Abstract
Background. The purpose of the present study was to assess the feasibility of using miR-126 in the urine as a biomarker for diabetic nephropathy. Methods. miRNAs were extracted from the urine samples of T2DM patients with diabetic nephropathy (DN; n = 92), T2DM without DN (n = 86), and 85 healthy volunteers using quantitative reverse transcriptase polymerase chain reaction (real-time polymerase chain reaction) analysis. Stability of urinary miR-126 and factors that affected the stability were assessed. A subgroup analysis was also carried out to compare the urinary miR-126 level in T2DM patients well controlled by the treatment versus those who were not well controlled. Results. Urinary miR-126 was stable when the urine samples were kept at room temperature for extended period of time, 4°C, −20°C, and −80°C for up to 12 hours or subjected to 10 freeze-and-thaw cycle. Urinary miR-126 was significantly higher in T2DM patients with DN (5.76 ± 0.33 versus 3.25 ± 0.45 in T2DM patients without DN). Successful treatment significantly reduced urinary miR-126 in T2DM patients with DN to 3.89 ± 0.52 (P < 0.05). Conclusion. miR-126 in the urine is stable and it could be used as a biomarker of DN and to monitor the treatment response.
1. Introduction
MicroRNAs (miRNAs) are small, endogenous noncoding RNAs, 21–25 nucleotides in length [1, 2]. Alteration in serum/plasma levels of miRNAs is closely associated with many diseases, including differentiation, inflammation, allergic reactions, diabetes, and several types of cancer [3–6]. For example, plasma miR-208 has been used as a biomarker of acute myocardial infarction [7, 8]. Plasma miR-122 increases during drug-induced liver injury [9–11]. In addition to their presence in cells/tissues and in plasma, miRNAs are also present in other body fluids, including saliva, urine, tears, amniotic fluid, and breast milk. The type and concentration of these miRNAs in body fluids have also been correlated with disease types and pathological processes and can potentially be used as disease biomarkers to monitor physiological and/or pathological states [12–14].
Diabetic nephropathy (DN) is a microvascular complication of type 2 diabetes mellitus (T2DM) and a major cause of end-stage renal disease (ESRD) [15]. Key pathological features of DN include glomerular basement membrane thickening, mesangial expansion podocyte effacement, and glomerular sclerosis [16]. Clinically, DN is often manifested as microalbuminuria and progressive glomerular dysfunction.
A wide variety of biomarkers, including creatinine, kidney enzymes, cystatin C, and injury molecules, have been developed for early detection of acute kidney injury (AKI) [17]. Recent studies implicated miRNAs in DN. For example, several types of miRNAs are upregulated in the kidneys of diabetic mice [18]. Decreased levels of miR-200c, miR-141, miR-429, and miR-192 have been reported in patients with systemic lupus erythematosus than in healthy controls [19].
miR-126 has whole bunch of effects/functions reported, including endothelial cell biology, cancer, cardiovascular disease, Parkinson, DM [20–23]. Lower plasma miRNA-126 has been associated with a loss of endothelial miR-126 expression in patients with T2DM [24].
In the present study, we examined miR-126 concentration in the urine of T2DM patients with versus without DN and the change of urine miR-126 after treatment in T2DM patients with DN. Prior to the formal experiments, a preliminary stability study was conducted to examine the feasibility of using urine miR-126 as a biomarker.
2. Subjects and Methods
2.1. Study Participants and Diagnostic Criteria
The current study included 92 T2DM patients with DN, 86 T2DM patients without DN, and 85 healthy volunteers who were matched for age, body mass index (BMI), and gender. Blood pressure was measured by two independent staff members blinded to the study grouping with a standard sphygmomanometer. Hypertension was defined as systolic pressure of 140 mmHg or greater, and/or diastolic pressure of 90 mmHg or greater, or when patients had a clear history of hypertension. Patients with a history of coronary artery disease, cerebrovascular accident, or intermittent claudication were excluded from the study. Hypervolemic subjects were also excluded. An informed consent was obtained from all subjects.
