Abstract
Importance
To effectively guide interventions aimed at reducing mortality in low-volume hospitals, the underlying mechanisms of the volume-outcome relationship must be further explored. Reducing mortality after major post-operative complications may represent one point along the continuum of patient care that could significantly impact overall hospital mortality.
Objective
To determine whether increased mortality at low-volume hospitals performing cardiovascular surgery is a function of higher post-operative complication rates or of less successful rescue from complications.
Design
We utilized patient-level data on Medicare beneficiaries undergoing coronary artery bypass grafting, aortic valve repair, or abdominal aortic aneurysm repair. For each operation, we first divided hospitals into quintiles of procedural volume. We then assessed hospital risk-adjusted rates of mortality, major complications, and “failure to rescue” (i.e., case fatality among patients with complications) within each volume quintile.
Setting
Medicare fee-for-service beneficiaries age 65 to 99.
Participants
A total of 119,434 Medicare beneficiaries undergoing one of three major cardiovascular operations between 2005 and 2006.
Exposure
Hospital procedural volume.
Main Outcome Measure
Hospital rates of risk-adjusted mortality, major complications, and failure to rescue.
Results
For each operation, hospital volume was more strongly related to failure to rescue rates than to complication rates. For example, patients undergoing aortic valve replacement at very low-volume hospitals (lowest quintile) were 12% more likely to have a major complication than those at very high-volume hospitals (highest quintile), but 57% more likely to die if a complication occurs.
Conclusion and Relevance
High-volume and low-volume hospitals performing cardiovascular surgery have similar complication rates but disparate failure to rescue rates. While preventing complications is important, hospitals should also consider interventions aimed at quickly recognizing and managing complications once they occur.
Although the volume-outcome relationship is well-recognized in cardiovascular surgery, minimal progress has been made at reducing the mortality disparity between high and low-volume centers. However, cardiovascular disease is distinguished from many other clinical entities by a long history of standardization in processes of care. For instance, in 1987 the Northern New England Cardiovascular Disease Study Group created a patient registry with the aim to “develop and exchange information concerning the treatment of cardiovascular disease.”1 The Group harnessed benchmarking and continuous quality improvement initiatives to disseminate gains in clinical care. Furthermore, their data has been used by accreditation bodies such as The Joint Commission and the American Heart Association to create practice guidelines.2,3 For instance, the latter has recommended hospitals use volume thresholds to reduce morbidity and mortality.4 Although increased surgeon and hospital volume significantly improves outcomes in cardiovascular surgery, the benefit is substantially less pronounced than in other specialties.5,6 This may suggest that for cardiovascular surgery, standardization of perioperative care has left less room for improvement.
However, differences in mortality may not be solely a function of volume. Recent research has identified failure to rescue (mortality after a major complication) as one system-level factor influencing post-operative mortality.7 Prior studies of gastrectomy, pancreatectomy, and esophagectomy demonstrate little variation in hospital post-operative complication rates but striking variation in failure to rescue rates.8 The latter measure reflects a hospital’s capacity to expeditiously diagnose and appropriately manage complications. In turn, this capacity is largely dictated by the ability of teams to work cohesively during times of crisis.9 Thus, poor performing hospitals may have the opportunity to improve outcomes through better teamwork.
In this context, we sought to examine the effect of failure to rescue on mortality. We hypothesize that failure to rescue will account for excess mortality at low-volume hospitals. We used two years of data from the US Medicare population for three high-risk cardiovascular operations.
Methods
Data source and patient population
We used the Centers for Medicare and Medicaid Services’ Medicare Provider Analysis and Review (MedPAR) files for 2005 and 2006 to obtain patient-level data. MedPAR includes all claims submitted by hospitals for inpatient services provided to Medicare beneficiaries. It catalogues age, gender, race, admission and discharge dates, 30-day mortality, and the International Classification of Disease 9th Revision, Clinical Modification (ICD-9-CM) codes for both primary and secondary diagnoses.
