Abstract
Myelination by oligodendrocytes is a highly specialized process that relies on intimate interactions between the axon and oligodendrocyte. Astrocytes also have an important part in facilitating myelination in the CNS, however, comparatively less is known about how they affect myelination. This review therefore summarizes the literature and explores lingering questions surrounding differences between white matter and grey matter astrocytes, how astrocytes support myelination, how their dysfunction in pathological states contributes to myelin pathologies and how astrocytes may facilitate remyelination. We propose that astrocytes in the white matter are specialized to facilitate myelination and myelin maintenance by clearance of extracellular ions and neurotransmitters and by secretion of pro-myelinating factors. Aditionally, astrocytes-oligodendrocyte coupling via gapjunctions is crucial for both myelin formation and maintenance, due to K+ buffering and possibly metabolic support for oligodendrocytes via the panglia syncytium. Dysfunctional astrocytes aberrantly affect oligodendrocytes, exemplified by a number of leukodystrophies in which astrocytic pathology is known as the direct cause of myelin pathology. Conversely, in primary demyelinating diseases, such as multiple sclerosis, astrocytes may facilitate remyelination. We suggest that specific manipulation of astrocytes could help prevent myelin pathologies and successfully restore myelin sheaths after demyelination.
Introduction to astrocytes
Distributed throughout the brain’s grey and white matter, under the dura and around cerebral vasculature, astrocytes comprise the most abundant and diverse type of glial cell in the CNS. Many key regulatory functions in maintaining brain homeostasis have been pinpointed to astrocytes. Astrocytic endfeet processes cover more than 90% of the cerebral vasculature and play a crucial role in formation and maintenance of the blood-brain barrier (BBB). The BBB is immensely selective and protects the brain from entry of toxic substances and influx of ions, such as K+ and Ca2+, thereby regulating the extracellular environment (Abbott et al., 2006). Astrocytes take up K+ and neurotransmitters such as glutamate that is increased in the interstitial fluid during high frequency firing of neurons. By keeping extracellular K+ and glutamate levels low astrocytes facilitate fast repetitive neurotransmission (Walz, 2000, Oliet et al., 2001). Astrocytes also control the dynamics of cerebral blood flow in order to increase the availability of oxygen and glucose and thereby accommodate changes in neuronal activity (Zonta et al., 2003, Takano et al., 2006, Iadecola and Nedergaard, 2007). During high levels of neuronal activity astrocytes also play a pivotal role in maintaining pH despite ionic changes (Chesler and Kaila, 1992). Astrocytes are the only cells in the CNS that store glycogen and these depots may serve as a source of energy in support of neurons’ metabolic needs during hypoglycemia (Wang and Bordey, 2008, Belanger et al., 2011). The metabolic role of astrocytes is further emphasized by their production of sterols and lipoproteins. The BBB is impermeable to many lipid-soluble molecules, including cholesterol and lipoproteins. Astrocytes produce and secrete cholesterol and distribute it using lipid carriers, e.g. Apolipoprotein E, as vehicles (Dietschy and Turley, 2004). Another way astrocytes may supply energy to other cells in the CNS is via facilitating the transport and exchange of soluble substrates between the cerebrospinal fluid (CSF) and the interstitial fluid (ISF) of the brain parenchyma (Iliff et al., 2012, Iliff et al., 2013, Iliff and Nedergaard, 2013).
In addition to their role in maintaining stable physiological conditions in the CNS, astrocytes are considered to be critical in specialized functions, such as such as control of respiratory rate by ATP release (Gourine et al., 2010), regulation of the sleep wake cycle (Blutstein and Haydon, 2013), and the facilitation of learning and memory (Han et al., 2013). Although they are not electrically excitable, astrocytes are capable of detecting and modulating neuronal activity. Astrocytes may tweak neuronal action potentials by release of glutamate, ATP and D-serine at the synapse, however, this might only take place early in development (Panatier et al., 2006, Araque, 2008, Nedergaard and Verkhratsky, 2012, Sun et al., 2013). One study also implicated astrocytes as active modulators of axonal propagation of action potentials (Sasaki et al., 2011). Astrocytes also play an active and specific role in both formation and pruning of synapses (Ullian et al., 2001, Christopherson et al., 2005, Eroglu et al., 2009, Dodla et al., 2010). Common between mammalian species, astrocytes comprise a gap-junction coupled network of non-overlapping domains. Each domain covers a significant region of neuronal synapses. Such an organization suggests that astrocytes may play important roles in integrating and processing complex cognitive tasks, as local neural activity in a single domain may be detected by astrocytes that then communicate with coupled cells to coordinate a wide-spread response. Most of the studies describing astrocytic function have been performed on rodents; however, considerable differences exist between human and rodent astrocytes. Compared to rodents, human astrocytes are 16 times larger in volume, conduct calcium waves at a 4 fold increased velocity and extend 10 times more primary processes (Oberheim et al., 2006, Oberheim et al., 2012). Intriguingly, engraftment of human astrocytes in rodents results in significant enhancements over wild type control animals in behavior analysis of learning and memory, directly tying astrocytic functions to cognitive powers (Han et al., 2013). The increased complexity of human astrocytes, the numbers of synapses contacted and the speed of Ca2+ waves are possibly contributing to heightened intelligence compared to rodents. Other glial cells, namely oligodendrocytes are also believed to contribute to the superior cognitive functions in humans, given that the volume of white matter in humans constitutes 50–80% of the brain while only 14% of the rodent brain (Zhang and Sejnowski, 2000). Whether human astrocytes facilitate myelination more than rodent astrocytes is still unknown, however, here we review the current knowledge on how astrocytes may affect myelination in normal physiological conditions and in disease.
Astrocyte heterogeneity and general characteristics
Given the ample functions of astrocytes it is not surprising that they are a heterogeneous class of cells. Developmentally, astrocytes originate from several sources and can vary in proliferation rates and in response to brain injury (Bardehle et al., 2013). The main sources of mature astrocytes during development are the radial glia cell in the brain and several types of progenitor cells in the spinal cord (Vaccarino et al., 2007, Nash et al., 2011, Molofsky et al., 2012, Tsai et al., 2012). Astrocytes can be continually generated in the adult brain by subventricular stem cells (Molofsky et al., 2012) and a small fraction of dividing mature astrocytes, depending on the brain region (Emsley and Macklis, 2006). Surprisingly, the astrocytes originating from different progenitor cells stay in place and are not replenished by astrocytes in neighbor domains (Tsai et al., 2012). Experimental data supports the idea that astrocytes are heterogenous according to both their origin and by virtue of their environment (Emsley and Macklis, 2006, Wang and Bordey, 2008). It is possible that astrocytes adapt to the needs of their surrounding tissue. This could also be reflected by the fact that the sheer density of astrocytes differs between brain regions (Emsley and Macklis, 2006).