The diagnosis of T2DM was established based on the American Diabetes Association criteria [25], with a disease course of ≥5 years. Diagnosis criteria for DN were established on the basis of overt diabetic retinopathy plus macroalbuminuria at >300 mg/d. Retinopathy is demanded to be exist is to ensure that albuminuria is the outcome of diabetic nephropathy rather than a nondiabetic glomerulopathy. A renal biopsy would be the gold standard to distinguish between diabetic nephropathy and a nondiabetic glomerulopathy. But in diabetic patients, a renal biopsy is almost never taken, and many researches have indicated that retinopathy being present is a good alternative for differentiation between diabetic nephropathy and nondiabetic glomerulopathy in type 2 diabetic patients with albuminuria [26–28]. The study was approved by the Ethics Committee of the 4th Affiliated Hospital of Harbin Medical University.
2.2. Sample Collection and Preparation
The second morning urine samples were collected after overnight fasting to measure pH, glucose, blood content, and specific gravity as described previously [29]. Then urine samples (500 μL) were centrifuged briefly to remove cells and debris prior to miRNA extraction, using a mirVana PARIS Kit (Ambion, America). miRNAs were transcribed to cDNAs using RevertAidTM reverse transcriptase and miRNA-specific stem-loop primers (Applied Biosystems, America). Each reaction consisted of 17.5 μL dissolved RNA, 7.5 μL master mix (5 μL 5× Reaction Buffer, 2 μL miRNA-specific stem-loop primers, and 0.5 μL 200 U/μL reverse transcriptase), and 1 μL deoxynucleoside triphosphates. A blank control was used in each experiment to ensure that the PCR products were not contaminated.
2.3. Quantitative Reverse Transcriptase Polymerase Chain Reaction (Real-Time Polymerase Chain Reaction) Analysis
Quantitative real-time PCR was used to analyze the amount of miRNA in the urine samples. miR-126 was amplified using LightCycler 480 Probes Master kit (Roche Applied Science, Germany) and examined using TaqManTM MicroRNA hsa-miR-126-specific primers (Applied Biosystems). Real-time PCR was performed on a LightCycler 480 II RT-PCR System (Roche Applied Science, Germany) with the following condition: 94°C for 120 seconds followed by 45 cycles of 94°C for 20 seconds, 56°C for 10 seconds, and 70°C for 20 seconds, using the LightCycler 480 Probes Master kit (Roche Applied Science, Germany). Nontemplate reaction controls produced no detectable signals in any of the experiments. Each urine sample was extracted in triplicate, followed by simultaneous reverse transcription and triplicate analysis through real-time PCR. The microRNA level was quantified using the formula 2(50-Ct). Data are expressed in lg unit.
2.4. miRNA Stability
Urine samples were collected from 60 healthy individuals and placed in plain glass tubes. Aliquots from each urine specimen were subject to miRNA extraction immediately or after storage at room temperature, 4°C, −20°C, and −80°C for 3, 6, or 12 hours. Some of the aliquots were also subjected to up to 10 freeze-thaw cycles. Data are expressed as percentage to the control immediately after the extraction (without storage).
2.5. Effects of Urinary Albumin on miRNA Detection
Effects of albumin were examined by adding albumin standard solution containing urinary albumin, urinary transferring ferritin, urinary immunoglobulin IgG, urine microglobulin β, urine microglobulin α, and retinol binding protein to the urine samples to a concentration of the trace protein at 0–140 mg/L.
2.6. pH and Urinary miRNA Levels
Twelve urine samples were used for this study. The pH of the samples was adjusted to 4.5, 7.0, and 8.0, respectively, using 5 M HCl or 5 M NaOH. Levels of miR-126 in these aliquots were used to assess the effect of pH on miRNA stability.
All the DN patients received insulin, ACEI/ARE, control of blood pressure, and moderate exercise for about six months as previously described [30–34].
2.7. Statistical Analysis
Statistical analysis was performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). All variables are presented as mean ± SD upon normal distribution and as medians (lower and upper quartiles) otherwise. Gene expression level was log transformed prior to a one-way ANOVA statistical analysis. One-way ANOVA was used to compare levels of gene expression between groups. Paired samples t-test was used to compare changes of gene expression before and after the treatment. P values below 0.05 were considered statistically significant. All probability tests were two-tailed.
