To the Editor
The etiology of allergic disease is generally considered to be a complex polygenic process highly influenced by environmental exposures with mast cells playing a major effector role. Genome-wide association studies and candidate gene approaches have produced an array of allelic variants which impart risk for allergic diatheses. (E1) While identification of monogenic allergic disease and inherited mast cell disorders has remained less common, several heritable mutations have been identified which help illuminate some pathophysiologic mechanisms underlying the development of atopy in the general population. These include: SPINK5 mutations in Netherton syndrome (1); dominant negative STAT3 mutations in a hyper-IgE syndrome (2); and PLCG2 mutations in a form of familial cold urticaria. (3) Within the mast cell compartment, both germ line and somatic mutations in KIT have been identified in rare familial presentations of mastocytosis. (4, 5)
While seeking to characterize patients with severe, unique, inherited allergic phenotypes among patients referred for evaluation of severe allergic skin, airway or gastrointestinal disease, or systemic mastocytosis (SM), we identified nine atopic individuals with persistent elevations in serum basal tryptase levels in the absence of evidence for a clonal mast cell disorder. Thorough clinical evaluations were undertaken, total and fractionated serum tryptase levels were obtained, in vitro basophil activation was assayed, and bone marrow biopsies were performed in 5 of these index patients. For a complete description see the Methods section in this article’s Online Repository at www.jacionline.org. Elevated basal serum tryptase was found to segregate with distinct clinical features and it was noted that multiple family members of each index patient shared elements of this phenotype. Serum tryptase levels were obtained in all available family members and an autosomal dominant inheritance pattern of elevated basal total serum tryptase was revealed in 9 families. (Table I) The mean basal total serum tryptase obtained during a resting state was 21.6 ± 1.4 ng/mL among individuals with inherited tryptasemia (n = 33). Five family members’ tryptase levels were unable to be obtained; these data were excluded from statistical analysis, but are included with unaffected family members in Table E1 of this article’s Online Repository at www.jacionline.org. Tryptase fractionation in a subset of 18 affected patients from 8 of 9 families further revealed that mature tryptase in the serum was undetectable (<1 ng/mL). (see Table E2 in this article’s Online Repository at www.jacionline.org)
Table I.
Pedigrees, ages, and clinical phenotypes of affected individuals
Family 1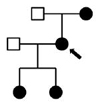
|
Family 2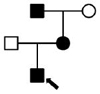
|
Family 3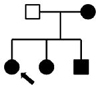
|
Family 4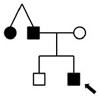
|
Family 5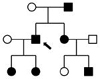
|
Family 6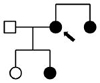
|
Family 7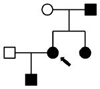
|
Family 8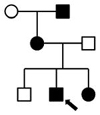
|
Family 9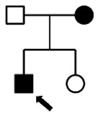
|
|
|---|---|---|---|---|---|---|---|---|---|
| Ages | 8, 10, 44, 70 |
11, 39, 69 |
6, 10,13, 41 |
21, 55, 55 |
17, 22, 23, 48, 57, 78 |
24, 43, 50 |
2, 26, 29, 53 |
12, 20, 44, 68 |
9, 46 |
|
| |||||||||
|
Symptoms No. affected |
|||||||||
|
| |||||||||
| Cutaneous | 4 | 1 | 4 | 3 | 5 | 2 | 2 | 2 | 2 |
|
| |||||||||
| Flushing | 1 | - | 2 | 3 | 4 | 1 | 1 | 1 | - |
| Urticaria | 4 | - | 3 | - | 5 | 2 | 2 | 1 | 1 |
| Angioedema | - | - | 1 | - | 3 | - | - | 1 | - |
| Pruritis | 4 | 1 | 2 | 1 | 5 | 1 | 1 | - | 2 |
|
| |||||||||
| Connective Tissue | 3 | 3 | 4 | 3 | 0 | 2 | 3 | 3 | 2 |
|
| |||||||||
| Hypermobility | 2 | 1 | 4 | 1 | - | 2 | 3 | 2 | - |
| Retained PrimaryTeeth | - | 3 | - | 1 | - | - | - | 1 | - |