We included all patients between the ages of 65 and 99 with ICD-9-CM codes for coronary artery bypass grafting (CABG), aortic valve repair (AVR), or abdominal aortic aneurysm repair (AAA). These operations were chosen as each has previously exhibited a volume-outcome relationship and all are associated with significant morbidity and mortality.9
Hospital Volume
First, we tabulated individual hospital volume for each of the three procedures. Second, we divided the hospitals into quintiles of procedure volume (Table 1).
Table 1.
Patient Characteristics by Hospital Volume
| Lowest volume hospitals |
Highest volume hospitals |
|
|---|---|---|
| CABG | (n=37,881) | (n=37,510) |
| Median age | 73.9 | 73.9 |
| Gender (% male) | 68.6% | 68.1% |
| Black race | 6.6% | 6.4% |
| 3+ comorbidities | 24.9% | 23.9% |
| AVR | (n=11,820) | (n=11,533) |
| Median age | 76.0 | 76.7 |
| Gender (%male) | 58.1% | 58.4% |
| Black race a | 4.5% | 2.7% |
| 3+ comorbidities | 21.5% | 18.0% |
| AAA | (n=10,541) | (n=10,149) |
| Median age | 75.2 | 75.8 |
| Gender (%male) | 72.1% | 75.9% |
| Black race a | 5.9% | 3.7% |
| 3+ comorbidities | 27.6% | 26.3% |
p-value <0.05
Hospital Mortality, Complications, and Failure to Rescue
Hospital mortality was defined as 30-day or in-hospital mortality. Using methods previously validated by The Complications Screening Program, we identified eight major postoperative complications by ICD-9-CM codes and divided them into medical and surgical groups.10 The medical group included: pulmonary failure, pneumonia, myocardial infarction, deep venous thrombosis/pulmonary embolism, acute renal failure, and gastrointestinal bleeding. The surgical group included: post-operative hemorrhage, surgical site infection (eTable 1). This method of coding complications has been shown to be in good agreement with patient-level medical records as measured by physician reviewers.11 We excluded myocardial infarction from our analysis of patients undergoing coronary artery bypass grafting because of the inability to assess the temporal relationship between infarction and operation. The overall complication rates using these methods have strong face validity and are consistent with previously published outcomes.9
Statistical Analysis
We began by calculating risk-adjusted mortality rates accounting for patient age, gender, race, urgency of operation, and pre-existing comorbidities (c-statistics across operations: 0.75-0.89). We obtained comorbidity data from secondary diagnosis codes using Elixhauser’s methods.12 We used logistic regression to calculate the probability of death for each patient. To estimate expected hospital mortality rates, we summated the probability of death by hospital. Finally, we generated each hospital’s operation-specific risk-adjusted mortality rate by multiplying the overall mean mortality rate for each operation by the hospital’s ratio of observed to expected mortality for that operation.
Failure to rescue (FTR) was defined as death in a patient after one or more of the listed complications. We determined hospital failure to rescue rates by evaluating the proportion of deaths among patients who developed at least one postoperative complication (numerator) relative to the total number of patients who developed a postoperative complication (denominator).
We used robust standard errors to adjust for clustered effects (non-independence of patients within hospitals). All analysis was performed using STATA 10.0 (College Station, TX). Our cut-off for statistical significance was a p-value of <0.05.
Results
Overall our final study cohort included 119,434 Medicare patients having 1 of 3 cardiovascular procedures between 2005 and 2006. With the exception of Black race, patients in the lowest and highest volume quintiles were similar with respect to preoperative risk factors (Table 1). We did not find evidence of a systemic racial bias across volume quintiles. This presumption is strengthened by very similar rates of expected mortality between the highest and lowest volume quintiles. During our study period, the median procedure volume at the lowest volume hospitals was 87 cases for CABG, 27 for AVR, and 14 for AAA. For the highest volume hospitals these volumes were 591, 274, and 169 cases respectively.
