Abstract
Vascular dementia is caused by various factors, including increased age, diabetes, hypertension, atherosclerosis, and stroke. Adiponectin is an adipokine secreted by adipose tissue. Adiponectin is widely known as a regulating factor related to cardiovascular disease and diabetes. Adiponectin plasma levels decrease with age. Decreased adiponectin increases the risk of cardiovascular disease and diabetes. Adiponectin improves hypertension and atherosclerosis by acting as a vasodilator and antiatherogenic factor. Moreover, adiponectin is involved in cognitive dysfunction via modulation of insulin signal transduction in the brain. Case-control studies demonstrate the association between low adiponectin and increased risk of stroke, hypertension, and diabetes. This review summarizes the recent findings on the association between risk factors for vascular dementia and adiponectin. To emphasize this relationship, we will discuss the importance of research regarding the role of adiponectin in vascular dementia.
1. Introduction
Vascular dementia is the second most common type of dementia, accounting for 15 to 20% of all cases of dementia [1]. It is characterized by cognitive impairment and cerebrovascular pathologies [2]. According to the World Alzheimer Report 2011, an estimated 36 million people worldwide were afflicted with dementia. This number is increasing twofold every 20 years and will likely reach 115 million people by 2050 [3, 4]. Among the subtypes of dementia, vascular dementia is important because it results from a variety of causes, including cerebrovascular dysfunction. Vascular dementia and cerebrovascular diseases have common risk factors including hypertension, insulin resistance, diabetes, obesity, hyperhomocystinemia, and hyperlipidemia [5–8]. Recent clinical-pathological studies have focused on cognitive impairment and increased risk of dementia in patients with cerebrovascular disease [2, 9, 10]. In addition, vascular dementia is the most severe form of vascular cognitive impairment (VCI) [2, 11], and it results from subclinical vascular brain injury and stroke. VCI reflects the full range of cognitive alterations due to vascular factors [12]. A previous study demonstrates that reducing vascular risk factors inhibits cognitive decline progression [12]. Type 2 diabetes mellitus (T2DM), a risk factor for vascular dementia, is a heterogeneous metabolic disease characterized by reduced insulin sensitivity and relative insulin deficiency. T2DM and dyslipidemia frequently coexist with vascular dementia [13]. Adiponectin is almost exclusively secreted by adipocytes, and it appears to act as a modulator of anti-inflammation and insulin-sensitizer [14]. Adiponectin has beneficial effects on endothelial cells and affects the progression of stroke, atherosclerosis, and hypertension [15–20]. Plasma adiponectin levels are decreased in patients with cardiovascular disease and several metabolic disorders [21]. Several studies reported an inverse relationship between plasma adiponectin and T2DM [22–26]. In this review, we examine current research regarding the relationship between risk factors for vascular dementia and adiponectin.
2. Risk Factors for Vascular Dementia
Vascular dementia is regarded as the most severe form of VCI characterized by the presence of clinical stroke or vascular brain injury as well as cognitive impairment [2, 11, 27]. Several studies suggest that the risk factors for vascular dementia are almost identical as the risk factors for VCI. Common risk factors in both animal models and humans include hypertension, insulin resistance, hyperlipidemia, hyperhomocystinemia, atherosclerosis, and diabetes [28–32]. Age is also a risk factor for vascular dementia, suggesting that dementia in patients after the age of 65 increased gradually [33]. In addition, cerebrovascular dysfunction is a risk factor because the cerebrovascular function is reduced in patients with dementia [34–42]. In addition, another study suggests that metabolic syndrome, including insulin resistance, hypertension, and dyslipidemia, is associated with cognitive decline, a typical feature of vascular dementia [30]. Figure 1 shows that vascular dementia risk factors include aging, diabetes, hypertension, atherosclerosis, and stroke (Figure 1).
Figure 1.
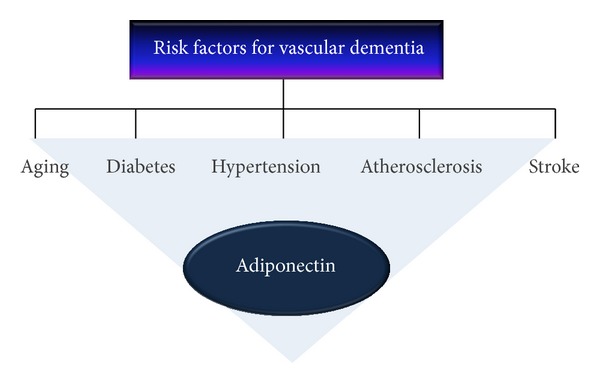
Risk factors for vascular dementia and adiponectin. Vascular dementia risk factors include aging, diabetes, hypertension, atherosclerosis, and stroke. Adiponectin is related to aging, diabetes, hypertension, atherosclerosis, and stroke by acting as a modulator or regulator in various mechanisms. Current researches have reported the role of adiponectin in diabetes, hypertension, atherosclerosis, and stroke.
3. Adiponectin
Adiponectin is one of the most abundant adipokines [43, 44]. It has significant sequence similarities with complement factor C1q, whose protein is termed Acrp30 because it is a 30 kDa adipocyte complement-related protein [45, 46]. Adiponectin is the protein produced by adipose's most abundant gene transcript 1 (APM1) gene, and APM1 gene is located on chromosome 3q27, a region associated with T2DM and metabolic syndrome susceptibility [47–50]. Several human genetic association studies emphasized that hypoadiponectinemia caused by the single nucleotides polymorphisms (SNPs) in APM1 gene is important to investigate the role of adiponectin in a variety of diseases [14, 51–54] including insulin resistance, T2DM, and metabolic syndrome, such as obesity [55]. In white French subjects, 2 SNPs in the promoter region of APM1 gene, SNP 11377 and SNP 11391, were strongly related to hypoadiponectinemia and T2DM [50]. In white German and North American subjects, the +276 G/T SNP was associated with obesity and insulin resistance [52, 56]. In Chinese subjects, the +276 G/T SNP was significantly involved in the coronary heart disease [57]. Adiponectin acts via binding its receptors, adiponectin receptor type 1 (AdipoR1) and type 2 (AdipoR2). AdipoR1 has a higher binding affinity to the globular form of adiponectin, whereas AdipoR2 has a higher binding affinity to full-length adiponectin [58]. Adiponectin binds to the C-terminal extracellular domain of AdipoR, and the N-terminal cytoplasmic domain interacts with APPL1 [59]. Adiponectin receptors are expressed in liver, hypothalamus, and brain vascular endothelial cells [60–62]. Adiponectin is associated with insulin resistance, obesity, T2DM, dyslipidemia, and cardiovascular diseases [63–70]. It is an effective insulin sensitizer [64, 71, 72], and it promotes peripheral insulin sensitivity [14] and inhibits liver gluconeogenesis [73]. Decreases in circulating adiponectin in the prediabetic state lead to insulin resistance [74]. Adiponectin activates AMP-activated protein kinase (AMPK), which activates insulin-independent glucose uptake by muscle, downregulates gluconeogenic enzymes, and increases muscle fatty acid oxidation [73]. Unlike other adipocyte-derived hormones, adiponectin gene expression and plasma concentration are inversely associated with body mass index (BMI) [75]. Reduced plasma adiponectin levels have also been reported in patients with coronary artery disease [19] as well as those with increased carotid intima media thickness [76]. Plasma adiponectin levels are inversely related to the platelet activation status of patients with cardiovascular risk factors [20]. Adiponectin suppresses platelet aggregation in hyperlipidemic rats by reversing the increase in inducible nitric oxide synthase expression while enhancing endothelial nitric oxide synthase activation [77, 78]. Current studies have reported the association between adiponectin and various diseases because adiponectin has multiple roles in glucose and lipid metabolisms and vascular system.
