Abstract
Background. A time dependent loss of dopaminergic neurons and the formation of intracellular aggregates of alpha synuclein have been reported in PD model flies. Methods. The progeny (PD flies) expressing human alpha synuclein was exposed to 25, 50, and 100 µM of curcumin mixed in the diet for 24 days. The effect of curcumin was studied on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of PD model flies. The activity of PD model flies was monitored by using Drosophila activity monitors (DAMs). For the estimation of oxidative stress, lipid peroxidation and protein carbonyl content were estimated in the flies brains of each treated groups. The cell death in Drosophila brain was analyzed by isolating brains in Ringer's solution placing them in 70% ethanol and stained in acridine orange to calculate the gray scale values. Results. The exposure of flies to 25, 50, and 100 µM of curcumin showed a dose dependent significant delay in the loss of activity pattern, reduction in the oxidative stress and apoptosis, and increase in the life span of PD model flies. Conclusion. Curcumin is potent in reducing PD symptoms.
1. Introduction
Parkinson's disease (PD) has been classified as a movement disorder and is characterized by the loss of dopaminergic neurons in substantia nigra [1]. The abnormal expression of alpha synuclein (αS) results in the formation of Lewy bodies: a pathological hallmark of PD [2]. The availability of various experimental models for PD, based on αS overexpression (mutant or wild form) in flies or mice, has led research scientists to study the effects of various compounds on the progression of PD symptoms [3]. Oxidative stress has been attributed as one of the important factors in progression of PD [4]. An emphasis has been given for the use of flavonoids to reduce the oxidative stress in the neurons [5–7]. Curcumin is the principal curcuminoid of the spice turmeric (Curcuma longa), a member of the ginger family [8]. Besides having a number of pharmacological properties [9], in our earlier study it was reported to delay the loss of climbing ability in the PD model flies [10]. In the present study, the effect of curcumin was studied on the life span, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of PD.
2. Materials and Methods
2.1. Drosophila Stocks
Transgenic fly lines that expresses wild-type human synuclein (h-αS) under UAS control in neurons “w[∗];P{w[+mC] = UAS-Hsap/SNCA.F}”5B and GAL4“w[∗];P{w[+mC] = GAL4-elavL}”3 were obtained from Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN). When the males of UAS (Upstream Activation Sequence)-Hsap/SNCA.F strains are crossed with the females of GAL4-elav. L (vice versa) the progeny will express the human alpha synuclein in the neurons [1].
2.2. Drosophila Culture and Crosses
The flies were cultured on standard Drosophila food containing 0.83% agar, 4.72% corn meal, 4.16% sugar, and 1.67% yeast at 25°C (24 ± 1) [11]. Crosses were set up as described in earlier published work [12]. The PD flies were exposed separately to different doses of curcumin (Sigma Aldrich, CAS 458-37-7) and mixed in culture medium at final concentration of 25, 50, and 100 μM. The PD flies were also exposed to 10−3 M of L-dopamine. The UAS-Hsap/SNC.F acts as a control. The control flies were also separately exposed to the selected doses of curcumin.
2.3. Activity Pattern
From the 12th day onwards, the activity of flies (males) in all treated groups was analysed by using Drosophila Activity Monitor (TriTek, USA). The activity was recorded every hour for a total of 267 hrs and the data was analysed by Actogram J software. The results were presented as a chi-square periodogram [13, 14].
2.4. Lifespan Determination
For the determination of lifespan the newly enclosed male flies (control and PD) were placed in culture tubes (10 flies per tube) containing 25, 50, and 100 μM of curcumin mixed in diet. The flies were transferred to new diet after every 3rd day and the number of dead flies were recorded at 3-day interval until the last one died [7].
