Abstract
The role of calreticulin (CRT) in host-parasite interactions has recently become an important area of research. Information about the functions of calreticulin and its relevance to the physiology of Entamoeba parasites is limited. The present work demonstrates that CRT of both pathogenic E. histolytica and nonpathogenic E. dispar species specifically interacted with human C1q inhibiting the activation of the classical complement pathway. Using recombinant EhCRT protein, we demonstrate that CRT interaction site and human C1q is located at the N-terminal region of EhCRT. The immunofluorescence and confocal microscopy experiments show that CRT and human C1q colocalize in the cytoplasmic vesicles and near to the surface membrane of previously permeabilized trophozoites or are incubated with normal human serum which is known to destroy trophozoites. In the presence of peripheral mononuclear blood cells, the distribution of EhCRT and C1q is clearly over the surface membrane of trophozoites. Nevertheless, the level of expression of CRT in situ in lesions of amoebic liver abscess (ALA) in the hamster model is different in both Entamoeba species; this molecule is expressed in higher levels in E. histolytica than in E. dispar. This result suggests that EhCRT may modulate some functions during the early moments of the host-parasite relationship.
1. Introduction
Calreticulin (CRT) is a highly conserved multifunctional protein that was originally identified as a major calcium-binding protein of the endoplasmic reticulum [1]. CRT has been detected in every eukaryotic cell, with the exception of erythrocytes. All CRT proteins contain three structural domains: a globular N-terminal domain, a proline-rich P domain, and an acidic C-terminal domain. The N-terminal domain is involved in protein-protein interactions, RNA-binding, and autoantibody binding. The P domain binds Ca2+ with high affinity and low capacity, while the C-terminal domain, which is the least conserved domain among CRTs, binds Ca2+ with low affinity [1, 2].
The role of CRT in host-parasite interactions has recently become an important area of research. CRT genes from a number of parasites (Trypanosoma, Leishmania, Entamoeba, Onchocerca, Schistosoma, and Haemonchus) have been cloned and sequenced, revealing approximately 50% identity with CRT human gene [3–8].
Although the functions of CRT are conserved in vertebrates, some CRT functions differ among parasites [9, 10]; parasite CRTs bind host C1q and inhibit C1q-dependent complement activation. Haemonchus contortus CRT binds host C-reactive protein and C1q; this interaction may inhibit the activation of the classical complement pathway [11]. The ectoparasite Amblyomma americanum secretes CRT during feeding, suggesting that the anticoagulant ability of CRT may prevent blood clotting and permit the parasite to feed on the host or induce host antiparasite responses [12]. The presence of CRT in the penetration gland cells of Schistosoma suggests that this molecule may be important for the host skin penetration [13].
Among protozoan parasites, the binding and inhibition of human C1q by CRT have been demonstrated in both Trypanosoma cruzi and T. carassii. T. cruzi and T. carassii CRT (TcCRT) bind human or fish C1q, respectively, and specifically inhibit the classical complement pathway. This suggests an evolutive conserved interaction between CRT and C1q [14, 15].
Previously, we reported the presence of CRT in E. histolytica (EhCRT). This protein induces an important immunogenic response in the human host. More than 90% of patients with amoebic liver abscess (ALA) develop high levels of serum antibodies against EhCRT [16]. We also reported the cloning of CRT gene in E. histolytica and the preparation of monospecific antibodies against recombinant CRT (rEhCRT); the immunohistochemical assays on trophozoites show that EhCRT is located in the cytoplasmic vesicles and in vesicles in close contact with the inner cytoplasmic membrane. In histopathological studies, on sections of experimental ALA in hamsters, EhCRT was clearly detected into the trophozoites and seems to be neither exposed in the surface of trophozoites nor exported into the hepatic tissue [8]. The binding of C1q to CRT in the surface of E. histolytica trophozoites has been recently reported after its activation in cell-to-cell interaction with Jurkat cells; authors mention that during erytrophagocytosis the CRT is located in the surface of trophozoites and in the phagocytic cups [17]. CRT in the surface of apoptotic human cells seems to function as a receptor for C1q allowing the phagocytosis of damaged cells. More so, the overexpression of crt gene is related to the presence of apoptosis inductors [18].
In mammals, translocation of CRT from the RE to the membrane can be mediated by the vesicular transportation from the RE to the Golgi, mediated by the SNARE-dependent fusion of exocytic vesicles with plasma membrane. Other possible mechanisms of translocation of CRT to the plasma membrane could be mediated by the ERP57 chaperone protein, albeit this mechanism is not yet totally demonstrated [19].
One of the indicators of virulence of E. histolytica trophozoites that has been cited over the years [20, 21] is resistance to the lytic action of human serum. The referred capacity of CRT to bind host C1q observed in some parasites has been considered as an evasion mechanism of the host immune response, impairing the lytic action of complement. In the case of E. histolytica, it is possible that resistance of virulent trophozoites to the lyses of human serum could be mediated by the C1q binding capacity of EhCRT.
In the present work, we tested the human C1q binding capacity of recombinant EhCRT and native CRT in an ELISA system in both pathogenic E. histolytica and nonpathogenic E. dispar species. We also demonstrated that CRT and C1q colocalize in the cytoplasmic vesicles and those near the surface membrane of previously permeabilized trophozoites. Besides, we tested the capacity of recombinant EhCRT to bind human C1q and, as a consequence, be able to inhibit the classical complement pathway in vitro. Results suggest a clear amoebicidal activity of human serum against trophozoites that can be inhibited indistinguishably in presence of recombinant or native EhCRT; the interaction of CRT-C1q evaluated was equal for both species of Entamoebas.
2. Material and Methods
2.1. Production of Recombinant EhCRT
Full-length rEhCRT and N- and C-terminal-domain proteins were expressed and purified as previously described [8, 22]. Briefly, the plasmid pBluescript-KS+ (pbKS+) was used to clone PCR products. We obtained three clones, which we refer to as pb-EhCRT, pb-EhCRT-N, and pb-EhCRT-C. These recombinant plasmids were subcloned into the prokaryotic expression vector pProEX HT-b (Gibco Life Technologies, Grand Island, NY, USA) to express the CRT constructed in fusion with a six-histidine tag on the NH2 end. Competent Escherichia coli BL21 cells were transformed with one of the recombinant plasmids. The expression of recombinant proteins rEhCRT, rEhCRT-N, and rEhCRT-C was induced with a final concentration of 1 mM isopropyl-β-D-thiogalactoside (IPTG). The QIAexpressionist system (Qiagen, Valencia, CA, USA) was used to purify recombinant proteins.
The cells were harvested by centrifugation at 3000 ×g for 12 min, and the bacterial pellet was resuspended in 5 mL of lysis buffer (8 M urea, 0.1 M NaH2PO4, and 0.1 M Tris-HCl, pH 8.0). The lysate was added to a 50% suspension of Ni-NTA agarose (Qiagen). The mixture was filtered through a filtration column (Qiagen), and the recombinant proteins were eluted with 8 M urea buffer, pH 4.5. The selected fractions were dialysed against 19 mM phosphate-buffered saline (PBS) to eliminate the urea.