4. Adiponectin, Aging, and Diabetes
4.1. Aging, Insulin Signal Transduction, and Adipocytokines
Recently, the number of elderly patients with dementia has been increasing rapidly [79]. One epidemiology study suggests an exponential increase in the incidence of dementia after the age of 65, doubling roughly every 5 years, such that greater than 50% of centenarians are expected to suffer from dementia [33]. Aging induces an oxidative redox shift by attenuating mitochondrial metabolism and changing glycolysis metabolism [80]. These alterations initiate a damaging pathway involving signaling molecules, transcription factors, and epigenetic transcriptional regulators [80, 81]. Among several important pathways for maintaining longevity, insulin sensitivity has been considered a key factor for the healthy aging phenotype in humans [82, 83] and mice [84, 85]. Several studies have reported that insulin and insulin growth factor-1 (IGF-1) receptor regulate the lifespan of mice [86, 87]. In humans, growth hormone (GH) and IGF-1 deficiencies are also associated with life expectancy [88]. Insulin sensitivity normally decreases during aging, and the prevalence of metabolic syndrome (MetS) and insulin resistance substantially increases [89, 90]. In elderly persons, decreased insulin receptor (IR) levels and impaired insulin signaling have been observed predominantly in the hippocampus cortex and choroid plexus [81]. Impaired insulin receptor binding promotes chronic insulin resistance [91]. Muller et al. [92] reported that IGF-I signaling deteriorated in the brains of aged mice. This study demonstrated that activation of the brain IGF-1R/Akt/GSK-3β pathway was evidently reduced although older mice have higher brain IGF-1R levels [92]. In humans, insulin sensitivity decreases with aging and the prevalence of T2DM increases with advancing age [89, 90]. Reduced mitochondrial function contributes to decline in glucose uptake with advancing age and leads to insulin resistance [93–97]. IGF-1 concentrations decline with age and are associated with age-related changes in body composition by both increasing fat mass and decreasing muscle mass [98–100]. Aging alters the function and number of adipose cells which cause alterations in the secretion and function of the adipocytokines such as leptin and adiponectin [101]. A recent study demonstrated that cellular senescence of adipose tissue causes insulin resistance [102]. Considering these evidences, aging alters the function of adipose cells, and alteration in secretion of adipocytokines attenuates insulin sensitivity.
4.2. Adiponectin and Insulin Signal Transduction
Insulin and IRs are ubiquitously expressed in the brain [81, 103] where insulin can reach levels 10- to 100-fold greater than in plasma, particularly in the hippocampus, cortex, hypothalamus, olfactory bulb, and pituitary [81, 104]. IRs are largely localized in neurons and are less abundant in glia [103, 105]. Insulin produced by pancreatic β-cells is transported by cerebrospinal fluid (CSF) to the brain where it crosses the blood-brain barrier (BBB) [106, 107]. Similar to IRs, IGF-1Rs are widely distributed in the brain [107, 108]. Insulin/IGF-1-mediated activation of Akt leads to GSK-3β inactivation, which triggers multiple cascades, including synthesis of proteins involved in neuronal glucose metabolism and antiapoptotic mechanisms [104, 109]. Regarding brain glucose metabolism, recent studies suggest that changes in circulating insulin levels modulate glucose transporter (GLUT) expression [110, 111]. Cerebral IRs and IGF-1Rs are involved in cortical and hippocampal synaptic plasticity, thereby affecting memory and learning [105, 112]. In brain, insulin contributes to memory function through regulation of neurotransmitter receptors and synaptic function [113, 114]. Additionally, insulin signal transduction also promotes neurite outgrowth and axonal regeneration in the brain [105, 112, 115]. In the brain, insulin resistance results from perturbation of insulin signal transduction, causing systemic hyperglycemia. Decreased insulin and IGF-1 have been observed in Alzheimer's disease brain [116, 117]. Also, decreased insulin receptor substrate (IRS) protein levels related to insulin resistance [118] are associated with cognitive decline in dementia [119]. Impaired insulin transduction aggravates features of Alzheimer's disease including formation of neurofibrillary tangle caused by the decreasing brain glucose level and the increase of amyloid β aggregation [104, 106, 118, 120–122]. In addition, insulin resistance is closely linked with other metabolic symptoms, including hypertension and hyperlipidemia [123]. Adiponectin directly regulates glucose metabolism and insulin sensitivity. Adiponectin, via activation of AMPK and adiponectin, stimulates GLUT4 translocation and glucose uptake [124]. Adiponectin receptors activate AMPK, PPAR-α, and p38 MAPK to increase insulin sensitivity [58, 125]. An adaptor protein, APPL1, binds to adiponectin receptors that activate the AMPK and p38 MAPK pathways [126]. In addition, adiponectin decreases insulin resistance by decreasing triglyceride content in obese mice [127]. Increased tissue triglyceride content has been reported to interfere with insulin-stimulated phosphatidylinositol (PI) 3-kinase activation and subsequent GLUT 4 translocation and glucose uptake, thus leading to insulin resistance. Adipose tissue deficiency or lipodystrophy is associated with insulin resistance and metabolic dysregulation [128]. Adiponectin knockout mice show impaired insulin secretion, and intravenous adiponectin injection into C57BL/6 mice induces insulin secretion [129, 130]. AdipoR1 and 2 double knockout mice have increased triglyceride levels in the liver and exhibit insulin resistance and glucose intolerance, suggesting that AdipoR1 and AdipoR2 regulate lipid and glucose homeostasis [14, 131]. In conclusion, adiponectin and adiponectin receptors improve insulin resistance by modulating triglyceride level and impaired insulin signal transduction. Thus, regulation of adiponectin is important impaired insulin signal transduction to improve and also adiponectin may contribute to the improvement of cognitive decline in dementia.