2.5. Lipid Peroxidation Assay
Lipid peroxidation assay in the brain homogenate was performed according to the procedure described by Siddique et al. [15]. Reagent 1 (R1) was prepared by dissolving 0.064 g of 1-methyl-2-phenylindole into 30 mL of acetonitrile to which 10 mL of methanol was added to bring the volume to 40 mL. The preparation of 37% HCl served as the reagent R2. The brains of flies were isolated under stereo zoom microscope in ice cold Tris HCl (20 mM) (10 brains/group; five replicates/group). Homogenate was prepared in Tris HCl and centrifuged at 3000 g for 20 min and subsequently the supernatant was collected. In a microcentrifuge tube 1300 μL of R1 was taken. A volume of 1 μL (supernatant) was added along with 300 μL of R2 vortexed and incubated at 45°C for 40 min. After incubation, the tubes were cooled in ice and centrifuged at 15,000 g for 10 min at 4°C and read at 586 nm.
2.6. Estimation of Protein Carbonyl Content
The protein carbonyl content was estimated according to the protocol described by Hawkins et al. [16]. The brain homogenate was diluted to a protein concentration of approx 1 mg/mL. About 250 μL of each diluted homogenate was taken in eppendorf centrifuge tubes separately. To it 250 μL of 10 mM 2,4-dinitrophenyl hydrazine (dissolved in 2.5 M HCl) was added, vortexed, and kept in dark for 20 min. About 125 μL of 50% (w/v) trichloroacetic acid (TCA) was added, mixed thoroughly, and incubated at −20°C for 15 min. The tubes were then centrifuged at 4°C for 10 min at 9000 rpm. The supernatant was discarded and the pellet obtained was washed twice by ice cold ethanol : ethyl acetate (1 : 1). Finally, the pellets were redissolved in 1 mL of 6 M guanidine hydrochloride and the absorbance was read at 370 nm.
2.7. Analysis of Cell Death in Drosophila Brain
The cell death in Drosophila brain was analyzed as per the method described by Mitchell and Staveley [17]. Flies (5 flies/treatment; 5 replicates/group) were placed in 70% ethanol in a 2 mL microcentrifuge tube for a minute. The brains were isolated in Ringer's solution under stereo zoom microscope. After removing the Ringer's solution about 100 μL of freshly prepared acridine orange (5 μg/mL) was added for 5 minutes. The brain was rinsed by Ringer's solution, immediately viewed, and photographed through fluorescent microscope (Optika, Italy). The image analysis program Image J (available online at http://rsb.info.nih.gov/ij/) was used to analyze the gray scale values for each brain.
2.8. Statistical Analysis
The statistical analysis was done using Statistica Soft Inc. The mean values of various fly groups were statistically compared using Student's t-test.
3. Results
The data collected for the male flies by Drosophila activity monitor (DAM) was analysed by chi-square periodogram. For control flies the number of peaks (significant) were more (Figures S1 (a) and (b)) compared to PD flies (Figures S2 (a) and (b)). A dose dependent significant delay in the loss of activity pattern was observed in the PD flies exposed to 25, 50, and 100 μM of curcumin (Figures S3–5, (a) and (b)). No change in the activity pattern of control flies exposed to 25, 50, and 100 μM of curcumin was observed (Figures S6–8, (a) and (b)). The PD flies exposed to 10−3 M of dopamine also showed a delay in the loss of activity (Figures S9 (a) and (b)) as compared to PD flies. The results obtained for the survival rate are shown in Figure 1. The survival rate was measured only in male flies. As is evident from Figure 1 the PD flies exposed to 25, 50, and 100 μM of curcumin showed a dose dependent significant increase in the life span as compared to unexposed PD flies. The control flies showed a life span of about 60 days. The results obtained for the estimation of lipid peroxidation are shown in Figure 2. The PD flies exposed to 25, 50, and 100 μM of curcumin showed a dose dependent significant decrease in the lipid peroxidation as compared to unexposed PD flies and control flies (Figure 2). The results obtained for protein carbonyl content are shown in Figure 3. A dose dependent significant decrease in the mean absorbance values was obtained in PD flies exposed to 25, 50, and 100 μM of curcumin as compared to unexposed PD and control flies (Figure 3). The unexposed PD flies showed the highest mean absorbance value as compared to control flies (Figure 3). The results obtained for cell death in the brains of PD flies was calculated as mean gray scale values shown in Figure 4. A significant dose dependent decrease in the mean gray scale values was obtained for the PD flies exposed to 25, 50, and 100 μM of curcumin as compared to unexposed PD and control flies (Figure 4). The PD flies exposed to 10−3 M of dopamine also showed a significant decrease in the mean gray scale value as compared to the unexposed PD flies and control flies (Figure 4).
