Narrative Abstract
Research to date demonstrates a relationship between exposure to ambient air pollutants and cardiovascular disease. Many studies have shown associations between short-term exposures to elevated levels of air pollutants and cardiovascular disease events, and several cohort studies suggest effects of long-term exposure on cardiovascular mortality, coronary heart disease events, and stroke. The biological mechanisms underlying this chronic exposure relationship are not entirely clear, but are hypothesized to include systemic inflammation, autonomic nervous system imbalance, changes in vascular compliance, altered cardiac structure, and development of atherosclerosis. The Multi-Ethnic Study of Atherosclerosis provides an especially well-characterized population in which to investigate the relationship between air pollution and cardiovascular disease and to explore these biological pathways. This paper reviews findings reported to date within this cohort, and summarizes the aims and anticipated contributions of a major ancillary study, the Multi-Ethnic Study of Atherosclerosis and Air Pollution.
Keywords: Air pollution, cardiovascular disease, subclinical atherosclerosis, progression
Introduction
Research to date suggests a causal relationship between exposure to ambient air pollutants and cardiovascular disease. While many investigations have focused on documenting the relationship between short-term exposures to elevated levels of air pollutants and the triggering of cardiovascular disease events in susceptible populations, several cohort studies also suggest effects of long-term exposure on cardiovascular disease risk. In particular, two landmark studies demonstrated relationships between fine particulate matter air pollution (particles <2.5 µm in diameter [PM2.5]) on risk of mortality when city-wide[1], and later county-wide[2], averages of air pollutant levels were assigned based on residential location. Subsequent cohort studies confirmed these initial findings, reporting associations between long-term exposures to PM2.5 and cardiovascular disease (CVD) mortality, coronary heart disease events and stroke.[3–8] It remains unclear exactly which biological mechanisms underlie this long-term relationship; hypotheses include, among others, systemic inflammation, autonomic nervous system imbalance, changes in vascular responsiveness and compliance, altered cardiac structure, and development of atherosclerosis.[9
The Multi-Ethnic Study of Atherosclerosis (MESA) provides a useful cohort in which to investigate the relationship between air pollution and CVD and these potential pathways. MESA was initiated by the National Heart, Lung, and Blood Institute (NHLBI) in July of 2000 to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in a multi-ethnic cohort of 6,814 men and women.[10] MESA participants were recruited from six US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County (Winston-Salem), North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Participants were aged 45 to 84 years at enrollment, with an approximately equal gender ratio, and were recruited from four ethnic/racial groups. MESA participants were free of recognized clinical cardiovascular disease at baseline.
The initial MESA exam occurred between 2000 and 2002, and measurements included coronary artery calcium (CAC) using computed tomography (CT); flow-mediated brachial artery endothelial vasodilation, carotid artery intima-medial wall thickness (IMT), and distensibility of the carotid arteries using ultrasonography; peripheral vascular disease using ankle and brachial blood pressures; retinal photography; magnetic resonance imaging (MRI); electrocardiography; and assessments of urinary albumin concentration, standard CVD risk factors, sociodemographic factors, life habits, and psychosocial factors. Subsequent exams, including repetitions of certain baseline measurements as well as new measures, were scheduled at approximately adjacent two-year intervals, with a fifth MESA exam beginning April 2010. Figure 1 depicts the biological pathways that have been proposed to underlie the relationship between air pollution and CVD, and includes outcome measurements that may be indicators of activity along these pathways. All outcome measures shown in this figure are available on some or all of the MESA study participants.
Figure 1.