2.2. Purification of Native EhCRT and EdCRT
Specific anti-rEhCRT IgG antibodies were obtained previously [8] and were used to purify native EhCRT and EdCRT by affinity chromatography. 20 mg of IgG anti-rEhCRT was bound to a Sepharose 4B column (Sigma Chemical Co., St Louis, MO, USA). A membrane-enriched E. histolytica or E. dispar extract was obtained as previously reported [23]. A 10 mg quantity of the respective antigen was applied to the column and incubated for 1 h. The column was washed with PBS, pH 7.5. This bound protein was eluted with 0.5 M glycine, pH 4.5, and 1 mL fractions were collected into 100 μL of 1.0 M Tris-HCl, pH 8.5, to neutralize the acidity of the elution buffer to preserve activity. Protein concentrations were determined using a Bradford Assay kit (Bio-Rad, Hercules, CA, USA).
2.3. Isolation of Human Lymphocytes
Peripheral mononuclear blood cell (PMBC) was isolated from fresh human blood obtained in heparinized tubes from human volunteers. Whole blood was centrifuged by gradient of Ficoll-Hypaque (Gibco BRL); the PMBC was separated and washed three times with PBS and used immediately for assays of interaction with trophozoites of E. histolytica or E. dispar (1 : 6 ameba/lymphocytes).
2.4. Interaction of EhCRT or EdCRT with Human C1q
Microtiter plates (EIA/RIA strip, Costar, Cambridge, MA, USA) were coated overnight at room temperature (RT) with 50 μL of 0–200 μM of full-length recombinant rEhCRT, rEhCRT-N, or rEhCRT-C or native nEhCRT or nEdCRT suspended in 0.1 M Na2CO3, pH 9.6. Each step was followed by three washes with 0.5% Tween 20/PBS. Nonspecific binding sites were blocked with 3% PBS/BSA, for 2 h at 37°C. After washing, 50 μL of a 1 : 10 dilution of NHS in PBS was added to each well and incubated for 2 h at 37°C. The plates were washed as before, and 50 μL of mouse anti-human C1q (1 : 500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to each well and incubated for 2 h at 37°C. The plates were washed again as before, and the antigen-antibody reaction was detected by incubation with HRP-conjugated goat anti-mouse IgG (1 : 1000) for 2 h at 37°C. The reaction was developed by the addition of 200 μL of ortho-phenylenediamine phosphate (OPD) (10 mg/mL), and the absorbance was measured at 490 nm in a microplate reader (ELx800, BioTek Instruments, Winooski, VT, USA).
2.5. Inhibition of C1q-Dependent Haemolytic Assays
For classical pathway complement activation, sheep red blood cells (SRBCs) were sensitized with rabbit anti-SRBC (1 : 400) (antibody ab50676, Abcam, Cambridge, MA, USA). A 500 μL aliquot of 107 antibody-sensitized erythrocytes (EAs) was incubated with normal human serum (NHS) diluted 1 : 10 in isotonic veronal-buffered saline containing 0.1 mM CaCl2, 0.5 mM MgCl2, 0.1% gelatine, and 1% glucose (GVB++) in a final volume of 1000 μL as a positive control. To assess the inhibition of complement activation, the NHS (1 : 10) was preincubated with 2 μg of native nEhCRT or nEdCRT, then added to EAs, and incubated for 1 h at 37°C. After the addition of 1 mL of cold GVB++, intact cells were centrifuged at 400 ×g for 15 min. Haemoglobin in the supernatant was measured at 550 nm with a DU-650 Spectrophotometer Beckman (Beckman, Danvers, MA, USA). Total haemolysis (100%) was measured by treating EAs with water. Background spontaneous haemolysis (0%) was determined by incubating EAs without serum. Haemolytic activity is expressed as a percentage of total haemolysis.
In a similar assay, 107 EAs were incubated with C1q-depleted human serum (Calbiochem, a division of Merck KGaA, Darmstadt, Germany) and then added to 2 μg of human C1q (Sigma). Haemolysis was calculated as before. To overcome the inhibitory effect of EhCRT on the classical complement pathway, we used IgG anti-EhCRT produced in mice.
2.6. Amoebicidal Activity of Human Serum
Axenic trophozoites of E. histolytica, E. dispar, or a virulent strain of E. histolytica, newly recovered from hamster livers [22], were harvested by centrifugation at 500 ×g for 10 min, washed twice with PBS, counted, adjusted to a cell density of 2 × 105, and incubated with TYIS-33 medium [24] added with (10, 20, 40, and 60%) NHS. The mixtures were incubated at 37°C for 15, 30, and 60 min; viability of trophozoites was estimated through the 2% trypan blue exclusion technique [25]; live trophozoites were counted in a haemocytometer. Cell counts were expressed as a percentage of dead cells. Heat-inactivated human serum was used as a negative control. To evaluate the inhibition of lyses due to interaction of EhCRT with the human C1q, 10 μg of rEhCRT was added to NHS incubating during 10 min; thereafter, the mixture was added to the trophozoites suspension. Lyses percentage was defined as the decrease in viable trophozoites in the presence of NHS compared with the heat-inactivated human serum control. Values were calculated as follows: (number of viable cell in control − number of viable cell in the presence of NHS)/viable cell in control) × 100. Results are the mean of three independent experiments with each E. dispar or E. histolytica species or virulent strain of E. histolytica.
2.7. Human C1q and EhCRT/EdCRT Colocalization
Trophozoites of E. histolytica or E. dispar were grown under axenic conditions using TYIS-33 or TYIS-2 [24], respectively, for 48 h. After incubation, the trophozoites were allowed to adhere to sterile glass cover slips for 2 h at 37°C and then fixed with 3.5% paraformaldehyde/PBS. Thereafter, cells were permeabilized or not with 0.1% (v/v) Triton X-100 and blocked with 3% BSA. Trophozoites were then incubated with 4 μg of C1q for 30 min. The slides were washed several times with PBS and incubated for 1 hr with specific rabbit anti-EhCRT (1 : 40 dilution) and mouse anti-human C1q antibodies (1 : 40). Thereafter, a mixture of secondary antibodies was used to reveal the antigen-antibody reactions (Alexa Fluor Cy5 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG, both 1 : 100) (Molecular Probes, Invitrogen, Eugene, OR, USA).
In a similar assay, trophozoites were incubated with NHS (1 : 10) or human peripheral mononuclear blood cells (PMBC) (1 : 6, trophozoites/lymphocytes ratio) for 60 min before incubation with 4 μg of C1q and finally processed for immunohistochemical assay as previously described [5]. Samples were examined by confocal microscopy (DM1RE-2, Leica Mikrosysteme, Wetzlar, Germany) using appropriate fluorescence emission filters. Images (z-series) were acquired with image-processing software (Leica, LCS Lite Profile Pro) using 0.5 μm steps. The images correspond to the maximum-intensity projection of the z-series.
