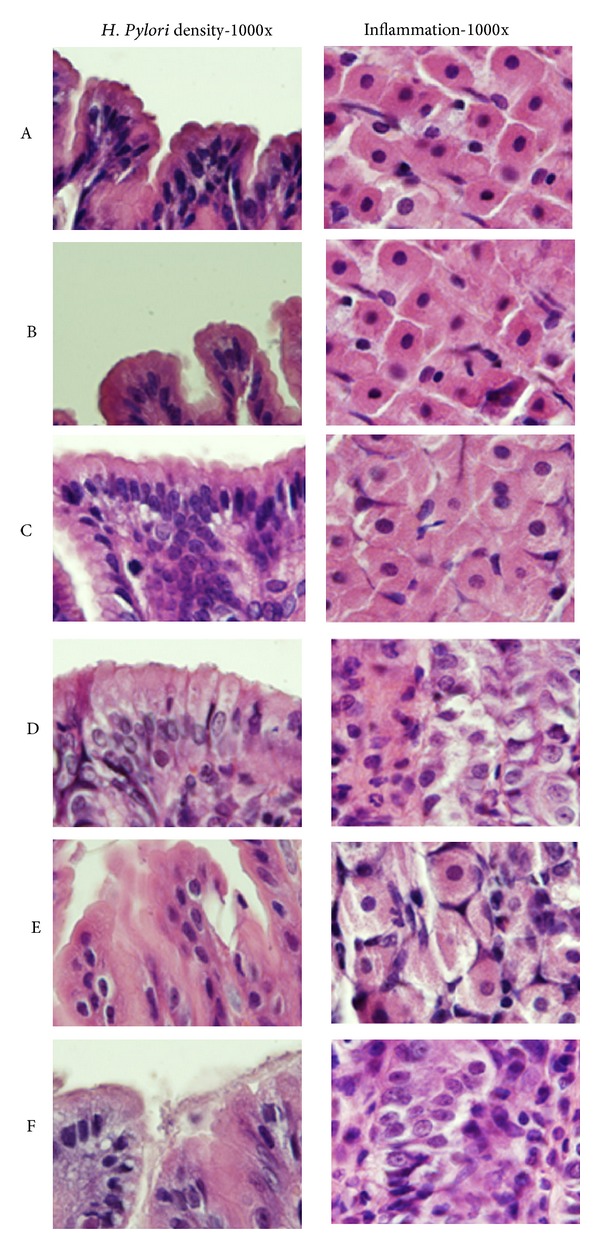
Histological changes of different groups are shown (H&E stain 1000x). We show the status of H. pylori density in right field and the status of inflammation in left field. There is no obvious inflammatory cells infiltration in groups A, B, and C, but obvious inflammatory cells infiltration was noted in groups D, E, and F. The densities of H. pylori were higher in group F.
The possible toxic effect of AgNP on gastric mucosa and its interaction with H. pylori were also surveyed. We showed the severity of inflammation of gerbil's mucosa according to the Sydney classification. There was no sign of inflammation noted in group A. However, all gerbils in groups B and C had mild monocyte infiltration but without neutrophil infiltration. The results of the inflammatory scores were 0 ± 0 (Gr A), 1.00 ± 0 (Gr B), 1.00 ± 0 (Gr C), 4.27 ± 1.10 (Gr D), 4.20 ± 1.82 (Gr E), and 4.83 ± 1.17 (Gr F). The proportions of moderate to severe inflammation were 0% (0/6), 0% (0/6), 0% (0/6), 54.5% (6/11), 53.3% (8/15), and 66.7% (4/6) in groups A, B, C, D, E, and F (Figures 5 and 6). The results revealed that AgNP alone did not have an acute toxic effect on gerbil's gastric mucosa. Besides these, there were no significant differences of inflammation among groups D, E, and F (P = 0.688).
Figure 5.