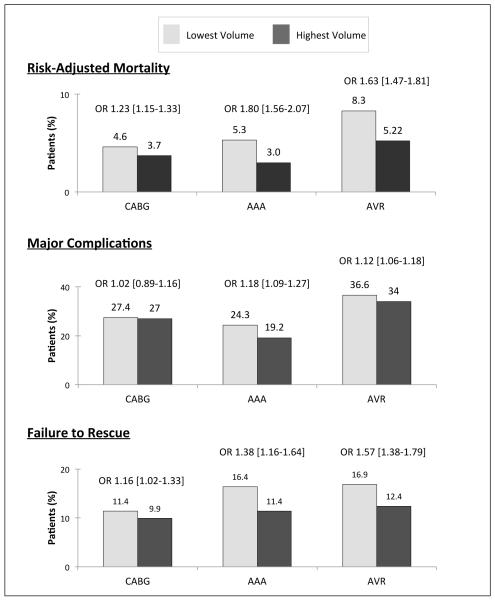
As compared with the highest volume hospitals, the lowest volume hospitals had significantly increased rates of risk-adjusted mortality for all three operations. Differences in the odds of major post-operative complications between the highest and lowest volume quintiles were small but statistically significant for AAA (odds ratio [OR] 1.18, 95% confidence interval [CI] 1.09-1.27) and for AVR (OR 1.12, 95% CI 1.06-1.18). In contrast, our results showed no statistically significant difference for CABG (OR 1.02, 95% CI 0.89-1.16) (Figure 1). When considering all three operations together, there were no statistically significant differences in major complications (OR 1.01, 95% CI 0.99-1.04). Table 2 details complication incidences for all operations combined. When evaluating the incidence of surgical complications alone (i.e. hemorrhage and surgical site infection) we found no statistically significant difference between the highest and lowest volume quintiles. In contrast, for medical complications, we found statistically significant differences in the incidence of pneumonia (OR 1.33, 95% CI 1.12-1.46) and pulmonary failure (OR 1.71, 95% CI 1.47-1.97).
Figure 1.
Hospital Rates of Risk-Adjusted Mortality, Major Complications, and Failure to Rescue.
Table 2.
Hospital Rates of Complication and Failure-to-Rescue by Volume, All Operations Combined
| Complication Incidence | Failure to Rescue | |||||
|---|---|---|---|---|---|---|
| Very Low Volume |
Very High Volume |
OR (95% CI) | Very Low Volume |
Very High Volume |
OR (95% CI) | |
| Any Major Complication | 27.8% | 27.5% | 1.01 [0.99 - 1.04] | 13.3% | 10.9% | 1.26 [1.17 - 1.35] |
| Medical Complications | ||||||
| Myocardial infarction a | 3.9% | 4.4% | 0.90 [0.82-1.00] | 19.1% | 12.9% | 1.58 [1.21 - 2.07] |
| Acute renal failure | 10.3% | 10.9% | 0.94 [0.82-1.07] | 18.5% | 15.0% | 1.28 [1.13 - 1.46] |
| VTE | 0.8% | 1.1% | 0.78 [0.63-0.96] | 12.4% | 9.9% | 1.30 [0.85 - 1.96] |
| Pneumonia | 2.3% | 1.7% | 1.33 [1.12-1.46] | 19.9% | 17.5% | 1.16 [0.90 - 1.50] |
| GI bleed | 1.0% | 1.0% | 1.06 [0.93-1.22] | 14.2% | 11.3% | 1.31 [0.90 - 1.89] |
| Pulmonary failure | 8.8% | 5.3% | 1.71 [1.47-1.97] | 20.2% | 21.4% | 0.93 [0.80 - 1.08] |
| Surgical Complications | ||||||
| Post-operative hemorrhage | 5.4% | 6.2% | 0.87 [0.76 - 0.99] | 11.4% | 9.0% | 1.30 [1.07 - 1.58] |
| Surgical site infection | 1.6% | 1.8% | 0.86 [0.74 - 1.00] | 13.7% | 7.6% | 1.93 [1.36 - 2.75] |
Excludes patients undergoing CABG secondary to the inability to make temporal inferences from Medicare data.
For failure to rescue, we found significantly lower rates among the highest volume hospitals. The odds ratio for failure to rescue ranged from 1.16 for CABG (95% CI 1.02-1.33) to 1.57 for AVR (95% CI 1.38-1.79) (Figure 1). When considering failure to rescue stratified by individual complications, we found statistically significant differences for both surgical complications. However for medical complications, differences were driven largely by myocardial infarction and acute renal failure (Table 2).