2.8. Experimental Amoebic Liver Abscess
Experimental acute ALA was produced in 100 g hamsters following a technique described by Tsutsumi et al. (1984) [26]. Briefly, 2.5 × 105 or 2 × 106 axenic trophozoites of EhVIR (newly recovered from hamster liver) or E. dispar, respectively, were inoculated into the portal vein of anesthetized hamsters. After 5, 15, and 30 min and 1, 3, and 9 hours, animals (5 hamsters at a time) were euthanized by an anaesthesia overdose. The liver was removed and fixed in 4% paraformaldehyde in PBS, followed by dehydration and paraffin embedding. Serial sections of 6 μm thickness were obtained and deparaffinized from tissue blocks; lesions and trophozoites were identified by hematoxylin/eosin stain. The sliced sections were used for immunohistochemical and reverse transcriptase real-time PCR (qRT-PCR) assays.
The institutional committee previously approved protocols for animal care. The institution fulfils all the technical specifications for the production, care, and use of laboratory animals and is certified by a National Law (NOM-062-ZOO-1999). All hamsters were handled according to the guidelines of the 2000 AVMA Panel of Euthanasia.
2.9. Immunochemical Detection of EhCRT and C1q in Amoebic Liver Abscess Lesions
Selected samples were blocked with 3% PBS/BSA solution and reacted with specific mouse anti-EhCRT antibody diluted 1 : 50 and in another slice with mouse anti-human C1q antibody (1 : 20); thereafter, slices were incubated at 4°C overnight. Antigen-antibody reaction was detected using 1 : 500 dilution of goat anti-mouse IgG antibody coupled to alkaline phosphatase (Zymed Laboratories, San Francisco, CA, USA); NBT/BCIP substrate (Roche Diagnostics GmbH; Mannheim, Germany) was used as the chromogen. Monoclonal mouse IgG1 antibody against Aspergillus niger glucose oxidase was used as the negative control (clone DAK-GO1, code number X09931, Dako, Glostrup, Denmark). To avoid cross-reaction with CRT from hamster hepatic tissue, anti-EhCRT antibodies were adsorbed with a lyophilized extract of hamster liver. The samples were counterstained with aqueous eosin.
2.10. Relative mRNA Quantification of EdCRT and EhCRT by qRT-PCR
The detection of CRT mRNA was carried out using a two-step in situ RT-PCR procedure as previously reported with some modifications [5]. Previously selected hamster liver tissue sections (3 sections after intraportal inoculation) were pretreated with 0.5 μg/μL proteinase K (Sigma Aldrich, St. Louis, MO, USA) and with 1 U/sample of DNase I, RNase-free (Roche Diagnostics GmbH, Mannheim, Germany). After washing with DEPC-treated water, reverse transcription was performed using SuperScript II reverse transcriptase following the manufacturer's specifications (Invitrogen, Carlsbad, CA, USA). Slides were incubated at 42° C for 2 h in a sealed humidified chamber.
The relative quantification (RQ) of the investigated samples by real-time PCR was performed using the previously synthesized cDNA in the in situ RT assays. For this purpose, a 7300 Applied Biosystems apparatus (Applied Biosystems, Carlsbad, CA, USA) and the Quantitect SYBR green PCR kit were used (Qiagen, Valencia, CA, USA).
qPCR was performed for 60 cycles of a 3-step PCR, including 10 seconds of denaturation at 95°C, a 30 sec primer-dependent annealing phase at 58°C, and a 10 sec template-dependent elongation at 72°C. The amplification of each template was performed in duplicate in one PCR run. The differential expression of the investigated genes was calculated as the normalized ratio to Ehβ-actin.
Results of the threshold cycle (Ct indicates number of cycles to which the amplified product is detected) dates were exported to an Excel sheet to calculate gene expression levels (RQ) using 2−ΔΔCt method described by Livak and Schmittgen (2001) [27].
2.11. Statistical Analysis
All values are expressed as the mean ± SD of at least three independent experiments. Statistical significance was determined with unpaired Student's t-test between each condition used (control against problem), and for comparisons of multiple groups with one-way analysis of variance (ANOVA), differences were considered statistically significant when P values were <0.05.
3. Results
3.1. CRT Binds Human C1q
To assess the interaction between EhCRT and human C1q, a direct binding ELISA was conducted. Figure 1 shows data of the interaction assay between rEhCRT (full-length molecule, N-terminal binding domain, or C-terminal binding domain), nEhCRT or nEdCRT, and human C1q. Differences observed in binding between the native EhCRT or EdCRT and the full-length rEhCRT or rEhCRT-N were not statistically significant. The interaction was dose dependent and saturable; the maximum absorbance was obtained when 100 μM of CRT was used; the OD remained constant in the presence of larger quantities of CRT. In contrast, the rEhCRT-C-terminal protein did not bind to human C1q.
Figure 1.
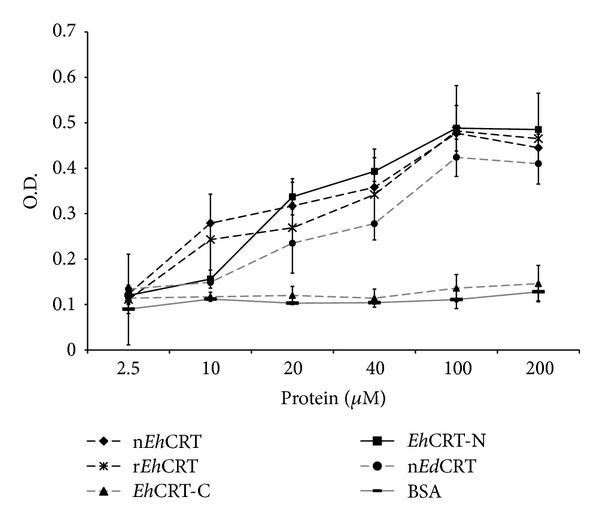
EhCRT/EdCRT interaction with human C1q (ELISA). Microtiter wells were coated with 2.5 to 200 μM of the EhCRT or EdCRT and incubated with 1 : 10 diluted normal human serum supplemented with 4 μg of human C1q; the interaction of EhCRT-C1q was revealed using an anti-human C1q monoclonal antibody produced in mice and then an anti-mouse IgG produced in goat conjugated to peroxidase. Values are the mean of three different assays performed in triplicate ± SD.
3.2. C1q-Dependent Haemolytic Assays
The ability of EhCRT to inhibit the activation of the classical complement pathway by binding to C1q was tested in a simple assay of inhibition of the haemolysis of SRBCs previously sensitized with antibody (EAs). Human serum was used as the source of C1q. The optimal dilution of the human serum was determined previously by a complement titration curve (data not shown); the optimal dilution of serum was 1 : 10. Figure 2 shows the values of inhibition of the activation of the classical complement pathway assays, in the presence of different concentrations of nEhCRT or nEdCRT. Both proteins inhibited the lysis of EAs in a dose-dependent manner as shown by the decrease in haemolytic activity (34–22% of baseline). By contrast, in the control assay (without CRT−) or in case we use the recombinant protein EhCRT-C, there was no significant decrease in haemolytic activity (95–86% of baseline) due to the absence of C1q binding site.
Figure 2.