4.3. Adiponectin, Diabetes, and Vascular Dementia
Diabetes characterized by reduced insulin sensitivity is associated with thrombosis, myocardial infarction, and cerebrovascular disease, which can lead to infarctions and white matter ischemia [132]. Macrovascular disease causes approximately 80% of mortality in patients with T2DM. The risk of vascular diseases in patients with T2DM is decreased by lowering the blood pressure of patients with hypertension [133–135]. In addition, diabetes and hypoglycemia are associated with cognitive impairment [136–138]. Yaffe et al., in a 4-year prospective study, suggested that older women with impaired fasting glucose levels performed poorly on cognitive tests compared to those with normal glycemia [139]. Considering these associations, diabetes may be regarded as a risk factor of vascular dementia. Adiponectin levels are elevated in type I diabetics compared with healthy controls [140]. Several studies have consistently found that increased adiponectin levels are associated with reduced risk for T2DM [22, 25, 26]. Hypoadiponectinemia has been considered an underlying mechanism of insulin resistance in T2DM [141–145]. In cross-sectional studies, plasma adiponectin concentrations were significantly lower in patients with diabetes [146]. In a 5-year follow-up study of 1096 nondiabetics, the association between adiponectin and T2DM was attenuated after adjustment for homeostatic model assessment of insulin resistance (HOMA-IR) and was eliminated after adjustment for insulin sensitivity. These data suggest that the antidiabetic effect of adiponectin is due to insulin sensitization [147]. Adiponectin predicts against diabetes onset, and diabetic patients always show lower plasma adiponectin levels compared to the general population [148]. Thus, adiponectin reduces the risk of diabetes by regulating insulin signal transduction and insulin resistance. Suppression of adiponectin aggravates diabetes as a risk for vascular dementia. Figure 2 shows that adiponectin stimulates the phosphorylation of AMPK and GLUT4 translocation and attenuates levels of triglyceride. As a result, adiponectin enhances glucose uptake and insulin sensitivity. This indicates that adiponectin reduces the risk of diabetes and vascular dementia (Figure 2).
Figure 2.
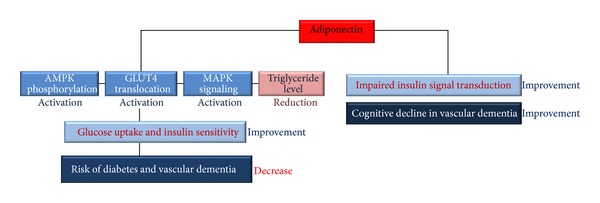
Adiponectin improves insulin sensitivity and reduces diabetes and vascular dementia risk. Adiponectin stimulates AMPK phosphorylation, GLUT4 translocation, and MAPK pathways. Adiponectin also reduces the levels of triglyceride. Consequentially, adiponectin increases glucose uptake and insulin sensitivity and reduces insulin resistance. In addition, adiponectin improves the cognitive decline by modulating impaired insulin signal transduction in vascular dementia brain. These mechanisms decrease the risk of diabetes and vascular dementia. Also, these mechanisms improve the memory dysfunction in dementia. AMPK: AMP-activated kinase, GLUT4: glucose transporter type 4, and MAPK: mitogen activated protein kinase.
5. Adiponectin, Hypertension, and Stroke
5.1. Adiponectin and Hypertension
Hypertension has been reported as the most common risk factor for stroke worldwide and has also been gradually recognized as a risk factor for dementia [149]. Arterial hypertension contributes to the development and progression of cerebrovascular disease [150]. Hypertension exposes the cerebral microvasculature to pulsatile pressure and flow that cause vascular endothelium and smooth muscle cell tearing [151]. Many cross-sectional and longitudinal studies have demonstrated that dementia and VCI are associated with hypertension [152–156]. Therefore, previous studies suggest that hypertension is the most important risk factor for cerebral vessel dysfunction, and it contributes to cognitive decline [157, 158]. Pulse pressure (PP), a marker of arterial stiffness, has been connected with the risk of cognitive decline [159] and AD [160, 161]. Elevated pulse pressure increases the risk of cognitive decline and impaired language abilities [162]. Decreased blood pressure (BP) is a clinical manifestation of dementia in elderly subjects [163, 164]. Endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) are crucial regulators of vascular homeostasis and, in particular, endothelial function [165, 166]. Endothelium-derived NO is a beneficial factor that promotes vasodilation and inhibits platelet aggregation, monocyte adhesion, and smooth muscle cell proliferation [167]. Adiponectin, acting via AdipoR1 and AdipoR2, promotes NO production through AMPK signaling pathway activation. AMPK activates eNOS through phosphorylation at Ser1177 and facilitates complex formation between eNOS and heat shock protein 90 (HSP-90), which is required for eNOS activation [167]. Adiponectin knockout mice have reduced endothelial NO levels in vessel walls [168]. Adiponectin inhibits the inflammatory response and causes vasodilatation largely through AMPK/eNOS [169–172]. Adiponectin-induced AMPK signaling promotes phosphatidylinositol 3-kinase-Akt signaling, leading to angiogenic growth factor synthesis [170, 173]. A recent study also suggests that adiponectin inhibits vascular endothelial growth factor- (VEGF-) induced ROS generation and has an antioxidant role in the vasculature [174]. These actions of adiponectin are also mediated via inhibition of growth factor-stimulated extracellular signal regulated kinase (ERK) signaling. In addition, several studies indicate that adiponectin plays a role in the regulation of microvascular network flow and function [175, 176]. Some clinical research demonstrates that plasma adiponectin levels are positively associated with arterial vasodilation [177]. Considering the role of adiponectin in vascular function, decreased adiponectin raises the risk of hypertension. Figure 3 shows that adiponectin increases AMPK phosphorylation and NO production. Platelet aggregation is decreased and vasodilation is increased due to NO production. Finally, adiponectin decreases the risk of hypertension and improves vascular cognitive impairment (Figure 3).
Figure 3.
