Abstract
Background
Measurement of serum thyroglobulin (Tg) is used to monitor patients after treatment for differentiated thyroid carcinoma (TC). Difficulty in using Tg as a biomarker of the recurrence of TC in many patients stems from the presence of endogenous anti-Tg autoantibodies (Tg-AAb), which can interfere with immunoassays (IA) and cause false-negative results.
Methods
Tg was enriched from serum samples using rabbit polyclonal anti-Tg antiserum and protein precipitation. Unrelated proteins were partially depleted in the process. Enriched proteins were then denatured, reduced, and digested with trypsin after the addition of a winged internal standard peptide. A Tg-specific tryptic peptide was purified by immunoaffinity extraction and analyzed by 2D-LC-MS/MS. Instrument cycle time was 6.5 min per sample.
Results
The lower limit of quantification was 0.5 ng/mL (0.76 fmol/mL of dimer). Total imprecision of triplicate measurements in serum samples over five days was less than 10%. Comparison with a commercial IA using serum samples free of Tg-AAb (n=73) showed Deming regression, IA= 1.00*LC-MS/MS-2.35, r=0.982, Sy,x=9.52. In a set of Tg-AAb positive samples tested negative for Tg using IA (n=71), concentrations determined by LC-MS/MS method were at or above 0.5 in 23% of samples (median 1.2, range 0.7–11 ng/mL).
Conclusions
The method has acceptable performance characteristics for use in clinical diagnostic applications. The most substantial disagreement between the methods was observed in Tg-AAb positive samples with concentration below 2 ng/mL (determined with LC-MS/MS). The affinity assisted enrichment strategy used for Tg in this method is applicable to other biomarkers that have endogenous autoantibodies.
Keywords: thyroglobulin, thyroid cancer, mass spectrometry, thyroglobulin auto-antibody
INTRODUCTION
Measurement of thyroglobulin (Tg) is commonly used for the follow-up of patients treated for differentiated thyroid carcinoma (TC). Because thyroid tissue is the only source of Tg, after total thyroidectomy and radioactive ablation, serum concentrations of Tg should decrease to very low or undetectable levels. A rise in the serum concentration of Tg is indicative of cancer recurrence (1, 2). In a retrospective assessment of the utility of multiple potential markers of the recurrence of TC, post-treatment Tg concentration was found to be the strongest independent predictor of the recurrence (3). The presence of endogenous anti-Tg autoantibodies (Tg-AAb) can mask the epitopes used by reagent antibodies in immunoassay for measurement of Tg, which can lead to falsely negative results (4–7). Tg autoantibodies were first described by Stokinger and Heidelberger (4); active research of Tg-AAb began over 50 years ago (8), but to date there are no commercially distributed immunoassays available that can overcome the interference of Tg-AAb in testing for Tg. To gauge the reliability of Tg measurements by immunoassay (IA), it is common practice to test every specimen analyzed for Tg for the presence of Tg-AAb and to perform a Tg recovery test in samples testing positive for Tg-AAb, although this approach can still miss-categorize false-negatives as “true-negatives” (9).
It has been hypothesized that there might be a causal, pathophysiological link between thyroid autoimmunity and the development of TC (9, 10). Importantly, approximately 25% of patients with TC and up to 10% of individuals without TC are positive for Tg-AAb. Unfortunately, the presence of Tg-AAb in these samples can interfere with quantification and lead to missed diagnoses of cancer recurrence. The only currently available methodology that could completely eliminate interference of Tg-AAb with the measurement of Tg appears to be mass spectrometry (11).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been increasingly used for quantifying peptides and proteins in biological samples (11–14). Commonly used sample preparation techniques that do not include targeted enrichment of analyte often cannot provide the necessary selectivity for highly complex samples, like protein digests; whereas immunoaffinity enrichment (IAE) can greatly enhance the detection and quantification of proteins and peptides (13).
LC-MS/MS has previously shown promise in the measurement of serum concentrations of Tg. A previously described method used proteolytic digestion, which cleaves serum proteins into peptides, followed by enrichment of Tg-specific peptides using anti-peptide antibodies, and quantitative analysis of the peptides with nano-LC-MS/MS (11, 12). It has been suggested that sensitivity required for reliable detection of the recurrence of TC should be close to 1 ng/mL (3), while limit of quantification of the previous method was 3 ng/mL (12). In addition, the previously published method had limited throughput and was insufficiently robust for use in routine diagnostic testing (i.e. involved nano-flow liquid chromatography).
The aim of this work was to develop a highly sensitive, robust method for quantification of Tg in serum and plasma samples—that would overcome interference of Tg-AAb—and to evaluate its performance. The novel method we have developed uses Tg enrichment, proteolytic digestion, IA enrichment of the targeted peptide, two dimensional (2D) chromatographic separation and MS/MS detection. The method has been fully validated according to CLSI guidelines and applied to the analysis of clinical samples. The potential caveats of the approach, enrichment of Tg, and IAE of the targeted peptides are discussed in regards to achieving the required limits of quantification, specificity, and robustness of the assay.
MATERIALS AND METHODS
Detailed methods are provided in Supplemental Materials.
