Abstract
Although there has been considerable interest in identifying potential correlates of cognitive change, results of past studies have been inconsistent. The present study incorporated a number of methodological features intended to maximize sensitivity to detect characteristics of individuals with different amounts of cognitive change. Cognitive change in five cognitive abilities was analyzed with second-order latent growth curve models applied to data from a moderately large sample of healthy adults ranging from 18 to 99 years of age (N’s of 4,802 with one occasion, 2,265 with two occasions, and 1,128 with three occasions). There was significant individual difference variance in the longitudinal changes in several cognitive abilities, even in separate analyses of individuals between 18 and 39, 40 and 64, and over 65 years of age. Potential correlates of change included measures of self-rated health, vision, mood, personality, and lifestyle. Most of the potential correlates of change had high reliability, and several analyses were based on even more reliable factors determined by the variance common to multiple measures. Despite favorable conditions for detecting correlates of change, there was little evidence that cognitive change was moderated by any of the variables examined. Possible reasons for the inconsistent results regarding correlates of cognitive change are discussed.
Because variables found to have significant correlations with cognitive change may be informative about the factors contributing to successful and unsuccessful aging, and perhaps even provide clues about the mechanisms involved in longitudinal change, there has been a great deal of interest in identifying correlates of the average level, and of the magnitude of change, in cognitive functioning in healthy adults. In fact, because of the potential to enhance quality of life in old age and possibly prolong the period of independent living, Hendrie et al. (2006, p. 13) suggested that “identification of factors that can help people maintain or enhance cognitive or emotional health becomes a major public health goal.”
A relatively large number of variables have been found to be correlated with measures of cognitive functioning in cross-sectional comparisons, but results from cross-sectional studies only indirectly reflect change, and do not allow analyses of individual differences in change because the age comparisons are derived from different people. The focus in the present report is on research investigating predictors of change in longitudinal studies of cognitive functioning. Of primary interest was the identification of characteristics of people with different patterns of cognitive change. Because there have been several recent reviews of the literature (e.g., Bielak, 2010; Clouston et al., 2013; Daffner, 2010; Daviglus et al., 2010; Depp, Vahia & Jeste, 2010; Hendrie et al. 2006; Hertzog et al., 2009; Miller et al., 2012; Plassman et al., 2010; Sofi et al., 2010), only a limited number of number of articles not included in the earlier reviews are discussed below. In order to organize the coverage, potential correlates of change are grouped into seven broad categories consisting of demographic characteristics, health, sensory ability, mood, personality and disposition, self-efficacy, and lifestyle.
Potential correlates of change
Demographic characteristics such as age, sex, and education have frequently been found to be related to cognition, and therefore are important variables to control when analyzing relations of other variables with cognitive change. More negative cognitive change at older ages has been reported in many studies (e.g., Lamar et al., 2003; Mitchell et al., 2012; Parisi et al., 2011; Ronnlund & Nilsson, 2006; van Dijk et al., 2008). A few studies have reported differential change in males and females (e.g., Parisi et al., 2011), but there are also numerous reports of no sex differences in change (e.g., Finkel et al., 2003; Lamar et al., 2003; Lovden et al., 2004), or mixed patterns in different cognitive variables (e.g., Mitchell et al., 2012; van Dijk et al., 2008). With respect to education, Hendrie et al. (2006, p. 22) concluded that “Higher levels of education were almost uniformly reported to be protective for both cognitive and emotional outcomes.” Although it is true that some studies have found less decline among individuals with higher levels of education (e.g., Parisi et al., 2011), other studies have found a relation of education with change only in some cognitive variables (e.g., Singh-Manoux et al., 2011), or have not found a relation between education and cognitive decline (e.g., Glymour et al., 2012; Karlamanga, et al., 2009; Mitchell et al., 2012; Tucker-Drob et al., 2009; van Dijk et al., 2008; Zahodne et al., 2011).
Health is a plausible correlate of cognitive change because a number of health conditions are known to affect level of cognitive functioning, and at least some of them could be associated with more rapid cognitive decline. Health status has been assessed in a variety of different ways, including various types of physical examinations, and counts of medications, diseases, or illness episodes. Because they are easy to obtain, the most common measures of health are subjective ratings of one’s health status. Although extremely simple, self-ratings of health have been found to be correlated with mortality (e.g., Idler & Benyamini, 1997; Mulunpalo et al. 1997; Singh-Manoux et al. 2007), physician visits (Mulupalo et al. 1997), and various biomarkers (Jylha et al. 2006).
Some studies have found poorer self-rated health to be associated with greater cognitive decline (e.g., Carmelli et al., 1997; Gold et al., 1995; Van Hooren et al. 2005; Wahlin et al., 2003), but other studies have found different patterns for different variables (e.g., Meijer et al., 2009; van Dijk et al., 2008), or have not found relations of self-rated health with cognitive change (e.g., Anstey et al., 2003; Hultsch et al., 1999; Small, Dixon & McArdle, 2011).
Because relevant information cannot be processed if it is not adequately registered, sensory ability could also be a factor moderating cognitive change. Indeed, several studies have reported significant correlations between change in sensory function and change in cognitive functioning (e.g., Anstey et al., 2003; Lindenberger & Ghisletta, 2009; Newson & Kemps, 2005; Sternang et al., 2010).
A relatively large number of studies have examined relations of mood on cognitive change. Three major hypotheses have been proposed regarding the relation between mood and cognitive functioning. One is that negative mood is not a cause of cognitive decline, but instead is a consequence of awareness of cognitive declines. A second hypothesis is that negative mood and cognitive change are both attributable to a third factor, such as vascular disease or reduced frontal lobe activity. The third hypothesis is most relevant to the issue of moderators of cognitive change because it postulates that negative mood influences subsequent cognitive change, perhaps because negative mood is associated with high levels of cortisol which contribute to dysregulation of the hypothalamic-pituitary-adrenal axis with negative consequences for hippocampal integrity and memory. There is consensus in the reviews that more depressive symptomatology is associated with more rapid cognitive decline (e.g., Daviglus et al., 2010; Hendrie et al., 2006; Hertzog et al., 2009). Significant relations of level of depressive symptoms to change in cognitive functioning have been reported in several recent studies (e.g., Bielak et al., 2011; Kohler et al., 2010; van den Kommer et al., 2013), but other studies have not found relations between baseline depressive symptoms and subsequent cognitive change (e.g., Jajodia & Bordes, 2011; Mortensen et al., 2012).
There is a moderately large literature documenting relations between aspects of personality and level of cognitive functioning at a single point in time (e.g., Soubelet & Salthouse, 2011; von Stumm & Ackerman, 2013). Personality and disposition have also been examined as potential correlates of change, in part because these characteristics could affect the amount and type of activity one pursues (e.g., Soubelet & Salthouse, 2010; von Stumm & Ackerman, 2013). Results with these variables have not been very consistent as significant relations of neuroticism on cognitive change have been reported in some studies (e.g., Chapman et al., 2011), but not in others (e.g., Sharp et al., 2010), and no effect of openness or other personality traits on change in cognitive ability was found in a recent study by Hogan et al. (2012).
Because less negative change might be expected among individuals with a more positive outlook regarding their own level of cognition, a few studies have examined relations of self-efficacy to cognitive change. As noted by Hertzog et al. (2009), the findings in this area have been mixed, although it should be noted that two recent studies reported significant correlations between change in subjective assessments of memory and change in objectively assessed memory (i.e., Mascherek & Zimprich, 2011; Parisi et al., 2011).
A large number of studies have examined relations between aspects of lifestyle and cognitive change. Many different types of lifestyle activities have been examined, but only cognitive activities and physical activities will be considered here. There has been enormous variation in how cognitive activities have been evaluated, as the assessments have ranged from presence or absence of participation in one or several activities, to the total number of activities in which one was engaged in a specified period, and to the number of hours per week engaged in activities classified as cognitively stimulating. Methodological issues associated with assessment of cognitive activity have been discussed by a number of reviewers (e.g., Bielak, 2010; Ghisletta et al., 2006; Hultsch et al., 1999; Salthouse, 2006; 2010; Salthouse et al., 2002), including the almost complete lack of information about the validity of the activity reports.
Reviewers of the literature on cognitive activity and cognitive change have differed in their interpretations of the results. For example, Hertzog et al., (2009, p. 22) suggested that: “Overall, these data strongly support the hypothesis that a higher level of engagement in mentally stimulating activity is associated with reduced loss of cognition in old age.” In contrast, Daviglus et al., (2010) were more cautious in stating that: “Limited but inconsistent evidence suggests that increased involvement in cognitive activities in later life may be associated with slower cognitive decline and lower risk for mild cognitive impairment.” Recent studies have also been mixed as some significant correlations between change in activity and change in cognition were reported in Small et al., (2012), but no relations of activity with cognitive change were reported in two other studies (e.g., Bielak et al., 2012; Mitchell et al., 2012).
As with the assessment of cognitive activity, there has been considerable variation in how physical activity has been assessed. For example, the evaluations have ranged from the presence or absence of any activity, to frequency of engagement in specific activities such as gardening or sailing, to estimates of metabolic expenditures across specific activities in MET units based on frequency, duration, and intensity (see Miller et al., 2012). Several reviewers have noted the weak validity of self-reports of physical activity (e.g., Atienza et al. 2011; Prince et al. 2008; Shephard, 2003), which may be attributable to influences of social desirability (Adams et al. 2005), memory limitations in the remembering frequency and duration of activities, and across-person variability in the interpretation of the nature of physical activity. Another parallel with the research on cognitive activity is discrepant interpretations of the existing evidence by reviewers. For example, Miller et al., (2012) claimed that “the association between exercise and preserved cognition during aging is clearly demonstrated,” and Sofi et al., (2010) stated that the results “…suggest a significant and consistent protection for all levels of physical activity against the occurrence of cognitive decline.” In contrast, other reviewers qualified their conclusions by suggesting that the evidence was “growing” (Hendrie et al., 2006), or was “preliminary” (Daviglus, et al., 2010). Results of recent studies have also been mixed as Clouston et al., (2013) found a correlation of physical activity at baseline with longitudinal change in cognition, but Lindwall et al. (2012) reported a relation of baseline physical activity with change only in a verbal fluency measure, and not in other cognitive measures.
Methodological Considerations
This brief overview indicates that each category of potential correlate of cognitive change has had inconsistent results. Furthermore, two reviews incorporating formal guidelines to evaluate the nature of the evidence concluded that the overall quality of evidence was low (Daviglus et al., 2010; Plassman et al., 2010). Future research investigating correlates of cognitive change should therefore incorporate as many desirable methodological features as possible. For example, the measurement of potential correlates should be sensitive, reliable, and valid. In addition, because the number of possible correlates is very large, instead of considering them separately and treating them as if they were all independent, relations among the variables should be identified to determine if the correlates form meaningful dimensions of individual differences, and if so, analyses should be carried out on measures of these dimensions and not simply on individual variables. Unique influences should also be investigated by considering groups of potential correlates simultaneously, rather than separately and independently.
The cognitive assessment should include several cognitive domains, with multiple indicators of each domain to emphasize effects on cognitive abilities rather than on individual variables that include test-specific influences and measurement error. As with the assessment of potential correlates, the measurement of cognitive functioning should be sensitive and reliable, with no restrictions attributable to measurement floors or ceilings. Furthermore, evidence of measurement invariance across occasions is desirable to ensure that any change is primarily quantitative and not qualitative. Measures of general cognition could be examined, but they may obscure differences in relations across cognitive domains, and there is little advantage of the enhanced reliability often associated with aggregate variables if the measures in each ability domain have high reliabilities.
The analytical methods should be sensitive to effects on change distinct from effects on level, which is not necessarily the case with all methods, such as those based on difference scores. In addition, age, sex and years of education should be included in the analyses to control for influences of these variables when examining the relations of primary interest. It is also important to consider variability of the measures of change in both the correlates and the cognitive variables because the critical factor affecting relations with other variables is not the magnitude of change, but instead the amount of reliable variance in change. That is, if there is little evidence of differential change, in the form of significant individual difference variance in the measures of change, one cannot expect to identify correlates of differences in change that do not exist (Hertzog & Nesselroade, 2003). An indirect indication of variability in change can be obtained from stability coefficients because high stability implies little variability in change. However, low stability is not sufficient to infer variability in change because a low stability coefficient could be a consequence of low reliability, and thus both short-term reliability and stability need to be considered in evaluating variance of change. Change variance can also be estimated directly with statistical models, such as the latent growth model employed in the present study.