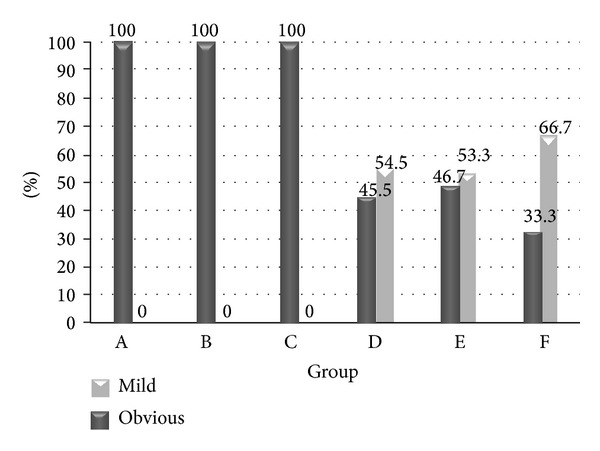
The inflammatory severities of gerbils' stomach in different groups are shown. All gerbils in groups A, B, and C showed mild inflammation only. The proportion of obvious inflammation was more than mild inflammation in groups D, E, and F. The difference was the most obvious in group F. However, there was no significant difference among these groups.
4. Discussion
The approaches to overcoming drug resistance include increasing the dosage and treatment duration of drugs and using multiple drugs or pretreatment with agents to reduce bacterial load. The agents used to decrease H. pylori load include probiotics, bismuth, or some herbs. Our study was designed to survey whether AgNP could reduce the H. pylori load in gerbils.
To the best of our understanding, the present investigation can be considered to be the first report of the anti-H. pylori activity of silver nanoparticles based on a gerbil model. A previous study showed that silver nanoparticles may have the effect of inhibiting urease activity of H. pylori [19]. However, these previous studies were under the evidence of in vitro studies. We chose 13 weeks as the intervention's duration according to our previous preliminary data (not published) of Mongolian gerbil's model. We found that H. pylori would induce obvious inflammation since this time point. So we expected that there might be obvious difference in different groups at this time point.
According to our results, AgNP could not completely inhibit the growth of H. pylori neither in low nor high concentrations of AgNP. But AgNP showed the potential effect of decreasing densities of H. pylori and also had dose-dependent response. This supported the notion that AgNP might be a bacteriostatic agent for H. pylori. Our results supported the finding of a previous study [19]. The effect of inhibition was directly related to AgNP/clay itself but not free silver ion in solution, because the synthesized AgNP/clay was nearly free of Ag+ leaching from the nanohybrid, even after a storage period of six months at room temperature.
Multiple investigations have been performed to show the antibacterial activity of metals and metals chelated with some ligands against H. pylori [22, 23]. According to a previous report, the charged clay could trap bacteria and the close interfacial interaction between bacteria and AgNP plays a cardinal role in inhibiting bacteria [15]. The contact and interaction between the AgNP/clay and the cell wall of bacterium is important to trigger a cytotoxic signal; unfortunately, H. pylori was colonized within the gel layer of gastric mucosa and we thought that the gel layer might interfere with the contact between H. pylori and AgNP/clay. This should be one of the reasons in which H. pylori was not inhibited obviously in our gerbil's model.
A previous study found that under anaerobic conditions, newly synthesized AgNP/clay still enabled suppression of cell growth as efficiently as under aerobic conditions [15]. So its effect would not be influenced by the microanaerobic environment in which H. pylori lives. Another possible challenge is the acidic environment in the stomach; one study revealed that the bactericidal effect would diminish in an acidic environment [24]. This might be one of the reasons that AgNP/clay only had bacteriostatic effect in the gerbil's stomach.
The bacterial load is an important factor for eradication of H. pylori. Previous studies demonstrated that decreasing the amount of H. pylori would increase the success rate of eradication [25]. They usually used bismuth as the agent for inhibiting H. pylori, but the side effects of bismuth were obvious and would decrease the compliance and success rates. Another method might use more complex regimens, such as sequential therapies, to achieve successful eradication by lower bacterial load. In our study, we found that AgNP had the effect of inhibiting H. pylori. Our study might provide another reliable way to help us eradicate H. pylori.