Expression levels of intermediate filament proteins vary distinctively in astrocytes. The abundance of filamentous proteins was amongst the very first characteristics of astrocytes that were noticed by researchers using histochemical stainings (Miller and Raff, 1984). The structural integrity of astrocytes is supported by several filamentous proteins including vimentin, desmin, synemin and Glial Acidic Fibrillary Protein (GFAP) (Dahl and Bignami, 1982, Pixley et al., 1984, Hirako et al., 2003). GFAP was identified as the most abundant intermediate filament expressed in astrocytes (Bignami et al., 1972, Schachner et al., 1977, Kimelberg, 2004). However, immunostainings for GFAP reveal neither the number nor the complexity of astrocytes, as up to 85% of astrocytic processes do not contain GFAP (Bushong et al., 2002). Usually, the finer processes are GFAP negative, however, a subset of astrocytes does not express GFAP at all. The cytoplasmic Ca2+ binding protein S100b is expressed in a larger proportion of astrocytes than GFAP, although it is not expressed in all astrocytes (Ogata and Kosaka, 2002). Whereas GFAP in the CNS is exclusively expressed by astrocytes, some studies have found s100b expression in a small proportion of neurons (Rickmann and Wolff, 1995, Yang et al., 1995) and oligodendrocytes (Vives et al., 2003, Zuo et al., 2004).
The majority of astrocytes are post mitotic at physiological conditions, and very active proliferation is usually associated with astrogliosis, e.g. induced by CNS damage (Nash et al., 2011). An overall 10% of astrocytes are cycling in the adult brain as measured by BrdU incorporation, however, the fraction of astrocytes undergoing cell division varies in different brain areas (Emsley and Macklis, 2006). Following injury astrocyte proliferation may be important for scar formation but the relevance of astrocyte proliferation under physiological condition is unknown (Bardehle et al., 2013). Uncertainty with regard to proliferation capabilities in the human, uninjured brain further highlights our lack of understanding of the importance of astrocyte proliferation in physiological states.
Despite their heterogeneity, astrocytes do have a number of characteristics in common. Electron microscopy reveals how the cytoplasm of astrocytes is distinguished from other cells due to their relatively scarce organelles. Besides from the intracellular filaments supporting the morphology of astrocytes as part of the cytoskeleton, the cytoplasm of astrocytes has a light appearance in the electron microscope (EM) compared to that of neurons and especially oligodendrocytes (Peters et al., 1991). Astrocytic filaments resemble neuronal filaments, however, unlike their neuronal counterparts the astrocytic filaments usually occur in bundles. As shown by immunohistochemistry, it is also observed on EM micrographs that white matter astrocytes contain larger amounts of filaments than protoplasmic astrocytes (Kettenmann and Ransom, 1995). The nuclei of astrocytes are usually irregular in shape and are finely granulated (Mori and Leblond, 1969). Ultrastructural analysis also reveals that mitochondria of white matter astrocytes are more elongated than those of grey matter astrocytes. The chromatin is condensed along the nuclear envelope and nuclear envelope pores are abundant (Peters et al., 1991). In terms of membrane properties, astrocytes are characterized by expression of a wealth of different K+ channels, causing their membrane to be leaky (Zhou et al., 2009). Consequently, the membrane resistance of astrocytes is ~10 MΩ, which is extremely low compared to other cell types in the CNS (Schools et al., 2006). Although some voltage-gated Na+ channels may be expressed by immature astrocytes (Kressin et al., 1995) the cell membranes of mature astrocytes are passive to changes in membrane potential and thus astrocytes are non-excitable. The resting membrane potential of astrocytes is −78 mV or below (Ransom and Sontheimer, 1992, Schools et al., 2006).
White matter versus grey matter astrocytes
Astrocytes have been divided into 9 groups based on morphology (Emsley and Macklis, 2006) and very distinctive differences are observed between grey and white matter astrocytes. The striking difference in morphology of astrocytes in the grey matter versus white matter gave rise to the two names: protoplasmic and fibrous astrocytes, respectively. In rodent white matter, the astrocytes’ cell bodies are small and their processes are aligned with the myelinated fibers, giving an elongated morphology (Fig 1). In grey matter, astrocytes are typically larger and have more sheath-like and branched processes, however, the more sheath-like processes are fine and not labeled by GFAP (Fig 1). Grey matter astrocytes may also be polarized, for example in the dentate gyrus, where astrocytes extend most of their processes towards the hippocampal fissure (Bushong et al., 2003). In humans, the overall morphology of fibrous and protoplasmic astrocytes is similar to those found in rodents, however, with some noticeable differences. Firstly, as reported by Oberheim et al, human astrocytes are significantly bigger than murine astrocytes (Oberheim et al., 2006, Oberheim et al., 2009). In addition, the human astrocytes, particularly in the grey matter, are much more complex in morphology than their rodent counterparts (Oberheim et al., 2006, Oberheim et al., 2009). The number of synapses that an individual astrocyte can span is therefore correspondingly larger and intracellular signaling through the astrocytes contacting synapses could potentially be facilitating transmission of information between neurons. The astrocytes originating from human-derived immature glial progenitor cells transplanted into rodent brains developed a complex morphology similar to what is observed for astrocytes in human brains (Han et al., 2013), indicating that size and morphology of astrocytes are determined by cell-intrinsic factors. However, the human astrocytes developed to adapt a protoplasmic and fibrous morphology according to their location in grey and white matter, respectively (Han et al., 2013). This is an example that astrocytes of the same origin adapt an intrinsic program in terms of size and morphology according to their environment. However, it is still unknown what factors in the grey and white matter are conducive for adaptation of the specific morphologies.
Figure 1.
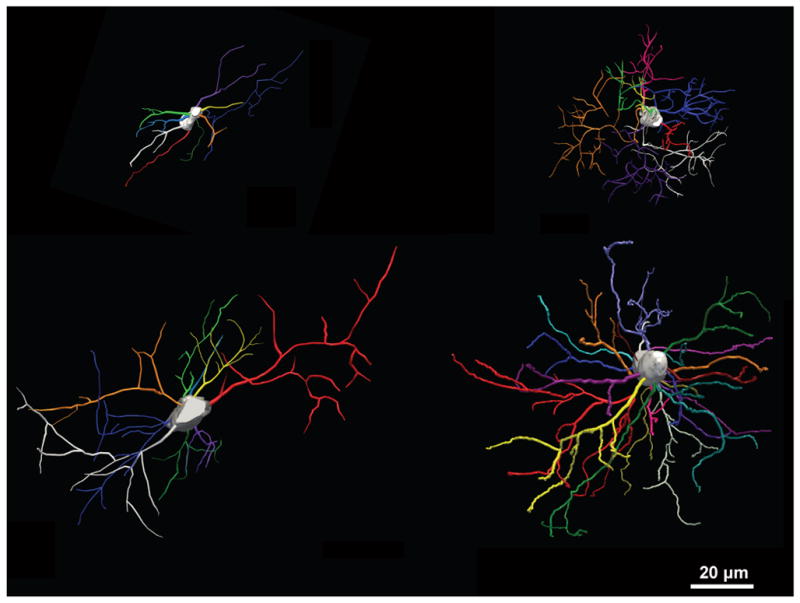
3D reconstruction of astrocytes stained for GFAP. Fibrous astrocyte in mouse corpus callosum (top left) and in human subcortical white matter (bottom left). Protoplasmic astrocyte in mouse cortex (top right) and human cortex (bottom right).