Preparation of reagents, standards, and quality control samples
Calibration standards containing Tg were purchased from Beckman Coulter (Fullerton, CA). Rabbit polyclonal anti-Tg antibody was purchased from Covance (Princeton, NJ). Serum quality control samples were pooled human serum samples. Internal standard (IS) “winged” peptide comprised the sequence PVPESKVIFDANAPV*AVRSKVPDS (V* [13C5; 15N]; mass shift 6 Da, RS synthesis Louisville, KY). Trypsin (purity 99%, activity 15,000 BAEE units/mg protein), formic acid (FA), dithiothreitol (DTT) and sodium deoxycholate (DOC) were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were of highest purity commercially available. Solvents were of HPLC grade, purchased from JT Baker (Phillipsburg, NJ).
Conjugation of antibody to magnetic beads
Custom polyclonal rabbit anti-peptide antibody (Covance, Princeton, NJ) was conjugated to Tosyl activated magnetic beads (DynaBeads M280, Life Technologies, Carlsbad, CA) according to the manufacturer’s recommendations.
Sample preparation
Sample preparation was performed on a liquid handler (epMotion, Eppendorf, Hamburg, Germany). Rabbit anti-Tg antibody (300 ng) was added to serum or plasma (500 µL). Proteins were precipitated with saturated ammonium sulfate solution. After centrifugation, the precipitates were reconstituted with water (300 µL), internal standard was added, and the samples were reduced at 60°C. Samples were diluted in NH4HCO3 and trypsinized (40 µg) for 4 h at 37°C. Preliminary experiments showed that DOC was critical for recovery of the targeted peptide. The denaturing, reduction and digestion conditions were optimized to ensure rapid, complete, and reproducible digestion of Tg. Preliminary experiments showed no effect of the cysteines alkylation on the recovery of the targeted peptide (data not shown); therefore cysteines were not alkylated in this method.
Magnetic beads were processed using a Magnetic Stand-96 (Life Technologies, Carlsbad, CA). Magnetic bead conjugates were added to the digests and incubated with agitation (20°C for 8 h). The beads were washed with PBS (pH 7.4), and the target peptide/IS were eluted with 25 mM glycine, pH 2 (75 µL). The elutions were transferred into a 96-well plate and injected (40 µL) on 2D LC-MS/MS. The overall time required for the sample preparation in a 96-well plate format was approximately 20 hours.
LC-MS/MS analysis
Two-dimensional HPLC separation was performed at 30°C using an HPLC system consisting of series 1260 and 1290 pumps (Agilent Technologies, Santa Clara, CA). A Zorbax XDB-CN 50x2.1, 5 µm HPLC column was used for the 1st dimension separation with gradient of mobile phases A 98% to 87%A in 1.3 min (A, 10 mM FA in water; B, 10 mM FA in acetonitrile); the 2nd dimension separation used a Poroshell 120EC-C18, 100x3, 2.7 µm column (both columns from Agilent Technologies) and a gradient of the same mobile phases 87 to 75%A in 2 min.
Quantitative analysis was performed on an API 5500 triple-quadrupole mass spectrometer with a V-spray ionization source operated in a positive ion, multiple reaction monitoring (MRM) mode. Mass transitions monitored in the method were m/z 636.36/1059.56, 636.86/1060.56, 636.36/912.49 and 636.36/541.35 for the VIFDANAPVAVR peptide, and m/z 639.34/1065.56, 639.84/1066.56, 639.34/918.48, 639.34/547.34, for the IS. The instrument settings were adjusted to maximize the sensitivity and the specificity of detection. Relevant instrument parameters can be found in the Supplemental Material. Total analysis time per sample was 6.5 min. Data were processed using software (Analyst 1.5.2).
Total protein concentration was measured by a spectrophotometric method using a NanoDrop™ 8000 (Thermo Scientific, Wilmington, DE); concentration of IgG and albumin were measured on an automated immunoassay analyzer (BN II, Dade Behring, Newark, DE) and Modular Analytics (Roche Diagnostics, Indianapolis, IN), respectively. The Tg-AAb test was performed on an automated immunoassay analyzer (IMMULITE 2000, Siemens, Tarrytown, NY).
Method validation
Method validation consisted of the evaluation of the imprecision, sensitivity, linearity, accuracy, recovery, carryover, ion suppression, and the establishment of reference intervals for Tg. Precision experiments used pools of human serum samples supplemented with Tg (AbD Serotec, Martinsri, Germany). Limits of quantification (LOQ) were determined as the lowest concentrations at which accuracy was within ±15%, imprecision was <15% and at which the ratio of mass transitions was maintained within ±30%. LOD was determined as the lowest concentration at which chromatographic peaks were present in all mass transitions and signal to noise ratio was >5. Linearity of the method was evaluated by analyzing seven samples prepared by mixing different proportions of two serum pools containing Tg at 5 and 1045 ng/mL (3.3 and 886 pmol/L).