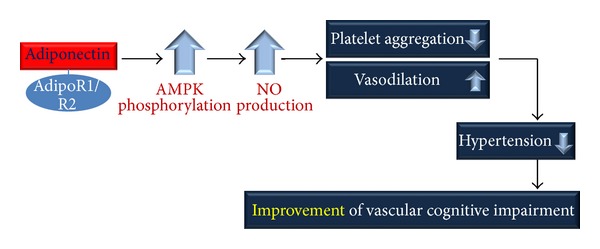
Adiponectin improves vascular cognitive impairment by stimulating NO production. Adiponectin, acting via AdipoR1 and AdipoR2, promotes AMPK phosphorylation and NO production. Increased NO reduces platelet aggregation and increases vasodilation. Consequentially, adiponectin decreases the risk of hypertension and improves vascular cognitive impairment. AMPK: AMP-activated kinase and NO: nitric oxide.
5.2. Adiponectin and Atherosclerosis
Atherosclerosis is a degenerative vessel disease that frequently affects large- to medium-sized arteries. In the brain, vessels of the circle of Willis are often involved [178]. Atherosclerotic plaques are prone to rupture with subsequent thrombosis [179, 180]. The thrombus resulting from plaque rupture leads to vessel occlusion or embolizes a smaller artery [181]. Atherosclerosis plaque rupture is related to inflammation, including secretion of cytokines and matrix-metalloproteinases, which are involved in vessel wall degradation [182–186]. Adiponectin plays the role of an antiatherogenic and anti-inflammatory modulator [18]. Several studies suggest that adiponectin inhibits many peptides and cytokines related to atherosclerosis progression [187]. Adiponectin inhibits monocyte adherence to TNF-α-stimulated endothelial cells by suppressing adhesion molecule expression [188, 189]. Adiponectin directly inhibits atherogenic molecules, such as intracellular adhesion molecule-1, vascular cellular adhesion molecule-1, and E-selectin, which are molecules associated with heightened leukocyte trafficking [189]. Adiponectin also attenuates expression of class A scavenger receptor in human macrophages and inhibits transformation of macrophages to foam cells [190]. The association between adiponectin and atherogenic factors indicates that regulation of adiponectin is important in atherosclerosis.
5.3. Adiponectin and Stroke
Several studies reported that stroke doubles the risk for dementia (poststroke dementia), and approximately 30% of stroke patients develop cognitive dysfunction within 3 years [191–194]. An association between stroke and dementia is also observed in patients younger than 50 years, and up to 50% of these patients exhibit cognitive deficits after a decade [195]. One of the first population-based studies to assess the relationship between stroke and dementia was conducted in Rochester, Minnesota [196]. Many stroke patients show gradual but continuous deterioration after a single-stroke lesion. This deterioration is characterized clinically by cognitive and behavioral dysfunction [197]. Several cross-sectional and retrospective case-control studies have reported an association between low adiponectin levels and increased stroke risk [16, 198–201]. In addition, adiponectin levels are associated with coronary heart disease such as coronary vascular disease [202, 203]. Several studies demonstrate that hypoadiponectinemia increases the prevalence of coronary vascular disease [189, 204]. Several studies demonstrated that adiponectin knockout (APN-KO) mice showed severe injuries during cerebral ischemia-reperfusion [205, 206], while adiponectin injected APN-KO mice were reduced pathological ischemia-induced damage [207]. Adiponectin blocks the interaction between the endothelial cells and leukocytes in ischemia-reperfusion and also inhibits the secondary inflammation in cerebral ischemia-reperfusion [208]. Adiponectin reduced the infarct size through nitric oxide synthase dependent mechanism in cerebral ischemic stroke mice model [209, 210]. In addition, adiponectin activates AMPK phosphorylation in cerebral ischemic stroke mice model [211]. Then, the activation of VEGF by the activated AMPK signaling promotes angiogenesis in cerebral ischemic brain [212, 213]. Considering the results of the above studies, adiponectin is associated with the risk of stroke and reduces cerebral ischemia induced damage. This may be due to the roles of adiponectin as an antiatherogenic modulator and a vasodilator in vascular system. Figure 4 shows that adiponectin decreases the expression of atherogenic molecules and plaque formation in blood vessels. Consequentially, adiponectin attenuates the risk of stroke and vascular dementia (Figure 4).
Figure 4.
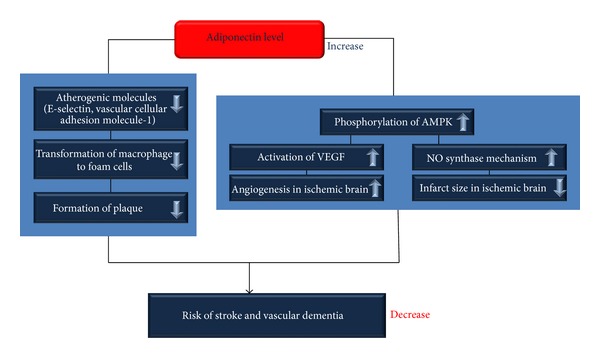
Adiponectin attenuates the risk of stroke and vascular dementia. Adiponectin decreases the expression of atherogenic molecules and formation of form cells in blood vessels. Adiponectin attenuates the risk of stroke by decreasing plaque formation in blood vessels. In addition, adiponectin binds with AdipoR1 and AdipoR2 and then activates the phosphorylation of AMPK. Increased AMPK phosphorylation promotes the activation of VEGF and NO synthase mechanism. As a result, adiponectin ameliorates angiogenesis in ischemic brain and reduces infarct size in ischemic brain. Consequentially, adiponectin decreases the risk of stroke and vascular dementia. AMPK: AMP-activated kinase, VEGF: vascular endothelial growth factor, and NO: nitric oxide.
6. Conclusions
Risk factors for vascular dementia include advanced age, diabetes, hypertension, atherosclerosis, and stroke. Adiponectin, an adipokine, acts as an antidiabetic and antiatherogenic regulator. Insulin sensitivity is a key cellular mechanism related to diabetes, cerebrovascular dysfunction, and cognitive decline. Adiponectin is involved in insulin sensitivity, and increased adiponectin levels improve impaired insulin signaling. Moreover, adiponectin affects the cerebrovascular function by stimulating NO production and inhibiting transformation of macrophages to foam cells. Specifically, we summarize the findings as follows.
Vascular dementia characterized by cognitive decline is associated with increased age because insulin receptors, which are related to cognitive function, decrease with age. Adiponectin is associated with age-related diseases, including cardiovascular disease and metabolic disease. Adiponectin is mediated via the activation of AMPK, and adiponectin stimulates GLUT4 translocation and glucose uptake. Moreover, binding between adiponectin and adiponectin receptors activates AMPK, PPAR-α, and p38 MAPK to increase insulin sensitivity. In addition, in clinical studies, an association between decreased adiponectin and diabetes was demonstrated. In conclusion, adiponectin improves impaired insulin signaling and improves cognitive decline as a typical feature of vascular dementia.