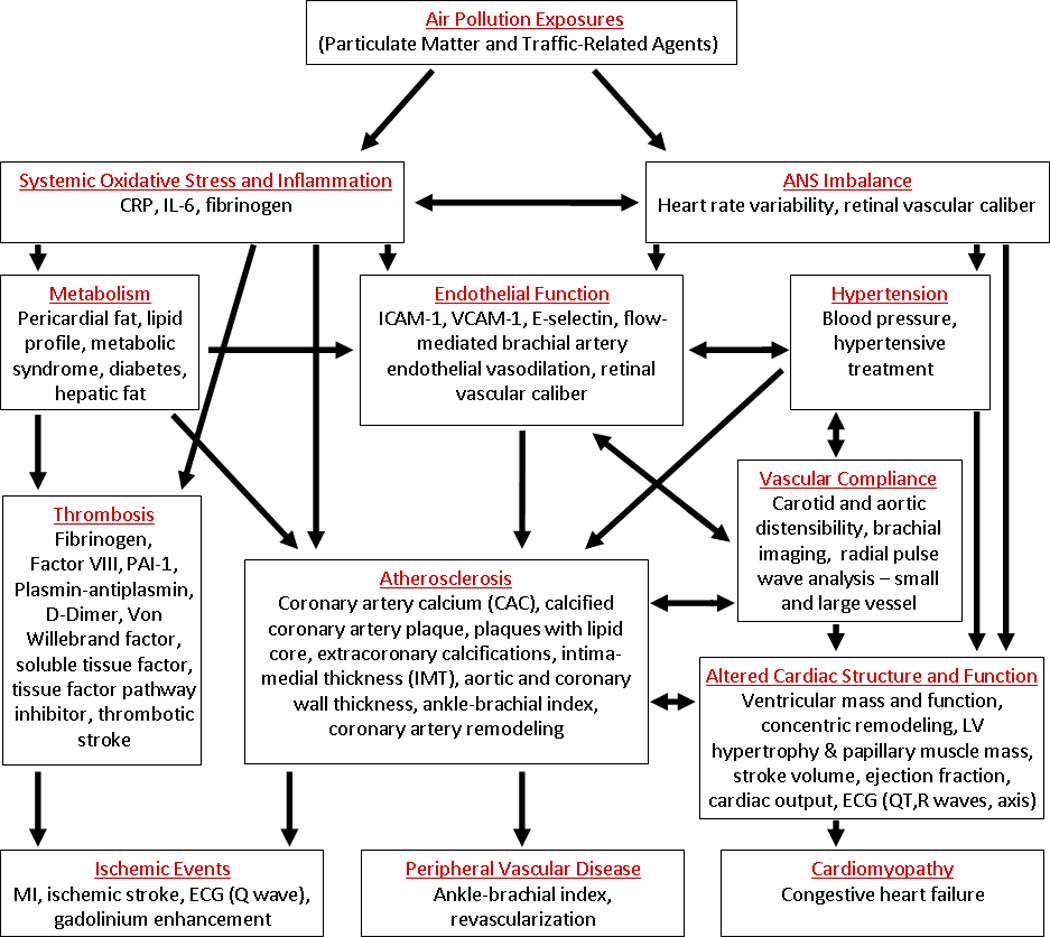
Physiological pathways by which air pollution may impact cardiovascular disease. General pathways/mechanisms are listed red, with potential indicators of these pathways listed below. All indicators listed are available as outcome measures in MESA.
As part of a project on effects of PM exposures on subclinical cardiovascular disease (R830543) funded in 2003 by the US Environmental Protection Agency (EPA), MESA investigators combined information from the Air Quality System (AQS), a national regulatory monitoring network that contains data collected by ~5000 EPA, state, local and tribal monitors, with information on other spatial covariates and recent and 20-year historical information on participants’ residential locations to estimate recent and long-term (20-year) exposures to air pollutants. Further, in 2004, the EPA funded a major new study within the MESA cohort, the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air, RD831697). This study leverages the NHLBI’s investment in MESA, and supplements that study with additional subjects, outcome measurements, and state-of-the-art cohort-specific air pollution exposure assessment for PM2.5oxides of nitrogen (NOX), and black carbon. Here we review the findings of initial work conducted as part of these two ancillary studies. These analyses have ranged from the basic science of gene association to clinical effects on blood pressure and heart rate variability. This manuscript describes the work to date on air pollution and CVD in MESA, organized by mechanistic pathway, and concludes with a description of MESA Air and its anticipated contributions.