The hypothesis that diversity in terms of morphology could be a reflection of specialized functions is supported by diversity in terms of protein expression profiles in grey matter compared to white matter astrocytes. Both surface markers and intracellular proteins are reported as differentially expressed in grey matter versus white matter astrocytes. The surface marker cluster of differentiation 44 (CD44) is expressed mainly by astrocytes, which express several splice variants of CD44. CD44 is a hyaluroran receptor, which is a highly abundant extracellular matrix glycosaminoglycan. CD44 is also expressed by astrocyte-committed glial progenitors (Bugiani et al., 2013). However, CD44 is expressed predominantly by fibrous astrocytes found in the brain’s white matter (Kaaijk et al., 1997). The functional relevance of the interactions between the extracellular matrix and astrocytes in white matter are not fully understood.
Other noteworthy proteins that are differentially expressed are filamentous proteins including vimentin and GFAP (Table 1). These intermediate filaments are highly expressed in white matter compared to grey matter (Goursaud et al., 2009), suggesting that astrocytes serve a specialized function with regards to structure amongst myelinated fibers. Besides from the structural function of GFAP, it has been suggested that GFAP expression correlates with glutamate transporter function. GFAP physically interacts with and might serve as an intracellular anchor for Glutamate Aspartate Transporter (GLAST) (Sullivan et al., 2007). The hypothesis is supported by the fact that GFAP knockout mice have decreased expression of GLAST (Hughes et al., 2004). A study showed that cultured astrocytes from white matter express higher levels of the glutamate transporters Glutamate Transporter 1 (GLT-1) and GLAST compared to astrocytes from grey matter (Goursaud et al., 2009). Not only is GFAP more highly expressed in white matter astrocytes, a large fraction of grey matter astrocytes does not express GFAP (Ludwin et al., 1976, Bushong et al., 2002).
Table 1.
Differences in general characteristics of grey and white matter astrocytes.
| Grey matter astrocytes | White matter astrocytes |
|---|---|
|
| |
| Low/no CD44 expression | High CD44 expression |
| Low GFAP expression | High GFAP expression |
| Low vimentin expression | High vimentin expression |
| Low nestin expression | High nestin expression |
| Highly branched morphology | Elongated, more simple morphology |
On the more physiological level, some differences between white and grey matter astrocytes have also been suggested. The cytoplasm of adjacent astrocytes is joined by homomeric gap junctions formed by Cx43 and to a lesser extent Cx30 through which Ca2+ waves are propagated in large networks of astrocytes (Haas et al., 2006, Orthmann-Murphy et al., 2008). This long-distance signaling between astrocytes propagates at a speed of 10–20 μm/s both in white and grey matter (Haas et al., 2006). However, dye injection experiments in acute brain slices showed that the coupling between astrocytes is remarkably different between grey and white matter astrocytes. In grey matter astrocytes are coupled in a network of an average of 94 cells that spanned 390 μm in diameter (Haas et al., 2006). In contrast, when the same experiment was performed on astrocytes in the corpus callosum the astrocytes showed coupling to few or no other astrocytes (Haas et al., 2006). However, dye injection followed by optical bleaching showed that the optic nerve contained the highest degree of coupled astrocytes of the areas investigated, namely 91% of the cells (Lee et al., 1994). Although the experiments were conducted using different methods, they suggest that the gapjunction coupling of astrocytes may be more varied in white matter.
As first demonstrated in the grey matter, astrocytes facilitate fast repetitive information transmission between neurons by clearing the extracellular space of neurotransmitters. Astrocytes are the main cell type expressing transporters for the main excitatory neurotransmitter, glutamate. The family of glutamate transporters consists of 5 genes which gene products are in the class of Excitatory Amino Acid Transporters (EAAT) (Danbolt, 2001). Of these, astrocytes express predominantly GLT-1 (EAAT2) and GLAST (EAAT1). That astrocytic uptake of glutamate is indeed vital for maintaining a physiological balance as demonstrated by Rothstein et al., who showed that knocking down GLAST and GLT-1 by siRNAs causes neurotoxicity (Rothstein et al., 1996). Accordingly, the activity of glutamate transporters per mg of freshly isolated tissue in grey matter is higher than for white matter (Hassel et al., 2003). However, when normalizing to the density of synapses in grey versus white matter, the glutamate transporter activity is significantly higher in corpus callosum than cortex (Hassel et al., 2003). Cell culture studies showed that the highest expression level of glutamate transporters is found in astrocytes isolated from white matter (Goursaud et al., 2009). In addition, the capacity for metabolism of glutamate to glutamine is higher in white matter astrocytes than grey matter astrocytes in vitro (Goursaud et al., 2009) further supporting the hypothesis that there is a need for more effective glutamate clearance in white matter. That a relatively higher glutamate uptake and metabolism is indeed an indicator that glutamate levels are more tightly controlled in white than grey matter is confirmed by the findings that the glutamate concentration in white matter is only half of what is found in the grey matter (Hassel et al., 2003). Overload of glutamate may cause excitotoxicity via ionotropic glutamate receptors on the neurons’ cell body or axons. Glutamatergic excitotoxicity may occur also in oligodendrocytes from activation of AMPA/kainate and NMDA receptor activation (Oka et al., 1993, Borges et al., 1994, Karadottir et al., 2005), suggesting that glutamate clearance by astrocytes in white matter might be needed in order to maintain healthy oligodendrocytes.
Astrocytes in developing and adult white matter
In early development astrocytes are generated from GFAP-expressing radial glial cells in addition to glia progenitors (Vaccarino et al., 2007); but they can be distinguished from mature astrocytes based on their morphology and their lack of ability to generate neurons or oligodendrocytes. GFAP-expressing radial glia are present in early pre-natal development, however, GFAP-expressing mature astrocytes in the grey and white matter are first detected at E16 (Miller et al., 1985, Sancho-Tello et al., 1995, Qian et al., 2000), around the time where OPCs have begun migrating throughout the brain from their originating locations (Fogarty et al., 2005, Vallstedt et al., 2005, Kessaris et al., 2006). Myelination in rodents is sparse before birth and is accelerated during early postnatal development. In the rat spinal cord the number of glial cells increase 6-fold during the first two post-natal weeks (Gilmore, 1971) and mature astrocytes appear in large numbers at the same developmental stage as axons are myelinated by oligodendrocytes (Qian et al., 2000). How astrocytes facilitate each step of myelination including proliferation of oligodendrocyte precursor cells, initial contact between oligodendrocytes and axons and myelination has been addressed by several studies.