Figure 1.
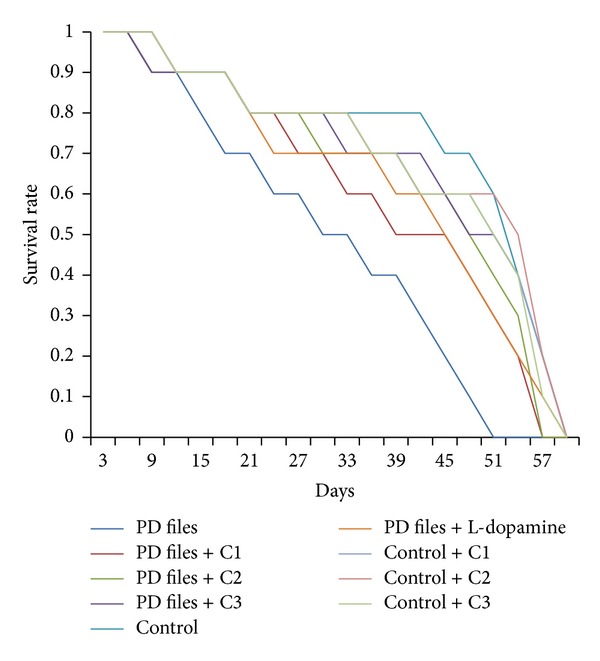
Effect of curcumin on survival rate measured in transgenic Drosophila melanogaster in various treated groups. (C1 = 25 μM curcumin; C2 = 50 μM curcumin; C3 = 100 μM curcumin).
Figure 2.
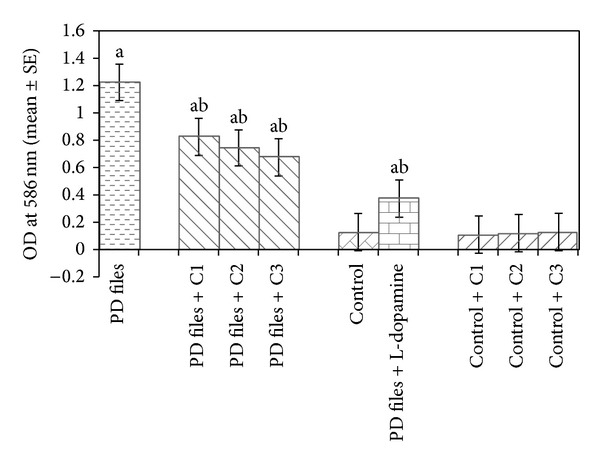
Effect of curcumin on lipid peroxidation measured in the brains of transgenic Drosophila melanogaster after 24 days of the exposure in treated groups. (C1 = 25 μM curcumin; C2 = 50 μM curcumin; C3 = 100 μM curcumin; asignificant with respect to control, P < 0.05; bsignificant with respect to PD model flies, P < 0.05).
Figure 3.
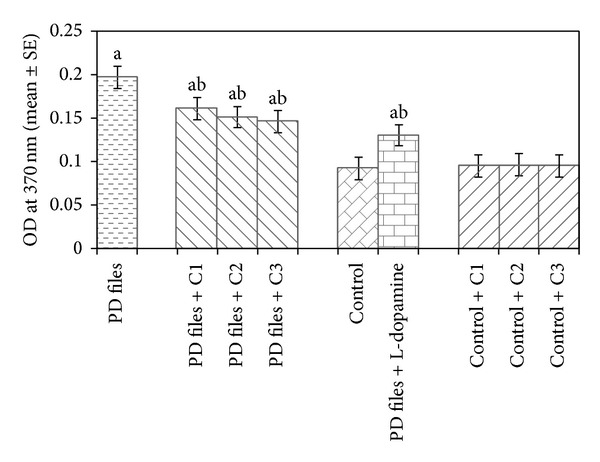
Effect of curcumin on protein carbonyl content measured in the brains of transgenic Drosophila melanogaster after 24 days of the exposure in various treated groups. (C1 = 25 μM curcumin; C2 = 50 μM curcumin; C3 = 100 μM curcumin; asignificant with respect to control, P < 0.05; bsignificant with respect to PD model flies, P < 0.05).