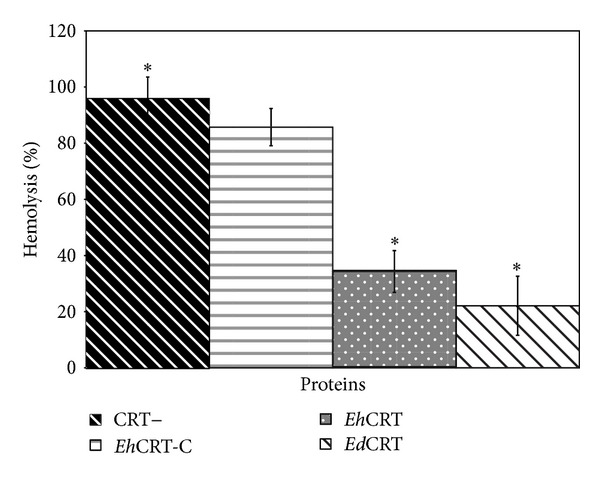
EhCRT inhibits classical pathway-mediated hemolysis. Different proteins of (CRT+) EhCRT or EdCRT; (CRT−) BSA or EhCRT-C was added to 1 : 10 dilution of NHS (as source of C1q), incubated 30 min at 37°C, and then added to 108 cell/mL of EA; the mixtures were incubated for 60 min at 37° C. After centrifugation, the OD (550 nm) of the supernatants was measured. The percentage of lyses was calculated using as reference the 100% lyses of erythrocytes in water. Values are the mean of three independent experiments ± SD. Differences between groups ∗ were compared through ANOVA test detecting statistical significance (P = 0.05).
To confirm that this activity is the result of the interaction of human C1q with EhCRT or EdCRT, we used C1q-depleted human serum; when this serum was added to human C1q in the presence of EhCRT, haemolysis was inhibited. Moreover, when EhCRT was pretreated with anti-CRT antibodies, EhCRT could not bind to C1q, and the activation of the classical complement pathway was restored (Figure 3).
Figure 3.
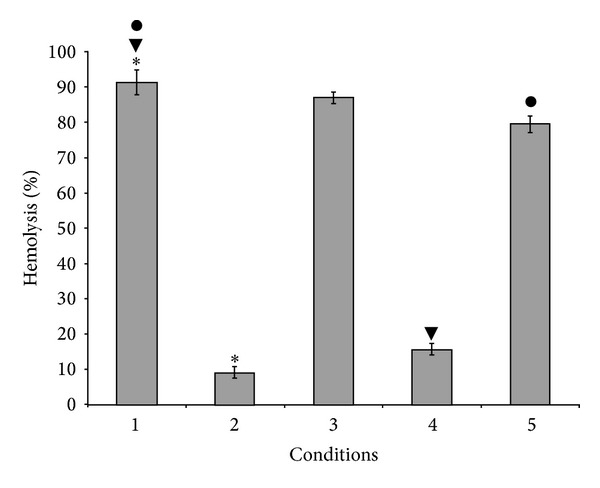
EhCRT-human C1q on the activation of classical complement pathway: hemolysis assay. 1: corresponding to NHS (positive control); 2: human C1q-depleted serum (NHSC1q−); 3: (NHSC1q−) + C1q; 4: (NHSC1q−) + C1q + EhCRT; 5: (NHSC1q−) + C1q + EhCRT + IgG anti-EhCRT. Assays were performed in triplicate; values are the mean of three different experiments ± SD. Differences between groups ∗, ●, and ▼ were compared through ANOVA test. Statistical significance (P = 0.012).
3.3. Amoebicidal Activity of Normal Human Serum
To test that human serum is indeed harmful to trophozoites through the action of serum complement, we previously titre the NHS; 40% of NHS was the optimal concentration to obtain reproducible results; proper time for interaction with trophozoites was 60 min. In these conditions, axenic E. histolytica HM1:IMSS (nonvirulent) and axenic E. dispar SAW760 strain are both susceptible to lysis by complement; however, E. dispar SAW760 is more susceptible than the virulent strain of E. histolytica; this strain showed a clear resistance to lyses (37%) (P < 0.005). The resistance of trophozoites to the lyses mediated by the rEhCRT-C1q binding is shown in Figure 4. Moreover, there is a clear reduction in the values of lyses of trophozoites in the presence of rEhCRT preincubated with NHS (40%). Differences with respect to controls (NHS without rEhCRT) were statistically significant (P < 0.004).
Figure 4.
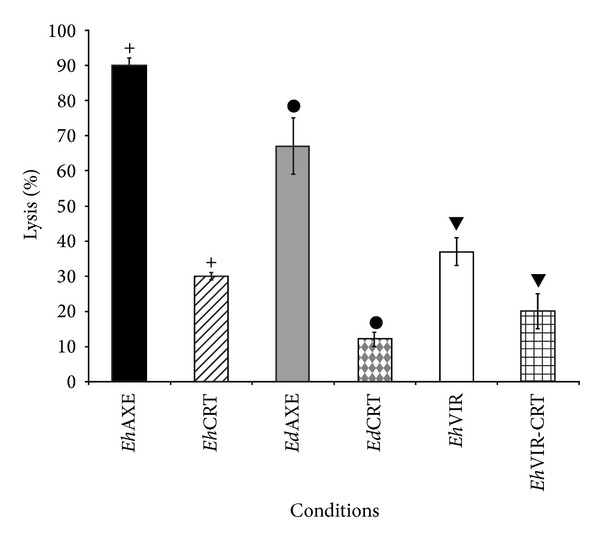
Amoebicidal activity of human serum. Trophozoites of E. histolytica and E. dispar were harvested by ice bath and then centrifuged at 500 g for 10 min, washed, counted, adjusted to a cell density of 2 × 105, and incubated with TYIS-33 medium added with 40% NHS at different times; viability was assessed by trypan blue exclusion technique. To estimate the inhibition of lyses due to interaction of EhCRT-C1q, 10 μg of rEhCRT was added to NHS incubating during 10 min, and the mixture was then added to trophozoites suspension. The percent of lyses was defined as the decrease of trophozoites viability in the presence of NHS compared with the heat-inactivated human serum control. Differences between groups +, ●, and ▼ were compared through Student's t-test detecting statistical significance (P < 0.05).
3.4. Human C1q and EhCRT/EdCRT Colocalization
To evaluate the interaction of EhCRT/EdCRT in trophozoites with human C1q, a colocalization assay was performed directly on trophozoites of both E. histolytica and E. dispar species by confocal microscopy. Both proteins clearly colocalized in previously permeabilized trophozoites; the fluorescent signal was detected in the cytoplasmic vesicles but was more concentrated near the cytoplasmic membrane (Figure 5); apparently, there are no differences in distribution of fluorescence in trophozoites between the two species. However, in nonpermeabilized trophozoites activated with peripheral mononuclear blood cells, the immunolocalization of CRT/C1q was detected on the surface membrane of trophozoites (Figure 6). Furthermore, when the trophozoites were incubated with NHS, the colocalization of both proteins was detected mainly in the cytoplasmic vesicles (Figure 7); this could be due to the destruction of trophozoites membranes induced by NHS.
Figure 5.