3. Results
3.1. Detection, Quantification, and Stability of miR-126 in Urine Samples
Using real-time PCR, miR-126 was within detection limit for all tested samples (Figure 1). miR-126 level in the urine did not change significantly after sample storage at room temperature for 3, 6, and 12 hr, respectively (5.65 (5.41–5.76), 5.70 (5.34–5.77), and 5.48 (5.28–5.60) versus 5.65 (5.40–5.79) immediately after extraction; P = 0.161; Figure 1(a)). Storage at 4°C, −20°C, and −80°C for 12 hours also did not affect the miR-126 measurement (5.65 (5.40–5.79), 5.72 (5.55–5.98), and 5.61 (5.33–5.89), resp., Figure 1(b)). Freezing and thawing up to 10 cycles prior to miRNA extraction did not affect the miR-126 levels (5.64 (5.58–5.84), 5.76 (5.58–6.00), 5.64 (5.54–5.82), and 5.67 (5.56–5.88) versus 5.68 (5.41–5.74), P = 0.643; Figure 1(c)). Sample pH variation (4.5–8.0) did not affect miR-126 level (5.70 (5.60–5.94), 5.67 (5.44–5.78), and 5.64 (5.44–5.96), resp., P = 0.635). Urinary miR-126 did not differ with varying albumin concentration (5.73 (5.57–5.87), 5.64 (5.43–5.95), and 5.81 (5.40–5.89), resp., P = 0.986).
Figure 1.
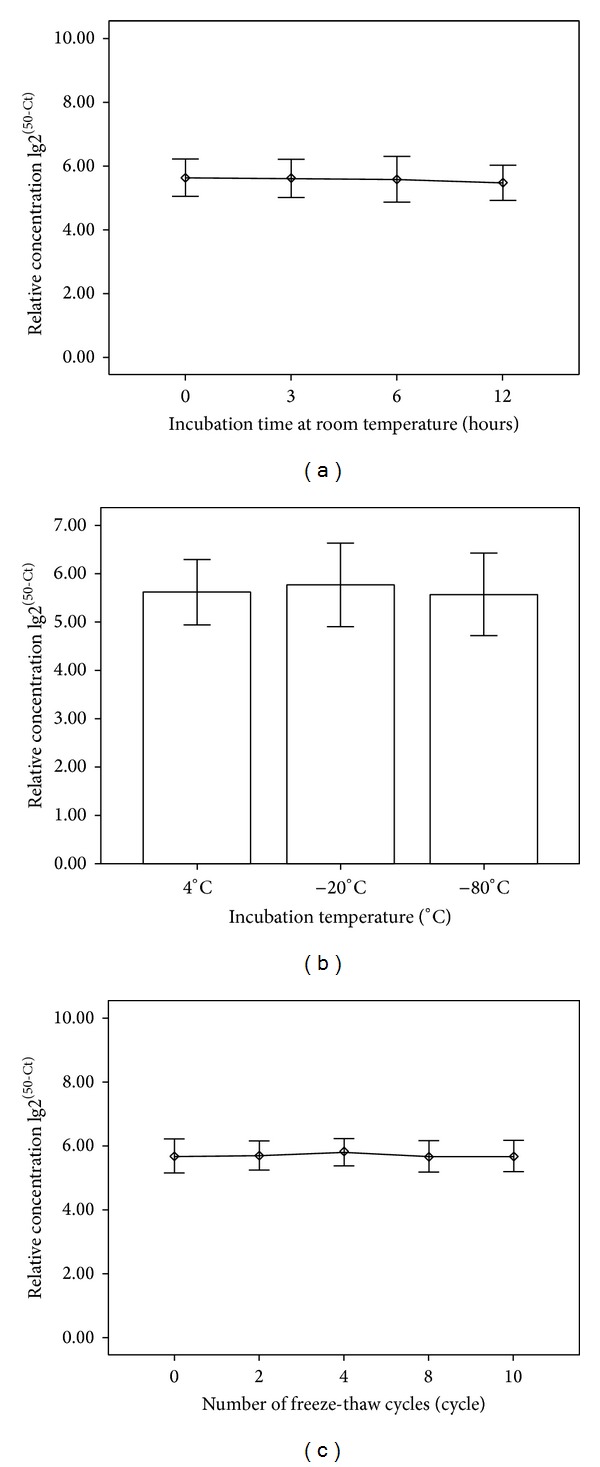
Characterization of miRNA stability in human urine. miRNA levels remain stable when urine is subjected to prolonged room temperature incubation (a), stored at 4°C, −20°C, −80° (b), or freeze-thawed up to multiple times (c). The P value is 0.161, 0.134, and 0.643, respectively, n = 60.