Astrocytes may control proliferation and migration of oligodendrocyte precursor cells (OPCs) as they are the main producer of platelet-derived growth factor-alpha (PDGF) in the CNS (Noble and Murray, 1984, Noble et al., 1988, Richardson et al., 1988). PDGF-alpha is also the most important survival factor for OPCs and inhibits differentiation of oligodendrocytes, thereby regulating the timing of myelination (McKinnon et al., 2005). Co-culture studies of retinal ganglion cells and optic nerve OPCs and astrocytes have demonstrated a role for astrocytes in facilitating alignment and adhesion of immature oligodendrocytes with unmyelinated axons (Meyer-Franke et al., 1999). The enhanced adhesion of MBP+ processes with axons induced by co-culturing with astrocytes was reproduced by adding endoneuroaminidase-N, that cleaves off polysialic acid (PSA) from NCAM (Rougon, 1993). This suggests that unmasking of neuronal NCAM by astrocytic enzymes is needed to initiate robust contact between oligodendrocytes and axons (Rutishauser, 1996, Kiss and Rougon, 1997). Downregulation of PSA is correlative with onset of myelination in rodents and humans, however, in vivo evidence that astrocyte-mediated cleavage of PSA initiates myelination is lacking (Bartsch et al., 1990, Nait Oumesmar et al., 1995, Fewou et al., 2007, Jakovcevski et al., 2007). A wealth of soluble factors secreted by astrocytes has also been implicated in enhancing myelination. Examples of these are neuregulin and cleavage of neuregulin by gamma-secretase (Wang et al., 2007, Taveggia et al., 2008, Watkins et al., 2008), brain-derived neurotrophic factor (BDNF) (Cellerino et al., 1997, Jean et al., 2008, Xiao et al., 2010), ciliary neurotrophic factor (CNTF)(Stankoff et al., 2002, Nash et al., 2011), insulin-like growth factor 1 (IGF-1)(Ballotti et al., 1987, Ye et al., 2002, Ye et al., 2004, Wang et al., 2007, Zeger et al., 2007) and osteopontin (Selvaraju et al., 2004), amongst others (Barnett and Linington, 2012). However, astrocytes also secrete factors implicated in inhibition of myelination, such as TGF-alpha, BMP2/4 and hyaluroran, as reviewed by Barnett and Linington (2012). Interestingly, a role for astrocytes in sensing activity of neurons and consequently instructing oligodendrocytes to myelinate have been suggested by Ishibashi et al. Using an in vitro model of myelination, they showed that leukemia inhibitory factor (LIF) is released from astrocytes in response to neuronal activity and axonal ATP release and that secretion of LIF by astrocytes increases myelination (Ishibashi et al., 2006). Similarly, application of the main excitatory neurotransmitter glutamate stimulates release of the pro-myelination growth factor BDNF from astrocytes in culture (Jean et al., 2008).
Evidence that physical contact between astrocytes and oligodendrocytes plays a crucial role in both myelination and continued support of white matter is indicated by the disease severity and progression of the myelin disease Pelizaeus-Merzbacher-like disease (PMLD) caused by mutations in genes encoding glial connexins (Orthmann-Murphy et al., 2007a). Connexin (Cx) 30 and Cx43 on astrocytes form gap junctions with oligodendrocytes’ Cx32 and Cx47, respectively (Orthmann-Murphy et al., 2007b), through which cytosolic components up to ~900 Da can flow (Bruzzone et al., 1996). The phenotype observed in connexin knockout mice recapitulates the pathology observed in patients. Elimination of gap-junction coupling of oligodendrocytes and astrocytes in mice produces pronounced white matter pathology including delayed myelination, vacuoles in the corpus callosum and optic nerve and death of oligodendrocytes in young adults (Odermatt et al., 2003, Tress et al., 2012). The importance of the pan-glia syncytium may be metabolic support of oligodendrocytes by astrocytes, as there is some evidence that suggests a unidirectional flow through gap junctions, whereby cytosolic contents originating from astrocytes are preferentially transported to the cytosol of oligodendrocytes (Robinson et al., 1993). However, it has been suggested that a major function of oligodendrocyte-astrocyte coupling is to buffer K+ which accumulates in oligodendrocytes following neurotransmission (Nagy and Rash, 2000). In white matter, oligodendrocytes are directly exposed to K+ released from active axons and astrocytes could potentially provide spatial buffering of K+ via direct flow into their cytoplasm via gap junctions.
The function of the structural integrity provided by astrocytes has been tested by genetic deletion of GFAP. Surprisingly, GFAP knockout mice are viable. Considering the many functions of astrocytes it was an even more surprising finding that the GFAP knockout mice reveal more extensive abnormalities in white matter than in grey matter. Hypomyelination as well as ultrastructural myelin pathologies including loosening of myelin sheaths are very prominent features in the CNS of GFAP knockout mice (Liedtke et al., 1996). It has been proposed that deletion of GFAP might have other consequences than alterations of structural support in the CNS such as reduced glutamate clearance as GFAP physically interacts with GLAST (Sullivan et al., 2007). Although the underlying mechanisms of pathology in the GFAP knockout mice are not fully understood, the developmental myelin pathologies in these mice demonstrate that normal development of oligodendrocytes is supported by astrocytes.
In summary, the majority of experimental evidence points to astrocytes as being crucial in facilitating normal myelination during development. In terms of maintaining healthy oligodendrocytes throughout adulthood astrocytes play crucial roles in maintaining the right environment for oligodendrocytes and perhaps also ion buffering and metabolic supply. However, as many experiments that demonstrate a role for astrocytes in myelination by secretion of soluble factors have been performed in vitro additional studies are needed to obtain a more detailed knowledge on the role of astrocytes in myelination in vivo.
The role of astrocytes in age-related white matter changes
Aging in humans is accompanied by increased rates of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. However, even in healthy individuals a substantial cognitive decline in terms of learning and memory deficits is a general consequence of old age (Vinkers et al., 2005). Surprisingly, although neuronal changes occur, investigations of primate brains showed that the number of neurons was constant from young adults to aged animals, indicating that cognitive decline as an effect of aging is not caused by neuronal loss (Peters et al., 1994, Peters et al., 1996). Further investigations showed gross changes in myelin of aged monkeys. The percentage of myelinated axons only decreases from 94 to 90% in the anterior commissure but the absolute number of myelinated axons declines dramatically (Sandell and Peters, 2003, Hinman and Abraham, 2007). A closer look at the myelin on the ultrastructural level revealed abnormalities with regards to structures of myelin sheaths and paranodes (Sandell and Peters, 2003). The numbers and proportions of glial cells were not affected by aging, however, the volume of astrocytic processes was greater in the aged monkeys (Sandell and Peters, 2002). One explanation for the expansion of astrocyte volume could be that astrocytes are simply filling the space left by the degenerated oligodendrocytes and axons, however, there are several indications that astrocytes might play an active role in the demise of white matter in aging. Increased astrocytic GFAP content, a changed secretion profile as well as an increase in general indicators of astrogliosis are known age-related changes of the mammal CNS (Sloane et al., 2000, Moore et al., 2011, Salminen et al., 2011). In the adult cerebral cortex only a small minority of astrocytes expresses GFAP and the cells are non-proliferative (Buffo et al., 2008). Following CNS injuries GFAP expression can be upregulated or induced and proliferation evoked in mature astrocytes, a phenotype acknowledged as reactive (Buffo et al., 2008). Although there is a continuum of the reactive astrocyte phenotype according to severity, even mild forms of reactive astrocytes have been correlated with damage (Sofroniew and Vinters, 2010). In some instances reactive astrocytes may improve the outcome after CNS injury, although the reactive astrocyte phenotype is more commonly connected to deleterious outcomes (Sofroniew and Vinters, 2010). The underlying cause of astrogliosis during aging might be chronic low grade inflammation, as inflammation is increasing with aging (Franceschi et al., 2007). In addition, senescent and activated astrocytes may themselves contribute to inflammation by releasing pro-inflammatory cytokines (Li et al., 2011, Salminen et al., 2011). The evidence for inflammatory-induced astrogliosis remains controversial (Little and O’Callagha, 2001), however, the link between astrogliosis and white patter pathology is more clear. There is compelling evidence that aging is accompanied by a transformation of a proportion of astrocytes into reactive astrocytes, as measured by increased expression of GFAP. Although an increase in the number of GFAP-expressing astrocytes is not reliably observed in aged animals, the level of GFAP expression per cell is consistently increased (Sabbatini et al., 1999, Salminen et al., 2011). The level of GFAP-expression per cell increases disproportionately more in white matter than grey matter during aging, indicating that white matter astrocytes may be more affected than grey matter astrocytes. Although it is unclear if the increase in GFAP in the context of aging is a direct cause of myelin degeneration, an experimental model of Alexander Disease showed that accumulation of mutant GFAP is directly correlated with disease severity (Jany et al., 2013). This suggests that senescent-induced changes in astrocyte may contribute, if not drive, age-relaged myelin pathology.