Air Pollution and CVD Findings in the MESA Cohort
Inflammation
As the mechanism of particulate matter-induced cardiovascular events and acceleration of atherosclerosis has been hypothesized to involve generation of an inflammatory state, several studies have evaluated the association of inflammatory makers with particulate matter exposure. In MESA, the association between PM2.5 and C-reactive protein (CRP) was examined.[11] Data from the AQS monitoring system was used to estimate PM2.5 exposures for the prior day, prior 2 days, prior week, and prior 30 and 60 days. The 30 and 60 day mean exposures showed a weak positive association with CRP, though the confidence intervals were wide. After adjusting for appropriate person-level covariates, the relative increase in C-reactive protein (mg/liter) per 10 µg/m3 increase in fine particles was 3% (95% confidence interval [CI], −2, 10) for 30-day mean and 4% (95% CI, −3, 11) for a 60-day mean. It was concluded that fine particulate matter had little demonstrable effect. No association was seen between PM2.5 and interleukin-6. The finding of potentially some, albeit small and not statistically significant, increase in CRP is partially concordant with the findings of other investigators. [12–14] Of the studies that have examined this relationship, Peters[12] and Seaton[13] found more significant increases in CRP while Pope’s findings[14] were largely isolated to one particular subject in their sampling group
Autonomic Nervous System (ANS) Imbalance
Heart rate variability (HRV) is a marker of a healthy cardiovascular system. Reduction in HRV is associated with autonomic nervous system (ANS) imbalance and is seen in patients with diabetes, metabolic syndrome, and established coronary artery disease.[15] The mechanism for reduction in HRV appears to be related to connections between cardiac myocytes and hence connexins and gap junctions have been implicated. Previously, a number of investigators have shown epidemiologic data linking particulate matter exposure to decreased heart rate variability. [16–25] Only a few studies have been conducted in population-based cohorts.[20, 22, 23] Several of these studies found associations between PM exposure and autonomic dysfunction to be stronger among persons with type-2 diabetes.[20, 22, 24]
MESA investigators evaluated the effects of PM2.5 on HRV and effect modification by metabolic syndrome including central obesity, type-2 diabetes and hypertension.[26] In this study, PM2.5 concentrations were obtained from AQS monitor data for the 60 days before the day on which HRV measures were obtained, and average periods were assessed for the 1, 2, 7, 30, and 60 days prior to HRV measurement. After controlling for confounding variables, PM2.5 was associated with a 2.1% decrease in the root mean square of successive differences (rMSSD) (95%CI, −4.2, 0.0). The association was stronger among individuals with metabolic syndrome compared to those without, with an interquartile range elevation in 2-day PM2.5 associated with a 6.2% decrease in the rMSSD (95% CI, −9.4, −2.9) as compared to almost no change found in those without metabolic syndrome. These findings support the notion that the autonomic nervous system may well play a role in the mechanism of PM2.5-induced cardiovascular complications, especially when accompanied by the metabolic syndrome.
Hypertension
Inhaled particles may down-regulate nitric oxide synthase and affect autonomic dysfunction, and both mechanisms could increase blood pressure. Since nitric oxide is a vasodilator, reduced bioavailability would increase blood pressure, as would increased autonomic tone -- particularly sympathetic tone -- resulting in vascular vasoconstriction. Previously, a repeated measures study showed an association between particle inhalation and increasing blood pressure amongst cardiac patients.[27
In the MESA cohort, investigators employed AQS monitors to estimate PM2.5 exposures for the preceding 1, 2, 7, 30, and 60 days, and roadway location data were used to estimate local exposures to traffic-related pollutants.[28] Both systolic blood pressure (SBP) and pulse pressure were positively associated with exposure to PM2.5. The observed long-term associations were more dramatic than the short-term. A10-µg/m3 increase in PM2.5 30-day mean was associated with a 1.12 mmHg higher pulse pressure (95% CI, 0.28, 1.97) and a 0.99 mmHg higher SBP (95% CI, −0.15, 2.13). This difference in SBP was roughly equivalent to a 1.5 to 3.5 year aging affect seen in the cohort. The results were generally not statistically significant for mean arterial blood pressure and diastolic blood pressure.
Vascular Function
On a variety of scales, changes in vascular function can be assessed and hypothesized to play a role in air pollution’s effects, including the observed blood pressure findings. Animal studies have suggested the microvasculature may be particularly important.[29] In a recent study of the relationship between fine particulate matter exposures and the microvasculature in MESA, Adar et al. evaluated central retinal arteriolar and venular equivalents as measured by digital retinal photography.[30] Outdoor concentrations of PM2.5 were estimated at each participants’ home for the 2 years preceding the clinical exam using an early MESA Air exposure model, and short-term concentrations were assigned using AQS measurements on the day preceding the clinical exam. After adjusting for appropriate confounders, central retinal arteriolar equivalents were found to be narrower among persons residing in regions with increased long-term levels of PM2.5 and on days with high short-term levels of PM2.5. In joint exposure models, −0.8 µm (95% CI, −1.1, −0.5) and −0.4 µm (95% CI, −0.8, 0.1) decreases in central retinal arteriolar equivalents per interquartile increases in long- and short-term PM2.5 levels were observed.