Comment
This is the first study to investigate the association between the volume-outcome relationship and failure to rescue in cardiovascular surgery. Echoing prior research, our study found that the highest volume hospitals have dramatically lower risk-adjusted mortality rates but only modestly higher complication rates. 6,9 This difference in overall mortality may be explained by substantially the higher mortality after serious complications (i.e. failure to rescue) observed at the lowest volume hospitals. These findings suggest that failure to rescue is a potential mechanism for the higher mortality rates at low-volume hospitals.
In cardiovascular surgery, there is little research toward explaining the mechanisms driving the volume-outcomes effect. Many prior studies of this effect in cardiovascular surgery have focused on parsing out the relative contributions of hospital and surgeon volumes.5,13 For instance, Birkmeyer and colleagues found that surgeon volume explained 100% of the survival benefit seen at high-volume hospitals for aortic valve replacement, 57% for elective repair of abdominal aortic aneurysms, and 49% for coronary artery bypass grafting. In another study, Peterson et al found low and high-volume centers have similar reoperation, renal failure, prolonged ventilation, stroke, and sternal wound infection rates, but, low-volume centers have mortality rates 1.5 times greater than high-volume centers.6 However, that study was limited only to patients undergoing CABG.
In non-cardiovascular surgery, there have been significant strides toward determining the mechanism of the volume-outcome effect. Ghaferi et al evaluated rates of risk-adjusted mortality and complications between high-volume and low-volume hospitals performing gastrectomy, pancreatectomy, and esophagectomy.8 That study yielded two significant results. First, a volume-outcome effect for high-risk cancer surgery. Second, despite similar rates of major complications, patients undergoing surgery at high-volume centers were 2-3 times more likely to survive a complication than those at low-volume centers. Those findings led to further studies examining the association between patient outcomes, hospital volume, and hospital macro-system characteristics (e.g. nurse-to-patient ratios, intensivist physician staffing, the presence of residency/fellowship training programs, and technology availability).8,14 Although prior studies of FTR have reported rates ranging from 15% (trauma) to more than 30% (lower extremity amputations), no authorities have posited an “acceptable” FTR rate.9,15 In our cohort, the FTR rate at high-volume hospitals was 10.9% and at low-volume hospitals 13.3%. Were low-volume hospitals to have the same FTR rate as high-volume hospitals, we estimate that 487 deaths would have been prevented during our two year study period.
Our study adds to the volume-outcome literature by identifying failure to rescue as a clinical pathway contributing to higher mortality at low-volume cardiovascular centers. While the differences in failure to rescue and mortality rates were sizable between high-volume and low-volume hospitals, the magnitude was less than the two to three fold variation previously reported for high risk cancer operations.8 One explanation for the attenuation of the volume-mortality relationship in cardiovascular surgery, compared to oncologic operations, is the significant standardization in perioperative processes of care. For example, in CABG patients, the most recent studies have found a three-fold increase in the use of anti-lipid therapy – a cost-effective and well-documented means of reducing perioperative mortality.16,17 However, the persistence of the volume-outcome effect suggests that gains made through perioperative measures may have already plateaued. In this event, further strides may be possible through applying similar standardization in post-operative care. For instance, with acute myocardial infarction, treatment delays have been shown to dramatically increase the odds of mortality.18,19,20 Studies of novel hospital based strategies such as empowering emergency medicine physicians to directly activate the cardiac catherization laboratory or transmitting triage electrocardiograms to the on-call interventional cardiologist’s smartphone, have reduced door-to-balloon time by 30%.21,22 In cases of acute stroke, a joint venture between the departments of emergency medicine, neurology, and laboratory services which implemented Toyota’s “Lean” manufacturing principles reduced treatment times by 33%.23 One pervasive theme in each of these interventions is synergy through interdisciplinary communication.