Statistical power to detect possible differences in cognitive change also needs to be considered in studies investigating correlates of cognitive change (e.g., von Oertzen et al. 2010). One way to think of the power to detect differences in cognitive change is to assume that there are two levels of the potential correlate, with equal numbers of participants at each level. Within a framework such as this it is possible to determine the number of participants in each group necessary to detect a given effect size for a difference in change with a specified degree of power. As an example, the sample size needed to achieve .8 power with a two-tailed significance level of .01 for a medium (i.e., Cohen’s d of .5) effect on change is 96 per group, and 586 participants per group would be needed to detect a small (i.e., Cohen’s d of .2) effect on change.
In addition to the size of the sample, characteristics of the participants in the sample are also important. For example, if individuals with cognitive impairments, either at baseline or emerging during the course of the longitudinal evaluation, are included in the analyses the results may reflect effects of disease processes as much or more than effects of normal aging. These individuals are obviously interesting for other questions, but their inclusion could distort inferences about what occurs in healthy aging.
It is also desirable to obtain information about the representativeness of the initial sample, and of the selectivity of attrition in the longitudinal sample. Individuals who continue to participate in longitudinal studies frequently have higher scores at the initial occasion than individuals who do not continue, and this can affect the generalizability of the results (Salthouse, in press-b). Selective attrition is also an important consideration in analyses of change because estimates of change, and correlates of change, can be distorted if selective attrition results in a restriction in the range of variation of the potential correlate or of the measures of cognitive functioning.
Finally, much of the prior research concerned with correlates of change has involved adults over about 65 years of age, and thus relatively little information is available about correlates of cognitive change at younger ages. This is unfortunate because different patterns might be expected at different ages if increased age is associated with shifts in the direction, magnitude, or causes of cognitive change. For example, different correlations of change might be expected at different ages if the change is primarily positive at young ages because of greater retest effects, and is primarily negative at older ages because of greater maturation-related effects. It is also possible that influences accumulate over time, such that the effects of the correlate are only pronounced at older ages.
Present Study
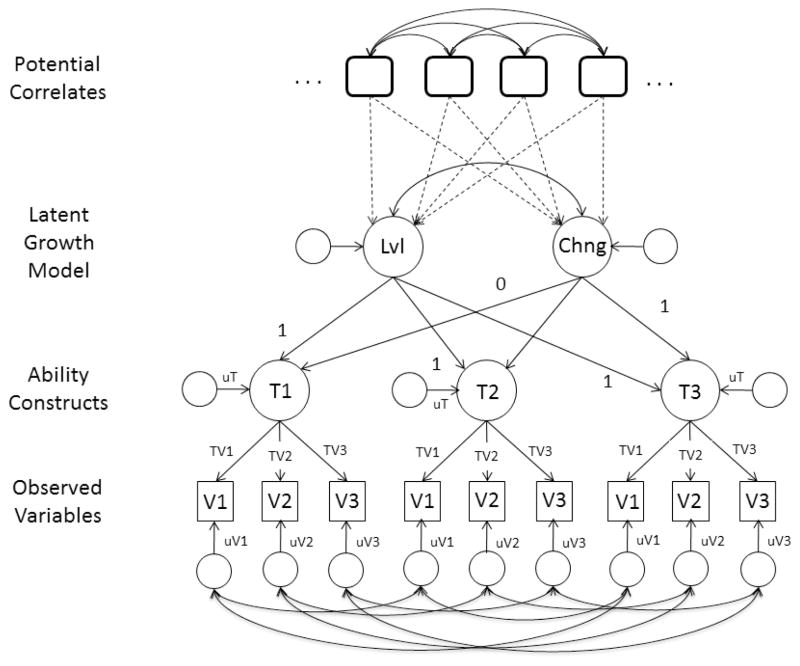
The current project incorporated the characteristics just described in an investigation of correlates of cognitive change. The initial sample consisted of a total of 4,802 adults, of whom 2,265 returned for a second measurement occasion, and 1,128 returned for a third measurement occasion. Longitudinal change was examined in five cognitive abilities, with each ability represented by either three or four different tests. Thirty potential correlates of change ranging from measures of sensory ability to aspects of lifestyle were examined both independently, and in simultaneous analyses. Because it may not be meaningful to study change as a quantitative phenomenon if the nature of the construct shifts from one occasion to the next, longitudinal measurement invariance for each cognitive ability construct was examined first. Means and variances of the latent level and latent change parameters were next examined among adults between 65 and 99 years of age, and among adults between 18 and 39 and 40 and 64 years of age. The former group corresponds to the typical age range of prior studies in which correlates of change have been reported, and the latter two groups allow the comparisons to be extended to younger ages. Cognitive change was analyzed with second-order (sometimes referred to as multiple-indicator) latent growth curve models in which the latent level and latent change constructs correspond to the second level, with latent constructs based on three or four variables for each cognitive ability at each occasion representing the first level (cf. Figure 1). Finally, relations of potential correlates with latent level and latent change parameters were examined in the three age groups.
Figure 1.
Illustration of the second-order latent growth model used in the analyses of longitudinal change. Unlabeled paths were freely estimated, and others were either constrained to the specified value or to be the same for relations with the same label. The paths in dotted lines represent the influences of the predictors on the latent level and latent change variables.
Method
Participants
Research participants were recruited from newspaper advertisements, flyers, and referrals from other participants. Approximately 81% of the participants were Caucasian, about 11% African American, and the remainder distributed across other ethnicities, or reporting more than one ethnicity. Demographic characteristics of the participants in the three age groups as a function of number of occasions are summarized in Table 1, with the right-most column containing the differences between numbers of occasions expressed in d units of effect size. The correlations of the demographic variables and composite cognitive ability scores with age are also reported in the table. It can be seen that increased age was associated with slightly poorer self-ratings of health, but higher levels of education.
Table 1.
Descriptive characteristics of the participants with different numbers of occasions in three age groups
| Number of occasions | d | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1–2 | 1–3 | 2–3 | |
| Number | ||||||
| 18–39 | 876 | 254 | 182 | NA | NA | NA |
| 40–64 | 1115 | 557 | 659 | NA | NA | NA |
| 65–99 | 546 | 326 | 287 | NA | NA | NA |
| Age | ||||||
| 18–39 | 26.6 (5.7) | 27.4 (6.7) | 28.8 (7.0) | .10 | .64^ | .23 |
| 40–64 | 53.2 (6.8) | 52.9 (6.4) | 52.9 (6.7) | .01 | .02 | .00 |
| 65–99 | 75.1 (7.2) | 74.4 (6.8) | 72.3 (5.7) | −.05 | −1.09^ | −.74^ |
| Proportion Females | ||||||
| 18–39 | .61 | .66 | .68 | NA | NA | NA |
| 40–64 | .69 | .68 | .73 | NA | NA | NA |
| 65–99 | .59 | .60 | .56 | NA | NA | NA |
| Self-Rated Health | ||||||
| 18–39 | 2.0 (0.8) | 2.0 (0.8) | 2.2 (0.9) | .01 | .25^ | .21 |
| 40–64 | 2.2 (0.9) | 2.1 (0.9) | 2.1 (0.9) | −.02 | −.06 | .01 |
| 65–99 | 2.4 (0.9) | 2.3 (0.9) | 2.3 (0.9) | −.05 | −.08 | .01 |
| Age correlation | .16* | .15* | .06 | |||
| Health-Related Limitations | ||||||
| 18–39 | 1.4 (0.7) | 1.4 (0.7) | 1.5 (0.8) | .05 | .13 | .03 |
| 40–64 | 1.7 (0.9) | 1.7 (1.0) | 1.7 (0.9) | .03 | .00 | .03 |
| 65–99 | 2.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) | .00 | .01 | .00 |
| Age correlation | .27* | .23* | .18* | |||
| Education | ||||||
| 18–39 | 15.2 (2.4) | 14.8 (2.4) | 14.6 (2.2) | −.13 | −.30^ | −.06 |
| 40–64 | 15.7 (2.8) | 15.7 (2.6) | 16.0 (2.6) | .00 | .15 | .17^ |
| 65–99 | 15.9 (2.9) | 16.1 (2.8) | 16.2 (3.0) | .03 | .04 | .00 |
| Age correlation | .14* | .21* | .22* | |||
| Est. IQ | ||||||
| 18–39 | 109.1 (12.9) | 108.6 (12.4) | 105.4 (15.4) | .01 | −.32^ | −.24 |
| 40–64 | 107.9 (14.8) | 110.3 (14.6) | 112.0 (15.1) | .22^ | .62^ | .10 |
| 65–99 | 106.9 (13.8) | 109.1 (13.1) | 112.5 (13.0) | .17^ | .99^ | .36^ |
| Age correlation | −.06* | .01 | .17* | |||
| T1–T2 Interval (years) | ||||||
| 18–39 | 3.5 (2.2) | 2.5 (1.1) | NA | NA | −1.38^ | |
| 40–64 | 3.6 (2.0) | 2.7 (1.2) | NA | NA | −2.84^ | |
| 65–99 | 2.9 (1.4) | 2.4 (1.0) | NA | NA | −1.10^ | |
| Age correlation | −.11* | −.05 | ||||
| T2–T3 Interval (years) | ||||||
| 18–39 | 3.3 (1.5) | NA | NA | NA | ||
| 40–64 | 3.2 (1.4) | NA | NA | NA | ||
| 65–99 | 3.0 (1.3) | NA | NA | NA | ||
| Age correlation | −.08 | |||||
| Memory | ||||||
| 18–39 | .44 (.75) | .39 (.73) | .33 (.77) | −.02 | −.08 | −.03 |
| 40–64 | −.10 (.79) | .02 (.77) | .17 (.73) | .21^ | 1.13^ | .33^ |
| 65–99 | −.68 (.81) | −.46 (.76) | −.20 (.73) | .49^ | 2.26^ | .71^ |
| Age correlation | −.49* | −.41* | −.26* | |||
| Speed | ||||||
| 18–39 | .67 (.74) | .62 (.70) | .62 (.73) | −.03 | −.02 | .00 |
| 40–64 | −.07 (.71) | −.03 (.69) | .18 (.68) | .03 | 1.20^ | .79^ |
| 65–99 | −.87 (.76) | −.78 (.70) | −.52 (.60) | .09 | 1.53^ | .96^ |
| Age correlation | −.65* | −.61* | −.51* | |||
| Vocabulary | ||||||
| 18–39 | −.31 (.87) | −.31 (.88) | −.49 (.86) | .00 | .18^ | −.21 |
| 40–64 | −.03 (.92) | .12 (.87) | .29 (.84) | .25^ | 1.26^ | .34^ |
| 65–99 | .12 (.77) | .24 (.73) | .41 (.65) | .18 | 1.06^ | .57^ |
| Age correlation | .20* | .24* | .35* | |||
| Reasoning | ||||||
| 18–39 | .50 (.75) | .46 (.75) | .25 (.89) | −.01 | −.44^ | −.32^ |
| 40–64 | −.08 (.83) | .07 (.81) | .14 (.79) | .29^ | .62^ | .06 |
| 65–99 | −.62 (.81) | −.50 (.74) | −.26 (.73) | .15 | 1.24^ | .57^ |
| Age correlation | −.48* | −.43* | −.24* | |||
| Space | ||||||
| 18–39 | .57 (.91) | .45 (.90) | .30 (1.0) | −.11 | −.41^ | −.12 |
| 40–64 | −.15 (.77) | −.03 (.76) | .08 (.79) | .23^ | .83^ | .16 |
| 65–99 | −.54 (.61) | −.49 (.60) | −.33 (.68) | .05 | .71^ | .37^ |
| Age correlation | −.51* | −.43* | −.27* | |||
Note: NA indicates that the value is not applicable. Health was a self-rating on a scale from 1 for excellent to 5 for poor, and Health Limitations was a rating of health-related activity limitations on a scale from 1 for none to 5 for a great deal. MMSE is the Mini Mental State Exam (Folstein, Folstein & McHugh, 1975). Est. IQ is estimated IQ (see text for details).
indicates that the mean difference was significant at p<.01.
Representativeness
In a recent study (Salthouse, in press-a) both the present test battery and the Wechsler Adult Intelligence Scale IV (Wechsler, 2008) test battery were administered to 90 adults between 20 and 80 years of age, which allowed estimates of full scale IQ scores to be derived in the current participants. Because IQ scores are age-adjusted, the estimation procedure consisted of partialling age from the raw scores to create residual scores, determining the best prediction of IQ from the residual scores, and then using the resulting regression equation to estimate IQ in the sample of 90 adults who performed both batteries. The most parsimonious regression equation with good prediction of IQ (i.e., R2 = .86) was: = 109.32 + 2.47 (series completion residual) + 1.54 (antonym vocabulary residual) + 1.78 (paper folding residual). This equation was applied to all of the current participants with relevant data to generate estimated IQ values.