Vascular Compliance
Increased vascular stiffness may also lie on the mechanistic pathway between air pollution exposures and cardiovascular risk, and stiffer arteries are associated with both higher pulse pressure and adverse ventricular remodeling. MESA investigators assessed the relationship between long-term (20-year) exposure to PM2.5 and PM10 and arterial stiffness as measured by Young’s modulus from carotid artery ultrasound and large and small artery vessel compliance from radial artery pulse-wave.[31] Twenty year-exposures were estimated based on a space-time model that combined historical AQS and residential history information with other spatial covariates to impute PM exposures for each month over the 20 years prior to outcome measurement.[32] However, the authors did not find an association between particulate matter exposure and any of these measures of arterial stiffness.
Urinary Albumin Excretion
Urinary albumin levels are used as a screening tool to evaluate renal function, especially in the setting of risk factors for microvascular impairment as in diabetes. Higher levels of urinary albumin result from glomerular changes, and albumin levels are well-correlated with microvascular dysfunction.[33] O’Neill et al. investigated the relationship between exposure to particulate matter and urinary albumin excretion.[34] PM2.5 and PM10 concentrations were estimated based on measurements at AQS monitors 1 month, 2 months, and 20 years prior to urine collection. Creatinine-adjusted albumin excretion was not found to be significantly associated with particulate matter exposures; per 10 µg/m3 increment of chronic PM10 exposure, the mean difference in the log of the urinary albumin/creatinine ratio was −0.02 (95% CI, −0.07, 0.03) adjusted for person-level covariates.
Altered Cardiac Structure and Function
Given the association between particulate matter and hypertension, a next step is to take this further and look at the actual effect on heart muscle. To this end, attention turned to studying the association of left ventricular mass (LVM) measured by cardiac MRI with air pollution.[35] In this study, investigators compared MESA participants living within 50 meters of a major roadway to those further than 150 meters away. Participants living very near to major roadways were observed to have a 1.4 g/m2 (95% CI, 0.3, 2.5) higher LVM index compared to those with residences rather away. This magnitude of increase in LVM is equivalent to the association with a 5.6 mmHg increase in systolic blood pressure in this cohort. Left ventricular systolic function, as estimated by ejection fraction, was not different comparing the two groups. Despite the effect of LVM being possibly equated to a 5.6 mmHg greater systolic blood pressure, there was no interaction between roadway proximity and hypertension (p=0.69 for interaction) or use of any antihypertensive medications (p=0.89 for an interaction). Although no association was found with estimated PM2.5 concentrations, the linkage between LVM and traffic-related air pollution is important since increased LVM is strongly associated with both heart failure and heart failure severity in patients with heart failure.[36
After establishing that proximity to traffic-related air pollution was associated with increased LVM, investigators evaluated 12 potential candidate genes that may play in a role of the ventricular hypertrophy.[37] The genes selected (ACE, ADRB2, AGT, AGTR1, ALOX15, EDN1, GRK4, PTGS1, PTGS2, TLR4, VEGFA, and VEGFB) were so chosen based on hypothesized role of their products on the mechanism for increased LVM. In this study, two genes with potentially compelling mechanisms stood out. Tag SNPs in the type 1 angiotensin II receptor (AGTR, rs680136) and arachidonate 15-lipoxygenase (ALOX15, rs2664593) genes were each significantly (q<0.2) associated with 9–10% differences in the association between LVM and residential proximity to major roadways. AGTR1 is particularly compelling because of well established impacts on inflammation and vasoconstriction.[38] Likewise, ALOX15 is felt to play a role in vascular inflammation and particularly in oxidative stress, the latter frequently implicated in the mechanism of particulate matter induced cardiovascular complications.[39
Atherosclerosis
Although particulate matter has consistently been associated with cardiovascular clinical events that are hallmarks of atherosclerotic disease, the proposed association with the development of atherosclerosis has not been confirmed. MESA is particularly well-suited to study the extent of subclinical atherosclerosis. There has been a significant effort over the last decade to develop methods of detecting subclinical atherosclerosis using imaging techniques. Carotid intima-medial thickness (IMT) and coronary artery calcium scoring (CAC) are two such measures that have been deemed promising, and these two measures are primary outcomes of interest within the MESA study. MESA investigators have evaluated the association of 20-year exposure to particulate matter with IMT, CAC, and ankle-brachial artery indices measured later in adulthood, at the end of the 20 year period.[40] The authors found that there was a small measureable effect on IMT, but not coronary calcium or ankle-brachial artery index. Even the effect on carotid IMT was small with the greatest relative difference between the 90th and the 10th percentiles risk being 1.04 (95%CI, 1.01, 1.07) for residentially stable subjects exposed to PM10 after control for coronary risk factors.