Our study has some important limitations. First, it may not be widely generalizable given the study population included only Medicare patients aged 65 and older. This issue is somewhat lessened by the fact that patients within this age group account for a significant proportion of persons undergoing surgery for AAA, CABG, and AVR. Second, our risk-adjustment model may have been biased by unobserved differences in patient factors. This is an inherent problem of the use of administrative data. To reduce potential bias, we used standard, well-accepted risk-adjustment techniques to minimize statistically significant differences in comorbidities, gender, and race between volume quintiles. Third, as with all administrative datasets, the utility of ICD-9 codes in determining post-operative complications is somewhat limited. To mitigate this issue, we used prior methodology by Iezzoni et al to choose a subset of complications unlikely to be caused by factors other than the operation at hand.10,11 For instance, we excluded mortality from myocardial infarction in patients undergoing CABG. The final list of complications is consistent with published work derived from prospectively collected clinical data.9
Our findings not only reaffirm the previously described volume-outcome effect for cardiovascular surgery but also demonstrate failure to rescue is an important component of the mechanism underlying this relationship. This suggests that developing a post-operative complication is not irrevocably fatal. Although the critical opportunity may be further along the continuum of patient care than previously thought, it still relies upon quickly recognizing and treating complications. In devising quality improvement strategies for low-volume hospitals, future studies should first examine why high-volume hospitals are better able to execute such rescues.
Supplementary Material
eTable 1. ICD-9-CM Codes Used to Define Individual Complications
Acknowledgements
Funding: This study was supported by a career development award to Dr. Dimick from the Agency for Healthcare Research and Quality (K08 HS017765), a grant to Dr. Birkmeyer from the National Cancer Institute (R01 CA098481), and Ruth L. Kirschstein National Research Service Award training grants to Dr. Ghaferi from the National Cancer Institute (T32 CA009672), and Dr. Gonzalez from the National Heart, Lung, and Blood Institute (T32 HL076123-09).
Footnotes
Authors’ Contributions: Andrew A. Gonzalez – analysis and writing
Justin B. Dimick – editing
John D. Birkmeyer – editing
Amir A. Ghaferi – data collection, analysis, writing, editing
Disclosure of Potential Conflicts of Interest: Drs. Gonzalez and Ghaferi have no disclosures. Drs. Dimick and Birkmeyer are consultants and equity owners in ArborMetrix – an Ann Arbor-based healthcare analytics and information technology firm. ArborMetrix was not, in whole or in part, involved in the collection or analysis of any data presented herein.
References
- 1.Northern New England Cardiovascular Disease Study Group [Accessed April 4, 2013];About NNECDSG. 2012 Available at: http://www.nnecdsg.org/about.htm.
- 2.Range JR. [Accessed April 4, 2013];Q & A Guide for Disease-Specific Care Certification. The Joint Commission. Available at: http://www.jointcommission.org/assets/1/18/2012_DSC_Cert_Guide.pdf.
- 3.Fonarow GC, Gregory T, Driskill M, et al. Hospital certification for optimizing cardiovascular disease and stroke quality of care and outcomes. Circulation. 2010;122(23):2459–69. doi: 10.1161/CIR.0b013e3182011a81. doi:10.1161/CIR.0b013e3182011a81. [DOI] [PubMed] [Google Scholar]
- 4.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of thoracic and cardiovascular surgery. 2012;143(1):4–34. doi: 10.1016/j.jtcvs.2011.10.015. doi:10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon Volume and Operative Mortality in the United States. NEJM. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. doi:10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 6.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291(2):195–201. doi: 10.1001/jama.291.2.195. doi:10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, Failure to Rescue, and Mortality With Major Inpatient Surgery in Medicare Patients. Ann Surg. 2009;250(6):1029–1034. doi: 10.1097/sla.0b013e3181bef697. doi:10.1097/SLA.