The role of astrocytes in white matter pathology
Congenital dysmyelinating disorders
Leukodystrophies encompass the genetically determined white matter disorders and are characterized by abnormal myelin formation (Schiffmann and van der Knaap, 2009). Despite the fact that these diseases are categorized as myelin pathologies there are several examples of leukodystrophies where astrocytes have been identified as the underlying cause of disease. Among those, Alexander Disease is a clear example of how astrocytic dysfunction can compromise the development and integrity of myelin. Alexander Disease can manifest from early infancy to late childhood and symptoms may include seizures, spasticity and intellectual disability in the affected patients (Gordon, 2003, Liem and Messing, 2009, Sawaishi, 2009). The underlying pathology results from mutations in the GFAP gene leading to toxic gain of function. Astrocytes in Alexander Disease develop a reactive phenotype including hypertrophic GFAP+ processes (Fig 2a–b). The mutant GFAP forms characteristic intracytoplasmic aggregates known as Rosenthal fibers, which can be seen on hematoxylin and eosin (H&E) stain and electron microscopy (Liem and Messing, 2009, Messing et al., 2012). Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is another leukodystrophy where astrocytes play a central role in the pathogenesis. The disease commonly affects infants and leads to progressive spasticity and ataxia (Ridder et al., 2011). The clinical pathologies were directly linked to mutations in a specific gene, named MLC1. MLC1 protein is mainly expressed in astrocyte endfeet surrounding blood vessels (Boor et al., 2005). In vitro studies showed that the formation of vacuoles found in vivo can be reproduced in astrocytes cultures with mutations in the MLC1 gene (Duarri et al., 2011), implying that the role of astrocytes in maintaining BBB integrity is crucial for myelination. Vanishing white matter (VWM) disease is another leukoencephalopathy where dysmorphic astrocytes have been described. It predominantly manifests in early childhood but can arise in all ages. VWM disease presents with episodes of rapid clinical deterioration triggered by stress such as minor head trauma or fever and may lead to death (van der Knaap et al., 2006). Pathohistological findings include increased proliferation and maturation defects in astrocytes. Abnormal composition of intermediate filament network, with predominance of the GFAP delta isoform, and general upregulation of heat shock proteins suggest that the astrocytes are metabolically stressed (Bugiani et al., 2011). The five genes linked to this pathology encode different subunits of the eukaryotic initiation factor eIF2B (Maletkovic et al., 2008). However, eIF2B is expressed in both astrocytes and oligodendrocytes and thus the primary role of astrocytes in VWM remains undefined (Bugiani et al., 2011).
Figure 2.
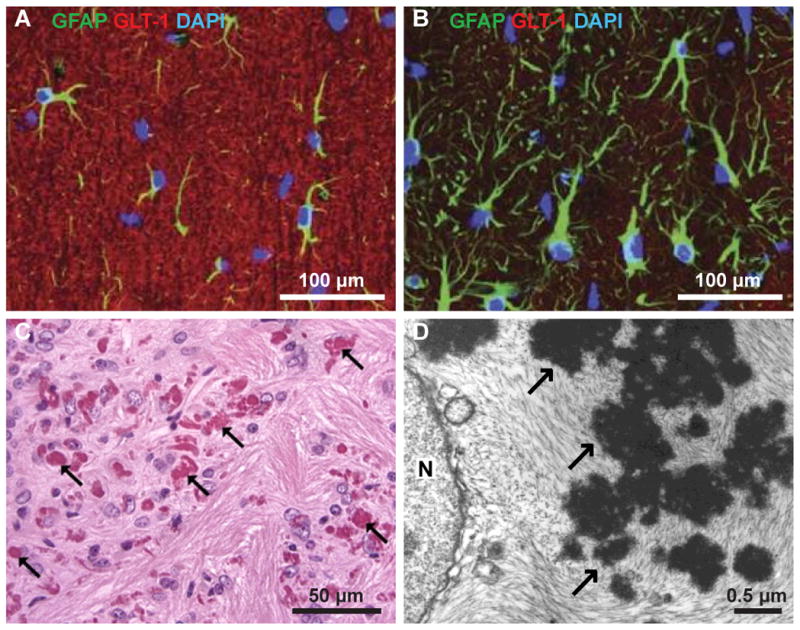
A: Astrocytes in the hippocampus of wild type mouse express GFAP (green) and GLT-1 (red). B: Astrocytes in the hippocampus of Alexander Disease model GFAP-R23H mouse have hypertrophic GFAP-positive processes (green) and express low levels of GLT-1 (red); from Tian et al., Neuropathol Exp Neurol (2010). C: Hematoxylin and eosin stain stain of periventricular white matter showing Rosenthal fibers (arrows) in Alexander Disease model R76H/+ mouse; from Hagemann et al., J Neurosci (2006). D: Electron micrograph of post-mortem tissue from a 17 month old child with Alexander Disease showing an astrocyte with Rosenthal fibers (arrows) in the cytoplasm next to the nucleus (N); from Eng et al., J Neurosci Res (1998).
Autoimmune myelopathies
The primary demyelinating disease multiple sclerosis (MS) is characterized by immune-mediated attacks leading to loss of mature oligodendrocytes and myelin. Astrogliosis is a hallmark of demyelinated lesions in MS and formation of an astroglia scar is often observed in chronic demyelinated lesions (Wu and Raine, 1992). Astrogliosis is thought to create a non-permissive environment for remyelination, however, it is believed to be a secondary effect of demyelination rather than the cause of disease (Holley et al., 2003). Conversely, in neuromyelitis optica (NMO), another autoimmune demyelinating disorder in some ways similar to MS, the disease is caused by autoimmune response directed against astrocytes. NMO is an inflammatory demyelinating disorder that predominantly affects the optic nerves and spinal cord and courses with rapid progression of disability (Wingerchuk et al., 1999). Although initially thought to be a subtype of MS, the finding of serum reactivity targeting Aquaporin 4 (AQP4), absent in MS, supports the distinct humoral pathogenesis directed against astrocytes (Jarius and Wildemann, 2010). Anti-AQP4 antibodies were first described by Lennon and colleagues in 2004 in parallel with a striking loss of AQP4 in optic nerve and spinal cord lesions (Lennon et al., 2004). The AQP4 antibody titer correlates with disease activity and lesions occur predominantly in areas of high AQP4 expression. AQP4 is expressed exclusively in astrocytes’ endfeet processes lining the cerebral vasculature. Experimental evidence suggests that APQ4 antibody activates complement, induces necrotic cell death in astrocytes and impairs BBB function and glutamate homeostasis. The combination of these effects is thought to result in demyelination (Jarius and Wildemann, 2010).