3.2. Elevated Urinary miR-126 in T2DM Patients with DN
The demographic and baseline clinical data of the study subjects are summarized in Table 1. Urinary miR-126 was significantly higher in T2DM patients with DN (5.76 ± 0.33) than that in T2DM patients with no DN (3.76 ± 0.38) or in the healthy subjects (3.25 ± 0.45) (Figure 2 and Table 1, P = 0.002). Interestingly, urinary miR-126 level (3.38 ± 0.51) in T2DM/DN patients with a urinary albumin of 2000 mg/L and above was comparable to non-DN (3.25 ± 0.45).
Table 1.
Clinical characteristics of T2DM patients with and without DN.
| Characteristics | Healthy controls (85*) | DM without DN (86) | DN before treatment (92) | DN after treatment (92) | P 1 | P 2 | |
|---|---|---|---|---|---|---|---|
| Well controlled (80) | Not well controlled (12) | ||||||
| Sex (M : F) | 36 : 49 | 47 : 39 | 57 : 35 | 50 : 30 | 7 : 5 | 0.62 | 0.75 |
| Age (years ± SD) | 52.1 ± 5.6 | 49.3 ± 4.7 | 50.3 ± 7.4 | 51.2 ± 6.3 | 49.5 ± 8.5 | 0.72 | 0.83 |
| BMI Kg/m2 | 24.07 ± 1.21 | 23.45 ± 1.02 | 23.17 ± 0.98 | 24.58 ± 1.16 | 23.66 ± 1.32 | 0.68 | 0.83 |
| SBP (mmHg) | 128.4 ± 10.6 | 129.7 ± 13.2 | 130.5 ± 11.7 | 127.8 ± 12.6 | 129.8 ± 12.9 | 0.89 | 0.93 |
| DBP (mmHg) | 78.2 ± 8.7 | 79.1 ± 6.9 | 80.4 ± 8.5 | 78.4 ± 7.4 | 75.3 ± 9.3 | 0.92 | 0.87 |
| Fasting glucose (mmol/L) | 3.98 ± 0.77 | 8.37 ± 3.46 | 5.32 ± 0.25 | 4.81 ± 2.32 | 4.54 ± 3.17 | <0.05 | 0.79 |
| HbA1c (%) | 5.31 ± 0.32 | 8.61 ± 0.75 | 6.26 ± 0.54 | 6.07 ± 0.61 | 6.72 ± 0.45 | <0.05 | 0.82 |
| AlbU (g/L) | 8.32 ± 3.5 | 10.32 ± 2.8 | 437.2 ± 164.6 | 21.36 ± 8.3 | 389.7 ± 45.2 | <0.05 | <0.05 |
| Cys-c (mg/L) | 0.62 ± 0.17 | 0.71 ± 0.21 | 1.48 ± 1.15 | 0.67 ± 0.08 | 1.45 ± 0.78 | <0.05 | <0.05 |
| UREA (mmol/L) | 4.24 ± 1.32 | 3.98 ± 1.27 | 6.78 ± 1.90 | 4.02 ± 1.13 | 5.79 ± 1.82 | <0.05 | <0.05 |
| CREA (mmol/L) | 53.44 ± 8.26 | 59.56 ± 7.48 | 86.43 ± 21.34 | 54.67 ± 7.14 | 84.23 ± 19.43 | <0.05 | <0.05 |
| miR-126 [lg2(50-Ct)] | 3.25 ± 0.45 | 3.76 ± 0.38 | 5.76 ± 0.33 | 3.89 ± 0.52 | 5.24 ± 0.47 | <0.05 | <0.05 |
*Indicates numbers of subjects in the group. DM: diabetes mellitus; DN: diabetic nephropathy; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb Alc-A: glycosylated hemoglobin; AlbU: urine albumin; Cys-c: serum cystatin C; CREA: serum creatinine; P 1: comparison to healthy controls, DM without DN, and DN before treatment. P 2: comparison between DN patients well controlled versus not well controlled by the treatment.
Figure 2.