In addition to coronary artery calcium, Allen et al. conducted a cross-sectional analysis of abdominal aortic calcification as measured by CT within the MESA cohort.[41] The authors observed a slightly elevated, but non-significant, increased risk of aortic calcification (Relative Risk (RR) =1.06, 95% CI, 0.96, 1.16) with a 10 µg/m3 contrast in PM2.5. Greater effects were observed among participants with less exposure misclassification, such as those with long-term residence near a PM2.5 monitor (RR=1.11, 95% CI, 1.00, 1.22).
Next Steps: MESA Air
MESA is well positioned to answer many of the outstanding questions about the biological mechanisms by which air pollution exposures result in cardiovascular risk. Myriad study outcomes are available at numerous points along the potential pathways, and additional data are available to ensure appropriate confounder control. The research conducted to date has informed our understanding of the relationship between air pollution and cardiovascular disease, and has contributed to the available literature on the potential mechanisms underlying this relationship. However, the analyses to date have been cross-sectional in design and all have been limited by significant uncertainty in the air pollutant exposure estimates employed.
Large uncertainty in estimates of air pollution exposure is by no means restricted to studies occurring in the MESA cohort. In general, the collective research on long-term exposure to air pollution and cardiovascular disease is affected by measurement error and potential misclassification in exposure assessment. Further, no chronic air pollution epidemiology studies to date have been able to account for potentially important individual-level factors, such as the amount of time spent indoors or the infiltration efficiencies of outdoor pollutants into indoor environments.[42
In 2003, the Environmental Protection Agency developed a competitive application process to engage researchers in the design and implementation of a study specifically to reduce the uncertainty in understanding the relationship between air pollution exposure and cardiovascular disease.[43] This process resulted in the investment of substantial new resources for MESA Air, initiated in 2004. MESA Air is using this unusual dedication of resources to combine state-of-the-art epidemiology inherent in the parent MESA study with state-of-the-art exposure estimation. MESA Air can prospectively assess the relation between individual-level assessment of long-term air pollution exposures and both the progression of subclinical atherosclerosis and the incidence of cardiovascular disease events. Recognizing the importance of characterizing fine-scale variation in pollutant concentrations within metropolitan areas, MESA Air assigns an individual-level, temporally-resolved estimate of exposure to ambient pollution to each study participant.
MESA Air also supplements the parent MESA study with additional subjects to increase exposure heterogeneity. From 2005–2007, approximately 300 new participants were recruited from two geographic areas in the Los Angeles basin (Santa Monica/Coastal LA County and Rubidoux/Riverside County) and one in the New York City region (Rockland County). The Coastal LA area represents an upwind location relative to the city center, and was selected to represent a lower PM2.5 contrast within the LA basin. Riverside County was selected to represent a downwind location relative to the urban center, and as an area with some of the highest pollution levels in the nation. Rockland County, New York is upwind of the New York City region, which allows capture of similar regional scale pollution to those participants in northern Manhattan and the southern Bronx without the urban contribution. With the inclusion of these regions, the MESA Air communities provide a wide range of exposure characteristics across a variety of pollutants.
In addition to these new participants, MESA Air adds new outcome measures to the MESA study. In MESA Exam 5, currently scheduled from April 2010 through October 2011, MESA Air is adding 3600 additional CT scans and ultrasounds for CAC and IMT determination to allow measurement of progression of subclinical atherosclerosis for a full 10 years, concurrent with the relevant time period of exposure.
Perhaps most significantly, MESA Air adds state-of-the-art exposure estimation to MESA. Between July 2005 and July 2009, MESA Air deployed 7,420 two-week air samples throughout the six MESA cities and the three additional areas of new recruitment.[44] These samples were analyzed for PM2.5light absorbing carbon, NOXNO2NO, O3SO2and trace elements, and ancillary studies collected samples analyzed for elemental and organic carbon, endotoxin, and PM10-2.5 at a subset of locations. In addition to the measurements collected directly by the project, MESA Air employs AQS monitoring data, geographic data such as roadway density and land use, and dispersion model outputs in exposure models to identify seasonal and shorter-term time trends, to capture key sources of spatial variability, and to account for underlying spatial and spatio-temporal correlation.