Selective attrition
The Virginia Cognitive Aging Project (VCAP) is an on-going longitudinal study in which new participants are recruited each year, and prior participants are invited to return after an average interval of about 3 years. Because approximately 800 of the individuals in the present sample participated for the first time within the last 3 years, they have not yet been invited to return for a second occasion. More information on the reasons for the attrition among the eligible participants are reported in Salthouse (in press-b).
The data in Table 1 are informative about the selectivity of the longitudinal participants relevant to the initial sample. Among the adults between 18 and 39 years of age, participants with more occasions were older than participants with fewer occasions, but the reverse was the case for adults between 65 and 99 years of age. There were relatively small differences in self-rated health and years of education associated with number of occasions, but participants in the two older groups with two or more occasions had higher estimated IQs and composite cognitive ability scores at the first occasion than participants with only one occasion. This pattern was reversed in participants between 18 and 39 years of age, which is likely attributable to greater mobility among the highest-ability young individuals.
Although the participants who returned on subsequent occasions tended to have higher levels of cognitive performance on the first occasion than those who did not return, it is important to note that this does not necessarily limit the generalizability of the results regarding correlates of change. That is, selective attrition would not necessarily affect generalizability if the magnitude of longitudinal change was similar across different levels of initial ability. In fact, little or no relations between initial ability and magnitude of longitudinal change were reported by Salthouse (2012) after controlling influences associated with regression toward the mean, and Salthouse (in press-b) recently found that the estimates of imputed change for participants who did not return for a second occasion were similar to the observed values for participants who did return. In addition, analyses conducted in the present data revealed no significant differences between participants with two or three occasions on the magnitude of change from the first to the second occasion. That is, between-group t-tests were conducted on the composite score differences from T1 to T2 in each cognitive domain, and all of the t-test values comparing participants with two or three occasions were less than 1.3, with effect sizes (in d units) ranging from .00 to .03.
Change Analyses
Change was analyzed with the second-order latent growth model portrayed in Figure 1. The boxes in the figure represent measured (manifest) variables, and the circles represent unmeasured (latent) variables. Some of the latent variables represent the level at each occasion (T1, T2, and T3), others represent the level (Lvl) or change (Chng) across occasions, and still others represent residual (unexplained) variance. The possibility of variable-specific change was accommodated by specifying covariances among the residuals at each occasion for a given variable. It should be noted that the level (Lvl) construct is determined equally by performance in all three occasions, whereas the change (Chng) construct was determined progressively more by scores on later occasions. The basis coefficients for the three occasions representing the latent change variable were set to 0 and 1 for the first and third occasions, respectively, with the coefficient for the middle occasion estimated from the data.
Advantages of the model in Figure 1 over other methods of analyzing change are that the latent variables representing level and change theoretically have no measurement error because only systematic variance can be shared, and estimates of means and variances of the level and change variables are available as well as the relations between them. Furthermore, rather than only analyzing data from individuals with complete data, missing data were handled by assuming that the data were missing at random, and using the full information maximum likelihood (FIML) algorithm in the AMOS (Arbuckle, 2007) modeling program. The FIML procedure uses all available data in the analyses, which not only increases precision and yields less biased estimates than analyses based on complete cases, but by including data from individuals only tested once, it also provides some adjustment for longitudinal selectivity. Unlike imputation procedures, in which estimates of the missing data are first derived and then the analyses conducted on the combined original and imputed data, FIML procedures handle the missing data and estimate parameters and standard errors in a single step (Graham, 2009; Schafer & Graham, 2003).
Because estimates based on participants with complete data could differ from those based on the FIML procedure, the same types of latent change analyses were also conducted on the sample of 1128 participants who had data on all three longitudinal occasions. Although these analyses were less powerful than the primary analyses because of the smaller sample size, the results were very similar to those in the primary analyses. For example, of the 306 possible predictors of cognitive change (9 each for the 30 variables and 4 factors), 11 were significant in the FIML analyses in Tables 7 and 9, and 9 were significant in the parallel analyses of the sample with data on all three occasions.
Table 7.
Standardized relations of predictors of latent level and latent change in different cognitive abilities, controlling age, sex, and education
| Level | d | Change | d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O |
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O | |
| Average Health (Higher scores indicate poorer health) | ||||||||||||
| Memory | −.07 | −.12* | −.00 | −.02 | .11^ | .07 | −.14 | .06 | −.13 | .04 | −.06 | −.01 |
| Speed | −.08 | −.24* | −.19* | −.12^ | .05 | −.08 | .02 | .10 | .17 | .01 | .05 | .05 |
| Vocabulary | −.05 | −.11* | −.11* | −.05 | .02 | −.03 | .06 | .22 | −.06 | .03 | −.07 | −.03 |
| Average Vision (Higher scores indicate poorer vision) | ||||||||||||
| Memory | −.09* | −.16* | −.10* | .00 | .11^ | .06 | −.03 | .23 | −.06 | .02 | .02 | .00 |
| Speed | −.11* | −.16* | −.18* | .02 | .02 | .06 | .08 | .22 | .03 | .00 | −.02 | −.02 |
| Vocabulary | −.11* | −.09* | −.10* | .05 | .00 | .06 | .05 | .01 | −.08 | .00 | .00 | .00 |
| CES-D (Depressive Symptoms) | ||||||||||||
| Memory | −.08 | −.09* | −.07 | .00 | .00 | .00 | .02 | −.10 | −.05 | −.02 | .00 | −.02 |
| Speed | −.07 | −.14* | −.09* | −.06 | .02 | −.03 | −.04 | −.01 | −.06 | .01 | −.03 | −.01 |
| Vocabulary | −.03 | −.07* | −.05 | −.03 | .01 | −.02 | −.09 | .17 | −.06 | .07 | −.07 | −.02 |
| Anxiety | ||||||||||||
| Memory | −.01 | −.08* | −.06 | −.07 | .02 | −.04 | −.03 | −.01 | .12 | .01 | .04 | .05 |
| Speed | −.01 | −.13* | −.07 | −.09^ | .02 | −.09 | −.04 | .11 | −.02 | .03 | −.03 | .00 |
| Vocabulary | .04 | −.04 | −.03 | −.07 | .01 | −.06 | −.25 | .13 | .07 | .09 | .00 | .06 |
| PANAS-Positive (Positive Mood) | ||||||||||||
| Memory | −.12* | −.08* | −.05 | .03 | .02 | .05 | .01 | −.10 | −.07 | −.02 | −.02 | −.03 |
| Speed | −.05 | .05 | −.00 | .08 | −.04 | .04 | −.14 | .02 | .19 | .04 | .07 | .10^ |
| Vocabulary | −.17* | −.13* | −.13* | .02 | .01 | .03 | .23 | −.03 | −.04 | −.04 | −.02 | −.05 |
| PANAS-Negative (Negative Mood) | ||||||||||||
| Memory | −.10* | −.15* | −.15* | −.04 | −.05 | −.08 | −.52* | .17 | .03 | .11^ | −.01 | .08 |
| Speed | −.03 | −.11* | −.13* | −.08 | −.05 | −.11^ | −.22 | .11 | −.06 | .08 | −.05 | .02 |
| Vocabulary | −.09* | −.14* | −.19* | −.07 | −.08 | −.14^ | .08 | .15 | −.11 | .01 | −.07 | −.06 |
| Dysexecutive Questionnaire | ||||||||||||
| Memory | −.08 | −.09* | −.05 | .00 | .02 | .01 | −.37 | −.01 | −.03 | .06 | −.01 | .05 |
| Speed | −.08 | −.13* | −.07 | −.03 | .04 | .00 | −.12 | −.05 | −.09 | .02 | −.02 | .00 |
| Vocabulary | −.01 | −.06* | −.08* | −.05 | −.02 | −.06 | −.12 | .04 | .02 | .02 | .02 | .03 |
| Emotional Stability | ||||||||||||
| Memory | −.01 | .06 | .06 | .05 | .01 | .06 | .14 | .13 | .01 | −.02 | −.01 | −.02 |
| Speed | .03 | .08* | .01 | .04 | −.06 | −.02 | .10 | .02 | .03 | −.02 | .01 | −.01 |
| Vocabulary | −.01 | .02 | .02 | .03 | .00 | .03 | .07 | −.12 | −.00 | −.03 | .02 | −.01 |
| Extraversion | ||||||||||||
| Memory | .03 | .02 | .05 | −.02 | .04 | .02 | −.15 | −.16 | −.03 | .01 | .01 | .01 |
| Speed | −.01 | .06 | .08 | .06 | .02 | .08 | .12 | −.12 | .08 | −.05 | .05 | −.01 |
| Vocabulary | −.01 | −.03 | −.02 | −.02 | .01 | −.01 | .22 | .05 | .19 | −.03 | .07 | .04 |
| Openness | ||||||||||||
| Memory | .21* | .21* | .22* | −.02 | .01 | −.01 | .52* | −.24 | −.11 | −.12^ | −.01 | −.12 |
| Speed | .11* | .16* | .16* | .03 | .01 | .04 | −.02 | −.12 | −.05 | −.01 | .01 | −.01 |
| Vocabulary | .37* | .34* | .38* | −.02 | .01 | −.02 | .07 | −.19 | −.03 | −.02 | .07 | −.01 |
| Agreeableness | ||||||||||||
| Memory | .11* | .09* | .06 | −.03 | −.02 | −.05 | .15 | −.01 | −.03 | −.02 | −.01 | −.03 |
| Speed | .01 | .11* | .03 | .08 | −.06 | .02 | .05 | .05 | .17 | −.00 | .05 | .04 |
| Vocabulary | .07 | .07* | .04 | .01 | −.04 | −.03 | −.15 | .35 | .09 | .05 | .02 | .05 |
| Conscientiousness | ||||||||||||
| Memory | .04 | −.00 | .04 | −.03 | .03 | .00 | .08 | .15 | −.08 | .01 | −.06 | −.04 |
| Speed | .06 | .18* | .08 | .11^ | −.08^ | .04 | .23 | .07 | .06 | −.04 | .01 | −.03 |
| Vocabulary | −.04 | .01 | .01 | .04 | .01 | .05 | −.02 | −.13 | .02 | −.03 | .01 | .01 |
| Busyness | ||||||||||||
| Memory | .02 | .05 | −.02 | .01 | −.04 | −.03 | −.18 | −.12 | −.00 | .01 | .01 | .02 |
| Speed | .01 | .03 | −.05 | .02 | −.06 | −.04 | .43 | −.07 | −.02 | −.09^ | .00 | −.08 |
| Vocabulary | −.04 | −.04 | −.06 | −.01 | −.01 | −.02 | −.29 | .06 | −.15 | .05 | −.05 | −.01 |
| Routine | ||||||||||||
| Memory | .04 | .05 | −.08 | .01 | −.09^ | −.08 | .13 | .33 | .18 | .02 | .01 | .03 |
| Speed | −.01 | .10* | −.01 | .08 | −.08 | −.00 | .26 | −.04 | .14 | −.05 | .04 | −.01 |
| Vocabulary | .01 | .10* | −.01 | .08^ | −.09^ | −.02 | .53 | .10 | −.01 | −.05 | −.01 | −.05 |
| Need for Cognition | ||||||||||||
| Memory | .22* | .16* | .17* | −.07 | .00 | −.06 | .01 | −.04 | −.07 | .00 | −.04 | −.03 |
| Speed | .19* | .14* | .12* | −.03 | −.02 | −.04 | .03 | −.03 | .04 | −.00 | .01 | .00 |
| Vocabulary | .26* | .21* | .21* | −.05 | −.02 | −.06 | −.08 | −.06 | .00 | .02 | .00 | .01 |