The method was compared with the Access™ Beckman Coulter DxI800 Tg IA performed at ARUP Laboratories. Samples included those that tested negative for Tg-AAb (n=73), and samples that tested positive for Tg-AAb (n=113). Samples with concentration of Tg-AAb below 20 IU/mL were considered as Tg-AAb negative. In the Tg-AAb positive samples concentration of Tg-AAb was 20-3000 IU/mL and the Tg recovery was 5–97%. Ratio of the concentrations determined from multiple mass transitions was used for evaluation of the specificity of analysis (15).
Magnetic bead enrichment recovery was determined by performing affinity enrichment of a pool of digested serum samples containing 80 ng/mL of Tg. Linearity of sample dilution (using Tg-negative serum) was evaluated by analyzing a serum sample containing over 3000 ng/mL of Tg (determined with LC-MS/MS).
The effects of lipemia, hemolysis and icterus was evaluated by analyzing pools of normal serum, lipemic, hemolized and ichteric samples ‘as is’, and mixed in ratio 1:1; the observed concentrations were compared with concentrations measured in the individual samples. Ion suppression was evaluated using the post column infusion method (16). Tube-type studies were performed using blood collected in potassium EDTA, sodium heparin, serum and serum separator tubes. Storage stability of thyroglobulin was evaluated at room temperature, at 4°C, and at −20°C after 1, 2, 3, 4, 8, 14, 21 and 28 days of storage.
The reference interval study for Tg was performed with samples from self-reported healthy adult volunteers (25 men and 25 women) and 140 samples from children aged 1, 4, 7, 10, 13, and 16 years old (10 samples from each gender of each age). All studies with human samples were approved by the Institutional Review Board of the University of Utah.
Differences in concentrations among the groups in the experiments for evaluation of different types of collection tubes and problem samples types were evaluated using ANOVA.
RESULTS
Mass transitions were selected from product ion mass spectrum of the Tg-specific peptide (VIFDANAPVAVR) and the IS peptide (Figure 1 and Supplemental Figure 1). Free and AAb-bound Tg was enriched from serum samples using Affinity-Assisted Enrichment (AAE), which consisted of the addition of an anti-Tg antibody to the samples, incubation, and precipitation of the Tg-Ab complexes with saturated ammonium sulfate. The supernatants (not containing Tg) were discarded and the precipitates containing Tg were further processed. Effects of the concentration of the rabbit antibody and the amount of ammonium sulfate on distribution of Tg, total protein, albumin, and IgG content in the supernatants and the precipitates were evaluated. Supplemental Figures 2 and 3 demonstrate distribution of Tg, total protein, IG and albumin between the supernatants and the precipitates.
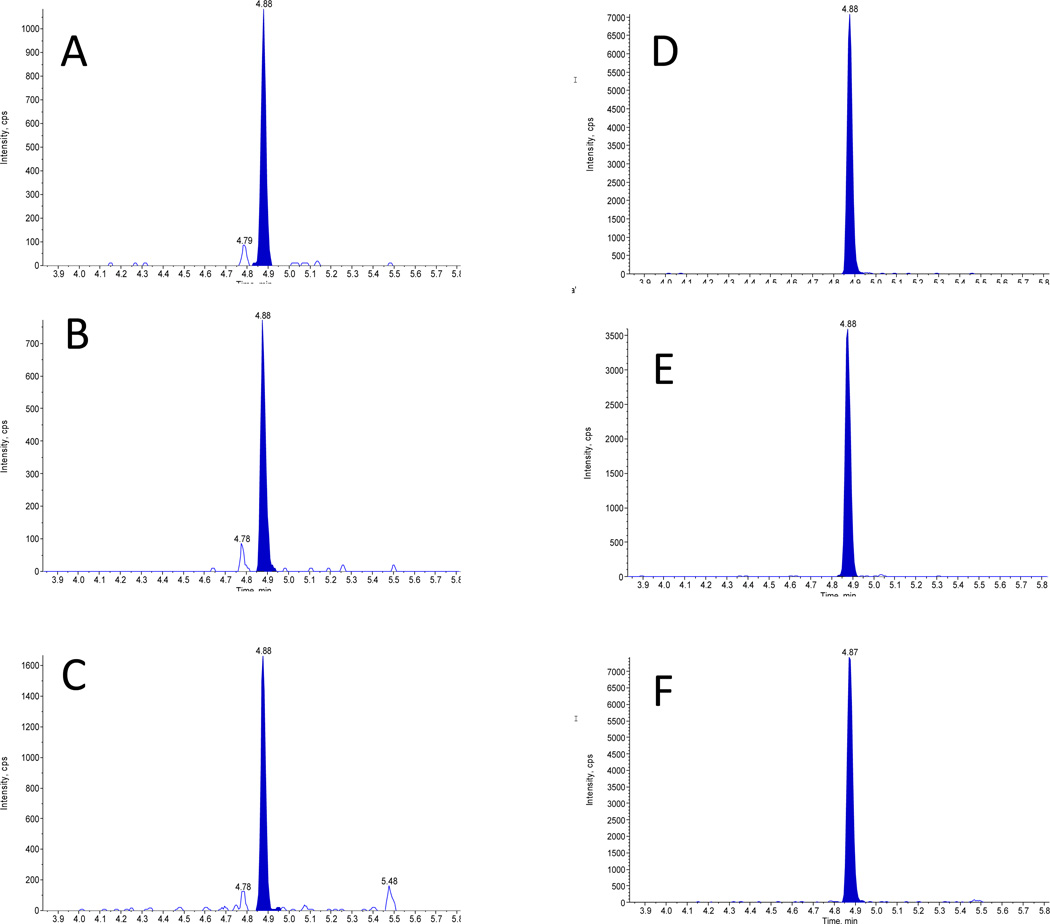
Figure 1.