Vascular dementia characterized by cerebrovascular dysfunction is associated with hypertension, atherosclerosis, and stroke. Adiponectin stimulates NO production through the AMPK signaling pathway. Adiponectin also plays the role of an antiatherogenic modulator. Adiponectin inhibits atherogenic molecules and attenuates the transformation of macrophages to foam cells. In conclusion, adiponectin improves vascular dysfunction and alleviates the progression of hypertension, atherosclerosis, and stroke as risk factors for vascular dementia.
Taken together, adiponectin attenuates the risk of vascular dementia and ameliorates vascular dementia-related pathologies including cerebrovascular dysfunction and cognitive decline which resulted from impaired insulin transduction and neuroinflammation.
In this review, we summarized the current research regarding the association between risk factors for vascular dementia and adiponectin. Considering the relationship between adiponectin and risk factors for vascular dementia including aging, diabetes, hypertension, atherosclerosis, and stroke, we suggest that further studies are necessary to examine the role of adiponectin in vascular dementia. Moreover, we emphasize that the regulation of adiponectin levels and receptors of adiponectin would be important for the prevention and treatment of vascular dementia.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MEST) (2012-0005440). This work was also supported by the Brain Korea 21 Plus Project for Medical Science, Yonsei University.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MMB. Incidence of dementia: does gender make a difference? Neurobiology of Aging. 2001;22(4):575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wortmann M. Dementia: a global health priority—highlights from an ADI and World Health Organization report. Alzheimer's Research & Therapy. 2012;4(5, article 40) doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer's Association. 2012 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Archives of Neurology. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. The American Journal of Geriatric Pharmacotherapy. 2008;6(2):100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Honjo K, Black SE, Verhoeff NP. Alzheimer's disease, cerebrovascular disease, and the β-amyloid cascade. The Canadian Journal of Neurological Sciences. 2012;39(6):712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- 8.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident alzheimer disease: a systematic review of the literature. Alzheimer Disease and Associated Disorders. 2009;23(1):1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 10.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136(9):2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 12.Hachinski VC, Bowler JV, Loeb C, Roman GC, Tatemichi TK. Vascular dementia. Neurology. 1993;43(10):2159–2161. doi: 10.1212/wnl.43.10.2159-a. [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 14.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of Clinical Investigation. 2006;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimabukuro M, Higa N, Asahi T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. The Journal of Clinical Endocrinology & Metabolism. 2003;88(7):3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 16.Chen MP, Tsai JC, Chung FM, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(4):821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 17.Snijder MB, Heine RJ, Seidell JC, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women the hoorn study. Diabetes Care. 2006;29(11):2498–2503. doi: 10.2337/dc06-0952. [DOI] [PubMed] [Google Scholar]
- 18.Jansson P-A, Pellmé F, Hammarstedt A, et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. The FASEB Journal. 2003;17(11):1434–1440. doi: 10.1096/fj.02-1132com. [DOI] [PubMed] [Google Scholar]
- 19.Dunajska K, Milewicz A, Jȩdrzejuk D, et al. Plasma adiponectin concentration in relation to severity of coronary atherosclerosis and cardiovascular risk factors in middle-aged men. Endocrine. 2004;25(3):215–221. doi: 10.1385/endo:25:3:215. [DOI] [PubMed] [Google Scholar]
- 20.Shoji T, Koyama H, Fukumoto S, et al. Platelet activation is associated with hypoadiponectinemia and carotid atherosclerosis. Atherosclerosis. 2006;188(1):190–195. doi: 10.1016/j.atherosclerosis.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Saltevo J, Laakso M, Jokelainen J, Keinänen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Levels of adiponectin, C-reactive protein and interleukin-1 receptor antagonist are associated with insulin sensitivity: a population-based study. Diabetes/Metabolism Research and Reviews. 2008;24(5):378–383. doi: 10.1002/dmrr.831. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Lowe GDO, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30(5):1200–1205. doi: 10.2337/dc06-2416. [DOI] [PubMed] [Google Scholar]
- 23.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Annals of Internal Medicine. 2008;149(5):307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in a Canadian Aborigine population: the Sandy Lake Health and Diabetes Project. Diabetes Care. 2008;31(7):1410–1415. doi: 10.2337/dc08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabák AG, Brunner EJ, Miller MA, et al. Low serum adiponectin predicts 10-year risk of type 2 diabetes and hba1c independently of obesity, lipids, and inflammation: Whitehall II study. Hormone and Metabolic Research. 2009;41(8):626–629. doi: 10.1055/s-0029-1216359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Löwel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. Journal of the American College of Cardiology. 2006;48(7):1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Experimental Gerontology. 2012;47(11):816–824. doi: 10.1016/j.exger.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. Journal of Cardiovascular Pharmacology. 2010;55(4):308–316. doi: 10.1097/fjc.0b013e3181d89670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metabolism. 2008;7(6):476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faraci FM. Protecting against vascular disease in brain. American Journal of Physiology: Heart and Circulatory Physiology. 2011;300(5):H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. International Journal of Stroke. 2012;7(1):61–73. doi: 10.1111/j.1747-4949.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- 33.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71(5):337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 34.Claassen JAHR, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early alzheimer disease: a pilot study using transcranial doppler. Journal of Alzheimer’s Disease. 2009;17(3):621–629. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao YZ, Zhang JJ, Liu H, Wu GY, Xiong L, Shu M. Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer's disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Current Neurovascular Research. 2013;10(1):49–53. doi: 10.2174/156720213804806016. [DOI] [PubMed] [Google Scholar]
- 36.Luckhaus C, Flüß MO, Wittsack H-J, et al. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. NeuroImage. 2008;40(2):495–503. doi: 10.1016/j.neuroimage.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 37.Mentis MJ, Horwitz B, Grady CL, et al. Visual cortical dysfunction in Alzheimer’s disease evaluated with a temporally graded “stress test” during PET. American Journal of Psychiatry. 1996;153(1):32–40. doi: 10.1176/ajp.153.1.32. [DOI] [PubMed] [Google Scholar]
- 38.Niedermeyer E. Alzheimer disease: caused by primary deficiency of the cerebral blood flow. Clinical EEG and Neuroscience. 2006;37(3):175–177. doi: 10.1177/155005940603700303. [DOI] [PubMed] [Google Scholar]
- 39.Ruitenberg A, den Heijer T, Bakker SLM, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Annals of Neurology. 2005;57(6):789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 40.Sabayan B, Jansen S, Oleksik AM, et al. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Research Reviews. 2012;11(2):271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Fukuyama H, Yamauchi H, et al. Regional cerebral blood flow abnormalities in nondemented patients with memory impairment. Journal of Neuroimaging. 2002;12(2):112–118. doi: 10.1111/j.1552-6569.2002.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 42.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Reviews Neuroscience. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 43.Achike FI, To N-HP, Wang H, Kwan C-Y. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clinical and Experimental Pharmacology and Physiology. 2011;38(1):1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clinical Science. 2006;110(3):267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 45.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of Biological Chemistry. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Current Biology. 1998;8(6):335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 47.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-α . Cytokine and Growth Factor Reviews. 2003;14(5):447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 48.Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori Y, Otabe S, Dina C, et al. Genome-wide search for type 2 diabetes in Japanese affected sib-pairs confirms susceptibility genes on 3q, 15q, and 20q and identifies two new candidate loci on 7p and 11p. Diabetes. 2002;51(4):1247–1255. doi: 10.2337/diabetes.51.4.1247. [DOI] [PubMed] [Google Scholar]