Figure 4.
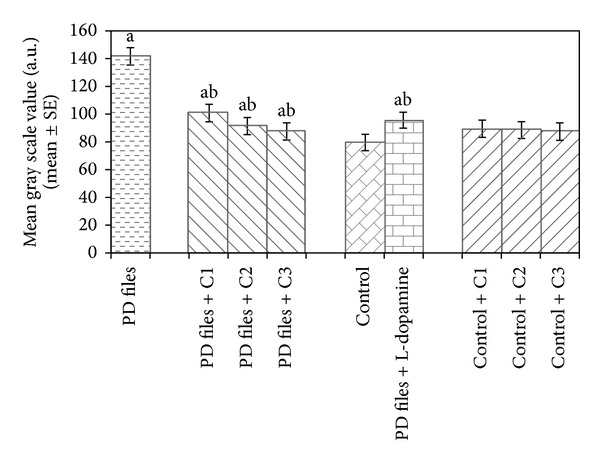
Effect of curcumin on mean gray scale value measured in the brains of transgenic Drosophila melanogaster after 24 days of the exposure in various treated groups. (C1 = 25 μM curcumin; C2 = 50 μM curcumin; C3 = 100 μM curcumin; asignificant with respect to control, P < 0.05; bsignificant with respect to PD model flies, P < 0.05).
4. Discussion
The results of the present study reveal that the exposure of PD flies to 25, 50, and 100 μM of curcumin showed a dose dependent significant delay in the loss of activity pattern, reduction in lipid peroxidation, protein carbonyl content, apoptosis, and increase in the life span. Oxidative stress as a result of the accumulation of alpha synuclein has been reported in neurons of PD model flies [18]. It remains still unclear that the degenerating neuron itself or misfolded proteins directly causes toxicity during the progression of PD [19, 20]. In our earlier studies with the same fly models, various plant extracts and flavonoids have been reported to delay the loss of climbing activity and reduced oxidative stress [21–25]. Flavonoids have been reported to show improvements in cognition function possibly by protecting vulnerable neurons or by stimulating neuronal regeneration [26, 27]. In our present study, treatment of curcumin has shown reduction in lipid peroxidation and protein carbonyl content in the brains of PD model flies. This protection is attributed to an antioxidant nature of curcumin [28, 29]. Recent findings have suggested that flavonoids have a remodelling effect on the nature of α-synuclein fibrils, converting them into nontoxic, smaller amorphous aggregates, thus preventing the formation of reactive oxygen species [30]. On the other hand, an antioxidant nature of the curcumin is attributed to its unique conjugated structure that includes two methoxylated phenols [31]. It has been reported to inhibit the generation of reactive oxygen species (ROS) responsible for DNA and membrane damage [32]. Although the animals are well acquainted with the self-defense mechanism, an enhancement in stress beyond the capacity of an animal to cope up may result in cellular damage leading to the cell death [33]. The exposure of PD flies to curcumin showed a dose dependent decrease in the mean gray scale values, thus confirming an antiapoptotic activity of curcumin [34]. In earlier studies, curcumin has shown the neuroprotection in the 6-OHD model PD due to its antioxidant potential and its capability to penetrate into the brain [35]. It has been reported to alleviate αS-induced toxicity, reduce ROS level, and protect cell against apoptosis [36]. The aggregation of αS in the brain has been implicated as a crucial step in the formation of Lewy bodies and curcumin has antifibrillogenic and fibril-destabilizing properties, thus inhibiting the formation of alpha synuclein fibrils [37, 38]. In Drosophila curcumin have been reported to extend life span in a gender and genotype specific manner [39, 40]. In our present study, the life span and pattern activity were studied on male PD flies. There are reports on the life span extension of curcumin in mice [41] and C. elegans [42]. This extension is due to the neuroprotective and antiageing properties of curcumin [43]. The current pharmacotherapeutic approaches for PD involve improvement in striatal dopamine. The therapies involving natural antioxidants/plant products may be used as adjunct therapy [44]. The results obtained in our present study and our earlier study, in which the alginate-curcumin nanocomposite was studied using the same PD fly strain, results in neuroprotective effects [45]. Tetrahydrocurcumin has been reported to extend the life span of Drosophila and reduce the oxidative stress by regulating O-type forkhead domain transcription factor (FOXO) [46]. Despite having apoptotic properties in various cancerous cell lines there are reports of having antiapoptotic properties of curcumin that corroborate with the findings of our study [47–51]. Cancer cells can accumulate higher intercellular, cellular concentrations of vitamins/antioxidants than normal cells due to loss of homeostatic controls. The high concentration of the antioxidants can alter cancer cell metabolisms and cell signaling [52]. The present study was carried out using Drosophila as a model of PD expressing human wild type α-synuclein in neuron of fly and consequent locomotor dysfunction [1]. The present fly model mimics the motor impairments associated with PD and can be used to study whether or not a variety of compounds or drugs mixed in the fly culture medium have the neuroprotective potential [53].