Chromatogram of patient sample containing 5 ng/mL of thyroglobulin.
Mass transitions of the VIFDANAPVAVR peptide m/z 636.36/1059.56 (A), 636.36/912.49 (B), 636.36/541.35 (C); and the internal standard m/z 639.34/1065.56 (D), 639.34/918.48 (E), 639.34/547.34 (F).
Results of the evaluation of the assay imprecision are summarized in Table 1. Within-assay imprecision at the evaluated concentrations was <10% and imprecision of Tg measurements in QC samples analyzed over 20 days was <14%. The LOQ and LOD of the method were 0.5 and 0.25 ng/mL (0.76 and 0.38 fmol/mL of the Tg dimer), respectively. The method was linear up to 1045 pg/mL with inaccuracy at the highest level <10%. No analyte was detected in an injection of a blank sample immediately after a standard containing 1045 ng/mL (1.58 pmol/mL) of Tg. Recovery of the immunoaffinity extraction of the VIFDANAPVAVR peptide was 52.5%. Concentrations observed in the samples from the dilution integrity experiment agreed with each other within 6.8%. Evaluation of Tg stability indicated degradation over 28 days of storage by 44%, 22% and 13% when stored in polypropylene tubes at room temperature, 4°C and −20°C, respectively (p-values for the difference in slopes of linear regression lines of 0.006 and 0.545, for RT vs. 4°C, and 4°C vs. −20°C, respectively); three freeze thaw cycles did not affect the concentration of Tg (Supplemental Figure 4). Considering these data, samples should be analyzed within a month after collection if stored frozen at −20°C and three days if stored refrigerated, or kept at −70°C for a long term storage. Analysis of samples collected in four different types of collection tubes did not show statistically significant difference in the concentration within an individual (n=5, Supplemental Figure 5). Tg concentrations in lipemic, hemolized and ichteric samples mixed in ratios 1:1 with a normal serum pool, were measured as 50% of the total value observed in the individual samples used for preparing the mixtures (Supplemental Table 1). This suggests that lipemia, hemolysis and icterus do not affect performance of this method. No ion suppression was observed at the retention time of the VIFDANAPVAVR peptide and no interfering peaks were detected in over 3000 patient samples analyzed by the method. A ratio of qualifier to quantifier ions outside of ±30% or the presence of split peaks was considered as evidence of the presence of interferences.
Table 1.
Within-run, between-run and total imprecision of LC-MS/MS method for thyroglobulin.
| Sample | Concentration, ng/mL |
Within-run, % |
Between- run/day, % |
Total, % |
|---|---|---|---|---|
| Low 1# | 2.1 | 6.75 | 3.67 | 7.69 |
| Low QC 1* | 2.3 | na | 13.9 | na |
| Low 2# | 5.7 | 6.87 | 5.96 | 9.10 |
| Medium QC 2* | 6.5 | na | 10.5 | na |
| Medium# | 14.8 | 6.56 | 5.40 | 8.50 |
| High# | 399 | 3.56 | 1.71 | 3.95 |
| High QC* | 172.8 | na | 3.5 | na |
Samples analyzed in three replicates per day over five days
Samples analyzed in one replicate per day, over 20 days
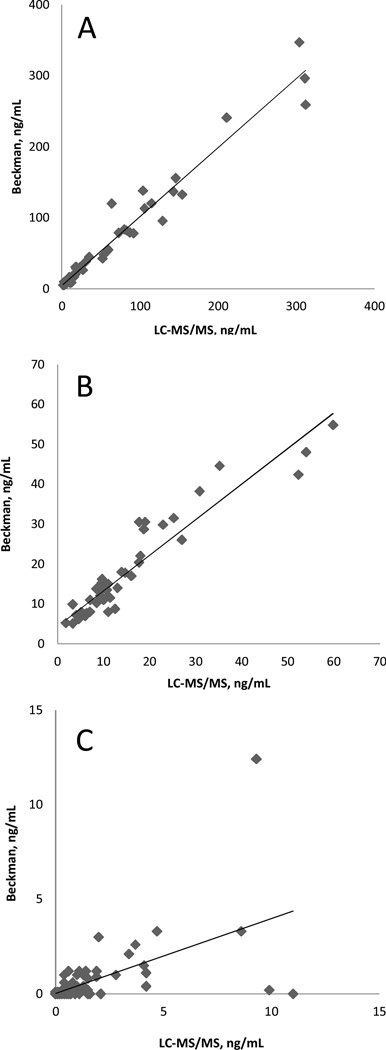
For method comparison, we used the Beckman Access immunoassay analyzer. Deming regression equations for the method comparison using Tg-AAb negative samples (n=73) were IA=1.00*LC-MS/MS-2.3, r=0.982, Sy,x=9.5 (concentrations 0–350 ng/mL), and IA=1.01*LC-MS/MS-2.1, r=0.956, Sy,x=2.8 (concentrations <60 ng/mL, n=56) (Figure 2). A set of 113 Tg-AAb was tested by both methods; concentrations of Tg measured by LC-MS/MS were higher than values determined by IA (Figure 2C), IA=0.53*LC-MS/MS −0.1, r=0.586, Sy,x = 1.1; the disagreement was especially severe at concentrations of Tg below 2 ng/mL. Among 71 Tg-AAb positive samples with concentration below 0.5 ng/mL (using Access analyzer) in 16 samples (23%) concentrations determined by LC-MS/MS method were at or above 0.5 ng/mL (median 1.2 ng/mL, range 0.7–11 ng/mL). In the Tg-AAb positive samples there was no association between the Tg-AAb concentration and the Tg concentration determined with LC-MS/MS method (Supplemental Figure 6).