Sampson et al.[45] describes a first iteration of the hierarchical spatio-temporal model developed as part of MESA Air to estimate outdoor PM2.5 concentrations at each participant’s residence, using a multi-step pragmatic estimation procedure, and Szpiro et al.[46] presents a similar model for predicting residential NOX concentrations, employing a unified estimation approach based on efficient maximum likelihood calculations. Ultimately, all of these estimates will incorporate exposure variation due both to pollution infiltration into each participant’s residence and participant-specific time-activity patterns.
With one exception (Adar et al.[30]), the studies previously described have not used pollutant estimates generated through MESA Air, as these “next-generation” estimates only recently became available. The previous studies have either used distance to major roadway as a proxy for exposure to traffic-related air pollution, have used data from the nearest AQS monitors, or have employed modeled long-term exposures developed to estimate outdoor concentrations based on AQS data and spatial covariates.[32] Moving forward, estimates developed by MESA Air will be available for all MESA investigators, and the use of these new estimates will substantially reduce the uncertainty associated with estimating air pollution exposures in the cohort. The availability of enhanced pollutant estimates provided by MESA Air, which will be available for the full cohort in 2012 to coincide with conclusion of the fifth MESA clinical examination, will enable more complete assessment of the pathways already examined. It is even possible that some of the early outcomes reported on in this review will be re-visited with the new exposure estimates.
MESA Air is designed to provide the most advanced approach feasible to understanding the relationship between air pollution and cardiovascular disease. The range of exposures under study is extremely relevant – the annual averages observed are largely at or below regulatory levels.[47–50] Both the clinical and subclinical health measures included in this study are cutting-edge, and reflect multiple pathways and different levels of severity within the same participant. The multi-ethnic study design improves the generalizability of the results. Sufficient populations with key putative risk factors are included, and the many outcomes available will illuminate possible mechanistic pathways underlying a relationship that, while generally acknowledged, is still somewhat uncertain.
Table 1.
Summary of studies of air pollution and cardiovascular disease in MESA.
| Author/Year | Outcome | Result (95% CI) |
|---|---|---|
| Diez Roux et al. 2006 | C-reactive protein | 3% (−2, 10) increase for 30-d mean PM2.5 4% (−3, 11) increase for 60-d mean PM2.5 |
| Park et al. 2010 | Heart rate variability | 2.1% (−4.2, 0.0) decrease in root mean square of successive differences for interquartile range increase in 2-day average PM2.5 (stronger in people with metabolic syndrome) |
| Adar et al. 2010 | Central retinal arteriolar and venular equivalents |
−0.8 µm (−1.1, −0.5) and −0.4 µm (−0.8, 0.1) decreases in central retinal arteriolar equivalents and 0.9 µm (0.4, −1.4) and 0.4 µm (0.3, 1.1) increases in central retinal venular equivalents per interquartile increases in long- and short-term PM2.5 levels |
| Auchincloss et al. 2008 | Systolic blood pressure, pulse pressure |
SBP: 0.99 mmHg (0.15, 2.13) increase for 30-d mean PM2.5 Pulse pressure: 1.12 mmHg (0.28, 1.97) increase for 30 d-mean PM2.5 |
| O’Neill et al. 2010 | Arterial stiffness | No significant associations; for example, large and small artery vessel compliance values were 0.0 (−0.8, 0.8) and 0.2 (−0.6, 1.0), respectively, for annual average nearest monitor PM2.5 |
| O’Neill et al. 2008 | Urinary albumin excretion | No significant associations; mean difference in log of urinary albumin/creatinine ratio of −0.02 (−0.07, 0.03) chronic PM10 exposure |
| Van Hee et al. 2009 | Left ventricular mass | 1.4 g/m2 increase in LVM for participants living closer to a major roadway |
| Van Hee et al. 2010 | Left ventricular mass (gene-environment interactions) |
Tag SNPs in the type 1 angiotensin II receptor and arachidonate 15-lipoxygenase genes were each significantly (q < 0.2) associated with 9–10% differences in the association between LVM and proximity to major roadways |
| Diez Roux et al. 2008 | Coronary artery calcium, intima-medial thickness |
Relative risk of 1.04 (1.01, 1.07) for carotid IMT for participants exposure to PM10 with coronary risk factors; No significant effect for CAC |
| Allen et al. 2009 | Aortic calcification | Relative risk of 1.06 (0.96, 1.16) for abdominal aortic calcification with a 10 µg/m3 increase in PM2.5 |
Acknowledgment
The research described here was supported by National Heart, Lung, and Blood Institute contracts N01-HC-95159 through N01-HC-95165, N01-HC-95169, R01 HL077612, HL070151, HL071205, HL071251-52, HL071258-59; U.S. Environmental Protection Agency Science to Achieve Results R830543 and RD831697; and National Institute of Environmental Health Sciences grants P50ES015915, P30ES07033, and K24ES013195.