In primary demyelinating disorders remyelination is needed for functional recovery. Several studies have investigated different aspects of the role of astrocytes in remyelination. Blakemore et al. tested how transplantation of astrocytes influenced remyelination in a study where endogenous astrocytes were killed using a gliatoxin (Blakemore et al., 2003). Although a proportion of the lesions with transplanted astrocytes were remyelinated as efficiently as the lesions largely devoid of astrocytes, remyelination was compromised in number of lesions with exogenous astrocytes. In addition, when comparing remyelination within lesions, there was no difference in the area of the lesion where astrocyte processes were present. However, a similar study using transplantation of astrocytes into demyelinated lesions showed that astrocytes enhanced remyelination by endogenous oligodendrocyte precursor cells (Franklin et al., 1991). In MS, astrocytes have a role in both phagocytosis of myelin debris which is necessary for efficient remyelination (Kotter et al., 2006) and antigen presentation which in the case of MS is disease-promoting (Lee et al., 1990). Also in terms of secreted factors astrocytes have been ascribed both enhancing and inhibiting roles in relation to remyelination (Nair et al., 2008). In chronic demyelinated lesions astrocytes express elevated levels of both CD44 and its ligand hyaluronan and this inhibits maturation of oligodendrocyte precursor cells (Back et al., 2005). However, it is unknown if this seemingly aberrant response from astrocytes in MS could is part of MS pathogenesis or a secondary response to MS pathology. Furthermore, the role of CD44 is unclear as a global knockout of CD44 showed worsened outcomes of EAE (Flynn et al., 2013). Amongst the basis for the worse outcomes of EAE in CD44 deficient mice was increased breakdown of the blood-brain barrier.
Similar to myelination in development, astrocyte secreted factors may also enhance remyelination (Barnett and Linington, 2012). In addition, a recent study indicated a specific metabolic role for astrocytes in remyelination by oligodendrocytes. A conditional knockout of the iron efflux transporter ferroportin (Fpn) under the GLAST promoter two weeks before induction of remyelination decreased both OPC proliferation and remyelination (Schulz et al., 2012). These results indicate that iron homeostasis is vital for oligodendrocytes ability to remyelinate axons and that astrocytes play an essential role in oligodendrocyte iron metabolism. Given that gapjunctions between astrocytes and oligodendrocytes appear to be crucial for myelination and survival of oligodendrocytes, it would not be surprising if gapjunction coupling also is important for remyelination (Odermatt et al., 2003, Orthmann-Murphy et al., 2007a, Tress et al., 2012). Expression of genes encoding oligodendrocyte and astrocytes connexins are dysregulated in MS patients, especially in chronic demyelinated lesions the glia connexins Cx43 and Cx47 are downregulated (Markoullis et al., 2012).
Ultimately, the balance between opposing effects of astrogliosis and normal functions of astrocytes may therefore be determining if astrocytes inhibit or enhance remyelination in demyelinating diseases such as MS. As reactive astrocytes is a common denominator in Alexander Disease and chronic demyelinated MS lesions, a potential strategy with regards to ameliorating astrogliosis-induced pathology would be to simply reduce astrogliosis. Using lentivira encoding short hairpin RNA against GFAP or Vimenting, Desclaux et al obtained promising results in terms of nerve regeneration in a spinal cord injury model (Desclaux et al., 2009).
Conclusion
In the mammalian CNS, astrocytes have been attributed countless of vital roles to development of neurons, e.g. formation and pruning of synapses, as well as in the development or repair of acute CNS trauma or chronic neurodegenerative diseases. This review was focused on a topic that has received less attention, namely the role of astrocytes in differentiation and maintenance of oligodendrocytes as well as repair in myelin diseases. The review of existing literature testifies to the plentiful roles of astrocytes in the CNS with relevance to oligodendrocytes, ranging from survival, proliferation and migration of OPCs to secreted factors regulating myelination. The central role of astrocytes in the leukodystrophies Alexander Disease, MLC and VWM is evidence that dysfunctional astrocytes may directly cause myelin abnormalities and breakdown. PMLD where gapjunctions between oligodendrocytes and astrocytes are compromised demonstrate that the panglia syncytium is crucial for myelin formation and maintenance. NMO is an example how targeted destruction of astrocytes around blood vessels lead to myelin pathology. Astrocytes can also positively enhance myelination and remyelination, as shown by both in vitro and in vivo studies. In conclusion, there is a potential scope for treatment of developmental myelin diseases and adult demyelinating disorders by manipulating astrocyte functions. Strategies in disease treatment could include identification and administration of astrocytic secreted agents that promote myelination and remyelination, or gene therapies that could reduce astrogliosis. Thus, treatments for CNS diseases converge on manipulating astrocytes and may in some cases provide a multifunctional solution in the forefront of combating neurodegenerative diseases.