Figure 2.
Results of the LC-MS/MS method comparison with Access™ analyzer (Beckman Coulter) using Tg-AAb negative (A, B) and Tg-AAb positive samples (C). A: concentrations 0–350 ng/mL IA=0.99*LC-MS/MS-3.1, r=0.980, Sy,x=14.8; B: concentrations <60 ng/mL. IA=0.94*LC-MS/MS-3.7, r=0.946, Sy,x=4.2; C: IA=0.53*LC-MS/MS −0.1, r=0.586, Sy,x = 1.1.
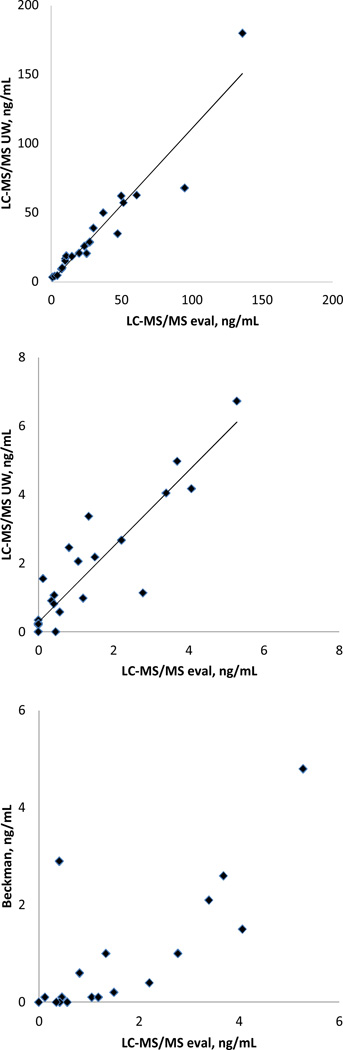
The evaluated LC-MS/MS method was also compared with LC-MS/MS method of University of Washington (12) using a set of Tg-AAb negative (n=21) and Tg-AAb positive (n=29) samples (Figure 3). The comparison showed good agreement between the methods for both sets of samples. For Tg-AAb negative samples LC-MS/MSUW=1.17*LC-MS/MSeval. −1.81, r=0.951, Sy,x=8.14; for the Tg-AAb positive samples LC-MS/MSUW=1.23*LC-MS/MSeval. + 0.15, r=0.917, Sy,x=0.475.). Poor agreement at concentrations (below 2 ng/mL of Tg) was observed between concentrations determined with LC-MS/MS assays and the Beckman Access immunoassay.
Figure 3.
Results of comparison of the evaluated LC-MS/MS method with LC-MS/MS method of the University of Washington (12) using (A) Tg-AAb negative (n=21), (B) Tg-AAb positive samples (n=29), and (C) comparison with Access™ analyzer (Beckman Coulter) using Tg-AAb positive samples (samples corresponding to the results shown on pane B). A: LC-MS/MSUW=1.17* LC-MS/MSeval. −1.81, r=0.951, Sy,x=8.14; B: LC-MS/MSUW=1.23*LC-MS/MS eval. + 0.15, r=0.917, Sy,x=0.475.)
Reference intervals of Tg established with this method were: 5.7–45.5 ng/mL(8.6–68.9 pmol/L) in 1–4 year old; 1.4–36.3 ng/mL (2.1–55 pmol/L) in 7–17 year old; 1.1–36.7 ng/mL (1.7–55.6 pmol/L) in18 and older.
DISCUSSION
We have developed a method for measuring Tg that overcomes interference of Tg-AAb. The method is based on the enrichment of all forms of Tg (free and AAb-bound), followed by proteolytic digestion, IAE and 2D-LC-MS/MS analysis of the Tg-specific peptide. The assay consisted of the following steps: (i) addition to serum samples of anti-Tg Ab; (ii) enrichment of Tg through precipitation of the Tg-Ab and Tg-AAb complexes; (iii) re-dissolving the precipitate with solvent containing IS, (iv) denaturing, reduction and proteolytic digestion, (v) enrichment of the targeted peptide using anti-peptide antibody conjugated to magnetic beads, (vi) removal of non-specifically bound peptides by washing the beads, (vii) elution of the targeted peptide, followed by (viii) LC-MS/MS analysis.