Supplementary Material
Chi-square periodogram for the control and treated groups.
Acknowledgments
The Grant (no. F.30-1/2013(SA-II)/RA-2012-14-GE-UTT-858), received as UGC Research Award (2012-14) from the University Grants Commission, New Delhi, to Dr. Yasir Hasan Siddique, is thankfully acknowledged. The authors are thankful to the Chairman, Department of Zoology for providing the laboratory facilities. The flies for the experiments were purchased from Bloomington Drosophila Stock Centre, Department of Biology, Indiana University, Bloomington, IN, USA.
Conflict of Interests
The authors declare that they have no conflict of interests concerning this paper.
References
- 1.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 2.Uversky VN, Li J, Souillac P, et al. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of α-synuclein assembly by β- and γ-synucleins. Journal of Biological Chemistry. 2002;277(14):11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 3.Celotto AM, Palladino MJ. Drosophila: a “model” model system to study neurodegeneration. Molecular Interventions. 2005;5(5):292–303. doi: 10.1124/mi.5.5.9. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz-Soriano V, Paricio N. Drosophila models of Parkinson’s disease: discovering relevant pathways and novel therapeutic strategies. Parkinson’s Disease. 2011;2011:14 pages. doi: 10.4061/2011/520640.520640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naoi M, Maruyama W. Future of neuroprotection in Parkinson’s disease. Parkinsonism and Related Disorders. 2001;8(2):139–145. doi: 10.1016/s1353-8020(01)00028-1. [DOI] [PubMed] [Google Scholar]
- 6.Lü J-M, Nurko J, Weakley SM, et al. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Medical Science Monitor. 2010;16(5):RA93–RA100. [PMC free article] [PubMed] [Google Scholar]
- 7.Long J, Gao H, Sun L, Liu J, Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila parkinson’s disease model. Rejuvenation Research. 2009;12(5):321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- 8.Stagos D, Amougias G, Matakos A, Spyrou A, Tsatsakis AM, Kouretas D. Chemoprevention of liver cancer by plant polyphenols. Food and Chemical Toxicology. 2012;50(6):2155–2170. doi: 10.1016/j.fct.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: biological actions and medicinal applications. Current Science. 2004;87(1):44–53. [Google Scholar]
- 10.Siddique YH, Ara G, Jyoti S, Afzal M. Protective effect of curcumin in transgenic Drosophila melanogaster model of Parkinson’s disease. Alternative Medicine Studies. 2012;2, article e3:7–9. [Google Scholar]
- 11.Siddique YH, Ara G, Jyoti S, Afzal M. The dietary supplementation of nordihydroguaiaretic acid (NDGA) delayed the loss of climbing ability in Drosophila model of Parkinson’s disease. Journal of Dietary Supplements. 2012;9(1):1–8. doi: 10.3109/19390211.2011.630716. [DOI] [PubMed] [Google Scholar]
- 12.Siddique YH, Naz F, Jyoti S, Afzal M. Protective effect of apigenin in transgenic Drosophila melanogaster model of parkinson’s disease. Pharmacologyonline. 2011;3:790–795. [Google Scholar]