| Life Satisfaction | ||||||||||||
| Memory | .10* | .07 | .00 | −.03 | −.05 | −.06 | .13 | .17 | −.02 | .00 | −.02 | −.02 |
| Speed | .04 | .13* | .02 | .07 | −.08 | −.02 | .20 | .08 | .09 | −.03 | .03 | −.01 |
| Vocabulary | .02 | .02 | .01 | .00 | .00 | .00 | .37 | −.10 | .09 | −.09^ | .05 | −.02 |
| Memory Rating | ||||||||||||
| Memory | .17* | .12* | .18* | −.06 | .06 | .00 | −.05 | −.08 | −.23* | −.00 | −.08 | −.07 |
| Speed | .05 | .08* | .09* | .03 | .01 | .04 | .03 | .11 | .03 | .01 | −.01 | .00 |
| Vocabulary | .05 | .03 | .05 | −.02 | .01 | −.00 | −.18 | −.09 | .17 | .02 | .08 | .09 |
| Thinking Rating | ||||||||||||
| Memory | .15* | .08* | .10* | −.07 | .02 | −.04 | −.10 | .25 | −.22 | .05 | −.11^ | −.05 |
| Speed | .06 | .08* | .02 | .01 | −.05 | −.03 | −.11 | −.04 | −.11 | .02 | −.03 | −.01 |
| Vocabulary | .09* | .07* | .08 | −.02 | .00 | −.01 | .07 | −.09 | −.05 | −.03 | −.01 | −.02 |
| Cognitive Activities | ||||||||||||
| Memory | −.06 | .00 | .08* | .05 | .07 | .14^ | .19 | −.09 | −.08 | −.07 | −.02 | −.07 |
| Speed | .04 | .08* | .15* | .02 | .09^ | .14^ | −.04 | −.12 | −.09 | −.02 | −.02 | −.06 |
| Vocabulary | −.07* | −.01 | .13* | .05 | .11^ | .20^ | .04 | −.05 | −.26* | .00 | −.09^ | −.11^ |
| Walking | ||||||||||||
| Memory | −.10* | −.03 | −.03 | .07 | .00 | .06 | .03 | .06 | .14 | .00 | .04 | .04 |
| Speed | .02 | .00 | −.01 | −.02 | −.02 | −.03 | −.09 | .13 | .03 | .04 | −.01 | .03 |
| Vocabulary | −.01 | −.01 | .08 | .00 | .11^ | .09 | −.06 | .03 | −.08 | .02 | −.04 | −.02 |
| Yard Work | ||||||||||||
| Memory | .03 | .03 | −.04 | .00 | −.05 | −.03 | −.25 | .09 | .12 | .09 | .02 | .09 |
| Speed | .04 | .05 | −.02 | .00 | −.07 | −.05 | −.38* | −.07 | .01 | .11^ | .02 | .10^ |
| Vocabulary | .01 | .02 | −.05 | .00 | −.07 | −.05 | −.16 | −.15 | −.08 | .02 | .00 | .01 |
| Calisthenics | ||||||||||||
| Memory | −.12* | −.08* | .02 | .01 | .07 | .09 | −.16 | .18 | −.03 | .06 | −.04 | .01 |
| Speed | −.03 | −.01 | −.03 | .01 | −.02 | −.02 | −.15 | .37* | .06 | .10^ | −.04 | .06 |
| Vocabulary | −.07 | −.03 | −.05 | .01 | −.02 | −.02 | −.06 | .06 | −.02 | .02 | −.01 | .00 |
| Running | ||||||||||||
| Memory | .06 | −.05 | −.03 | −.07 | −.01 | −.06 | .09 | .15 | .07 | .02 | .02 | .02 |
| Speed | .03 | .02 | .05 | .00 | .05 | .06 | −.28 | .31* | .05 | .11 | −.02 | .06 |
| Vocabulary | .00 | −.01 | −.04 | −.01 | −.04 | −.05 | .56* | −.10 | .02 | −.10^ | .02 | −.03 |
| Aerobics | ||||||||||||
| Memory | −.01 | −.02 | −.05 | −.00 | −.03 | −.03 | −.07 | .20 | .04 | .04 | −.02 | .03 |
| Speed | .01 | .06 | .05 | .03 | −.00 | .03 | −.14 | .08 | −.11 | .05 | −.06 | .00 |
| Vocabulary | −.05 | −.01 | −.03 | .04 | −.01 | .03 | .06 | .03 | −.20 | −.00 | −.08 | −.07 |
| Swimming | ||||||||||||
| Memory | −.05 | −.02 | −.01 | .01 | .01 | .02 | −.06 | .05 | .05 | .01 | .01 | .02 |
| Speed | −.00 | −.02 | .03 | −.02 | .04 | .02 | −.05 | .06 | .01 | .02 | −.01 | .02 |
| Vocabulary | −.08* | −.00 | .04 | .04 | .04 | .12^ | −.02 | −.08 | −.04 | −.02 | .00 | −.02 |
| Tennis | ||||||||||||
| Memory | −.03 | −.00 | .04 | .02 | .03 | .05 | −.20 | .13 | −.00 | .05 | −.02 | .04 |
| Speed | −.01 | .01 | .05 | .01 | .02 | .04 | .08 | −.03 | .15 | −.02 | .04 | .02 |
| Vocabulary | −.09* | −.03 | .04 | .04 | .05 | .13^ | −.01 | .11 | −.14 | .02 | −.06 | −.05 |
| Rowing | ||||||||||||
| Memory | −.03 | −.01 | −.01 | .02 | −.01 | .01 | .14 | .02 | −.04 | −.03 | −.02 | −.04 |
| Speed | −.04 | −.01 | −.01 | .03 | −.01 | .02 | .08 | .05 | −.07 | −.02 | −.04 | −.04 |
| Vocabulary | −.06 | .01 | −.04 | .08 | −.05 | .02 | .16 | −.02 | −.08 | −.04 | −.03 | −.05 |
| Cycling | ||||||||||||
| Memory | −.03 | −.06 | .04 | −.04 | .09 | .06 | .52* | .13 | .04 | −.07 | .00 | −.04 |
| Speed | −.03 | −.02 | .01 | −.01 | .03 | .03 | −.22 | −.26 | .02 | −.01 | .05 | .04 |
| Vocabulary | −.02 | .00 | −.02 | .01 | −.02 | −.01 | .42* | .09 | .04 | −.04 | .00 | −.02 |
| Sports | ||||||||||||
| Memory | −.04 | .01 | −.02 | .03 | −.02 | .01 | −.21 | .01 | .06 | .03 | .03 | .05 |
| Speed | .00 | −.02 | .00 | −.01 | .01 | .00 | −.12 | .10 | .05 | .04 | .00 | .04 |
| Vocabulary | −.09* | .01 | −.03 | .08^ | −.03 | .05 | .06 | −.06 | .09 | −.02 | .04 | .02 |
| All Physical Activity | ||||||||||||
| Memory | −.09* | −.03 | −.04 | .02 | .00 | .03 | −.17 | .18 | .17 | .05 | .02 | .09 |
| Speed | .01 | .03 | .01 | .02 | −.02 | .00 | −.19 | .14 | .04 | .07 | .00 | .09 |
| Vocabulary | −.09* | −.00 | −.02 | .05 | −.02 | .03 | .05 | −.10 | −.13 | −.02 | −.02 | −.06 |
Note:
p<.01. Estimates of effect sizes (d) of the group difference are derived from the standard errors of the unstandardized coefficients.
indicates that the difference in raw regression coefficients was significant at p<.01.
Table 9.
Simultaneous relations (standardized) of predictors of latent level and latent change in different cognitive abilities, controlling age, sex, and education
| Level | d | Change | d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O |
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O | |
| F1-Negative Affect (Higher scores correspond to lower levels of negative affect) | ||||||||||||
| Memory | −.04 | .04 | .06 | .04 | .02 | .06 | −.14 | .38 | −.29 | .05 | −.11^ | −.06 |
| Speed | −.05 | .12 | −.02 | .09^ | −.08 | .01 | −.20 | −.19 | .05 | .01 | .04 | .04 |
| Vocabulary | −.15* | −.04 | −.04 | .07 | −.01 | .06 | .38 | −.19 | −.25 | −.06 | −.04 | −.08 |
| F2-Openness (Higher scores correspond to higher levels of openness) | ||||||||||||
| Memory | −.02 | −.03 | −.07 | −.00 | −.02 | −.02 | .34 | −.14 | .30 | −.04 | .07 | .03 |
| Speed | .09 | .14 | .07 | .02 | −.03 | −.01 | .43 | −.02 | .33 | −.06 | .06 | .02 |
| Vocabulary | .05 | .06 | .09 | .00 | .01 | .02 | .16 | .26 | −.43 | .01 | −.10 | −.08 |
| F3-Self Efficacy (Higher scores correspond to higher self ratings of memory and thinking) | ||||||||||||
| Memory | .30* | .24* | .34* | −.05 | .06 | .01 | .11 | −.10 | −.50 | −.02 | −.10^ | −.10 |
| Speed | .12 | .03 | .14 | −.04 | .06 | .02 | −.15 | .29 | −.20 | .04 | −.07 | −.02 |
| Vocabulary | .30* | .20* | .22* | −.05 | .00 | −.05 | .03 | −.31 | .62* | −.03 | .14^ | −.11^ |
| F4-Busy (Higher scores correspond to greater self-reported busyness) | ||||||||||||
| Memory | −.08 | −.04 | .05 | .02 | .06 | .08 | −.39 | .09 | −.19 | .04 | −.06 | −.02 |
| Speed | −.02 | −.03 | .07 | −.01 | .07 | .06 | −.23 | .09 | .03 | .04 | −.00 | .04 |
| Vocabulary | −.09 | −.09* | −.03 | −.02 | .05 | .03 | .09 | .00 | −.24 | −.01 | −.07 | −.06 |
Note:
p<.01. Estimates of effect sizes (d) of the group difference are derived from the standard errors of the unstandardized coefficients.
indicates that the difference in raw regression coefficients was significant at p<.01.
Cognitive variables
Cognitive functioning was assessed with 16 tests selected to represent five cognitive abilities; word knowledge (vocabulary), inductive reasoning (reasoning), spatial visualization (space), episodic memory (memory), and perceptual speed (speed). Identical test versions were used at each longitudinal occasion. All of the individual test variables had coefficient alpha and test-retest reliabilities of .7 or higher, and loadings of .7 or greater on their respective ability factors. The measures are briefly described in the appendix, and more details, including sources of the tests, are contained in other publications (e.g., Salthouse, 2004; Salthouse & Ferrer-Caja, 2003; Salthouse et al., 2008). Scores at each occasion were converted to z-scores based on the means and standard deviations of the scores at the first occasion. Ability constructs were formed at each occasion from the three or four (for vocabulary) measures established to have high loadings on the relevant ability factor. For some analyses composite scores were created by averaging the z-scores for the measures representing each ability.
Potential correlates of change
Self-rated health was assessed with two questions; “how would you rate your health at the current time” (on a scale from 1 for excellent to 5 for poor), and “how much are your daily activities limited in any way by your health or health-related problems?” (on a scale from 1 for not at all to 5 for a great deal). Visual acuity in both the right and left eyes was assessed with the Lighthouse Near Visual Acuity Test while the participants were wearing any prescribed corrective lenses. The denominator of the Snellen ratio was used as the measure of acuity.
Additional questions asked the participant to evaluate his or her own memory and thinking abilities. The memory rating was the average of three ratings of memory compared to the average individual, to the best it has ever been, and in terms of problems experienced, on scales from 1 for very poor or much worse to 7 for very good or much better. The thinking rating was the average of two ratings of thinking and reasoning relative to earlier in life and in terms of problems in day-to-day life on scales from 1 for much worse or interferes a lot, to 7 for much better or does not interfere.
Other potential correlates were obtained from questionnaires completed by the participants at home. Depressive symptoms were assessed with the Center for Epidemiological Studies-Depression scale (Radloff, 1977), and trait anxiety was assessed with the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1983). The Big 5 Personality traits were assessed with the International Personality Item Pool questionnaire (Goldberg, 1999; 50-item version). Dispositions were assessed with the Satisfaction with Life Scale (Diener et al., 1985), and with the 18-item version of the Need for Cognition Questionnaire (Cacioppo et al., 1996). Mood was assessed with the Positive and Negative Affect Scale (Watson et al., 1988), and self-reported problems with executive functioning were assessed with the Dysexecutive questionnaire (Wilson et al., 1996). The Martin and Park (2003) busyness scale was administered to assess self-perceived busyness and routineness of one’s lifestyle.
Two locally developed questionnaires were designed to assess aspects of lifestyle related to cognitive and physical activity. The cognitive activity questionnaire (Salthouse, Berish & Miles, 2002) asked participants to indicate the number of hours they devoted to each of 22 activities, and to rate the cognitive demands of the activities. The measure of cognitive activity used in the analyses was the number of hours per week devoted to the seven activities with the highest average ratings of cognitive demands (i.e., reading newspapers, using a computer, driving a car, reading non fiction, working crossword puzzles, handling finances, and writing).