Many patients have Tg-AAb that can interfere with the quantification of Tg by immunoassays. Gentile et al. identified nineteen epitopes on the Tg molecule, which could participate in autoantibody binding (17). In samples of patients treated for TC there is a need for high sensitivity accurate measurement of total concentration of Tg (free and AAb-bound) as a means of detecting the recurrence of TC. Inter-individual difference in the epitopes and the affinity of Tg-AAb towards Tg, make it impossible to reliably and accurately measure Tg in every sample containing AAb using immunoassays (9).
The novel AAE strategy used in this method essentially adheres to the old saying, “if you can’t beat ‘em…join ‘em.” It relies on the conversion of all Tg in biological samples to an Ab-bound (and AAb-bound) form, followed by precipitation of the Tg-antibody complexes with ammonium sulfate. A large fraction of non-targeted blood proteins are simultaneously depleted (up to 70%) during the enrichment step. Partial enrichment of Tg was also observed in samples not containing Tg-AAb (Supplemental Figure 3). This likely occurs through co-precipitation of Tg non-specifically bound to IG. In samples that did not contain IG, Tg did not precipitate with ammonium sulfate and remained in solution (data not shown). Addition of polyclonal anti-Tg antibody to patient samples was found to enhance the efficiency of enrichment, likely due to antibody binding to free Tg, which resulted in the enhanced partitioning of the antibody-Tg complexes to the IG-containing fraction. The AAE therefore allowed the precipitation of free and AAb-bound Tg in a single fraction, while (i) reducing sample complexity prior to the digestion, (ii) the amount of trypsin used, and (iii) the complexity of the resulting tryptic digests.
Multidimensional separations are widely used in the analysis of small molecules (18–21). Using the same concept, a 2D separation was optimized for the targeted peptide using two chromatographic columns with complementary retention mechanisms under UPLC conditions. Optimal selectivity was achieved by combining 1st-D column with weak retention with a 2nd-D column that strongly retained the peptide. The separation conditions were optimized to provide a good separation from other endogenous peaks, while maintaining a short analysis time. While the instrumentation and the method utilizing multi-dimensional chromatography are more complex, 2D separations have number of advantages over separation using single analytical column, (a) in a well-developed method orthogonal mechanisms of chromatographic separation on the 1st and 2nd columns could remove peaks that would remain unresolved using single retention mechanism; (b) peaks eluting outside of the window of the peak transfer from the 1st to the 2nd column are directed to waste; (c) reduced potential for ion suppression; (d) longer life of analytical column, and consequently more robust methods; and finally (e) reduced analysis time (reconditioning of the 1st column takes place while separation takes place on the 2nd column, and vice versa) (19–21).
Digestion of Tg with trypsin produces over 400 peptides, among which three were suggested as suitable for quantitative measurements of Tg (11, 12). In this method, peptide VIFDANAPVAVR was used as a surrogate marker for quantification of Tg. Advantages of the use of VIFDANAPVAVR peptide for quantitative measurement of Tg are (i) the quantitative yield of the peptide from tryptic digestion, and (ii) the absence in its sequence of amino acids that could be post-translationally modified. One of the difficulties of “bottom-up” proteomics approaches in the quantification of endogenous proteins in human serum lies in the extreme variability of trypsin digestion between experiments (22, 23). In attempt to minimize this variability, we employed a novel approach using a “winged” peptide, a stable-isotope-labeled analog of the VIFDANAPVAVR peptide synthesized with six amino acids of the sequence of Tg concatenated at each end. The use of a structurally matched IS containing amino acids beyond the tryptic digestion sites, led to improved assay precision (data not shown). This is likely due to the fact that the internal standard undergoes tryptic digestion in a similar fashion to the intact Tg in patient samples. While the “winged” peptide-IS does not provide the full spectrum of benefits embodies by a fully stable isotope-labeled protein-analog IS, it does compensate for digestion variability to some extent. Evaluation of the kinetics of trypsin digestion of Tg and the “winged” peptide showed some difference in the rate of the release of the targeted peptide and the stable isotope-labeled peptide-analog (data not shown). A similar approach, using an extended sequence peptide-IS was recently employed by Zhang, et al. (24). The IS peptide in the method (24) contained an extra amino acid within the sequence of the targeted peptide; it is unclear how this modification would affect antibody binding to the IS during the IAE step.