Astrocytes facilitate myelination
Dysfunctional astrocytes may lead to myelin pathologies
Astrocytes may be required for efficient remyelination in demyelinating diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of references
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Araque A. Astrocytes process synaptic information. Neuron glia biology. 2008;4:3–10. doi: 10.1017/S1740925X09000064. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature medicine. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Ballotti R, Nielsen FC, Pringle N, Kowalski A, Richardson WD, Van Obberghen E, Gammeltoft S. Insulin-like growth factor I in cultured rat astrocytes: expression of the gene, and receptor tyrosine kinase. The EMBO journal. 1987;6:3633–3639. doi: 10.1002/j.1460-2075.1987.tb02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nature neuroscience. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Linington C. Myelination: Do Astrocytes Play a Role? The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2012 doi: 10.1177/1073858412465655. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Kirchhoff F, Schachner M. Highly sialylated N-CAM is expressed in adult mouse optic nerve and retina. Journal of neurocytology. 1990;19:550–565. doi: 10.1007/BF01257243. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain research. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Gilson JM, Crang AJ. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Experimental neurology. 2003;184:955–963. doi: 10.1016/S0014-4886(03)00347-9. [DOI] [PubMed] [Google Scholar]
- Blutstein T, Haydon PG. The Importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61:129–139. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor PK, de Groot K, Waisfisz Q, Kamphorst W, Oudejans CB, Powers JM, Pronk JC, Scheper GC, van der Knaap MS. MLC1: a novel protein in distal astroglial processes. Journal of neuropathology and experimental neurology. 2005;64:412–419. doi: 10.1093/jnen/64.5.412. [DOI] [PubMed] [Google Scholar]
- Borges K, Ohlemeyer C, Trotter J, Kettenmann H. AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience. 1994;63:135–149. doi: 10.1016/0306-4522(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. European journal of biochemistry/FEBS. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M, Boor I, van Kollenburg B, Postma N, Polder E, van Berkel C, van Kesteren RE, Windrem MS, Hol EM, Scheper GC, Goldman SA, van der Knaap MS. Defective glial maturation in vanishing white matter disease. Journal of neuropathology and experimental neurology. 2011;70:69–82. doi: 10.1097/NEN.0b013e318203ae74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, van der Knaap MS, Boor I. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain : a journal of neurology. 2013;136:209–222. doi: 10.1093/brain/aws320. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Examination of the relationship between astrocyte morphology and laminar boundaries in the molecular layer of adult dentate gyrus. The Journal of comparative neurology. 2003;462:241–251. doi: 10.1002/cne.10728. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Molecular and cellular neurosciences. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Dahl D, Bignami A. Immunohistological localization of desmin, the muscle-type 100 A filament protein, in rat astrocytes and Muller glia. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1982;30:207–213. doi: 10.1177/30.3.7037941. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Desclaux M, Teigell M, Amar L, Vogel R, Gimenez YRM, Privat A, Mallet J. A novel and efficient gene transfer strategy reduces glial reactivity and improves neuronal survival and axonal growth in vitro. PloS one. 2009;4:e6227. doi: 10.1371/journal.pone.0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. Journal of lipid research. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dodla MC, Mumaw J, Stice SL. Role of astrocytes, soluble factors, cells adhesion molecules and neurotrophins in functional synapse formation: implications for human embryonic stem cell derived neurons. Current stem cell research & therapy. 2010;5:251–260. doi: 10.2174/157488810791824520. [DOI] [PubMed] [Google Scholar]
- Duarri A, Lopez de Heredia M, Capdevila-Nortes X, Ridder MC, Montolio M, Lopez-Hernandez T, Boor I, Lien CF, Hagemann T, Messing A, Gorecki DC, Scheper GC, Martinez A, Nunes V, van der Knaap MS, Estevez R. Knockdown of MLC1 in primary astrocytes causes cell vacuolation: a MLC disease cell model. Neurobiology of disease. 2011;43:228–238. doi: 10.1016/j.nbd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron glia biology. 2006;2:175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewou SN, Ramakrishnan H, Bussow H, Gieselmann V, Eckhardt M. Down-regulation of polysialic acid is required for efficient myelin formation. The Journal of biological chemistry. 2007;282:16700–16711. doi: 10.1074/jbc.M610797200. [DOI] [PubMed] [Google Scholar]
- Flynn KM, Michaud M, Madri JA. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. The American journal of pathology. 2013;182:1322–1336. doi: 10.1016/j.ajpath.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. Journal of neurocytology. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- Gilmore SA. Neuroglial population in the spinal white matter of neonatal and early postnatal rats: an autoradiographic study of numbers of neuroglia and changes in their proliferative activity. The Anatomical record. 1971;171:283–291. doi: 10.1002/ar.1091710208. [DOI] [PubMed] [Google Scholar]
- Gordon N. Alexander disease. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2003;7:395–399. doi: 10.1016/j.ejpn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud S, Kozlova EN, Maloteaux JM, Hermans E. Cultured astrocytes derived from corpus callosum or cortical grey matter show distinct glutamate handling properties. Journal of neurochemistry. 2009;108:1442–1452. doi: 10.1111/j.1471-4159.2009.05889.x. [DOI] [PubMed] [Google Scholar]
- Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cerebral cortex. 2006;16:237–246. doi: 10.1093/cercor/bhi101. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell stem cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Boldingh KA, Narvesen C, Iversen EG, Skrede KK. Glutamate transport, glutamine synthetase and phosphate-activated glutaminase in rat CNS white matter. A quantitative study. Journal of neurochemistry. 2003;87:230–237. doi: 10.1046/j.1471-4159.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- Hinman JD, Abraham CR. What’s behind the decline? The role of white matter in brain aging. Neurochemical research. 2007;32:2023–2031. doi: 10.1007/s11064-007-9341-x. [DOI] [PubMed] [Google Scholar]
- Hirako Y, Yamakawa H, Tsujimura Y, Nishizawa Y, Okumura M, Usukura J, Matsumoto H, Jackson KW, Owaribe K, Ohara O. Characterization of mammalian synemin, an intermediate filament protein present in all four classes of muscle cells and some neuroglial cells: co-localization and interaction with type III intermediate filament proteins and keratins. Cell and tissue research. 2003;313:195–207. doi: 10.1007/s00441-003-0732-2. [DOI] [PubMed] [Google Scholar]
- Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathology and applied neurobiology. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Brain research Molecular brain research. 2004;124:114–123. doi: 10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature neuroscience. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. The Journal of clinical investigation. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke; a journal of cerebral circulation. 2013;44:S93–95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Mo Z, Zecevic N. Down-regulation of the axonal polysialic acid-neural cell adhesion molecule expression coincides with the onset of myelination in the human fetal forebrain. Neuroscience. 2007;149:328–337. doi: 10.1016/j.neuroscience.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jany PL, Hagemann TL, Messing A. GFAP expression as an indicator of disease severity in mouse models of Alexander disease. ASN neuro. 2013;5:e00109. doi: 10.1042/AN20130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nature reviews Neurology. 2010;6:383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- Jean YY, Lercher LD, Dreyfus CF. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron glia biology. 2008;4:35–42. doi: 10.1017/S1740925X09000052. [DOI] [PubMed] [Google Scholar]
- Kaaijk P, Pals ST, Morsink F, Bosch DA, Troost D. Differential expression of CD44 splice variants in the normal human central nervous system. Journal of neuroimmunology. 1997;73:70–76. doi: 10.1016/s0165-5728(96)00167-1. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nature neuroscience. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochemistry international. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Rougon G. Cell biology of polysialic acid. Current opinion in neurobiology. 1997;7:640–646. doi: 10.1016/s0959-4388(97)80083-9. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin K, Kuprijanova E, Jabs R, Seifert G, Steinhauser C. Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia. 1995;15:173–187. doi: 10.1002/glia.440150210. [DOI] [PubMed] [Google Scholar]
- Lee SC, Moore GR, Golenwsky G, Raine CS. Multiple sclerosis: a role for astroglia in active demyelination suggested by class II MHC expression and ultrastructural study. Journal of neuropathology and experimental neurology. 1990;49:122–136. doi: 10.1097/00005072-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim WT, Cornell-Bell AH, Sontheimer H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia. 1994;11:315–325. doi: 10.1002/glia.440110404. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D, Hoi Yu AC. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Current Alzheimer research. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Liem RK, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. The Journal of clinical investigation. 2009;119:1814–1824. doi: 10.1172/JCI38003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AR, O’Callagha JP. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–618. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. The Journal of comparative neurology. 1976;165:197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Maletkovic J, Schiffmann R, Gorospe JR, Gordon ES, Mintz M, Hoffman EP, Alper G, Lynch DR, Singhal BS, Harding C, Amartino H, Brown CM, Chan A, Renaud D, Geraghty M, Jensen L, Senbil N, Kadom N, Nazarian J, Yuanjian F, Zuyi W, Hartka T, Morizono H, Vanderver A. Genetic and clinical heterogeneity in eIF2B-related disorder. Journal of child neurology. 2008;23:205–215. doi: 10.1177/0883073807308705. [DOI] [PubMed] [Google Scholar]