Abbreviations
- AQS
Air Quality System
- ANS
Autonomic Nervous System
- CVD
Cardiovascular Disease
- CRP
C-reactive protein
- CT
Computed Tomography
- CAC
Coronary Artery Calcium
- CI
Confidence Interval
- EPA
Environmental Protection Agency
- HRV
Heart Rate Variability
- IMT
Intima-Medial Thickness
- LVM
Left Ventricular Mass
- PM10
Particulate Matter Less than 10 Microns in Aerodynamic Diameter
- PM2.5
Particulate Matter Less than 2.5 Microns in Aerodynamic Diameter
- MRI
Magnetic Resonance Imaging
- MESA
Multi-Ethnic Study of Atherosclerosis
- MESA Air
Multi-Ethnic Study of Atherosclerosis and Air Pollution
- NHLBI
National Heart, Lung, and Blood Institute
- NOX
Oxides of Nitrogen
- rMSSD
Root Mean Square of Successive Differences
- RR
Relative Risk
- SBP
Systolic blood pressure
Contributor Information
Edward A. Gill, Department of Medicine, University of Washington, Seattle, WA.
Cynthia L. Curl, Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, WA.
Sara D. Adar, Department of Epidemiology, University of Michigan, Ann Arbor, MI.
Ryan W. Allen, Department of Health Sciences, Simon Fraser University, Burnaby, BC.
Amy H. Auchincloss, Department of Epidemiology and Biostatistics, Drexel University, Philadelphia, PA.
Marie S. O’Neill, Department of Epidemiology and Environmental Health Sciences, University of Michigan, Ann Arbor, MI.
Sung Kyun Park, Department of Epidemiology, University of Michigan, Ann Arbor, MI.
Victor C. Van Hee, Department of Medicine, University of Washington, Seattle, WA.
Ana V. Diez Roux, Department of Epidemiology, University of Michigan, Ann Arbor, MI.
Joel D. Kaufman, Departments of Environmental and Occupational Health Sciences, Epidemiology, Medicine, University of Washington, Seattle, WA.
References
- 1.Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 4.Hoek G, Brunekreef B, Goldbohm S, et al. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 5.Ostro B, Lipsett M, Reynolds P, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California teachers study. Environ Health Perspect. 2010;118:363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehring U, Heinrich J, Kramer U, et al. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 2006;17:545–551. doi: 10.1097/01.ede.0000224541.38258.87. [DOI] [PubMed] [Google Scholar]
- 7.Rosenlund M, Berglind N, Pershagen G, et al. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 2006;17:383–390. doi: 10.1097/01.ede.0000219722.25569.0f. [DOI] [PubMed] [Google Scholar]
- 8.Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Diez Roux AV, Auchincloss AH, Astor B, et al. Recent exposure to particulate matter and C-reactive protein concentration in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;164:437–448. doi: 10.1093/aje/kwj186. [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Frohlich M, Doring A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 13.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope CA, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerritsen J, Dekker JM, TenVoorde BJ, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease - The Hoorn study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 16.Creason J, Neas L, Walsh D, et al. Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol. 2001;11:116–122. doi: 10.1038/sj.jea.7500154. [DOI] [PubMed] [Google Scholar]
- 17.Devlin RB, Ghio AJ, Kehrl H, et al. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. European Respiratory Journal. 2003;21:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 18.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 19.Holguin F, Tellez-Rojo MM, Hernandez M, et al. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology. 2003;14:521–527. doi: 10.1097/01.ede.0000081999.15060.ae. [DOI] [PubMed] [Google Scholar]
- 20.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: The VA Normative Aging Study. Environmental Health Perspectives. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. American Journal of Respiratory and Critical Care Medicine. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 22.Whitsel EA, Quibrera PM, Christ SL, et al. Heart rate variability, ambient particulate matter air pollution, and glucose homeostasis: the environmental epidemiology of arrhythmogenesis in the women's health initiative. Am J Epidemiol. 2009;169:693–703. doi: 10.1093/aje/kwn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao D, Duan Y, Whitsel EA, et al. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 2004;159:768–777. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- 24.Dubowsky SD, Suh H, Schwartz J, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environmental Health Perspectives. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adar SD, Gold DR, Coull BA, et al. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 26.Park SK, Auchincloss AH, O'Neill MS, et al. Particulate air pollution, metabolic syndrome, and heart rate variability: the multi-ethnic study of atherosclerosis (MESA) Environ Health Perspect. 2010;118:1406–1411. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 28.Auchincloss AH, Diez-Roux AV, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) . Environmental Health Perspectives. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurkiewicz TR, Porter DW, Barger M, et al. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environmental Health Perspectives. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adar SD, Klein R, Klein BE, et al. Air pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA) Plos Medicine. 2010;7:e1000372. doi: 10.1371/journal.pmed.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neill MS, Diez-Roux AV, Auchincloss AH, et al. Long-term exposure to airborne Particles and arterial stiffness: The Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. doi: 10.1289/ehp.0901524. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghunathan T, Diez Roux AV, Chen W. Predicting cumulative particulate matter exposure using space-time models and historical monitor data. Epidemiology. 2006;17:S250. [Google Scholar]
- 33.Strain WD, Chaturvedi N, Bulpitt CJ, et al. Albumin excretion rate and cardiovascular risk: could the association be explained by early microvascular dysfunction? Diabetes. 2005;54:1816–1822. doi: 10.2337/diabetes.54.6.1816. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill MS, Diez-Roux AV, Auchincloss AH, et al. Airborne particulate matter exposure and urinary albumin excretion: the Multi-Ethnic Study of Atherosclerosis. Occup Environ Med. 2008;65:534–540. doi: 10.1136/oem.2007.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Hee VC, Adar SD, Szpiro AA, et al. Exposure to traffic and left ventricular mass and function: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2009;179:827–834. doi: 10.1164/rccm.200808-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 37.Van Hee VC, Adar SD, Szpiro AA, et al. Common genetic variation, residential proximity to traffic exposure, and left ventricular mass: the multi-ethnic study of atherosclerosis. Environ Health Perspect. 2010;118:962–969. doi: 10.1289/ehp.0901535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gradman AH. Evolving understanding of the renin-angiotensin-aldosterone system: pathophysiology and targets for therapeutic intervention. Am Heart J. 2009;157:S1–S6. doi: 10.1016/j.ahj.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Dogne JM, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends in Pharmacological Sciences. 2005;26:639–644. doi: 10.1016/j.tips.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Diez Roux AV, Auchincloss AH, Franklin TG, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–675. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- 41.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20:254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman JD. Does air pollution accelerate progression of atherosclerosis? J Am Coll Cardiol. 2010;56:1809–1811. doi: 10.1016/j.jacc.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.USEPA. Epidemiologic Research on Health Effects of Long-term Exposure to Ambient Particulate Matter and Other Air Pollutants. Washington DC: 2003. Retrieved from http://epagov/ncer/rfa/current/2003_pm_epi.html. [Google Scholar]
- 44.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43:4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampson P, Szpiro A, Sheppard L, et al. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. BE Press University of Washington Biostatistics Working Paper. 2009;353 [Google Scholar]
- 46.Szpiro AA, Sampson PD, Sheppard L, et al. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2010;21:606–631. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.USEPA. [[cited 2010 1/11/11]];National Ambient Air Quality Standards (NAAQS) 2010 Available from: http://www.epa.gov/oar/criteria.html.
- 48.EUROPA. [[cited 2011 1/11/11]];European Commission Environment Air Quality Standards. 2010 Available from: http://ec.europa.eu/environment/air/quality/standards.htm.
- 49.WHO. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide -- Global Update 2005 -- Summary of Risk Assessment. Geneva, Switzerland: 2005. [Google Scholar]
- 50.HealthCanada. [[cited 2011 1/11/11]];National Ambient Air Quality Objectives (NAAQOs) 2010 Available from: http://www.hc-sc.gc.ca/ewh-semt/air/out-ext/reg-eng.php.