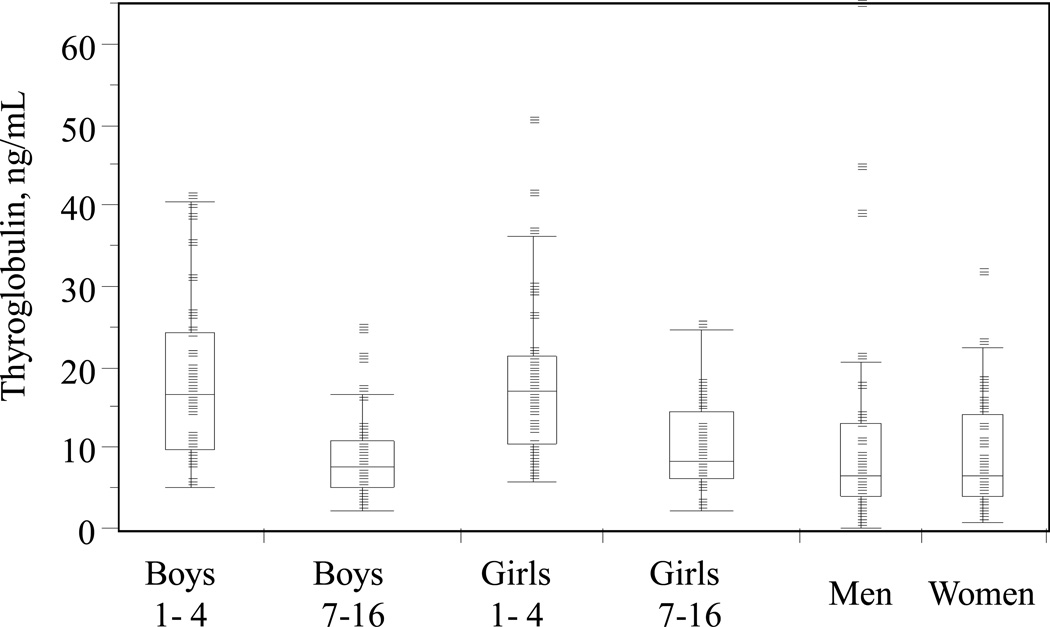
No significant differences were found in distribution of concentrations of Tg between men and women, and boys and girls within the same age groups; because of this we established single reference intervals (nonparametric, central 95% of the distribution) within the age groups in both genders. Distribution of concentrations of Tg in children and adults is shown on Figure 4. A statistically significant difference was observed between distributions of concentration in children of age groups 1–4 year old and 7–17 year old. The observed distributions of concentrations and age-dependent differences are in agreement with the reference intervals established using Beckman Access analyzer (25), supporting good agreement between the LC-MS/MS method and the immunoassay in samples negative for Tg-AAb.
Figure 4.
Distribution of concentrations of thyroglobulin in healthy children and adults.
Good agreement was observed between two comparative LC-MS/MS methods for both, Tg-AAb negative and Tg-AAb positive samples. Poor agreement at concentrations of Tg below 2 ng/mL was observed between the LC-MS/MS methods and the Beckman Access immunoassay, suggesting that presence of Tg-AAb predominantly affects measurements of Tg in Tg-AAb positive samples containing less than 2 ng/mL of Tg.
Future studies (in which clinical information on the participants will be available) will be needed for assessment of the clinical sensitivity, clinical specificity, and receiver operator characteristic of this and other LC-MS/MS methods; clinical sensitivity and clinical specificity of LC-MS/MS methods will also need to be compared to available immunoassays.
CONCLUSIONS
We have developed a high sensitivity IAE 2D-LC-MS/MS method for the quantitative analysis of Tg in human serum and plasma in presence of endogenous Tg-AAb. The method was validated to CLSI specifications and showed acceptable performance for use in clinical applications. The AAE strategy developed in this method includes enrichment of Tg and partial depletion of unrelated proteins, which allowed sensitive and accurate measurement of total concentration of Tg (free and AAb-bound) in serum and plasma. The AAE strategy developed here is generally applicable to other biomarkers for which autoantibodies can be present in clinical samples. The use of 2D-LC-MS/MS reduced the complexity of chromatograms and enhanced the sensitivity and specificity of the assay. In samples free of Tg-AAb, we observed good agreement between this LC-MS/MS method and an immunoassay. Poor agreement between the methods was observed in Tg-AAb positive samples, the most substantial disagreement was observed in samples with concentration by LC-MS/MS below 2 ng/mL. Good agreement was observed between LC-MS/MS methods for both, Tg-AAb negative and Tg-AAb positive samples. Poor agreement at concentrations of Tg below 2 ng/mL was observed between the LC-MS/MS methods and the Beckman Access immunoassay, suggesting that presence of Tg-AAb predominantly affects measurements of Tg in Tg-AAb positive samples containing less than 2 ng/mL of Tg. From our data, it appears that the lower Tg concentrations observed in AAb positive samples are likely caused by interference of Tg-AAb with the IA. In a set of Tg-AAb positive samples tested negative for Tg using IA, concentrations determined by this method were at or above 0.5 in 23% (median 1.2 ng/mL, range 0.7 – 11 ng/mL), which could fundamentally alter approach for monitoring patients treated for differentiated thyroid carcinoma.