A previous study in vitro demonstrated that urease inhibitory activity increased linearly with increased concentration of AgNP. So we tried two different doses of AgNP/clay (1% weight versus 0.1% weight) to survey the dose-dependent response. On the other hand, the possible toxic effect of AgNP was an important concern of this study. Many studies have revealed AgNP to have mild toxicity against several cell lines and the possible mechanism of these toxic effects is under survey [26–28], so our study also surveyed the toxic effect on gastric mucosa. In our results, no obvious mucosal damage was found in groups B and C. According to previous study [6], the paper clearly demonstrated that fed silver nanoparticles were not absorbed by animal. In our study, we did not find any evidence about AgNP absorbed by gastrointestinal mucosa. However, further survey and longer observation period would be needed for application of AgNP in human.
There were some limitations of our study. One was the duration of feeding AgNP. To our best knowledge, there is no previous similar design reported in Mongolian gerbil's model, so we did not know the optimal intervention period. It might need longer therapeutic period for obtaining obvious inhibitory effect. Another limitation was the unequal number of gerbils used in these groups. However, we used more gerbils in groups D and E (fed both H. pylori and AgNP). The number per group of gerbils used in previous studies was around six to ten, so the number of gerbils used in our study groups A, B, C, and F was enough for analysis. Besides this, we kept feeding gerbils during the experiment and the various ions in the food might have interaction with AgNP/clay. So the effect of AgNP/clay on H. pylori was possibly diminished.
In summary, our study showed that AgNP/clay would be a potential and safe agent for decreasing the amount of H. pylori. It should be helpful for eradication of H. pylori infection and needs further survey on the method given.
Acknowledgments
This work was supported by Excellence for Cancer Research Center Grant DOH102-TD-C-111-002, Department of Health, Executive Yuan, Taiwan, National Science Council (NSC-97-2321-B-037-002-MY3), Kaohsiung Medical University Hospital (KMUH100-0I01 and KMUH99-9R32), and Kaohsiung Municipal Hsiao-Kang Hospital (kmhk-97-021).
Conflict of Interests
All the authors have no conflict of interests to declare.
References
- 1.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56(6):772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo C-H, Hsu P-I, Kuo F-C, et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. Journal of Antimicrobial Chemotherapy. 2013;68(1):222–228. doi: 10.1093/jac/dks361. [DOI] [PubMed] [Google Scholar]
- 3.Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Archives of Internal Medicine. 1999;159(14):1562–1566. doi: 10.1001/archinte.159.14.1562. [DOI] [PubMed] [Google Scholar]
- 4.Monica PD, Lavagna A, Masoero G, Lombardo L, Crocella L, Pera A. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Alimentary Pharmacology & Therapeutics. 2002;16(7):1269–1275. doi: 10.1046/j.1365-2036.2002.01244.x. [DOI] [PubMed] [Google Scholar]
- 5.Amin M, Iqbal MS, Hughes RW, et al. Mechanochemical synthesis and in vitro anti-Helicobacter pylori and uresase inhibitory activities of novel zinc(II)-famotidine complex. Journal of Enzyme Inhibition and Medicinal Chemistry. 2010;25(3):383–390. doi: 10.3109/14756360903179518. [DOI] [PubMed] [Google Scholar]
- 6.Chiao SH, Lin SH, Shen CI, et al. Efficacy and safety of nanohybrids comprising silver nanoparticles and silicate clay for controlling Salmonella infection. International Journal of Nanomedicine. 2012;7:2421–2432. doi: 10.2147/IJN.S31594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrivastava T, Ramachandran R. Mechanistic insights from the crystal structures of a feast/famine regulatory protein from Mycobacterium tuberculosis H37Rv . Nucleic Acids Research. 2007;35(21):7324–7335. doi: 10.1093/nar/gkm850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Wiley B, McLellan J, Xiong Y, Li Z-Y, Xia Y. Optical properties of Pd–Ag and Pt–Ag nanoboxes synthesized via galvanic replacement reactions. Nano Letters. 2005;5(10):2058–2062. doi: 10.1021/nl051652u. [DOI] [PubMed] [Google Scholar]