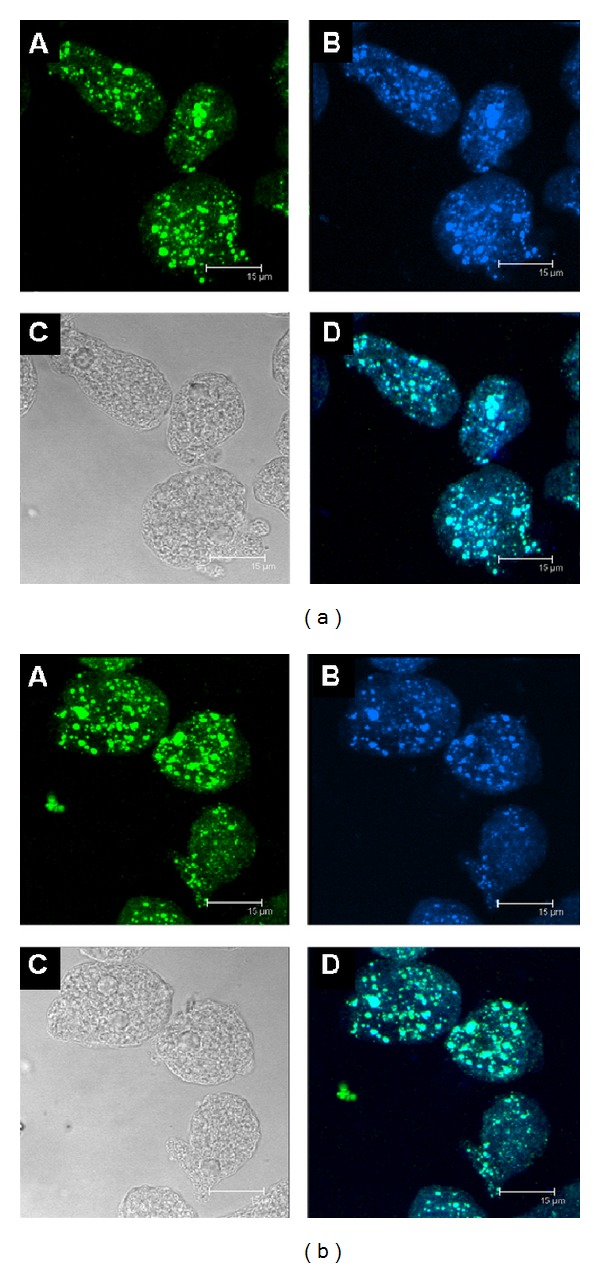
Confocal microscopy assay: colocation of EhCRT or EdCRT and human C1q. Panels (a) and (b) represent different patterns of immunodetection when using E. histolytica or E. dispar trophozoites, respectively; A: rabbit anti-EhCRT and Alexa Fluor 350-conjugated secondary antibody; B: trophozoites reacted with mouse anti-human C1q and with anti-mouse Alexa Fluor 488; C: representing the differential interference contrast (DIC); D: colocation of EhCRT with the C1q human protein (Channel Mergin). The micrographs showed the maximal projection of the z-series. Scale bar represents 15 μm.
Figure 6.
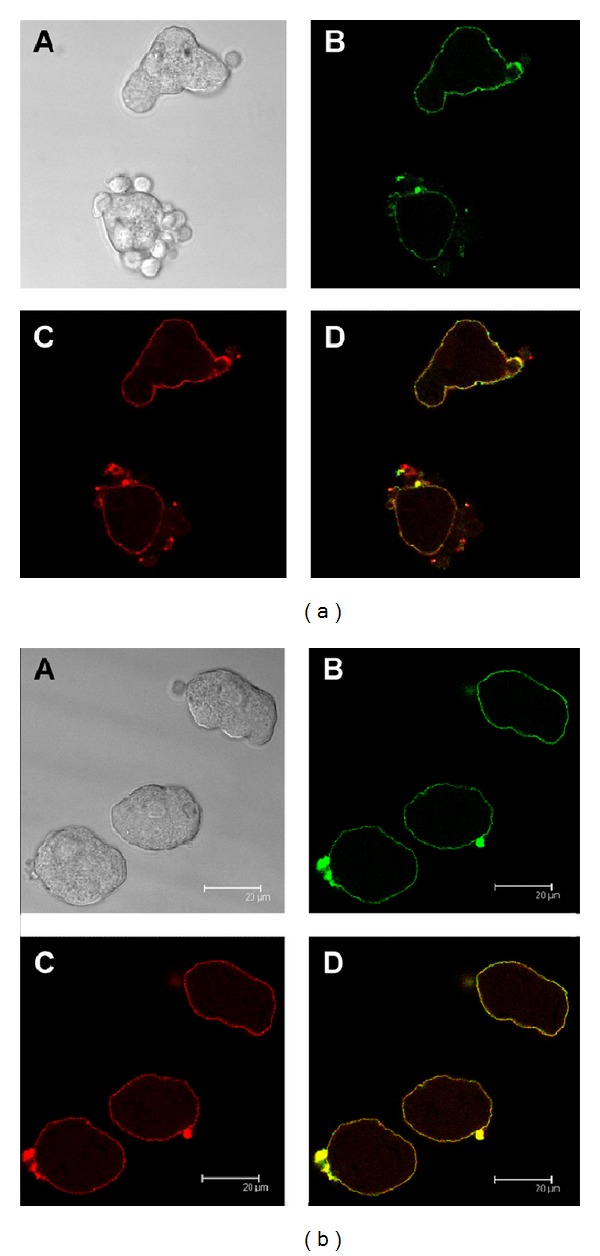
Colocalization of EhCRT or EdCRT and human C1q after interaction with PMBC. C1q and EhCRT or EdCRT colocalization by confocal microscopy of (a) E. histolytica; (b) E. dispar trophozoites. Trophozoites were grown under axenic conditions and incubated during 30 min with PMBC; thereafter, C1q was added and incubated for 30 min. Then, the mixture was added with specific primary antibodies, anti-rabbit EhCRT IgG and mouse anti-human C1q IgG, respectively. The reaction was revealed with Alexa Fluor 555 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG. The micrographs show the maximal projection of the z-series. Scale bar represents 20 μm. A: phase contrast microscopy; B: EhCRT (red); C: C1q (green); D: merge.
Figure 7.