| Arched Palate | 1 | 1 | 2 | - | - | 1 | 1 | - | 2 |
| Bone/Vascular abnormality1 | 2 | - | - | 3 | - | - | 1 | 2 | - |
|
| |||||||||
| Atopy | 4 | 3 | 4 | 3 | 6 | 3 | 2 | 4 | 2 |
|
| |||||||||
| Anaphylaxis | - | 2 | 1 | 2(1†) | 3 | - | 1 | 1† | - |
| Eczema | 3 | 1 | 3 | - | 3 | - | 1 | 2 | 2 |
| Asthma | 4 | 2 | 2 | 2 | 4 | 1 | 2 | 1 | 1 |
| Environmental Allergy | 4 | 2 | 2 | 2 | 4 | 3 | 1 | 3 | 2 |
| Food Allergy | 3 | 2 | 1 | 1 | 4 | - | 1 | 1 | - |
| Drug Allergy | 3 | 1 | 25 | 1 | 5 | 2 | 2 | 1 | - |
|
| |||||||||
| Gastrointestinal | 4 | 3 | 4 | 3 | 5 | 2 | 3 | 4 | 0 |
|
| |||||||||
| Episodic Pain | 2 | - | 2 | 3 | 3 | 2 | 3 | 2 | - |
| IBS/Episodic Diarrhea | 1 | 14 | 3 | - | 4 | 2 | 2 | 1 | - |
| GERD | 4 | 1 | 3 | 2 | 3 | 1 | 2 | 2 | - |
| Eosinophilic Esophagitis | 1 | 2 | - | 1 | - | - | - | - | - |
| Failure to Thrive in Infancy | 1 | - | 3 | - | - | - | - | - | - |
|
| |||||||||
| Neuropsychiatric | 4 | 1 | 3 | 2 | 3 | 2 | 4 | 3 | 2 |
|
| |||||||||
| Dysautonomia2 | 4 | - | 3 | - | 2 | 1 | - | - | - |
| Anxiety/Depression | 4 | 1 | - | 1 | 2 | - | - | 2 | - |
| Behavioral Disorder | 2 | 1 | 2 | - | 1 | 1 | 1 | - | - |
| Chronic Pain3 | 2 | - | 1 | 2 | 2 | 1 | 3 | 1 | - |
| Developmental Delay | 1 | 1 | - | - | - | - | 1 | - | 2 |
indicates hymenoptera hypersensitivity;
abormalities include: pectus excavatum, plagio-/brachycephaly, torticollis, femoral anteversion, tibial torsion, congenital mitral valve insufficiency, tortuous/ectatic intracranial vessels, and scoliosis;
principally manifest as Postural Orthostatic Tachycardic Syndrome (POTS);
arthralgia, fibromyalgia, and vulvodynia;
encopresis;
immediate hypersensitivity reaction to radiocontrast media in one patient
Prominent among features segregating with basal serum tryptase elevations were symptoms consistent with chronic and episodic mast cell degranulation, with 26/33 reporting episodic urticaria, flushing, and/or cramping abdominal pain, frequently associated with urgency and/or diarrhea. These symptoms could be unprovoked, or triggered by heat, exercise, vibration, emotional stress, non-specific foods, or minor physical trauma. A history of anaphylaxis was reported in 10/33 individuals from six different families; seven occurring with exposure to foods, two due to insect stings, and another without an identified trigger. Additional triggers in three individuals with food-mediated anaphylaxis included stress/heat/exercise (n = 2) and anesthesia (n = 1). (Table I) One individual (Family 1) with normal serum tryptase (6.6 ng/mL) had a history of anaphylaxis; this reaction was due to hymenoptera envenomation. (see Table E1 in this article’s Online Repository at www.jacionline.org)
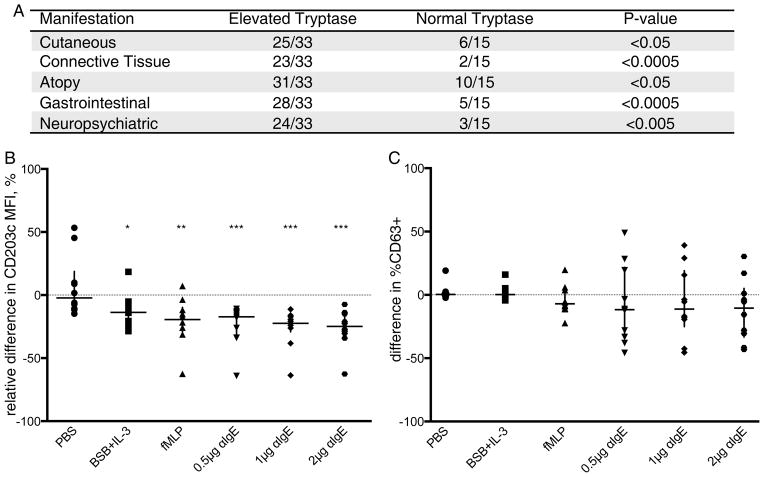
Gastrointestinal manifestations, whether chronic or episodic, were another prominent feature seen in 28/33 individuals with elevated tryptase; these included eosinophilic gastrointestinal disease, gastroesophageal reflux disease (GERD), tenesmus, fecal urgency, irritable bowel syndrome (IBS), and diarrhea. Atopic symptoms were present in 31/33 tryptasemics, with environmental allergies and asthma being reported amongst 28/33 affected individuals. Connective tissue abnormalities were present in 23/33 individuals with elevated tryptase from 8/9 families, and chronic musculoskeletal pain was present among 11/33 tryptasemic individuals from 6/9 families. Autonomic dysfunction, manifest as postural orthostatic tachycardia syndrome (POTS) was reported in 10/33 affected individuals and a neuropsychiatric diagnosis was present in 17/33 affected individuals. (Table I) Each of these five clusters of clinical characteristics (cutaneous, connective tissue, GI, atopic, neuropsychiatric) were independently and significantly associated with elevated tryptase. (Figure 1A) For additional clinical characterization of the families see the Results section in this article’s Online Repository at www.jacionline.org.