In an attempt to increase the validity of the self reports of physical activity, items in the physical activity questionnaire asked about the number of times per month and the duration each time engaged in specific activities (i.e., walking, yard work, calisthenics, running, aerobics, swimming, tennis, rowing, cycling, and sports). The participants were also given an opportunity to list other activities, and among those mentioned were weight lifting, yoga, dance, and sex. However, because they were not systematically assessed from everyone, these other activities were not included in the present analyses. The measure of physical activity for each primary activity was the estimated hours per month, derived by multiplying the frequency per month by the time at each occasion. In addition, the total number of hours per month engaged in all activities, derived by summing the hours in the ten specified activities, was used as an additional measure of physical activity.
Results1
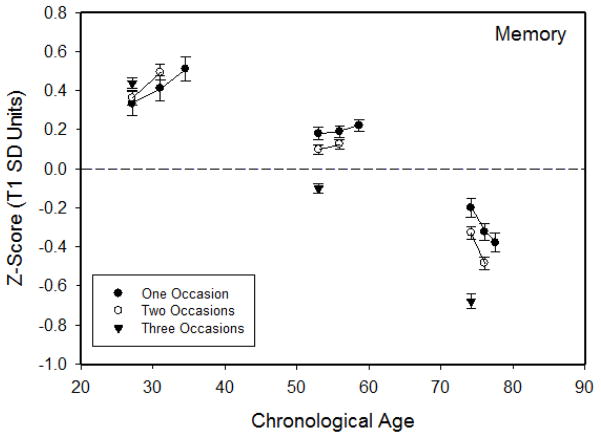
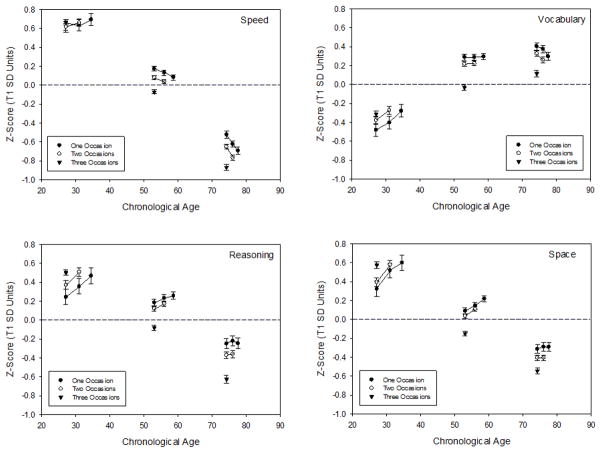
Composite scores across occasions
Composite scores at each occasion for participants with complete data for different numbers of occasions are plotted in Figure 2 for memory, and in Figure 3 for the other cognitive domains. Notice that the values were lower with increased age for each cognitive domain except vocabulary. Consistent with the selective attrition results, with the exception of the youngest group, the means were higher for participants with more occasions. It can also be seen that the lines connecting the means across successive occasions were flat for reasoning and space in the older group, which suggests that there was little mean change in these cognitive abilities for adults in the sample over 65 years of age.
Figure 2.
Means (and standard errors) of the composite memory score at each occasion for participants with one, two, or three occasions in adults in three age groups.
Figure 3.
Means (and standard errors) of the composite scores in four cognitive domains at each occasion for participants with one, two, or three occasions in three age groups.
Reliability and stability
An initial set of analyses examined properties of the cognitive variables at different levels of aggregation to determine the level that might be most meaningful in the analyses of change. For each individual variable, composite variable, and latent variable, correlations were computed between scores on two sessions in the first occasion as an estimate of immediate test-retest reliability, and between the first and third occasion as an estimate of long-term (approximately 6 years) stability. Data reported in Salthouse and Tucker-Drob (2008) were used to compute the short-term retest correlations because in that study 56 participants between 18 and 39 years of age, 113 participants between 40 and 64 years of age, and 58 participants between 65 and 99 years of age performed identical versions of the tests on a second session approximately one week after the initial session.
Correlations from these analyses are reported in Table 2, where it can be seen that most of the reliabilities were above .70, and were similar in the three age groups. The reliabilities were higher for composite scores than for individual variables, and were highest for latent variables. Estimates of stability from the first to the third occasion were lowest for individual variables and highest for latent variables, but unlike reliabilities, the stabilities were generally lower for participants age 65 to 99 than for participants in the younger groups.
Table 2.
Estimates of first occasion reliability (Rel), stability correlation between the first and third occasion (r13), and reliability of the difference between the first and third occasion (D13Rel) for individual variables, composite variables, and latent variables in three age groups
| Age 18–39 | Age 40–64 | Age 65–99 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rel | r13 | D13Rel | Rel | r13 | D13Rel | Rel | r13 | D13Rel | |
| Memory | |||||||||
| Recall | .64 | .66 | −.06 | .78 | .55 | .51 | .79 | .51 | .57 |
| Paired Associates | .71 | .71 | .00 | .73 | .61 | .31 | .78 | .61 | .44 |
| Logical Memory | .79 | .68 | .34 | .79 | .61 | .46 | .72 | .54 | .39 |
| Composite | .84 | .81 | .16 | .86 | .71 | .52 | .86 | .68 | .56 |
| Latent variable | .99 | .89 | .91 | .97 | .85 | .80 | .96 | .73 | .85 |
| Speed | |||||||||
| Digit Symbol | .85 | .81 | .21 | .88 | .74 | .54 | .87 | .68 | .59 |
| Pattern Comparison | .88 | .59 | .71 | .71 | .52 | .40 | .80 | .60 | .50 |
| Letter Comparison | .80 | .63 | .46 | .78 | .59 | .46 | .85 | .59 | .63 |
| Composite | .92 | .77 | .65 | .88 | .74 | .54 | .91 | .69 | .71 |
| Latent variable | 1.00 | .89 | 1.00 | .96 | .85 | .73 | .95 | .81 | .74 |
| Vocabulary | |||||||||
| Definition | .90 | .84 | .38 | .91 | .74 | .65 | .85 | .57 | .65 |
| Picture | .96 | .90 | .60 | .91 | .86 | .36 | .92 | .69 | .74 |
| Synonym | .86 | .78 | .36 | .84 | .85 | −.07 | .85 | .69 | .52 |
| Antonym | .89 | .72 | .61 | .81 | .79 | .10 | .77 | .70 | .23 |
| Composite | .96 | .92 | .50 | .94 | .92 | .25 | .95 | .79 | .76 |
| Latent variable | .99 | .98 | .50 | .99 | .98 | .50 | 1.00 | .90 | 1.00 |
| Reasoning | |||||||||
| Matrix Reasoning | .83 | .75 | .32 | .75 | .66 | .26 | .62 | .64 | −.06 |
| Shipley | .83 | .82 | .06 | .87 | .80 | .35 | .78 | .70 | .27 |
| Letter Sets | .76 | .71 | .17 | .83 | .65 | .51 | .69 | .65 | .11 |
| Composite | .89 | .87 | .15 | .91 | .86 | .36 | .84 | .82 | .11 |
| Latent variable | .96 | .93 | .43 | .97 | .95 | .40 | .97 | .94 | .50 |
| Space | |||||||||
| Spatial Relations | .74 | .88 | −1.17 | .82 | .81 | .05 | .81 | .71 | .34 |
| Paper Folding | .79 | .78 | .05 | .72 | .65 | .20 | .65 | .54 | .24 |
| Form Boards | .84 | .76 | .33 | .77 | .62 | .39 | .59 | .59 | .00 |
| Composite | .91 | .89 | .18 | .89 | .84 | .31 | .82 | .75 | .28 |
| Latent variable | 1.00 | .95 | 1.00 | 1.00 | .95 | 1.00 | .95 | .87 | .62 |
| Medians | |||||||||
| Individual Variables | .83 | .76 | .33 | .80 | .66 | .38 | .79 | .63 | .41 |
| Composite Variables | .91 | .87 | .18 | .89 | .84 | .36 | .86 | .75 | .56 |
| Latent Variables | .99 | .93 | .91 | .97 | .95 | .73 | .96 | .87 | .74 |
Note: Rel. refers to test-retest reliability over a period of about 1 week with data from Salthouse and Tucker-Drob (2008), r13 is the (stability) correlation between scores on the first and third longitudinal occasion, and D13Rel is estimated reliability of the difference between the T1 and T3 scores as computed from [(Rel. − r13)/(1−r13)].
Stability is inversely related to amount of change, and therefore high stability implies small individual differences in change. However, low stability does not necessarily imply large individual differences in change because reliability also needs to be considered when interpreting the stabilities. One method of incorporating both reliability and stability information involves estimating the reliability of the difference between scores on the first and third occasion with the formula:
assuming equal reliabilities at each occasion (see Cohen & Cohen, 1982, p. 69). Because the stability coefficients indicate the proportion of variance in the T3 score shared with the T1 score, one minus the stability coefficient indicates the proportion of T3 variance not predicted from the initial score that could be associated with change. The formula can therefore be interpreted as providing an estimate of the proportion of reliable variance at T3 that is potentially attributable to change.
Although the values can only be considered approximations, the estimates of difference score reliability are clearly much higher for latent variables than for either individual variables or composite variables. Even though composite variables are aggregates and have higher reliability than individual variables, the estimated reliabilities of the T1 to T3 differences for composite scores were modest. Because they had the highest reliabilities at the initial occasion, as well as for the T1 to T3 differences, the subsequent analyses focused on latent variables.
Measurement invariance across longitudinal occasions
Longitudinal measurement invariance (e.g., Ferrer et al., 2008) across the three occasions was examined separately for each cognitive domain in the three age groups. The analyses were based on latent variables with either three or four (for vocabulary) manifest variables at each occasion. Model 1 corresponds to configural invariance in which there were across-time correlations of the factors and of the residuals for each variable, but no constraints on the parameter estimates at each occasion. Model 2 corresponds to weak factor invariance, and differs from Model 1 in that the factor loadings were constrained to be equal at each occasion. Model 3 corresponds to strong factor invariance, and differs from Model 2 in that intercepts (means of the manifest variables) were also constrained to be equal across occasions. Finally, Model 4 corresponds to strict factor invariance, and differs from Model 3 in that unique variances for the variables were also constrained to be equal at each occasion.
Results of the invariance analyses for each cognitive ability in the three age groups are reported in Table 3. Values for Model 1 (configural invariance) are presented in the first row within each set, where it can be seen that this model had excellent fits to the data in each cognitive domain. The difference in the Χ2 test indicated significant loss of fit when progressively more constraints were imposed, particularly when intercepts of the observed variables were specified to be equal across time (Model 3). However, it is important to note that the absolute fit was quite good (i.e., CFI >.95, RMSEA < .05) for all models, including the strict factor invariance model incorporating all constraints. It therefore seems reasonable to conclude that although the measurement properties of the cognitive ability constructs were not identical across occasions, they were nevertheless very similar.
Table 3.