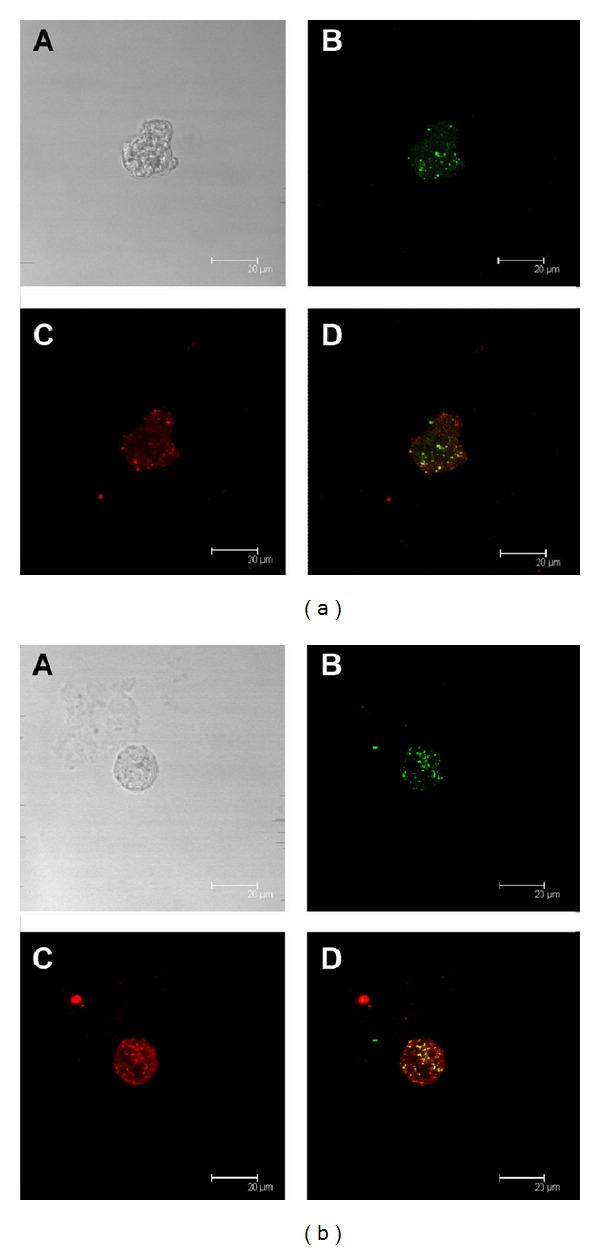
Colocalization of EhCRT or EdCRT and human C1q after interaction with NHS: human C1q and EhCRT or EdCRT colocalization estimated by confocal microscopy of (a) E. histolytica; (b) E. dispar trophozoites. Trophozoites were grown under axenic conditions and were allowed to adhere to sterile glass cover slips. Trophozoites were incubated with normal human serum (NHS) (source of C1q) and then fixed with 3.5% paraformaldehyde/PBS; thereafter, trophozoites were incubated with specific primary antibodies, rabbit anti-EhCRT IgG and mouse anti-human C1q IgG, respectively. The reaction was revealed with Alexa Fluor 555 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG. The micrographs show the maximal projection of the z-series. Scale bar represents 20 μm. A: phase contrast microscopy; B: EhCRT (red); C: C1q (green); D: merge.
3.5. Immunochemical Detection of EhCRT and C1q in Amoebic Liver Abscess Lesions
Representative sections of hepatic tissue obtained at 30 min and 3 h after the intraportal inoculation of E. histolytica virulent trophozoites or E. dispar are shown in Figures 8(a) and 8(b), respectively. The immunodetection of CRT and C1q in the trophozoites established in the hepatic tissue is evident and displays a similar distribution on trophozoites as observed in the confocal microscopy assays (Figure 5). The immunohistochemical signals, both anti-EhCRT and anti-C1q, were displayed in different size cytoplasmic vesicles. In some trophozoites, signals are apparently located on the cell surface membrane in both Entamoebas. It is clear that trophozoites apparently do not secrete or export the CRT protein into the hepatic tissue. The negative control and secondary antibody did not show background reactivity. These assays can be found in the Supplementary Material available online at http://dx.doi.org/10.1155/2014/127453.
Figure 8.
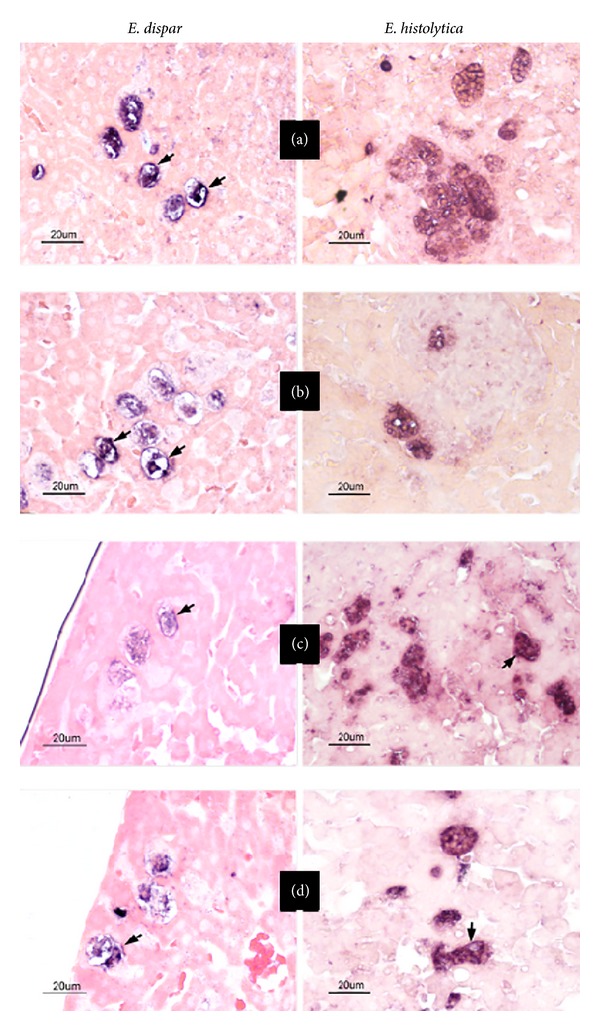
Immunohistochemical staining of EhCRT or EdCRT and C1q in situ. Representative images of immunohistochemical detection of CRT and C1q in amoebic liver abscess sections of livers from hamsters inoculated with E. histolytica HM1:IMSS virulent trophozoites and E. dispar SAW760 trophozoites and sacrificed at different times after inoculation for 30 min (a, b) and 3 h (c, d) (representative times). (a and c) Tissue section stained with mouse IgG against EhCRT and (b and d) section stained with mouse IgG against C1q. Scale bar represents 20 μm. The control assays of negative and secondary antibody were unstained; they are included as Supplementary Material.
3.6. Relative mRNA Quantification of EdCRT and EhCRT by qRT-PCR
The relative quantification (RQ) of EhCRT or EdCRT mRNA expression is shown in Figure 9. The values correspond to the relative expression of cDNA into ALA specimens assayed by qPCR. The EdCRT was expressed for a short period of time after inoculation (15 min RQ = 1.5) but it started to decline quickly for the remaining time resulting in lower levels than baseline. In contrast, EhCRT increases at 30 min (RQ = 2) reaching a peak after 60 min (RQ = 5) between 3 h (RQ = 4.5); however, it decreased to values close to baseline thereafter. The level of expression of EhCRT in comparison with EdCRT was statistically significant (P = 0.04).
Figure 9.