- 13.Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila . Journal of Visualized Experiments. 2010;(43) doi: 10.3791/2157.e2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosato E, Kyriacou CP. Analysis of locomotor activity rhythms in Drosophila . Nature Protocols. 2006;1(2):559–568. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- 15.Siddique YH, Mujtaba SF, Jyoti S, Naz F. GC-MS analysis of Eucalyptus citriodora leaf extract and its role on the dietary supplementation in transgenic Drosophila model of Parkinson’s disease. Food and Chemical Toxicology. 2013;55:29–35. doi: 10.1016/j.fct.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radical Biology and Medicine. 2009;46(8):965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell KJ, Staveley BE. Protocol for the detection and analysis of cell death in the adult Drosophila brain. Drosophila Information Services. 2006;93:118–121. [Google Scholar]
- 18.Giasson BI, Ischiropoulos H, Lee VM-Y, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radical Biology and Medicine. 2002;32(12):1264–1275. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- 19.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 20.Abbott RA, Cox M, Markus H, Tomkins A. Diet, body size and micronutrient status in Parkinson’s disease. European Journal of Clinical Nutrition. 1992;46(12):879–884. [PubMed] [Google Scholar]
- 21.Ray B, Bisht S, Maitra A, Maitra A, Lahiri DK. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc) in the neuronal cell culture and animal model: implications for Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;23(1):61–77. doi: 10.3233/JAD-2010-101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan S, Jyoti S, Naz F, et al. Effect of L-ascorbic acid on the climbing ability and protein levels in the brain of Drosophila model of Parkinson’s disease. International Journal of Neurosciences. 2012;122:704–709. doi: 10.3109/00207454.2012.709893. [DOI] [PubMed] [Google Scholar]
- 23.Siddique YH, Jyoti S, Naz F. Effect of epicatechin gallate dietary supplementation on transgenic Drosophila model of Parkinson’s disease. Journal of Dietary Supplements. 2014;11(2):121–130. doi: 10.3109/19390211.2013.859207. [DOI] [PubMed] [Google Scholar]
- 24.Siddique YH, Ara G, Jyoti S, Afzal M. Effect of capsaicin on the climbing ability in Drosophila model of Parkinson’s disease. The American Journal of Drug Discovery and Development. 2012;2(1):50–54. [Google Scholar]
- 25.Pal R, Miranda M, Narayan M. Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochemical and Biophysical Research Communications. 2011;404(1):324–329. doi: 10.1016/j.bbrc.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radical Biology and Medicine. 2001;30(6):583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 27.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes and Nutrition. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y. Protective effects of curcumin against rotenone and salsolinnol-induced toxicity: implications for Parkinson’s disease. Neurotoxicity Research. 2014;25:81–89. doi: 10.1007/s12640-013-9433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad SN, Muralidhara Neuroprotective effect of geraniol and curcumin in an acrylamide model of neurotoxicity in Drosophila melanogaster. Relevance to neuropathy. Journal of Insect Physiology. 2014;60:7–16. doi: 10.1016/j.jinsphys.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Bieschke J, Russ J, Friedrich RP, et al. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(17):7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda T, Maekawa T, Hidaka K, Bando H, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: analysis of oxidative coupling products from curcumin and linoleate. Journal of Agricultural and Food Chemistry. 2001;49(5):2539–2547. doi: 10.1021/jf001442x. [DOI] [PubMed] [Google Scholar]
- 32.Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochimica et Biophysica Acta. 1994;1224(2):255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 33.Ryter SW, Kim HP, Hoetzel A, et al. Mechanism of cell death in oxidative stress. Anti-Oxidant Radical Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 34.Chan W-H, Wu H-J. Anti-apoptotic effects of curcumin on photosensitized human epidermal carcinoma A431 cells. Journal of Cellular Biochemistry. 2004;92(1):200–212. doi: 10.1002/jcb.20059. [DOI] [PubMed] [Google Scholar]