Measurement invariance results for the five ability constructs in three age groups
| df | Age 18–39 | Age 40–64 | Age 65–99 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Χ2 | CFI | RMSEA | Χ2 | CFI | RMSEA | Χ2 | CFI | RMSEA | ||
| Memory | ||||||||||
| Model 1 | 19 | 33 | .994 | .024 | 32 | .998 | .017 | 39 | .993 | .030 |
| Model 2 | 23 | 38 | .994 | .022 | 39 | .997 | .017 | 41 | .994 | .026 |
| Model 3 | 27 | 59 | .987 | .030 | 52 | .996 | .020 | 69 | .985 | .037 |
| Model 4 | 33 | 67 | .986 | .028 | 60 | .995 | .019 | 82 | .982 | .036 |
| Speed | ||||||||||
| Model 1 | 19 | 73 | .978 | .047 | 38 | .997 | .021 | 67 | .987 | .047 |
| Model 2 | 23 | 96 | .970 | .049 | 45 | .997 | .020 | 80 | .985 | .046 |
| Model 3 | 27 | 102 | .969 | .046 | 89 | .990 | .031 | 106 | .979 | .050 |
| Model 4 | 33 | 117 | .965 | .044 | 106 | .989 | .031 | 119 | .977 | .047 |
| Vocabulary | ||||||||||
| Model 1 | 43 | 140 | .986 | .042 | 243 | .988 | .045 | 178 | .974 | .052 |
| Model 2 | 49 | 158 | .985 | .041 | 259 | .988 | .043 | 181 | .975 | .048 |
| Model 3 | 55 | 268 | .970 | .054 | 296 | .986 | .043 | 209 | .970 | .049 |
| Model 4 | 63 | 288 | .968 | .052 | 310 | .965 | .041 | 270 | .960 | .053 |
| Reasoning | ||||||||||
| Model 1 | 19 | 41 | .993 | .030 | 30 | .999 | .016 | 22 | .999 | .011 |
| Model 2 | 23 | 60 | .988 | .035 | 38 | .998 | .016 | 25 | .999 | .008 |
| Model 3 | 27 | 93 | .979 | .043 | 80 | .993 | .029 | 27 | 1.00 | .002 |
| Model 4 | 33 | 102 | .978 | .040 | 93 | .992 | .028 | 38 | .998 | .012 |
| Space | ||||||||||
| Model 1 | 19 | 48 | .993 | .034 | 80 | .992 | .037 | 18 | 1.00 | .000 |
| Model 2 | 23 | 56 | .992 | .033 | 85 | .992 | .034 | 19 | 1.00 | .000 |
| Model 3 | 27 | 128 | .975 | .053 | 147 | .984 | .044 | 28 | 1.00 | .005 |
| Model 4 | 33 | 133 | .975 | .048 | 150 | .984 | .039 | 30 | 1.00 | .000 |
Note: df is degrees of freedom, CFI is Comparative Fit Index, and RMSEA is Root Mean Squared Error of Approximation. CFI values greater than about .90 and RMSEA values less than about .08 are often considered to reflect a reasonably good fit (Kline, 2005). Model 1 is configural invariance, Model 2 is weak factor invariance (equal factor loadings), Model 3 is strong factor invariance (equal intercepts), and Model 4 is strict factor invariance (equal unique variances).
Measurement of level and change
The latent growth model portrayed in Figure 1 was fit to the data with each cognitive ability in each age group. Fit statistics (reported in the first three columns of Table 4) with all combinations of abilities and age groups indicated that the model had excellent fits to the data, with all CFI > .98 and RMSEA < .06, and medians of .99 and .02, respectively.
Table 4.
Results of latent growth model analyses on each cognitive ability
| Χ2/df | CFI | RMSEA | Level | Level Var. | Change | Change Var. | Change-T2 | Level-Change | |
|---|---|---|---|---|---|---|---|---|---|
| Memory | |||||||||
| 18–39 (Y) | 0.91 | 1.00 | .000 | .30* | .41* | .17* | .01 | .71* | .72 |
| 40–64 (M) | 1.37 | .998 | .013 | .01 | .42* | .08* | .06* | .55* | −.22 |
| 65–99 (O) | 1.53 | .994 | .021 | −.34* | .46* | −.19* | .23* | .57* | −.17 |
| d Y-M | NA | NA | NA | −.30^ | .00 | −.06^ | .03 | −.02 | −.06^ |
| d M-O | NA | NA | NA | −.39^ | .04 | −.27^ | .14^ | .00 | −.03 |
| d Y-O | NA | NA | NA | −.68^ | .04 | −.30^ | .16^ | −.04 | −.10^ |
| Speed | |||||||||
| 18–39 (Y) | 3.21 | .971 | .041 | .57* | .41* | .11* | .11* | .36* | .00 |
| 40–64 (M) | 3.21 | .989 | .031 | .00 | .44* | −.06* | .06* | .25 | −.10 |
| 65–99 (O) | 2.32 | .989 | .034 | −.69* | .48* | −.18* | .17* | .54* | −.05 |
| d Y-M | NA | NA | NA | −.39^ | .02 | −.09^ | −.03 | −.01 | −.08^ |
| d M-O | NA | NA | NA | −.81^ | .03 | −.13^ | .11^ | .06 | .00 |
| d Y-O | NA | NA | NA | −1.44^ | .06 | −.25^ | .05 | .06 | −.13^ |
| Vocabulary | |||||||||
| 18–39 (Y) | 3.15 | .981 | .041 | −.32* | .61* | .20* | .03 | .49* | −.09 |
| 40–64 (M) | 4.93 | .986 | .041 | .07* | .77* | .04* | .02 | .36* | −.27* |
| 65–99 (O) | 4.28 | .962 | .053 | .19* | .59* | −.09* | .10* | .23* | −.18 |
| d Y-M | NA | NA | NA | .26^ | .08^ | −.15^ | −.01 | −.02 | −.02 |
| d M-O | NA | NA | NA | .12^ | −.14^ | −.19^ | .13^ | −.03 | −.01 |
| d Y-O | NA | NA | NA | .57^ | −.02 | −.35^ | .11^ | −.10^ | −.05 |
| Reasoning | |||||||||
| 18–39 (Y) | 2.22 | .988 | .030 | .26* | .43* | .19* | .04 | .49* | −.05 |
| 40–64 (M) | 1.44 | .998 | .014 | .04 | .57* | .10* | .03 | .69* | −.21 |
| 65–99 (O) | 1.17 | .998 | .012 | −.46* | .64* | −.05 | .06 | −.14 | −.06 |
| d Y-M | NA | NA | NA | −.14^ | .07^ | −.06^ | −.01 | .03 | −.02 |
| d M-O | NA | NA | NA | −.52^ | .06 | −.18^ | .04 | −.14^ | .02 |
| d Y-O | NA | NA | NA | −.75^ | .17^ | −.23^ | .02 | −.11^ | −.01 |
| Space | |||||||||
| 18–39 (Y) | 2.38 | .989 | .032 | .63* | .62* | .19* | .05 | .61* | .04 |
| 40–64 (M) | 1.90 | .996 | .020 | −.06* | .37* | .12* | .01 | .53* | .10 |
| 65–99 (O) | 0.89 | 1.00 | .000 | −.51* | .25* | .02 | .04 | .00 | −.08 |
| d Y-M | NA | NA | NA | −.44^ | −.12^ | −.05^ | −.03 | −.01 | .00 |
| d M-O | NA | NA | NA | −.54^ | −.14^ | −.15^ | .05 | −.10^ | −.03 |
| d Y-O | NA | NA | NA | −1.22^ | −.30^ | −.21^ | −.01 | −.11^ | −.02 |
Note:
p<.01. Estimates of effect sizes (d) of the group difference are derived from the standard errors of the unstandardized coefficients.
indicates that the difference in raw regression coefficients was significant at p<.01. NA indicates that the value is not applicable.
Table 4 also contains estimated means and variances of the latent level and latent change variables for the five abilities in the three age groups. The estimated standard errors were converted to standard deviations to allow computation of d values of effect sizes for the age group differences. As expected, there were large age differences in the level estimates, with progressively lower means at older ages for all cognitive abilities except vocabulary, where the direction of the age difference was reversed. The variances of the level estimates were similar across age groups, with the exception of larger values for reasoning and smaller values for space at older ages. All of the change estimates were more negative at older ages, with significant positive change in every ability in the 18–39 group, and significant negative change in memory, speed, and vocabulary in the 65–99 group.
The variances of the change estimates were small compared to the variances of the level estimates, but in the older group only the values for reasoning and space abilities were not significantly greater than zero. The estimates of change variance in memory and vocabulary were significantly larger in the 65–99 group than in the younger groups.
Entries in the column labeled Change-T2 are estimates of the basis coefficients representing the proportion of the interval between T1 and T3 that provided the best fit for a growth function. Most of the values were between about .3 and .6, indicating nearly equal change in the two intervals (T1 to T2 and T2 to T3). However, the coefficients for reasoning and space in the older group were small or negative rather than positive, which suggests that change in these domains may not have been systematic for participants 65 years and older.
Finally, the last column contains level-change relations. Nearly all of the estimates were small, and thus there was little evidence in these analyses that the magnitude of change was related to the level of that ability.
It is noteworthy that there was no significant change variance in the reasoning and space domains in any of the three age groups. These results are consistent with the very high stabilities and low estimated reliabilities of the 1–3 differences in Table 2. Because correlates of change cannot be expected when there is little systematic variance in change, the reasoning and space ability measures were not included in subsequent analyses.
Power Analyses
Statistical power was computed with the method outlined in the introduction in which the possible correlates were considered to be dichotomous and a two-group contrast was specified with sample sizes equal to one-half of the sample in each group (i.e., N = 656 in the 18–39 group, N = 1165 in the 40–64 group, and N = 579 in the 65–99 group). The analyses revealed that the power to detect a medium (d = .5) effect size with a two-tailed test and a significance level of .01 was 1.0 in each group, and the power to detect a small (d = .2) effect size was .85 in the 18–39 group, .99 in the 40–64 group, and .79 in the 65–99 group. In order to place this information in context, estimates of effect sizes were computed for a difference corresponding to 50% of the observed mean change. These effect sizes, and the corresponding power to detect a difference of that magnitude as significant (two-tailed alpha of .01), were: .90 and 1.00 for memory in the 18–39 group, .15 and .85 for memory in the 40–64 group, −.19 and .74 for memory in the 65–99 group, .16 and .62 for speed in the 18–39 group, −.12 and .62 for speed in the 40–64 group, −.22 and .88 for speed in the 65–99 group, .61 and 1.00 for vocabulary in the 18–39 group, .14 and .79 for vocabulary in the 40–64 group, and −15 and .49 for vocabulary in the 65–99 group. Note that because the mean changes were small and were associated with moderate variability, even a substantial difference equal to one-half of the observed change corresponds to a small effect size. Nevertheless, even with these small effect sizes, the statistical power in the present study was greater than .74 for the memory changes in all three groups and for the change in speed in the older group, and greater than .60 for all except change in vocabulary in the oldest group.
Analyses of potential correlates
Table 5 contains means, standard deviations, coefficient alphas, stability coefficients between the first and third occasion, and linear and quadratic age relations for each potential correlate. All coefficient alphas except that for self-rated health were above .7, indicating good internal consistency. No internal consistency values are reported for the cognitive activity measures because the total score is based on different types of activities (e.g., using a computer and driving a car), which could be inversely related to one another, or for the physical activity measures based on single scores.
Table 5.
Summary statistics for potential moderators of cognitive change
| Variable | N @ T1 | Mean | SD | Alpha | T1–T3 Corr. | Std. Coeff. | |
|---|---|---|---|---|---|---|---|
| Age | Age2 | ||||||
| Average Health | 4799 | 1.9 | 0.8 | .67 | .58 | .23* | .06* |
| Average Vision | 4753 | 52.2 | 39.3 | .71 | .39 | .42* | .00 |
| CES-D | 4630 | 11.6 | 8.7 | .90 | .57 | −.17* | −.01 |
| Anxiety | 3797 | 36.0 | 10.5 | .93 | .74 | −.25* | −.06* |
| PANAS-Positive | 3839 | 30.8 | 7.9 | .89 | .54 | .17* | −.04 |
| PANAS-Negative | 3839 | 13.3 | 5.0 | .89 | .47 | −.16* | −.01 |
| Dysexecutive Quest. | 3847 | 20.1 | 13.1 | .90 | .65 | −.21* | .05* |
| Emotional Stability | 3847 | 34.1 | 8.0 | .87 | .68 | .19* | .03 |
| Extraversion | 3847 | 31.9 | 7.6 | .85 | .74 | −.00 | .04 |
| Openness | 3847 | 36.3 | 6.4 | .79 | .71 | −.09* | .01 |
| Agreeableness | 3847 | 40.7 | 5.8 | .77 | .61 | .07* | −.08* |
| Conscientiousness | 3847 | 36.9 | 6.4 | .79 | .71 | .17* | −.10* |
| Busyness | 2559 | 20.7 | 5.6 | .88 | .58 | −.31* | −.16* |
| Routine | 2558 | 13.5 | 3.3 | .79 | .58 | .30* | −.07* |
| Need for Cognition | 4802 | 61.6 | 12.8 | .85 | .72 | −.06* | −.00 |
| Life Satisfaction | 3396 | 22.9 | 7.2 | .90 | .71 | .13* | .14* |
| Memory Rating | 4124 | 4.2 | 0.9 | .73 | .57 | −.15* | .08* |
| Thinking Rating | 3484 | 5.2 | 1.3 | .71 | .52 | −.10* | .08* |
| Cognitive Activities | 4705 | 37.4 | 22.5 | NA | .36 | −.06* | −.06* |
| Walking (74.5) | 3600 | 7.3 | 11.6 | NA | .23 | .00 | −.04 |
| Yard Work (60.7) | 3600 | 7.3 | 16.2 | NA | .35 | .13* | −.04 |
| Calisthenics (32.2) | 3599 | 2.2 | 7.2 | NA | .12 | −.09* | .10* |
| Running (22.4) | 3600 | 1.4 | 4.0 | NA | .30 | −.27* | .11* |
| Aerobics (15.9) | 3599 | 1.1 | 3.6 | NA | .23 | .02 | .03 |
| Swimming (21.0) | 3599 | 1.4 | 6.4 | NA | .20 | −.05* | .03 |
| Tennis (7.1) | 3597 | 1.0 | 26.6 | NA | .22 | .02 | .02 |
| Rowing (3.4) | 3598 | 0.1 | 1.5 | NA | .36 | .01 | .01 |
| Cycling (16.1) | 3597 | 1.4 | 9.6 | NA | .33 | −.03 | .02 |
| Sports (18.9) | 3591 | 2.7 | 11.0 | NA | .31 | −.03 | .08* |
| All Physical Activity (94.5) | 3586 | 25.1 | 28.8 | NA | .36 | −.03 | .04* |
Note: See text for descriptions of the variables. Cognitive activities are in hours per week, and physical activities are in hours per month. Numbers in parentheses for physical activity variables are the percentages of participants with non-zero values for that activity. NA indicates that the value is not applicable.
p<.01.