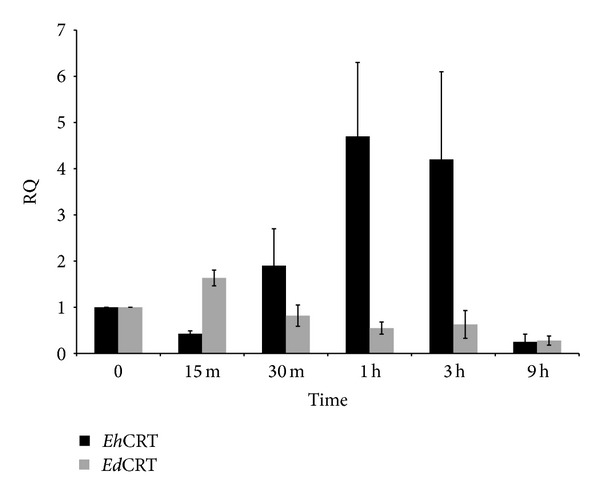
Relative quantification (RQ) of expression of mRNA for EhCRT and EdCRT. Reverse transcription real-time PCR was used to independently measure mRNA expression of EdCRT and EhCRT in trophozoites present in tissue sections of liver in hamsters after different times of postinoculation. The values represent the mean of three independent experiments. Differences between E. histolytica and E. dispar were compared through Student's t-test detecting statistical significance *(P = 0.005).
4. Discussion
E. histolytica and E. dispar are parasites whose natural host is the human being; their target organ is the large bowel. Therefore, they are in intimate contact with the local immune system. In human populations, the infection can be persistent or recurrent, self-limited, and usually asymptomatic (90% of cases), suggesting a balanced host-parasite interaction and some kind of immune response evasion mechanism. E. histolytica and E. dispar activate both classical and alternative pathways of serum complement [28, 29]. This activity is part of the immunological mechanisms induced by these protozoa during the early phases of host-parasite relationship. These mechanisms are among the main resistance mechanisms of hosts against parasite infection, but parasites have evolved alternative methods to evade immune attack and survive in the tissues of their host. One of these alternatives is the expression of an interesting and complex protein, calreticulin. This ubiquitous endoplasmic reticulum-associated protein has a large spectrum of functions, including the capacity to bind C1q, which is the first component of the classical serum complement pathway. CRT-C1q binding inhibits the activation of the complement cascade in different hosts; this mechanism has been considered an evasion mechanism of the immune response developed by a number of parasites [9, 10].
This evasion mechanism has been described in schistosomiasis, oncocercosis, trypanosomiasis [3, 5, 14], and now in amoebiasis. The interaction of CRT from T. cruzi (TcCRT) with human C1q is one of the most studied systems. In this protozoan, CRT not only allows the evasion of the immune response but also modulates it. Furthermore, the presence of CRT on the surface of the parasite increases its infectivity by binding to C1q promoting the early phagocytosis of the parasite [30, 31].
In contrast to T. cruzi, in our in vitro assays, E. histolytica and E. dispar trophozoites do not express CRT on the external surface and do not export CRT into tissues in the in vivo model of amoebic liver abscess in hamsters [5]. Recently, the presence of CRT was reported in cytoplasmic membranes of trophozoites previously activated with Jurkat cells [17] or concanavalin A-activated trophozoites [32]. In the present study, we demonstrate that trophozoites activated by human PBMC also show the presence of CRT in the surface membrane (Figure 6). In the in vivo experiment of amoebic liver abscess in hamsters, the localization of CRT was also observed on the surface of trophozoites (Figure 8).
EhCRT is highly immunogenic in humans, mice, and rabbits, suggesting that, in natural or experimental infection with E. histolytica, EhCRT is in some way exposed to the host immunocompetent cells. The mechanism of this interaction may be time dependent and may occur via surface expression and/or by exposure to apoptotic or dead trophozoites as was previously reported [16].
In human trypanosomiasis, the role of TcCRT as an immune evasion mechanism is easily understood because CRT is located on the surface of the trypomastigote in blood during the acute phase of infection and accessible to C1q. The role of Entamoeba CRT in pathogenicity is less clear. In intestinal amoebiasis, trophozoites are not totally exposed to complement system; apparently, the complement system only crosses to the mucosa membrane in conditions of disease as cancer, inflammatory bowel diseases, or autoimmune inflammatory intestinal diseases [33]. In amoebiasis infection, only in the case of invasive intestinal amoebiasis trophozoites are exposed to serum complement system. In the case of ALA, the trophozoites are indeed exposed to complement system. In this sense, trophozoites that express CRT on the surface membrane can bind C1q, induce the inhibition of the classical pathway of complement, and be protected from the lyses. This can be the case of our in vivo experimental model. During phagocytosis, C1q facilitates the binding to apoptotic epithelial cells by E. histolytica trophozoites [34]. Moreover, EhCRT has been detected in the uropods induced in trophozoites by concanavalin A [32, 35], which may function as another mechanism by which EhCRT/EdCRT is exposed to the host immune system. However, capping formation in animal models and in human amoebic invasive lesions has not been observed. Draws attention the over expression of Ehcrt gene in E. histolytica during first hours of host-parasite interaction in contrast with the E dispar specie, which do not over express this gene. This may suggest that over expression of crt gen could be a regulatory mechanisms that may allow the adaptation and survival of the parasite in the host tissues, as has been described in other parasites [31]. These are more suitable for the hostile environment in the liver. Besides, we cannot discard the possible selective pressure of serum complement over the infective trophozoites allowing the survival of complement resistant trophozoites in infected tissues. Finally, these trophozoites will be responsible for amoebic abscess development.
Supplementary Material
In this section can you see, the negative control and secondary antibody of confocal microscopy for colocalization of EhCRT and C1q in trophozoites of E. histolytica (figure 5-A). The negative control and secondary antibody of representative tissue slides of immunohistochemical detection of EhCRT and C1q in amebic liver abscess sections of livers from hamster inoculated whit E. histolytica virulent (figure 8-A).