Supplementary Material
Acknowledgement
We thank the ARUP Institute for Clinical and Experimental Pathology for supporting this project.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
List of abbreviations
- AAE
affinity assisted enrichment
- Ab
antibody
- DOC
sodium deoxycholate
- 2D
two dimensional
- IA
immunoassay
- IG
immunoglobulins
- IAE
Immunoaffinity enrichment
- IS
internal standard
- FA
formic acid
- MS
mass spectrometry
- MRM
multiple reaction monitoring
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- Tg
thyroglobulin
- Tg-AAb
thyroglobulin autoantibodies
- TC
differentiated thyroid carcinoma
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
References
- 1.Pacini F. Follow-up of Differentiated Thyroid Cancer. Eur J Nucl Med Mol Imaging. 2002;29:S492–S496. doi: 10.1007/s00259-002-0847-9. [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Ain KB. Thyroglobulin: A Specific Serum Marker for the Management of Thyroid Carcinoma. Clin Lab Med. 2004;24:29–47. doi: 10.1016/j.cll.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Pelttari H, Valimaki MJ, Loyttyniemi E, Schalin-Jantti C. Post-ablative serum thyroglobulin is an independent predictor of recurrence in low-risk differentiated thyroid carcinoma: a 16-year follow-up study. Eur J Endocrinol. 2010;163:757–763. doi: 10.1530/EJE-10-0553. [DOI] [PubMed] [Google Scholar]
- 4.Stokinger HE, Heidelberger M. A quantitative theory of the precipitin reaction: VI The reaction between mammalian thyroglobulins and antibodies to homologous and heterologous preparations. J Exp Med. 1937;66:251–272. doi: 10.1084/jem.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer CA. Challenges of serum thyroglobulin (Tg) measurement in the presence of Tg autoantibodies. J Clin Endocrinol Metab. 2004;89:3702–3704. doi: 10.1210/jc.2004-0986. [DOI] [PubMed] [Google Scholar]
- 6.Okosieme OE, Evans C, Moss L, Parkes AB, Premawardhana L, Lazarus JH. Thyroglobulin antibodies in serum of patients with differentiated thyroid cancer: relationship between epitope specificities and thyroglobulin recovery. Clin Chem. 2005;51:729–734. doi: 10.1373/clinchem.2004.044511. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, et al. American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 8.Blizzard RM, Hamwi GJ, Skillman TG, Wheeler WE. Thyroglobulin antibodies in multiple thyroid diseases. N Engl J Med. 1959;260:112–116. doi: 10.1056/NEJM195901152600302. [DOI] [PubMed] [Google Scholar]
- 9.Spencer CA. Clinical Utility of Thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC) J Clin Endocrinol Metab. 2011;96:3615–3627. doi: 10.1210/jc.2011-1740. [DOI] [PubMed] [Google Scholar]
- 10.Feldt-Rasmussen U, Rasmussen AK. Autoimmunity in differentiated thyroid cancer: Significance and related clinical problems. Hormones. 2010;9:109–107. doi: 10.14310/horm.2002.1261. [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle AN. Clinical tumor marker quantification with LC/MS/MS: is there hope?. Proceedings of 23rd Asilomar Conference on Mass Spectrometry; September 14–18, 2007; [Abstract] [Google Scholar]
- 12.Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54:1796–1804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass spectrometric. quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA) J Proteome Res. 2004;3:235–244. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- 14.Van den Broek I, Sparidans RW, Schellens JHM, Beijnen JH. Quantitative bioanalysis of peptides by liquid chromatography coupled to (tandem) mass spectrometry. J Chromatogr B. 2008;872:1–22. doi: 10.1016/j.jchromb.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Kushnir MM, Rockwood AL, Nelson GJ, Yue B, Urry FM. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin Biochem. 2005;38:319–327. doi: 10.1016/j.clinbiochem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 17.Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: what can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology. 2004;112:13–25. doi: 10.1111/j.1365-2567.2004.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giddings JC. Concepts and comparisons in multidimensional separation. J High Resol Chromatogr Commun. 1987;10:319–323. [Google Scholar]
- 19.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW. A tandem mass spectrometry assay for estrone and estradiol in serum of postmenopausal women, men and children. Am J Clin Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 20.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. LC-MS/MS assay for androstenedione, dehydroepiandrosterone and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 21.Kushnir MM, Ray JA, Rockwood AL, Roberts WL, La'ulu SL, Whittington JE, Meikle AW. Rapid Analysis of 25OH Vitamins D2 and D3 by LC-MS/MS and Association of Vitamin D and Parathyroid Hormone Concentrations in Healthy Adults. Am J Clin Pathol. 2010;134:148–156. doi: 10.1309/AJCPPIA7DFBT4GKS. [DOI] [PubMed] [Google Scholar]
- 22.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoofnagle AN. Quantitative clinical proteomics by liquid chromatography-tandem mass spectrometry: assessing the platform. Clin Chem. 2010;56:161–164. doi: 10.1373/clinchem.2009.134049. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Lai S, Zhang Y, Huang B, Li D, Ren Y. Multiple reaction monitoring-based determination of bovine α-lactalbumin in infant formulas and whey protein concentrates by ultra-high performance liquid chromatography-tandem mass spectrometry using tryptic signature peptides and synthetic peptide standards. Anal Chim Acta. 2012;727:47–53. doi: 10.1016/j.aca.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Owen WE, Bunker AM, Straseski JA, Roberts WL. Pediatric Reference Intervals for Thyroglobulin. Clin Chem. 2012;58:A176. [Abstract] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.