Figure 1. Phenotypic Characteristics and in vitro basophil activation of patients with inherited tryptase elevation.
A, Clinical traits and vibratory challenge results independantly and significantly associated with inherited elevations in basal serum tryptase; Fisher’s exact test. B, Relative difference of CD203c MFI in affected individuals normalized to paired controls, as a percent of paired control MFI, ((Patient-Control)/Control)*100). C, Difference in %CD63+ within the CD203c+ population of affected individuals, from paired controls. Data in C and D are plotted with median ± interquartile range. *P<0.05 **P<0.01 ***P<0.005; Wilcoxon-signed rank test.
Because some of the reported symptoms in tryptasemic individuals could have been consistent with the familial presentation of a clonal mast cell disorder, index patients in 5/9 families underwent bone marrow biopsy. A significant increase in mast cells was observed (P<0.05) (mean 9.6 cells/HPF, range 4–19.4) compared to normal volunteers (mean 2.5 cells/HPF, range 1–5). (see Figure E1 in this article’s Online Repository at www.jacionline.org) However none of the patients met the WHO established criteria for a diagnosis of SM or Monoclonal Mast Cell Activation (MMAS). (E2) Mast cell aggregates were not present, aberrant expression of CD2/CD25 was absent, and KIT D816V mutation testing was negative in all 5 patients. Spindled shaped mast cells (>25%) were observed in a single patient. In the 4 families in which a bone marrow biopsy was not performed, the clinical phenotype aligned with the cohort in which SM was excluded.
In vitro basophil activation was assessed in affected individuals from 6 of 9 families (n = 10). While basal CD203c expression was comparable to paired healthy controls, basophils from affected individuals demonstrated reduced activation, as measured by CD203c mean fluorescence intensity (MFI) following stimulation with anti-IgE or fMLP. (Figure 1B) However, CD63 induction within the CD203c+ basophil population, while variable, was not significantly different between patients and paired controls. (Figure 1C)
We report a series of families with elevated basal serum pro-tryptase following a dominant inheritance pattern associated with symptoms consistent with mast cell mediator release and additional non-atopic features. Phenotypic characteristics common in this syndrome include episodic flushing, pruritus, gastrointestinal symptoms and chronic musculoskeletal pain – symptoms frequently observed in SM. Additional characteristics not classically associated with inherited or somatic clonal mast cell diseases included urticaria, EoE, and a hypermobile connective tissue phenotype. Despite bone marrow findings showing increased numbers of mast cells among those patients sampled, a diagnosis of SM or MMAS was not established in these individuals. The mechanism resulting in impaired basophil activation in vitro remains unknown. Suppression of CD203c by endogenous histamine, (6) or impaired activation due to a refractory state, are possible explanations. (E3) Caution must be exercised with regard to generalization of the identified associations given the small sample size and preliminary nature of this report; additional discretion must be exercised with regard to those data which were derived from a sub-population of our cohort. Furthermore, several potential biases may exist in these data with regard to referral, detection, and recall. However tryptase is a robust biomarker and the finding of families with inherited elevations is novel.