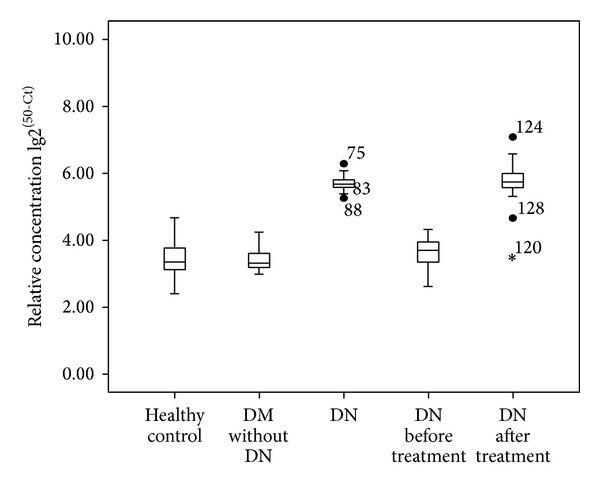
Urinary miR-126 levels of different crowds. Urinary miR-126 level in DN patients was higher than DM without DN patients and healthy controls, P = 0.002. However, there was no significant difference between the healthy control and DM without DN, P = 0.324. After a period of treatment, urinary miR-126 levels in DN patients were decreased.
3.3. Urinary miR-126 Correlated with Treatment Response in T2DM/DN Patients
Among the 92 patients treated, 80 patients responded to the treatments (patients with decreased urinary albumin level, HbA1c ≤ 7.0%, systolic pressure ≤ 140 mmHg, and diastolic pressure ≤ 90 mmHg) in comparison to the pretreatment baseline (Table 1; (3.89 ± 0.52 effective versus not effective treatment, n = 80, P < 0.05)). The remaining 12 responded poorly to the treatment, with no significant change of urinary albumin after the treatment (5.76 ± 0.33 before treatment versus 5.24 ± 0.47 after treatment) (Figure 2).
4. Discussion
miRNAs in serum and plasma are generally stable [35]. However, the stability of miRNAs in urine, especially during sample collection and preparation, has not been addressed. Our preliminary experiments showed that urinary miRNA (i.e., miR-126) is stable at room temperature, 4°C, −20°C, and −80°C within 12 hours. Also, repeated freezing and thawing up to 10 times did not affect the analysis. The presence of proteins in the urine, up to 140 mg/L, or pH variation (4.5–8.0) did not affect the apparent miRNA level in the urine. Accordingly, urinary miRNA-126 is suitable for use in clinical laboratory setting.
The main experiments in the current study demonstrated higher urinary miR-126 in T2DM patients with DN versus patients without DN. Effective treatment of DN patients significantly reduced the urinary levels of miR-126. These findings indicate that urinary miR-126 could be used as a biomarker for diabetic nephropathy and monitoring the progression and regression of kidney damage in patients with T2DM.
Our findings also suggested a possible role of miR-126 in the pathophysiology of renal damage in T2DM. miR-126 has been found to be highly enriched in endothelial cells and plays a pivotal role in maintaining endothelial homeostasis and vascular integrity [20–22]. Endothelial miR-126 in plasma has been reported to be lower in T2DM patients than in healthy individuals [25]. It is likely that the urine miRNA-126 originates from cells (secreted as exosomes) [36]. Upon leaving the cells, the miRNAs become associated with other molecules and thus protected from degradation. It is likely, as in the case of AlbU, that a RNA-induced silencing complex (RISC) containing miRNA may leak from injured epithelial cells of kidney or glomerular vascular endothelial cells into the urine. This could explain lower urinary miR-126 after effective treatment in the current study.
Also there are many deficiencies in our study. First, there is still no recognized internal reference standard for bodily fluids [37, 38]. We compared log transformation of (50-Ct) values in a given volume of 500 μL urine. The content of urine may be influenced by many factors which probably affect the urinary miRNAs level. Second, our experiments were an indicative research. The sample size was small; we did not study the correlation among the miR-126 level and the stages of DN. And it needs to be seen definitely in the further.