- 50.Vasseur F, Helbecque N, Dina C, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Human Molecular Genetics. 2002;11(21):2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 51.Leu H-B, Chung C-M, Lin S-J, Jong Y-S, Pan W-H, Chen J-W. Adiponectin gene polymorphism is selectively associated with the concomitant presence of metabolic syndrome and essential hypertension. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019999.e19999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stumvoll M, Tschritter O, Fritsche A, et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes. 2002;51(1):37–41. doi: 10.2337/diabetes.51.1.37. [DOI] [PubMed] [Google Scholar]
- 53.Kollias A, Tsiotra PC, Ikonomidis I, et al. Adiponectin levels and expression of adiponectin receptors in isolated monocytes from overweight patients with coronary artery disease. Cardiovascular Diabetology. 2011;10, article 14 doi: 10.1186/1475-2840-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51(2):536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 55.Bermudez VJ, Rojas E, Toledo A, et al. Single-nucleotide polymorphisms in adiponectin, AdipoR1, and AdipoR2 genes: insulin resistance and type 2 diabetes mellitus candidate genes. American Journal of Therapeutics. 2013;20(4):414–421. doi: 10.1097/MJT.0b013e318235f206. [DOI] [PubMed] [Google Scholar]
- 56.Menzaghi C, Ercolino T, Paola RD, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51(7):2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Tian H, Zhao Y, et al. Meta-analysis of the association between adiponectin gene +276 g/T polymorphisms and coronary atherosclerotic heart disease. Wei Sheng Yan Jiu. 2013;42(4):693–697. [PubMed] [Google Scholar]
- 58.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. The Journal of Biological Chemistry. 2003;278(4):2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 59.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. The American Journal of Clinical Nutrition. 2010;91(1):258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spranger J, Verma S, Göhring I, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55(1):141–147. [PubMed] [Google Scholar]
- 61.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metabolism. 2007;6(1):55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89(1):38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 63.Zoico E, di Francesco V, Mazzali G, et al. Adipocytokines, fat distribution, and insulin resistance in elderly men and women. The Journals of Gerontology A: Biological Sciences and Medical Sciences. 2004;59(9):935–939. doi: 10.1093/gerona/59.9.m935. [DOI] [PubMed] [Google Scholar]
- 64.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 65.Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27(6):1375–1380. doi: 10.2337/diacare.27.6.1375. [DOI] [PubMed] [Google Scholar]
- 66.Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. European Journal of Endocrinology. 2007;157(5):625–631. doi: 10.1530/EJE-07-0223. [DOI] [PubMed] [Google Scholar]
- 67.Blüher M, Williams CJ, Klöting N, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30(12):3110–3115. doi: 10.2337/dc07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blüher M, Bullen JW, Jr., Lee JH, et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. The Journal of Clinical Endocrinology & Metabolism. 2006;91(6):2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 69.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Reviews Immunology. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 70.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 71.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Current Diabetes Reports. 2003;3(3):207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 72.Lim S, Sung HC, Jeong I-K, et al. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. The Journal of Clinical Endocrinology & Metabolism. 2008;93(6):2263–2268. doi: 10.1210/jc.2007-2028. [DOI] [PubMed] [Google Scholar]
- 73.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 74.Stefan N, Vozarova B, Funahashi T, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–1888. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 75.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obesity Reviews. 2005;6(1):13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 76.Iglseder B, Mackevics V, Stadlmayer A, Tasch G, Ladurner G, Paulweber B. Plasma adiponectin levels and sonographic phenotypes of subclinical carotid artery atherosclerosis: data from the SAPHIR study. Stroke. 2005;36(12):2577–2582. doi: 10.1161/01.STR.0000190834.00284.fd. [DOI] [PubMed] [Google Scholar]
- 77.Wang W-Q, Zhang H-F, Gao G-X, Bai Q-X, Li R, Wang X-M. Adiponectin inhibits hyperlipidemia-induced platelet aggregation via attenuating oxidative/nitrative stress. Physiological Research. 2011;60(2):347–354. doi: 10.33549/physiolres.932044. [DOI] [PubMed] [Google Scholar]
- 78.Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release. Potential role in the metabolic syndrome. American Journal of Physiology: Endocrinology and Metabolism. 2010;298(5):E1072–E1077. doi: 10.1152/ajpendo.00728.2009. [DOI] [PubMed] [Google Scholar]
- 79.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's & Dementia. 2013;9(1):63.e2–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Experimental Gerontology. 2010;45(3):173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. Journal of Aging Research. 2012;2012:21 pages. doi: 10.1155/2012/384017.384017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kappeler L, Filho CDM, Dupont J, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biology. 2008;6(10) doi: 10.1371/journal.pbio.0060254.e254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franceschi C, Olivieri F, Marchegiani F, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mechanisms of Ageing and Development. 2005;126(2):351–361. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 84.Lorenzini A, Salmon AB, Lerner C, et al. Mice producing reduced levels of insulin-like growth factor type 1 display an increase in maximum, but not mean, life span. The Journals of Gerontology A: Biological Sciences and Medical Sciences. 2014;69(4):410–419. doi: 10.1093/gerona/glt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selman C, Lingard S, Choudhury AI, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. The FASEB Journal. 2008;22(3):807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 86.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 87.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 88.Besson A, Salemi S, Gallati S, et al. Reduced longevity in untreated patients with isolated growth hormone deficiency. The Journal of Clinical Endocrinology & Metabolism. 2003;88(8):3664–3667. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
- 89.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. The Journal of the American Medical Association. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi J, Nishimura K, Matoba M, Maekawa N, Mabuchi H. Generation and gender differences in the components contributing to the diagnosis of the metabolic syndrome according to the Japanese criteria. Circulation Journal. 2007;71(11):1734–1737. doi: 10.1253/circj.71.1734. [DOI] [PubMed] [Google Scholar]
- 91.Fulop T, Larbi A, Douziech N. Insulin receptor and ageing. Pathologie Biologie. 2003;51(10):574–580. doi: 10.1016/j.patbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Molecular and Cellular Neuroscience. 2012;49(1):9–12. doi: 10.1016/j.mcn.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Kim J-A, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circulation Research. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Current Diabetes Reports. 2008;8(3):173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 95.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. The Journal of Biological Chemistry. 2000;275(5):3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 97.Cortopassi GA, Shibata D, Soong N-W, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. The Journal of Clinical Endocrinology & Metabolism. 1997;82(5):1484–1491. doi: 10.1210/jcem.82.5.3930. [DOI] [PubMed] [Google Scholar]
- 99.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocrine Reviews. 1993;14(1):20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 100.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. The New England Journal of Medicine. 1990;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 101.Gulcelik NE, Halil M, Ariogul S, Usman A. Adipocytokines and aging: adiponectin and leptin. Minerva Endocrinologica. 2013;38(2):203–210. [PubMed] [Google Scholar]