Whether elevations in serum tryptase and mast cell numbers are primary or secondary phenomena is currently under investigation, however this phenotype appears to represent a distinct familial variant in the spectrum of mast cell disorders. This cohort shares many clinical features observed in both mastocytosis and Mast Cell Activation Syndrome (MCAS) and it remains uncertain how many patients diagnosed with MCAS may have dominantly inherited tryptasemia. (7) While symptoms of mast cell mediator release among multiple first-degree relatives of patients with diagnosed mast cell disorders have been described, (E4) the presence of connective tissue disease provides further distinction of this familial phenotype. Recently other previously characterized connective tissue syndromes (Loeys-Dietz, Marfan’s, and Ehlers-Danlos Syndromes) with some features common to our cohort were also found to be associated with atopic disease. (8, 9) Additional genetic study is ongoing, and as the dysfunctional pathways in these families are elucidated, we may gain additional insights into the hypermobile connective tissue phenotype, atopic disease, autonomic dysfunction, neuropsychiatric illness may be gained, and their interface with mast cell mediators.
Supplementary Material
Acknowledgments
Funding: This study was supported by the Division of Intramural Research of the NIAID, NIH; by NIH U19AI77435; and in part by grants from Food Allergy Research and Education (formerly the Food Allergy and Anaphylaxis Network [FAAN]), Food Allergy Project [FAP], Food Allergy Initiative [FAI]), the Buckeye Foundation, and the Campaign Urging Research for Eosinophilic Diseases (CURED) Foundation. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
We thank the patients, their families, and the healthy volunteers for participating in this study, as well as the clinical staff of the LAD and the University of Cincinnati for their efforts. We would also like to thank Alexandra Freeman for her collaboration and clinical efforts, as well as Deena Abdulazeez of VCU for performing the tryptase immunoassays.
Abbreviations used
- POTS
Positional Orthostatic Tachycardic Syndrome
- EDS
Ehlers-Danlos Syndrome
- LDS
Loeys-Dietz Syndrome
- SPINK5
serine peptidase inhibitor, kazal type 5
- PLAID
PLCG2-associated antibody deficiency and immune dysregulation
- PLCG2
phospholipase C, gamma 2
- STAT3
signal transducer and activator of transcription 3
- GERD
gastroesophageal reflux disease
- IBS
Irritable Bowel Syndrome
- EoE
eosinophilic esophagitis
- AD
atopic dermatitis
- SM
systemic mastocytosis
- WHO
World Health Organization
- BSB
basophil stimulation buffer
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- HPF
high power field
- MFI
mean fluorescence intensity
- PCR/RFLP
polymerase chain reaction/restriction fragment length polymorphism
- TGF-β
transforming growth factor beta
- PBMCs
peripheral blood mononuclear cells
Footnotes
Conflicts of Interest
The NIH authors declare no conflict of interest. LBS receives royalties from VCU that are collected from ThermoFisher for the tryptase UniCAP assay. MER has received consultancy fees from Immune Pharmaceuticals; has patents owned by CCHMC; may receive royalties from Teva Pharmaceuticals; and has stock/stock options in Immune Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chavanas S, Garner C, Bodemer C, Ali M, Teillac DH, Wilkinson J, et al. Localization of the Netherton syndrome gene to chromosome 5q32, by linkage analysis and homozygosity mapping. American journal of human genetics. 2000 Mar;66(3):914–21. doi: 10.1086/302824. Epub 2000/03/11.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. The New England journal of medicine. 2007 Oct 18;357(16):1608–19. doi: 10.1056/NEJMoa073687. Epub 2007/09/21.eng. [DOI] [PubMed] [Google Scholar]
- 3.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. The New England journal of medicine. 2012 Jan 26;366(4):330–8. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang X, Boxer M, Drummond A, Ogston P, Hodgins M, Burden AD. A germline mutation in KIT in familial diffuse cutaneous mastocytosis. Journal of medical genetics. 2004 Jun;41(6):e88. doi: 10.1136/jmg.2003.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanotti R, Simioni L, Garcia-Montero AC, Perbellini O, Bonadonna P, Caruso B, et al. Somatic D816V KIT mutation in a case of adult-onset familial mastocytosis. J Allergy Clin Immunol. 2013 Feb;131(2):605–7. doi: 10.1016/j.jaci.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Chirumbolo S, Brizzi M, Ortolani R, Vella A, Bellavite P. Inhibition of CD203c membrane up-regulation in human basophils by high dilutions of histamine: a controlled replication study. Inflammation research : official journal of the European Histamine Research Society [et al] 2009 Nov;58(11):755–64. doi: 10.1007/s00011-009-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. International archives of allergy and immunology. 2012;157(3):215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013 Apr 19; doi: 10.1016/j.jaci.2013.02.030. Epub 2013/04/24.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Science translational medicine. 2013 Jul 24;5(195):195ra94. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.