The estimates of stability from T1 to T3 were modest for self-rated health and objectively assessed visual acuity, PANAS negative mood, and self-rated thinking, but were above .55 for most other variables. The stability coefficients for the measures of cognitive and physical activities were very low, indicating little consistency from the first to the third occasion.
The age relations were generally as expected in that increased age was associated with poorer self-rated health and lower visual acuity (both indicated by higher numbers), lower self-ratings of depressive symptoms, anxiety, and negative mood, but higher self-ratings of positive mood and emotional stability, lower reported busyness, higher reported routine, and poorer ratings of one’s level of memory and thinking. Some quadratic age trends were significant, indicating acceleration of the age relations if the quadratic trend had the same sign as the linear trend, and flattening of the age relations if the sign was in the opposite direction.
Latent growth models were examined with each potential correlate to determine if there was significant variance in the change in the correlate. Many of the estimates of change variance were not significantly greater than zero, which implies very small individual differences in change in the correlate. Because most of the potential correlates had moderately high stability coefficients, the value at T1 was used as the predictor of level and change in cognitive abilities in all subsequent analyses. Although this precludes potentially informative analyses of the relations of correlate change with cognitive change, the measures at the first occasion were more reliable than the measures of change. In addition, assessment of the correlate at the first occasion minimizes ambiguity about reciprocal causation because subsequent cognitive change is unlikely to be the cause of the initial value of the correlate.
Correlates of Level and Change
An initial set of analyses examined demographic characteristics at T1 as simultaneous predictors of the latent level and latent change estimates in each cognitive domain, and standardized coefficients from these analyses are reported in Table 6.
Table 6.
Standardized relations of demographic predictors at T1 with level and change in cognitive abilities, and effect sizes (based on unstandardized coefficients)
| Level | d | Change | d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O |
Y 18–39 |
M 40–64 |
O 65–99 |
Y-M | M-O | Y-O | |
| Age | ||||||||||||
| Memory | −.31* | −.17* | −.47* | .15^ | −.31^ | −.10^ | −.38 | −.13 | −.18* | .04 | −.06 | −.01 |
| Speed | −.29* | −.33* | −.49* | −.03 | −.18^ | −.20^ | −.50* | −.15 | −.19* | .10^ | −.04 | .07 |
| Vocabulary | −.11* | .13* | −.11* | .30^ | −.30^ | .00 | −.54* | −.24* | −.10 | .07 | .09 | .07 |
| Sex (M=0, F=1) | ||||||||||||
| Memory | .10* | .22* | .26* | .12^ | .03 | .15^ | −.27 | .15 | .17* | .10^ | .04 | .13^ |
| Speed | .06 | .15* | .07 | .09^ | −.08 | .02 | .08 | .13 | .02 | .01 | −.03 | −.01 |
| Vocabulary | −.13* | .00 | .03 | .13^ | .03 | .17^ | −.06 | −.04 | .11 | .00 | .05 | .05 |
| Education | ||||||||||||
| Memory | .44* | .44* | .15* | −.06 | −.26^ | −.31^ | .55* | .02 | .08 | −.13^ | .04 | −.08 |
| Speed | .41* | .35* | .15* | −.07 | −.19^ | −.25^ | .05 | .03 | .00 | −.01 | −.01 | −.02 |
| Vocabulary | .58* | .57* | .45* | −.01 | −.24^ | −.23^ | −.06 | −.16 | −.19 | −.02 | −.05 | −.06 |
Note:
p<.01. Estimates of effect sizes (d) of the group difference are derived from the standard errors of the unstandardized coefficients.
indicates that the difference in raw regression coefficients was significant at p<.01.
With the exception of vocabulary ability in the 40-to-64 group, all of the relations of age with the level estimates were significantly negative, indicating lower levels at older ages. All of the relations of age with the latent change estimates were negative, but were significant only for some of the comparisons. However, the d values indicate that the differences in the unstandardized coefficients relating age to change were relatively small.
Females had higher average scores than males in memory, and also slightly higher levels of speed in the 40-to-64 group, and slightly lower levels of vocabulary in the 18–39 group. Longitudinal change in memory was less negative for females than for males in the 65–99 group, but not in the 18–39 group. More education was associated with higher levels of performance in each ability domain, although the relations were weaker in the 65–99 group than in either of the younger groups. Importantly, the only relation of education to change was more positive change in the 18–39 group.
Because most prior studies considered potential correlates of cognitive change in separate analyses, each potential correlate was initially examined individually with only age, sex, and education as covariates. Standardized coefficients from these analyses are presented in Table 7.
Most of the relations on the level coefficients were as expected, with higher levels of cognitive performance associated with better health, better vision, fewer depressive symptoms, less negative mood, lower self-reported executive (DEX) problems, higher openness, higher agreeableness, higher need for cognition, higher life satisfaction, more cognitive activities, and higher self-ratings of memory and thinking. However, the significant negative relations between positive mood and both memory and vocabulary were unexpected, as were the weak relations with emotional stability (the reverse of neuroticism). Only the relations with cognitive activity exhibited much of a difference across age groups, with more positive relations of reported cognitive activity on level of cognitive performance for the older age group in all three cognitive domains.
Only 9 predictors of change in Table 7 (out of 270, corresponding to a proportion of .03) were significant at p<.01, with five in the 18–39 group, two in the 40–64 group, and two in the 65–99 group. Furthermore, the effect sizes indicating differences between age groups in relations of the correlates were small, with d values ranging from −.12 to .11.
Because it is unlikely that the potential correlates were all independent of one another, the possibility of meaningful clusters of variables was investigated with exploratory factor analysis (principal axis factoring with promax rotation) on all 29 variables (excluding the sum of physical activities measure). Ten eigenvalues were greater than 1, but only four factors were interpretable in which the same variables had high loadings on the factors in all three age groups. The factor analysis results are summarized in Table 8.
Table 8.
Exploratory factor analysis (Principal axis factoring, 4 factors, promax rotation) on 29 potential correlates
| Variable | Neg. Affect | Openness | Self Effic. | Busy |
|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Anxiety | −.927 | −.314 | −.225 | .040 |
| CES-D | −.846 | −.256 | −.225 | .032 |
| Emotional Stability | .719 | .233 | .193 | −.046 |
| Dysexecutive Quest. | −.619 | −.403 | −.279 | .331 |
| Life Satisfaction | .636 | .207 | .225 | .090 |
| PANAS-Negative | −.612 | −.168 | −.205 | .127 |
| Conscientiousness | .441 | .344 | .129 | −.317 |
| Openness | .120 | .679 | .542 | −.036 |
| Need for Cognition | .155 | .562 | .504 | .026 |
| Agreeableness | .254 | .498 | .141 | −.117 |
| PANAS-Positive | .399 | .411 | .135 | .047 |
| Extraversion | .232 | .402 | .154 | .176 |
| Memory Rating | .333 | .292 | .609 | −.056 |
| Thinking Rating | .380 | .283 | .609 | −.055 |
| Routine | .357 | .055 | −.017 | −.331 |
| Busyness | −.239 | .169 | .040 | .268 |
| Running | −.025 | .048 | .148 | .247 |
| Sports | .034 | .023 | .002 | .241 |
| Calisthenics | .014 | .056 | .030 | .231 |
| Average Vision | .084 | −.040 | −.197 | −.167 |
| Tennis | .052 | .002 | .010 | .146 |
| Aerobics | .064 | .065 | .007 | .140 |
| Rowing | −.008 | −.003 | .039 | .120 |
| Cognitive Activities | .006 | .203 | .125 | .117 |
| Swimming | −.009 | .019 | −.016 | .116 |
| Cycling | .000 | .028 | .060 | .090 |
| Average Health | −.242 | −.205 | −.244 | −.076 |
| Yard Work | .088 | .035 | −.073 | .038 |
| Walking | .009 | .068 | −.029 | .001 |
| Factor Correlations | ||||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Age | .27* | .00 | −.25 | −.37* |
| Factor 1 | X | .40* | .33* | −.17* |
| Factor 2 | X | .64* | −.06* | |
| Factor 3 | X | .01 | ||
The first factor can be labeled negative affect because the highest positive loadings were with anxiety, depressive symptoms, negative mood from the PANAS, and the dysexecutive score. The second, third, and fourth factors can be labeled Openness, Self Efficacy, and Busyness, respectively, because those variables had the strongest loadings in each factor.
The four factor scores were next used as simultaneous predictors of the latent level and latent change variables in the memory, speed, and vocabulary domains, with age, sex, and education as control variables. Results of these analyses are presented in Table 9.
The only consistent relations across all three age groups were those of the self efficacy factor on the level of memory and vocabulary. Two other relations on the level parameters were significant, a negative relation of negative affect on vocabulary in the 18-to-39 group, and a negative relation of busyness on vocabulary in the 40-to-64 group. Of primary interest were the correlations of the factors with measures of cognitive change. Only one change relation was significant, and that was the positive relation between self efficacy and change in vocabulary in the 65-to-99 group.
Discussion
As noted in the introduction, prior research on correlates of cognitive change has been inconsistent, and thus it is important that additional research on this topic be as methodologically rigorous as possible. The present study has a number of strengths compared to prior studies in which correlates of cognitive change have been investigated. For example, cognitive functioning was assessed at the level of latent variables defined by scores on three or four separate cognitive tests, which increases the breadth of assessment and minimizes measurement error relative to assessments with single variables. In addition, measurement invariance analyses indicated that the cognitive ability constructs had similar meaning at each measurement occasion, which implies that the changes were primarily quantitative rather than qualitative. Furthermore, sensitive assessment of cognitive change based on a second-order latent growth model revealed that there was significant mean change, and significant variance in change, in the speed measure in all three age groups, in the memory measure in the 40-to-64 and 65-to-99 groups, and in the vocabulary measure in the 65-to-99 group (cf. Table 4). Because there was no significant change variance in the measures of reasoning and space, and high stability from the first to the third occasion, it was not meaningful to examine correlates of change in those abilities in the present study. In addition, the statistical power to detect small differences in change was above .74 for the measures of memory in all three age groups, for the vocabulary measure in the 40-to-64 and 65-to-99 groups, and for the speed measure in the older group. Most of the potential correlates had good coefficient alpha reliability, and in some analyses they were aggregated into factors that can be expected to be even more reliable. Although the sample of participants had a higher average level of functioning than that in a nationally representative normative sample, the magnitude of variability was similar, and there was little attenuation of the variability after attrition. In addition, unlike many earlier studies in which all of the participants were over 65 years of age, the participants spanned a wide age range.
Many of the potential correlates had significant relations with measures of the level of cognitive functioning. As in other reports, there were negative relations of the measures of cognitive functioning with depressive symptoms, anxiety, and negative mood, and positive relations with health, vision, openness, conscientiousness, need for cognition, life satisfaction, and self ratings of memory and thinking. There were also a few age differences in the pattern of relations as the female advantage for memory was greater in the two older groups, and the effects of education were weaker in the oldest group.
The major results with both individual variables and with the factors representing groups of related variables were the weak to non-existent correlations of cognitive change. Only three of the potential correlates were related to individual differences in change in memory, two in the 18–39 group, reflecting more negative memory change with higher values of negative mood, and more positive memory change with higher levels of cycling activity. The other relation with memory was in the 65–99 group, in which individuals with more negative ratings of their memory had more negative change in memory. Three predictors had significant relations with change in speed, and all were in the 40-to-64 group. Individuals with less time in yard work, greater time in calisthenics and greater time in running had more positive change in speed. The only significant predictor of change in vocabulary was in the 65-to-99 group in which, surprisingly, people with a greater reported time in cognitive activities had more negative change in vocabulary.