Recently studies show that levels of about 27 miRNAs are significantly upregulated or downregulated in different stages of untreated nephropathy when compared with urinary miRNAs from type 1 diabetes with persistent or intermittent microalbuminuria [39]. Urinary miR-126 may appear to be upregulated or downregulated in the progression of diabetic kidney disease. The early detection of its presence in urine may assist the prediction of the disease course. The threshold of detection of microRNAs by various amplification methods should be increased if we aim to employ miR-126 in urine as a biomarker for determining the severity of diabetic kidney disease and checking the progression of recovery during treatment.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biology. 2004;5(3, article R13) doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning MS, Kim AS, Prasad N, Levy SE, Zhang H, Andl T. Characterization of the merkel cell carcinoma miRNome. Journal of Skin Cancer. 2014;2014:9 pages. doi: 10.1155/2014/289548.289548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clinical Chemistry. 2009;55(11):1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 8.Wang G-K, Zhu J-Q, Zhang J-T, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European Heart Journal. 2010;31(6):659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starkey Lewis PJ, Dear J, Platt V, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54(5):1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 11.Kerr TA, Korenblat KM, Davidson NO. MicroRNAs and liver disease. Translational Research. 2011;157(4):241–252. doi: 10.1016/j.trsl.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clinical Chemistry. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzimagiorgis G, Michailidou EZ, Kritis A, Markopoulos AK, Kouidou S. Recovering circulating extracellular or cell-free RNA from bodily fluids. Cancer Epidemiology. 2011;35(6):580–589. doi: 10.1016/j.canep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids-the mix of hormones and biomarkers. Nature Reviews Clinical Oncology. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Declèves A-E, Sharma K. New pharmacological treatments for improving renal outcomes in diabetes. Nature Reviews. Nephrology. 2010;6(6):371–380. doi: 10.1038/nrneph.2010.57. [DOI] [PubMed] [Google Scholar]
- 16.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 17.Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clinical Toxicology. 2011;49(8):720–728. doi: 10.3109/15563650.2011.615319. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Koo S, White N, et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Research. 2004;32(22, article e188) doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Kwan BC-H, Lai FM-M, Chow K-M, Kam-Tao Li P, Szeto C-C. Expression of microRNAs in the urinary sediment of patients with IgA nephropathy. Disease Markers. 2010;28(2):79–86. doi: 10.3233/DMA-2010-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Developmental Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific MicroRNA miR-126 governs vascular integrity and angiogenesis. Developmental Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asgeirsdottir SA, van Solingen C, Kurniati NF, et al. Microrna-126 contributes to renal microvascular heterogeneity of vcam-1 protein expression in acute inflammation. American Journal of Physiology—Renal Physiology. 2012;302(12):1630–1639. doi: 10.1152/ajprenal.00400.2011. [DOI] [PubMed] [Google Scholar]
- 23.Metzinger-Le Meuth V, Andrianome S, Chillon JM, Bengrine A, Massy ZA, Metzinger L. microRNAs are dysregulated in the cerebral microvasculature of CKD mice. Frontiers in Bioscience (Elite edition) 2014;6:80–88. doi: 10.2741/e693. [DOI] [PubMed] [Google Scholar]
- 24.Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial MiR-126 and other microRNAs in type 2 diabetes. Circulation Research. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 25.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 26.Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clinical Nephrology. 2007;67(5):293–297. doi: 10.5414/cnp67293. [DOI] [PubMed] [Google Scholar]
- 27.Parving H-H, Gall M-A, Skott P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney International. 1992;41(4):758–762. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 28.Wong TYH, Choi PCL, Chun CS, et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25(5):900–905. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 29.Haubitz M, Good DM, Woywodt A, et al. Identification and validation of urinary biomarkers for differential diagnosis and evaluation of therapeutic intervention in anti-neutrophil cytoplasmic antibody-associated vasculitis. Molecular and Cellular Proteomics. 2009;8(10):2296–2307. doi: 10.1074/mcp.M800529-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parving H-H, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. The New England Journal of Medicine. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 31.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. The New England Journal of Medicine. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 32.Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. British Medical Journal. 2000;321(7274):1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossing K, Jacobsen P, Pietraszek L, Parving H-H. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care. 2003;26(8):2268–2274. doi: 10.2337/diacare.26.8.2268. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Diabetic nephropathy. Diabetes Care. 2002;25(supplement 1):S85–S89. [Google Scholar]
- 35.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 37.Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3(9) doi: 10.1371/journal.pone.0003148.e3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G, Chan ES, Kwan BC, et al. Expression of microRNAs in the urine of patients with bladder cancer. Clinical Genitourinary Cancer. 2012;10(2):106–113. doi: 10.1016/j.clgc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Argyropoulos C, Wang K, McClarty S, et al. Urinary MicroRNA proiling in the nephropathy of type 1 diabetes. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054662.e54662 [DOI] [PMC free article] [PubMed] [Google Scholar]