- Markoullis K, Sargiannidou I, Schiza N, Hadjisavvas A, Roncaroli F, Reynolds R, Kleopa KA. Gap junction pathology in multiple sclerosis lesions and normal-appearing white matter. Acta neuropathologica. 2012;123:873–886. doi: 10.1007/s00401-012-0978-4. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Waldron S, Kiel ME. PDGF alpha-receptor signal strength controls an RTK rheostat that integrates phosphoinositol 3′-kinase and phospholipase Cgamma pathways during oligodendrocyte maturation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3499–3508. doi: 10.1523/JNEUROSCI.5049-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5017–5023. doi: 10.1523/JNEUROSCI.5384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Shen S, Barres BA. Astrocytes induce oligodendrocyte processes to align with and adhere to axons. Molecular and cellular neurosciences. 1999;14:385–397. doi: 10.1006/mcne.1999.0788. [DOI] [PubMed] [Google Scholar]
- Miller RH, David S, Patel R, Abney ER, Raff MC. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Developmental biology. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes & development. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- Mori S, Leblond CP. Electron microscopic features and proliferation of astrocytes in the corpus callosum of the rat. The Journal of comparative neurology. 1969;137:197–225. doi: 10.1002/cne.901370206. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain research Brain research reviews. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cellular and molecular life sciences : CMLS. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait Oumesmar B, Vignais L, Duhamel-Clerin E, Avellana-Adalid V, Rougon G, Baron-Van Evercooren A. Expression of the highly polysialylated neural cell adhesion molecule during postnatal myelination and following chemically induced demyelination of the adult mouse spinal cord. The European journal of neuroscience. 1995;7:480–491. doi: 10.1111/j.1460-9568.1995.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Nash B, Ioannidou K, Barnett SC. Astrocyte phenotypes and their relationship to myelination. Journal of anatomy. 2011;219:44–52. doi: 10.1111/j.1469-7580.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A. Artifact versus reality--how astrocytes contribute to synaptic events. Glia. 2012;60:1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. The EMBO journal. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends in neurosciences. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. Journal of molecular neuroscience : MN. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Molecular and cellular neurosciences. 2007a;34:629–641. doi: 10.1016/j.mcn.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007b;27:13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cerebral cortex. 1994;4:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. Journal of neuropathology and experimental neurology. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Pixley SK, Kobayashi Y, de Vellis J. A monoclonal antibody against vimentin: characterization. Brain research. 1984;317:185–199. doi: 10.1016/0165-3806(84)90096-8. [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Sontheimer H. The neurophysiology of glial cells. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1992;9:224–251. doi: 10.1097/00004691-199204010-00005. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience. 1995;67:977–991. doi: 10.1016/0306-4522(94)00615-c. [DOI] [PubMed] [Google Scholar]
- Ridder MC, Boor I, Lodder JC, Postma NL, Capdevila-Nortes X, Duarri A, Brussaard AB, Estevez R, Scheper GC, Mansvelder HD, van der Knaap MS. Megalencephalic leucoencephalopathy with cysts: defect in chloride currents and cell volume regulation. Brain : a journal of neurology. 2011;134:3342–3354. doi: 10.1093/brain/awr255. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993;262:1072–1074. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rougon G. Structure, metabolism and cell biology of polysialic acids. European journal of cell biology. 1993;61:197–207. [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid and the regulation of cell interactions. Current opinion in cell biology. 1996;8:679–684. doi: 10.1016/s0955-0674(96)80109-8. [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Barili P, Bronzetti E, Zaccheo D, Amenta F. Age-related changes of glial fibrillary acidic protein immunoreactive astrocytes in the rat cerebellar cortex. Mechanisms of ageing and development. 1999;108:165–172. doi: 10.1016/s0047-6374(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. The European journal of neuroscience. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Sancho-Tello M, Valles S, Montoliu C, Renau-Piqueras J, Guerri C. Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia. 1995;15:157–166. doi: 10.1002/glia.440150208. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on the glial cells in the rhesus monkey optic nerve. The Journal of comparative neurology. 2002;445:13–28. doi: 10.1002/cne.10162. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. The Journal of comparative neurology. 2003;466:14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- Sawaishi Y. Review of Alexander disease: beyond the classical concept of leukodystrophy. Brain & development. 2009;31:493–498. doi: 10.1016/j.braindev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Schachner M, Hedley-Whyte ET, Hsu DW, Schoonmaker G, Bignami A. Ultrastructural localization of glial fibrillary acidic protein in mouse cerebellum by immunoperoxidase labeling. The Journal of cell biology. 1977;75:67–73. doi: 10.1083/jcb.75.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology. 2009;72:750–759. doi: 10.1212/01.wnl.0000343049.00540.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. Journal of neurophysiology. 2006;96:1383–1392. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- Schulz K, Kroner A, David S. Iron efflux from astrocytes plays a role in remyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4841–4847. doi: 10.1523/JNEUROSCI.5328-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraju R, Bernasconi L, Losberger C, Graber P, Kadi L, Avellana-Adalid V, Picard-Riera N, Baron Van Evercooren A, Cirillo R, Kosco-Vilbois M, Feger G, Papoian R, Boschert U. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Molecular and cellular neurosciences. 2004;25:707–721. doi: 10.1016/j.mcn.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hollander W, Rosene DL, Moss MB, Kemper T, Abraham CR. Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain research. 2000;862:1–10. doi: 10.1016/s0006-8993(00)02059-x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SM, Lee A, Bjorkman ST, Miller SM, Sullivan RK, Poronnik P, Colditz PB, Pow DV. Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: an identified role for GFAP. The Journal of biological chemistry. 2007;282:29414–29423. doi: 10.1074/jbc.M704152200. [DOI] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nature neuroscience. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7499–7518. doi: 10.1523/JNEUROSCI.0392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat Y, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J, Smith KM. Astroglial cells in development, regeneration, and repair. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13:173–185. doi: 10.1177/1073858406298336. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet neurology. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- Vinkers DJ, Stek ML, van der Mast RC, de Craen AJ, Le Cessie S, Jolles J, Westendorp RG, Gussekloo J. Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology. 2005;65:107–112. doi: 10.1212/01.wnl.0000167544.54228.95. [DOI] [PubMed] [Google Scholar]
- Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. The Journal of comparative neurology. 2003;457:404–419. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochemistry international. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Progress in neurobiology. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Colognato H, Ffrench-Constant C. Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia. 2007;55:537–545. doi: 10.1002/glia.20480. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- Wu E, Raine CS. Multiple sclerosis. Interactions between oligodendrocytes and hypertrophic astrocytes and their occurrence in other, nondemyelinating conditions. Laboratory investigation; a journal of technical methods and pathology. 1992;67:88–99. [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro-Signals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Yang Q, Hamberger A, Hyden H, Wang S, Stigbrand T, Haglid KG. S-100 beta has a neuronal localisation in the rat hindbrain revealed by an antigen retrieval method. Brain research. 1995;696:49–61. doi: 10.1016/0006-8993(95)00755-f. [DOI] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, DiAugustine RP, D’Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D’Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. Journal of neuroscience research. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D’Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8551–8564. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature neuroscience. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10999–11009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Book references
- Peters A, Palay SL, Webster HF, editors. The Fine Structure of the Nervous System. 3. Oxford University press; 1991. [Google Scholar]
- Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University press; 1995. [Google Scholar]