- 35.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radical Research. 2005;39(10):1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 36.Wang MS, Boddapati S, Emadi S, Sierks MR. Curcumin reduces α-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neuroscience. 2010;11, article 57 doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for α-synuclein fibrils in vitro . Journal of Neurochemistry. 2006;97(1):105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 38.Gadad BS, Subramanya PK, Pullabhatla S, Shantharam IS, Rao KS. Curcumin-glucoside, a novel synthetic derivative of curcumin, inhibits α-Synuclein oligomer formation: relevance to Parkinson’s disease. Current Pharmaceutical Design. 2012;18(1):76–84. doi: 10.2174/138161212798919093. [DOI] [PubMed] [Google Scholar]
- 39.Lee K-S, Lee B-S, Semnani S, et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster . Rejuvenation Research. 2010;13(5):561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 40.Soh JW, Marowsky N, Nichols TJ, et al. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila . Experimental Gerontology. 2013;48:299–239. doi: 10.1016/j.exger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Strong R, Miller RA, Astle CM, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid and medium-chain triglyceride oil on life span of genetically heterogenous mice. The Journals of Gerontology A, Biological Sciences and Medical Sciences. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao VH-C, Yu C-W, Chu Y-J, Li W-H, Hsieh Y-C, Wang T-T. Curcumin-mediated lifespan extension in Caenorhabditis elegans . Mechanisms of Ageing and Development. 2011;132(10):480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Bala K, Tripathy BC, Sharma D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology. 2006;7(2):81–89. doi: 10.1007/s10522-006-6495-x. [DOI] [PubMed] [Google Scholar]
- 44.Mythri RB, Srinivas Bharath MM. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Current Pharmaceutical Design. 2012;18(1):91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 45.Siddique YH, Khan W, Singh BR, Naqvi AH. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic Drosophila model of parkinson’s disease. ISRN Pharmocology. 2013;2013:8 pages. doi: 10.1155/2013/794582.794582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang L, Nakamura Y, Lim Y-M, et al. Tetrahydrocurcumin extends life span and inhibits the oxidative stress response by regulating the FOXO forkhead transcription factor. Aging. 2011;3(11):1098–1109. doi: 10.18632/aging.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J, Yu S, Zheng W, et al. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochemical Research. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Molecular Pharmacology. 2006;69(1):195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 49.Aktas C, Kanter M, Erboga M, Ozturk S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicology and Industrial Health. 2012;28(2):122–130. doi: 10.1177/0748233711407242. [DOI] [PubMed] [Google Scholar]
- 50.Toydemir T, Kanter M, Erboga M, Oguz S, Erenoglu C. Antioxidative, antiapoptotic, and proliferative effect of curcumin on liver regeneration after partial hepatectomy in rats. Journal of Toxicology and Industrial Health. 2013 doi: 10.1177/0748233712469658. [DOI] [PubMed] [Google Scholar]
- 51.Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochemical Pharmacology. 2008;76(11):1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Prasad KN, Hernandez C, Edwards-Prasad J, Nelson J, Borus T, Robinson WA. Modification of the effect of tamoxifen, cis-platin, DTIC, and interferon-α2b on human melanoma cells in culture by a mixture of vitamins. Nutrition and Cancer. 1994;22(3):233–245. doi: 10.1080/01635589409514349. [DOI] [PubMed] [Google Scholar]
- 53.Pendleton RG, Parvez F, Sayed M, Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson’s disease in Drosophila melanogaster . Journal of Pharmacology and Experimental Therapeutics. 2002;300(1):91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chi-square periodogram for the control and treated groups.