Acknowledgments
The authors are very grateful to Dr. Marco Antonio Santillán for his generous gift of SRBCs and to Mario Nequiz-Avendaño for the Entamoeba histolytica HM1:IMSS axenic culture. The authors are also highly grateful with Dr. Adolfo Martinez-Palomo and Technician Lizbeth Salazar Villatoro for providing them with the axenic culture of E. dispar SAW760 strain. The authors are indebted to Marco Gudiño and Mrs. Ma Lourdes Alonso De la Rosa for art design and to Mrs. Ma Elena Ortiz for secretarial assistance.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochemical Journal. 1999;344(2):281–292. [PMC free article] [PubMed] [Google Scholar]
- 2.Gold LI, Eggleton P, Sweetwyne MT, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. The FASEB Journal. 2010;24(3):665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcelain K, Colombo A, Molina MC, et al. Development of an immunoenzymatic assay for the detection of human antibodies against Trypanosoma cruzi calreticulin, an immunodominant antigen. Acta Tropica. 2000;75(3):291–300. doi: 10.1016/s0001-706x(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 4.Joshi M, Pogue GP, Duncan RC, et al. Isolation and characterization of Leishmania donovani calreticulin gene and its conservation of the RNA binding activity. Molecular and Biochemical Parasitology. 1996;81(1):53–64. doi: 10.1016/0166-6851(96)02676-x. [DOI] [PubMed] [Google Scholar]
- 5.González E, García de Leon MC, Meza I, et al. Tripanozoma cruzi calreticulin: an endoplasmic reticulum protein expressed by trophozoites into experimentally induced amoebic liver abscesses. Parasitology Research. 2011;108(2):439–449. doi: 10.1007/s00436-010-2085-6. [DOI] [PubMed] [Google Scholar]
- 6.Rokeach LA, Zimmerman PA, Unnasch TR. Epitopes of the Onchocerca volvulus RAL1 antigen, a member of the calreticulin family of proteins, recognized by sera from patients with onchocerciasis. Infection and Immunity. 1994;62(9):3696–3704. doi: 10.1128/iai.62.9.3696-3704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Gengehi N, El Ridi R, Abdel Tawab N, El Demellawy M, Mangold BL. A Schistosoma mansoni 62-kDa band is identified as an irradiated vaccine T-cell antigen and characterized as calreticulin. The Journal of Parasitology. 2000;86(5):993–1000. doi: 10.1645/0022-3395(2000)086[0993:ASMKBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Suchitra S, Joshi P. Characterization of Haemonchus contortus calreticulin suggests its role in feeding and immune evasion by the parasite. Biochimica et Biophysica Acta. 2005;1722(3):293–303. doi: 10.1016/j.bbagen.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Nakhasi HL, Pogue GP, Duncan RC, et al. Implications of Calreticulin function in parasite biology. Parasitology Today. 1998;14(4):157–160. doi: 10.1016/s0169-4758(97)01180-0. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira V, Molina MC, Valck C, et al. Role of calreticulin from parasites in its interaction with vertebrate hosts. Molecular Immunology. 2004;40(17):1279–1291. doi: 10.1016/j.molimm.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Naresha S, Suryawanshi A, Agarwal M, Singh BP, Joshi P. Mapping the complement C1q binding site in Haemonchus contortus calreticulin. Molecular and Biochemical Parasitology. 2009;166(1):42–46. doi: 10.1016/j.molbiopara.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Jaworski DC, Simmen FA, Lamoreaux W, Coons LB, Muller MT, Needham GR. A secreted calreticulin protein in ixodid tick (Ambylomma americanum) saliva. Journal of Insect Physiology. 1995;41(4):369–375. [Google Scholar]
- 13.Khalife J, Liu JL, Pierce R, Porchet E, Godin C, Capron A. Characterization and localization of Schistosoma mansoni calreticulin Sm58. Parasitology. 1994;108(5):527–532. doi: 10.1017/s0031182000077398. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira V, Valck C, Sánchez G, et al. The classical activation pathway of human complement system is specifically inhibited by calreticulin from Tripanosoma cruzi . Journal of Immunology. 2004;172(5):3042–3050. doi: 10.4049/jimmunol.172.5.3042. [DOI] [PubMed] [Google Scholar]
- 15.Oladiran A, Belosevic M. Trypanosoma carassii calreticulin binds host complement component C1q and inhibits classical complement pathway-mediated lysis. Developmental and Comparative Immunology. 2010;34(4):396–405. doi: 10.1016/j.dci.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez E, Rico G, Mendoza G, et al. Calreticulin–like molecule in trophozoites of Entamoeba histolytica HM1:IMSS. American Journal of Tropical Medicine and Hygiene. 2002;67(6):636–639. doi: 10.4269/ajtmh.2002.67.636. [DOI] [PubMed] [Google Scholar]
- 17.Vaithilingam A, Teixeira JE, Miller PJ, Heron BT, Huston CD. Entamoeba histolytica cell surface calreticulin binds human C1q and functions in amebic phagocytosis of host cells. Infection and Immunity. 2012;80(6):2008–2018. doi: 10.1128/IAI.06287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. Journal of Experimental Medicine. 2001;194(6):781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panaretakis T, Joza N, Modjtahedi N, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death and Differentiation. 2008;15(9):1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 20.Reed SL, Curd JG, Gigli I. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica . Journal of Immunology. 1986;136(6):2265–2270. [PubMed] [Google Scholar]
- 21.Braga LL, Ninomiya H, McCoy JJ, et al. Inhibition of the complement membrane attack complex by the galactose-specific adhesin of Entamoeba histolytica . Journal of Clinical Investigation. 1992;90(3):1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González E, Villegas-Sepúlveda N, Bonilla R, et al. Cloning and expression of Entamoeba histolytica calreticulin gene. Proceedings of the 5th International Congress on Tropical Medicine and International Health, MEDIMOND S.r.l. International Proceedings; 2007; pp. 43–49. [Google Scholar]
- 23.Ximénez C, Leyva O, Morán P, et al. Entamoeba histolytica: antibody response to recent and past invasive events. Annals of Tropical Medicine and Parasitology. 1993;87(1):31–39. doi: 10.1080/00034983.1993.11812737. [DOI] [PubMed] [Google Scholar]
- 24.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba . Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 25.Colingan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current Protocols in Immunology. John Wiley and Sons; 1997. Trypan blue exclusion test of cell viability. [Google Scholar]
- 26.Tsutsumi V, Mena-Lopez R, Anaya-Velazquez F, Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation. American Journal of Pathology. 1984;117(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Calderon J, Schreiber RD. Activation of the alternative and classical complement pathways by Entamoeba histolytica . Infection and Immunity. 1985;50(2):560–565. doi: 10.1128/iai.50.2.560-565.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walderich B, Weber A, Knobloch J. Sensitivity of Entamoeba histolytica and E. dispar patient isolates to human complement. Parasite Immunology. 1997;19(6):265–271. doi: 10.1046/j.1365-3024.1997.d01-208.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramírez G, Valck C, Ferreira VP, López N, Ferreira A. Extracellular Trypanosoma cruzi calreticulin in the host-parasite interplay. Trends in Parasitology. 2011;27(3):115–122. doi: 10.1016/j.pt.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Ramírez G, Valck C, Molina MC, et al. Entamoeba histolytica calreticulin: a novel virulence factor that binds complement C1 on the parasite surface and promotes infectivity. Immunobiology. 2011;216(1-2):265–273. doi: 10.1016/j.imbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Girard-Misguich F, Sachse M, Santi-Rocca J, Guillén N. The endoplasmic reticulum chaperone calreticulin is recruited to the uropod during capping of surface receptors in Entamoeba histolytica . Molecular and Biochemical Parasitology. 2008;157(2):236–240. doi: 10.1016/j.molbiopara.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Marks DJB, Seymour CR, Sewell GW, et al. Inflammatory bowel diseases in patients with adaptive and complement immunodeficiency disorders. Inflammatory Bowel Diseases. 2010;16(11):1984–1992. doi: 10.1002/ibd.21280. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira JE, Heron BT, Huston CD. C1q- and collectin-dependent phagocytosis of apoptotic host cells by the intestinal protozoan Entamoeba histolytica . Journal of Infectious Diseases. 2008;198(7):1062–1070. doi: 10.1086/591628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marquay Markiewicz J, Syan S, Hon C-C, Weber C, Faust D, Guillen N. A proteomic and cellular analysis of uropods in the pathogen Entamoeba histolytica . PLoS Neglected Tropical Diseases. 2011;5(4):p. e1002. doi: 10.1371/journal.pntd.0001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this section can you see, the negative control and secondary antibody of confocal microscopy for colocalization of EhCRT and C1q in trophozoites of E. histolytica (figure 5-A). The negative control and secondary antibody of representative tissue slides of immunohistochemical detection of EhCRT and C1q in amebic liver abscess sections of livers from hamster inoculated whit E. histolytica virulent (figure 8-A).