- 102.Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Medicine. 2009;15(9):1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 103.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neuroscience and Biobehavioral Reviews. 2000;24(8):855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 104.van der Heide LP, Ramakers GMJ, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Progress in Neurobiology. 2006;79(4):205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neuroscience and Biobehavioral Reviews. 2007;31(7):1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 106.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends in Endocrinology and Metabolism. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends in Pharmacological Sciences. 2002;23(6):288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 108.Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. Journal of Cellular and Molecular Medicine. 2011;15(9):1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fang X, Yu SX, Lu Y, Bast RC, Jr., Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends in Endocrinology and Metabolism. 2012;23(3):133–141. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cunnane S, Nugent S, Roy M, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27(1):3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiology of Learning and Memory. 2011;96(3):432–442. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 114.van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GMJ. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-D-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. Journal of Neurochemistry. 2005;94(4):1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- 115.Correia SC, Santos RX, Perry G, Zhu X, IMoreira P, Smith MA. Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Research Reviews. 2011;10(2):264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? Journal of Alzheimer’s Disease. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 117.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. Journal of Alzheimer’s Disease. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 118.Craft S, Asthana S, Newcomer JW, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Archives of General Psychiatry. 1999;56(12):1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 119.Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle-and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathologica. 2011;121(3):337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiology and Behavior. 2000;68(4):509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 121.Craft S, Asthana S, Schellenberg G, et al. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70(2):146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- 122.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 123.Cornier M-A, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocrine Reviews. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vu V, Bui P, Eguchi M, Xu A, Sweeney G. Globular adiponectin induces LKB1/AMPK-dependent glucose uptake via actin cytoskeleton remodeling. Journal of Molecular Endocrinology. 2013;51(1):155–165. doi: 10.1530/JME-13-0059. [DOI] [PubMed] [Google Scholar]
- 125.Song J, Lee JE. Adiponectin as a new paradigm for approaching Alzheimer's disease. Anatomy & Cell Biology. 2013;46(4):229–234. doi: 10.5115/acb.2013.46.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mao X, Kikani CK, Riojas RA, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature Cell Biology. 2006;8(5):516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 127.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 128.Leow MKS, Addy CL, Mantzoros CS. Clinical review 159: human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. The Journal of Clinical Endocrinology & Metabolism. 2003;88(5):1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 129.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. The Journal of Biological Chemistry. 2002;277(29):25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 130.Okamoto M, Ohara-Imaizumi M, Kubota N, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51(5):827–835. doi: 10.1007/s00125-008-0944-9. [DOI] [PubMed] [Google Scholar]
- 131.Yamaguchi N, Kukita T, Li Y-J, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans . FEMS Immunology & Medical Microbiology. 2007;49(1):28–34. doi: 10.1111/j.1574-695X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 132.Hofman A, Ott A, Breteler MMB, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam study. The Lancet. 1997;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 133.McAlister FA, Zarnke KB, Campbell NRC, et al. The 2001 Canadian recommendations for the management of hypertension—part two: therapy. Canadian Journal of Cardiology. 2002;18(6):625–641. [PubMed] [Google Scholar]
- 134.Jandeleit-Dahm K, Cooper ME. Hypertension and diabetes. Current Opinion in Nephrology and Hypertension. 2002;11(2):221–228. doi: 10.1097/00041552-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 135.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International. 2002;61(3):1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 136.McNay EC. The impact of recurrent hypoglycemia on cognitive function in aging. Neurobiology of Aging. 2005;26(supplement 1):S76–S79. doi: 10.1016/j.neurobiolaging.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 137.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 138.Cox DJ, Kovatchev BP, Gonder-Frederick LA, et al. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28(1):71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 139.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 140.Imagawa A, Funahashi T, Nakamura T, et al. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25(9):1665–1666. doi: 10.2337/diacare.25.9.1665. [DOI] [PubMed] [Google Scholar]
- 141.Zhu M, Miura J, Lu LX, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Experimental Gerontology. 2004;39(7):1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 142.Stefan N, Bunt JC, Salbe AD, Funahashi T, Matsuzawa Y, Antonio Tataranni P. Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. The Journal of Clinical Endocrinology & Metabolism. 2002;87(10):4652–4656. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 143.Krakoff J, Funahashi T, Stehouwer CDA, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26(6):1745–1751. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- 144.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. The Lancet. 2002;360(9326):57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 145.Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. American Journal of Physiology: Heart and Circulatory Physiology. 2005;288(5):H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 146.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 147.Hanley AJ, Wagenknecht LE, Norris JM, et al. Adiponectin and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family study. Diabetes Care. 2011;34(10):2231–2236. doi: 10.2337/dc11-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50(5):1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 149.Tu JV. Reducing the global burden of stroke: INTERSTROKE. The Lancet. 2010;376(9735):74–75. doi: 10.1016/S0140-6736(10)60975-0. [DOI] [PubMed] [Google Scholar]
- 150.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease—part 1: prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. The Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 151.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 152.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet Neurology. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 153.Staessen JA, Richart T, Birkenhäger WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49(3):389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 154.Nagai M, Hoshide S, Kario K. Hypertension and dementia. American Journal of Hypertension. 2010;23(2):116–124. doi: 10.1038/ajh.2009.212. [DOI] [PubMed] [Google Scholar]