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 8.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital Volume and Failure to Rescue With High-Risk Surgery. Med Care. 2011;49(12):1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in Hospital Mortality Associated with Inpatient Surgery. NEJM. 2009;361(14):1368–75. doi: 10.1056/NEJMsa0903048. doi:10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 10.Iezzoni LI, Daley J, Heeren T, et al. Identifying Complications of Care Using Administrative Data. Med Care. 1994;32(7):700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Weingart SN, Iezzoni LI, Davis RB, et al. Use of Administrative Data to Find Substandard Care: Validation of the Complications Screening Program. Med Care. 2000;38(8):796–806. doi: 10.1097/00005650-200008000-00004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10929992. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris D, Coffey RM. Comorbidity Measures for Use with Administrative Data. Med Care. 1998 Jan 36; doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kilic A, Shah AS, Conte JV, Baumgartner WA, Yuh DD. Operative outcomes in mitral valve surgery: Combined effect of surgeon and hospital volume in a population-based analysis. J Thoracic and Cardiovascular Surg. 2012 doi: 10.1016/j.jtcvs.2012.07.070. ari. doi:10.1016/j.jtcvs.2012.07.070. [DOI] [PubMed] [Google Scholar]
- 14.Joseph B, Morton JM, Hernandez-Boussard T, Rubinfeld I, Faraj C, Velanovich V. Relationship Between Hospital Volume, System Clinical Resources, and Mortality in Pancreatic Resection. J Amer Coll Surg. 2009;208(4):520–7. doi: 10.1016/j.jamcollsurg.2009.01.019. doi:10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Bukur M, Singer MB, Chung R, et al. Influence of Resident Involvement on Trauma Care Outcomes. Arch Surg. 2012;147(9):856–862. doi: 10.1001/archsurg.2012.1672. doi:10.1001/archsurg.2012.1672. [DOI] [PubMed] [Google Scholar]
- 16.Kulik A, Levin R, Ruel M, Mesana TG, Solomon DH, Choudhry NK. Patterns and predictors of statin use after coronary artery bypass graft surgery. J Thoracic and Cardiovascular Surg. 2007;134(4):932–938. doi: 10.1016/j.jtcvs.2007.05.039. doi:10.1016/j.jtcvs.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Lazar HL. Role of Statin Therapy in the Coronary Bypass Patient. Ann Thoracic Surg. 2004;78(2):730–40. doi: 10.1016/j.athoracsur.2003.12.041. doi:10.1016/j.athoracsur.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Rathore SS, Curtis JP, Nallamothu BK, et al. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807–b1807. doi: 10.1136/bmj.b1807. doi:10.1136/bmj.b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TY, Nallamothu BK, Krumholz HM, et al. Association of Door-In to Door-Out Time With Reperfusion Delays and Outcomes Percutaneous Coronary Intervention. JAMA. 2011;305(24):2540–2547. doi: 10.1001/jama.2011.862. doi:10.1001/jama.2011.862. [DOI] [PubMed] [Google Scholar]
- 20.Rathore SS, Curtis JP, Nallamothu BK, et al. Association of door-to-balloon time and mortality in patients > or =65 years with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiology. 2009;104(9):1198–203. doi: 10.1016/j.amjcard.2009.06.034. doi:10.1016/j.amjcard.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khot UN, Johnson ML, Ramsey C, et al. Emergency Department Physician Activation of the Catheterization Laboratory and Immediate Transfer to an Immediately Available Catheterization Laboratory Reduce Door-to-Balloon Time in ST-Elevation Myocardial Infarction. Circulation. 2007;116(1):67–76. doi: 10.1161/CIRCULATIONAHA.106.677401. doi:10.1161/CIRCULATIONAHA.106.677401. [DOI] [PubMed] [Google Scholar]
- 22.Chen KC, Yen DH, Chen CD, Young MS, Yin WH. Effect of Emergency Department In-Hospital Tele-Electrocardiographic Triage and Interventional Cardiologist Activation of the Infarct Team on Door-to-Balloon Times in ST-Segment-Elevation Acute Myocardial Infarction. Am J Cardiology. 2011;107(10):1430–1435. doi: 10.1016/j.amjcard.2011.01.015. doi:10.1016/j.amjcard.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota’s lean manufacturing principles and value stream analysis. Stroke. 2012;43(10):3395–3398. doi: 10.1161/STROKEAHA.112.670687. doi:10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9-CM Codes Used to Define Individual Complications