- 9.Magaña SM, Quintana P, Aguilar DH, et al. Antibacterial activity of montmorillonites modified with silver. Journal of Molecular Catalysis A: Chemical. 2008;281(1-2):192–199. [Google Scholar]
- 10.Niesz K, Grass M, Somorjai GA. Precise control of the Pt nanoparticle size by seeded growth using EO13PO30EO13 triblock copolymers as protective agents. Nano Letters. 2005;5(11):2238–2240. doi: 10.1021/nl051561x. [DOI] [PubMed] [Google Scholar]
- 11.Du W-L, Niu S-S, Xu Y-L, Xu Z-R, Fan C-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydrate Polymers. 2009;75(3):385–389. [Google Scholar]
- 12.Zayats M, Baron R, Popov I, Willner I. Biocatalytic growth of Au nanoparticles: from mechanistic aspects to biosensors design. Nano Letters. 2005;5(1):21–25. doi: 10.1021/nl048547p. [DOI] [PubMed] [Google Scholar]
- 13.Callegari A, Tonti D, Chergui M. Photochemically grown silver nanoparticles with wavelength-controlled size and shape. Nano Letters. 2003;3(11):1565–1568. [Google Scholar]
- 14.Yamamoto M, Kashiwagi Y, Nakamoto M. Size-controlled synthesis of monodispersed silver nanoparticles capped by long-chain alkyl carboxylates from silver carboxylate and tertiary amine. Langmuir. 2006;22(20):8581–8586. doi: 10.1021/la0600245. [DOI] [PubMed] [Google Scholar]
- 15.Su H-L, Chou C-C, Hung D-J, et al. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30(30):5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, Sepúlveda MS. Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine. 2011;6(5):879–898. doi: 10.2217/nnm.11.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaborska W, Krajewska B, Olech Z. Heavy metal ions inhibition of jack bean urease: potential for rapid contaminant probing. Journal of Enzyme Inhibition and Medicinal Chemistry. 2004;19(1):65–69. doi: 10.1080/14756360310001650237. [DOI] [PubMed] [Google Scholar]
- 18.Zaborska W, Krajewska B, Leszko M, Olech Z. Inhibition of urease by Ni2+ ions: analysis of reaction progress curves. Journal of Molecular Catalysis B: Enzymatic. 2001;13(4–6):103–108. [Google Scholar]
- 19.Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berrye: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori . International Journal of Molecular Sciences. 2012;13(8):9923–9941. doi: 10.3390/ijms13089923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Tada M, Nagi H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115(3):642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 21.Newman GR, Walker M, Hobot JA, Bowler PG. Visualisation of bacterial sequestration and bactericidal activity within hydrating Hydrofiber wound dressings. Biomaterials. 2006;27(7):1129–1139. doi: 10.1016/j.biomaterials.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Fang M, Chen J-H, Xu X-L, Yang P-H, Hildebrand HF. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. International Journal of Antimicrobial Agents. 2006;27(6):513–517. doi: 10.1016/j.ijantimicag.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Yuan P, He HP. Advances of Ag-type inorganic antibacterial agents’ research. Industrial Minerals & Processing. 2002;31(10):5–9. [Google Scholar]
- 24.Fabrega J, Fawcett SR, Renshaw JC, Lead JR. Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environmental Science & Technology. 2009;43(19):7285–7290. doi: 10.1021/es803259g. [DOI] [PubMed] [Google Scholar]
- 25.Wu D-C, Hsu P-I, Wu J-Y, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clinical Gastroenterology and Hepatology. 2010;8(1):36–41.e1. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asharani PV, Wu YL, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19(25) doi: 10.1088/0957-4484/19/25/255102.255102 [DOI] [PubMed] [Google Scholar]
- 27.Durán N, Marcato PD, de Conti R, Alves OL, Costa FTM, Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. Journal of the Brazilian Chemical Society. 2010;21(6):949–959. [Google Scholar]
- 28.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro . Environmental Health Perspectives. 2010;118(3):407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]