- 155.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging study. Neurobiology of Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 156.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging study. The Journal of the American Medical Association. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- 157.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathologica. 2010;119(4):421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 158.Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathologica. 2011;121(5):571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 160.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke. 2003;34(3):594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- 161.Lee AY, Jeong S-H, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Archives of Gerontology and Geriatrics. 2006;42(2):157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 162.Nation DA, Wierenga CE, Delano-Wood L, et al. Elevated pulse pressure is associated with age-related decline in language ability. Journal of the International Neuropsychological Society. 2010;16(5):933–938. doi: 10.1017/S1355617710000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. The Lancet. 1996;347(9009):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 164.Molander L, Gustafson Y, Lövheim H. Longitudinal associations between blood pressure and dementia in the very old. Dementia and Geriatric Cognitive Disorders. 2010;30(3):269–276. doi: 10.1159/000320252. [DOI] [PubMed] [Google Scholar]
- 165.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 166.Guzik TJ, Black E, West NE. Relationship between the G894T polymorphism (Glu 298 Asp variant) in endothelial nitric oxide synthase and nitric oxide-mediated endothelial function in human atherosclerosis. American Journal of Medical Genetics. 2001;100(2):130–137. doi: 10.1002/ajmg.1229. [DOI] [PubMed] [Google Scholar]
- 167.Zhu W, Cheng KKY, Vanhoutte PM, Lam KSL, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clinical Science. 2008;114(5-6):361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 168.Ouedraogo R, Gong Y, Berzins B, et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. The Journal of Clinical Investigation. 2007;117(6):1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. The Journal of Biological Chemistry. 2004;279(27):28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 170.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. The Journal of Biological Chemistry. 2003;278(45):45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 171.Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. The Journal of Biological Chemistry. 2004;279(2):1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kobayashi H, Ouchi N, Kihara S, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation Research. 2004;94(4):e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takahashi A, Kureishi Y, Yang J, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Molecular and Cellular Biology. 2002;22(13):4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovascular Research. 2008;78(2):376–384. doi: 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 175.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nature Clinical Practice: Cardiovascular Medicine. 2009;6(1):27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takaoka M, Nagata D, Kihara S, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circulation Research. 2009;105(9):906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 177.Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 178.Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathologica. 2007;113(1):13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 179.Stary HC. Natural history and histological classification of atherosclerotic lesions an update. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(5):1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 180.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 181.Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging study. Hypertension. 2004;44(1):29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 182.Deguchi J-O, Aikawa E, Libby P, et al. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112(17):2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 183.Deguchi J-O, Aikawa M, Tung C-H, et al. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114(1):55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 184.Dollery CM, Owen CA, Sukhova GK, Krettek A, Shapiro SD, Libby P. Neutrophil elastase in human atherosclerotic plaques production by macrophages. Circulation. 2003;107(22):2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 185.Larionov S, Dedeck O, Birkenmeier G, Thal DR. Expression of α2-macroglobulin, neutrophil elastase, and interleukin-1α differs in early-stage and late-stage atherosclerotic lesions in the arteries of the circle of Willis. Acta Neuropathologica. 2007;113(1):33–43. doi: 10.1007/s00401-006-0134-0. [DOI] [PubMed] [Google Scholar]
- 186.Liang J, Liu E, Yu Y, et al. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113(16):1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 187.Han SH, Quon MJ, Kim J-A, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. Journal of the American College of Cardiology. 2007;49(5):531–538. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 188.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 189.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 190.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 191.Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(12):3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leys D, Hénon H, Mackowiak-Cordoliani M-A, Pasquier F. Poststroke dementia. The Lancet Neurology. 2005;4(11):752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 193.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. The Lancet Neurology. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 194.Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MMB. Prestroke cognitive performance, incident stroke, and risk of dementia: the Rotterdam study. Stroke. 2008;39(1):36–41. doi: 10.1161/STROKEAHA.107.490334. [DOI] [PubMed] [Google Scholar]
- 195.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44(6):1621–1628. doi: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 196.Kokmen E, Whisnant JP, O’Fallon WM, Chu C-P, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996;46(1):154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 197.Grant I, Rochford J, Fleming T, Stunkard A. A neuropsychological assessment of the effects of moderate marihuana use. Journal of Nervous and Mental Disease. 1973;156(4):278–280. doi: 10.1097/00005053-197304000-00008. [DOI] [PubMed] [Google Scholar]
- 198.Stott DJ, Welsh P, Rumley A, et al. Adipocytokines and risk of stroke in older people: a nested case-control study. International Journal of Epidemiology. 2009;38(1):253–261. doi: 10.1093/ije/dyn215. [DOI] [PubMed] [Google Scholar]
- 199.Prugger C, Luc G, Haas B, et al. Adipocytokines and the risk of ischemic stroke: the PRIME study. Annals of Neurology. 2012;71(4):478–486. doi: 10.1002/ana.22669. [DOI] [PubMed] [Google Scholar]
- 200.Savopoulos C, Michalakis K, Apostolopoulou M, Miras A, Hatzitolios A. Adipokines and stroke: a review of the literature. Maturitas. 2011;70(4):322–327. doi: 10.1016/j.maturitas.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 201.Kim BJ, Lee S-H, Ryu W-S, Kim CK, Yoon B-W. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. Journal of the Neurological Sciences. 2012;312(1-2):117–122. doi: 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 202.Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90(5):528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Otsuka F, Sugiyama S, Kojima S, et al. Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. Journal of the American College of Cardiology. 2006;48(6):1155–1162. doi: 10.1016/j.jacc.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 204.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 205.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nature Medicine. 2005;11(10):1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tao L, Jiao X, Gao E, et al. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006;114(13):1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- 207.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nature Medicine. 2004;10(12):1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jung YS, Ha SK, Kim SD, Kim SH, Lim DJ, Choi JI. The role of adiponectin in secondary inflammatory reaction in cerebral ischemia. Journal of Cerebrovascular and Endovascular Neurosurgery. 2013;15(3):171–176. doi: 10.7461/jcen.2013.15.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase-dependent mechanisms. Circulation. 2008;117(2):216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 210.Song W, Huo T, Guo F, et al. Globular adiponectin elicits neuroprotection by inhibiting NADPH oxidase-mediated oxidative damage in ischemic stroke. Neuroscience. 2013;248:136–144. doi: 10.1016/j.neuroscience.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 211.Shen L, Miao J, Yuan F, et al. Overexpression of adiponectin promotes focal angiogenesis in the mouse brain following middle cerebral artery occlusion. Gene Therapy. 2013;20(1):93–101. doi: 10.1038/gt.2012.7. [DOI] [PubMed] [Google Scholar]
- 212.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. The Journal of Biological Chemistry. 2003;278(33):31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 213.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circulation Research. 2005;96(8):838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]