The patterns of relations were generally similar in the three age groups, and therefore there was little evidence that relations with cognitive functioning were restricted to the period of late adulthood. The primary exception was the cognitive activity variable, which had more positive relations with the levels of memory, speed and vocabulary in the two older age groups. These results indicate that among the middle-aged and older adults, people with more engagement in cognitively stimulating activities had higher levels of cognitive functioning than people with less engagement. Although these results are interesting, it is important to recognize that the causal direction of this relation is ambiguous because level of ability could have contributed to participation in stimulating activities rather than participation contributing to different levels of ability. Furthermore, the relations of cognitive activity were primarily apparent with measures of the level of functioning and the only relation with change in functioning was negative, and thus there was no support for the hypothesis that engagement in cognitively stimulating activities alters the rate of change in cognitive ability.
Despite numerous strengths, the present study failed to identify significant moderators of cognitive change. It is therefore important to consider factors that might be contributing to the inconsistencies in research concerned with correlates of cognitive change. Although a definitive answer is not yet available, at least six possibilities that might account for different patterns of results in studies investigating correlates of change are worth considering.
First, it is conceivable that the published literature is somewhat distorted because negative findings might have been less likely to have been published than positive findings. In addition, some of the positive outcomes that were reported could have been attributable to chance because not all studies adjusted the significance level for the number of statistical comparisons.
Second, a variety of cognitive measures have been included in the prior studies, and some of the differences in results may reflect effects on different aspects of cognitive functioning. There has also been considerable variation in the outcome variables as some studies have focused on incidence of pathological conditions such as dementia, whereas others have been concerned with continuous change in cognitive functioning in healthy adults.
Third, many different measures of potential correlates have been examined, and even when they were described with the same label, they may not have represented the same construct. For example, in some studies a subset of items from the original scales was used, which may not have had same reliability (because there were fewer items), or validity (because all facets of the construct may not have been represented) as the original scale. Activities have sometimes been assessed with a very small number of items, which might not have been very reliable or valid, particularly when evaluated with self reports. The assessments might also have differed qualitatively and not quantitatively as they have ranged from evaluation of presence or absence, to measures combining time and intensity in multiple activities. There has also been considerable variation with respect to when the correlate was assessed, such as current, past, recent, or cumulative across one’s lifetime, and in characteristics such as intensity and frequency.
Fourth, there have been many differences across studies in the composition of the samples, including the range of ages and of ability levels, and the magnitude and selectivity of attrition. Furthermore, in some studies the distribution of individuals with different numbers of measurement occasions was highly skewed, which implies that the change estimates were heavily influenced by a very small number of individuals from the initial sample. Some prior studies may also have included substantial proportions of individuals in early stages of dementia or terminal decline, which could have resulted in more negative mean change and/or greater variance in change relative to studies with only healthy adults.
Fifth, different analytical methods have been used to assess cognitive change, and some of the analyses of change may have been influenced by the mean level of performance, or by the relation of the correlate to the baseline scores (Glymour et al., 2005). Furthermore measurement equivalence was seldom examined to evaluate comparability of the cognitive constructs across different occasions.
Sixth, many of the analyses may have had low power to detect potentially interesting differences in cognitive change. To illustrate, the findings in the present study that a 50% difference in the change in memory corresponded to an effect size of only −.19 in the 65-to-99 group suggest that even large differences in cognitive change may be difficult to detect without very large sample sizes.
Importantly, few studies have reported whether there was significant variance in change, which is necessary to have correlations with other variables. Unfortunately, little information is currently available about the magnitude of change variance in longitudinal studies. However, it is noteworthy that one major study in which variance of cognitive change was examined over a period of 10 years found significant change variance in only 4 of 20 comparisons (i.e., five age groups with four cognitive measures each), and none of those was significant after eliminating participants who died or developed dementia during the interval (de Frias et al., 2007). If there is no evidence of differential change, it is unrealistic to expect to identify correlates of differences that do not exist.
Finally, the fact that there have been very few, if any, exact replications with the same measures and analytical procedures reinforces concerns raised about the role of “flexibility in designs, definitions, outcomes, and analytical modes” (Ioanndis, 2005) and “researcher degrees of freedom” (Simmons et al., 2011) in contributing to false positive results. As an example, although many studies have investigated the relation between physical activity and cognitive change, Salthouse (2010, p. 144) noted that the available studies differed in many respects, including the measures of cognitive functioning, the analytical procedures, and the methods used to assess physical activity, such as self-rating at baseline, change in self rating, or objectively assessed fitness. It may therefore be misleading to suggest that the studies are reporting the same result when they had so few features in common. Because longitudinal studies are expensive and time consuming, exact replications with longitudinal studies are rare. Nevertheless, three approximations to replications in longitudinal studies should be encouraged because they can be informative in examining the robustness of correlates of cognitive change: (1) comparing results across different subsamples within the same study, such as the three age groups in the present study; (2) comparing results across different cohorts recruited in different years (e.g., Small et al., 2012); and (3) using common models to analyze similar variables in different data sets (e.g., Hofer & Piccinin, 2009; Lindwall, et al., 2012; Mitchell et al., 2012).
It is not yet clear which, if any, of the preceding characteristics may have contributed to the different patterns of results regarding correlates of cognitive change in healthy adults. However, it is important to note that the present study had moderately large samples, assessment of multiple cognitive abilities at the level of latent variables which minimize measurement error, reliable assessment of potential correlates which were significantly related to many measures of the level of cognitive functioning, and powerful analytical methods which revealed significant variance in the change in memory, speed, and vocabulary. Although these features should have contributed to sensitive detection of correlates of change, there was little evidence in this study that aspects of lifestyle, mood, or personality moderate longitudinal change in cognitive functioning among healthy adults.
Much of the interest in correlates of cognitive change has been motivated by an interest in identifying possible targets for intervention. In a recent review of risk factors for cognitive decline, Plassman et al. (2010) concluded that “The current literature does not provide adequate evidence to make recommendations for interventions.” The results of the present study reinforce this conclusion because no consistent correlates of cognitive change could be identified. It is also important to recognize that even if significant correlations were found, drawing causal inferences from correlations should be done with great caution, and in particular, one should avoid implications that altering a correlate will necessarily alter the trajectory of cognitive change. As an example, a recent study reported that married individuals exhibited less memory decline than individuals who were not married (Mousavi-Nasab et al., 2012), but even if this finding were confirmed in other studies, marriage should not necessarily be advocated as an intervention to minimize cognitive decline.
In conclusion, the results of the present study suggest that in healthy adults increased age is associated with more negative change in several major cognitive abilities, and that there are significant individual differences in cognitive change, particularly among adults age 65 and older. However, there was little evidence of moderators of cognitive change across cognitive abilities, different age groups, or different analytical methods (e.g., with individual predictors or factors, and in both the FIML analyses and the analyses based on participants with data on all three occasions). Until a consistently replicated pattern based on methodologically strong studies has been established, therefore, the most reasonable conclusion at the current time may be that if these variables do moderate the rate of cognitive change, the effects are likely to be quite small.
Acknowledgments
The project was supported by Award Number R37AG024270 from the National Institute on Aging.
Appendix - Description of cognitive variables
Vocabulary
WAIS Vocabulary: Provide definitions of words.
Picture Vocabulary: Name the pictured object.
Antonym Vocabulary: Select the best antonym of the target word.
Synonym Vocabulary: Select the best synonym of the target word.
Reasoning
Matrix Reasoning: Determine which pattern best completes the missing cell in a matrix.
Shipley Abstraction: Determine the words or numbers that are the best continuation of a sequence.
Letter Sets: Identify which of five groups of letters is different from the others.
Spatial Visualization
Spatial Relations: Determine the correspondence between a 3-D figure and alternative 2-D figures.
Paper Folding: Determine the pattern of holes that would result from a sequence of folds and a punch through the folded paper.
Form Boards: Determine which combinations of shapes are needed to fill a larger shape.
Memory
Logical Memory: Recall idea units across three stories.
Word Recall: Recall words across four trials of the same word list.
Paired Associates: Recall response terms when presented with a stimulus item.
Speed
Digit Symbol: Use a code table to write the correct symbol below each digit.
Letter Comparison: Same/different comparison of pairs of letter strings.
Pattern Comparison: Same/different comparison of pairs of line patterns.
Footnotes
Because of the relatively large sample sizes, a significance level of .01 was used to determine statistical significance.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
There are no conflicts of interest.
References
- Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Hebert JR. The effect of social desirability and social approval on self reports of physical activity. American Journal of Epidemiology. 2005;161:389–398. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve analysis of late-life sensory and cognitive function over 8 years: Evidence for specific and common factors underlying change. Psychology and Aging. 2003;18:714–726. doi: 10.1037/0882-7974.18.4.714. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. AMOS (Version 7) Chicago: SPSS; 2007. Computer Program. [Google Scholar]
- Atienza AA, Moser RP, Perna F, Dodd K, Ballard-Barbash R, Troiano RP, Berrigan D. Self-reported and objectively measured activity related to biomarkers using NHANES. Medicine & Science in Sports & Exercise. 2011;43:815–821. doi: 10.1249/MSS.0b013e3181fdfc32. [DOI] [PubMed] [Google Scholar]
- Bielak AAM. How can we not “lose it” if we still don’t understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age – A mini-review. Gerontology. 2010;56:507–519. doi: 10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Anstey KJ, Christensen H, Windsor TD. Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychology and Aging. 2012;27:219–228. doi: 10.1037/a0024667. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Gerstorf D, Kiely KM, Anstey KJ, Luszcz M. Depressive symptoms predict decline in perceptual speed in older adulthood. Psychology and Aging. 2011;26:576–583. doi: 10.1037/a0023313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Feinstein JA, Jarvis WBG. Dispositional differences in cognitive motivation: The life and times of individuals varying in Need for cognition. Psychological Bulletin. 1996;119:197–253. [Google Scholar]
- Carmelli D, Swan GE, LaRue A, Eslinger PJ. Correlates of change in cognitive function in survivors from the Western Collaborative Group Study. Neuroepidemiology. 1997;16:285–295. doi: 10.1159/000109699. [DOI] [PubMed] [Google Scholar]
- Chapman B, Duberstein P, Tindle HA, Sink KM, Robbins J, Tancredi DJ, Franks P. Personality predicts cognitive function over 7 years in older persons. American Journal of Geriatric Psychiatry. 2012;20:612–621. doi: 10.1097/JGP.0b013e31822cc9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, Rubin MS, Hofer SM. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiological Reviews. 2013;35:33–50. doi: 10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Daffner KR. Promoting successful cognitive aging: A comprehensive review. Journal of Alzheimer’s Disease. 2010;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, SIlberg D, Trevisan M. National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer Disease and Cognitive Decline. Annals of Internal Medicine. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Lovden M, Lindenberger U, Nilsson LG. Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence. 2007;35:381–392. [Google Scholar]
- Depp C, Vahia IV, Jeste D. Successful aging: Focus on cognitive and emotional health. Annual Review of Clinical Psychology. 2010;6:527–550. doi: 10.1146/annurev.clinpsy.121208.131449. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Balluerka N, Widaman KF. Factorial invariance and the specification of second-order latent growth models. Methodology. 2008;4:22–36. doi: 10.1027/1614-2241.4.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology. 2003;39:535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Bickel JF, Lovden M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. Journal of Gerontology: Psychological Sciences. 2006;61B:253–261. doi: 10.1093/geronb/61.5.p253. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American Journal of Epidemiology. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Tzourio C, Dufouil C. Is cognitive aging predicted by one’s own or one’s parents’ educational level? Results from the three-city study. American Journal of Epidemiology. 2012;175:750–759. doi: 10.1093/aje/kwr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DP, Andres D, Etezadi J, Arbuckle TY, Schwartzman AE, Chaikelson J. Structural equation model of intellectual change and continuity and predictors of intelligence in older men. Psychology and Aging. 1995;10:294–303. doi: 10.1037//0882-7974.10.2.294. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. In: Mervielde I, Deary I, De Fruyt F, Ostendorf F, editors. Personality psychology in Europe. 7. Tilburg, the Netherlands: Tilburg University Press; 1999. pp. 7–28. [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, Yaffe K, Cuthbert BN, Edwards E, Wagster MV. The NIH Cognitive and Emotional Health Project: Report of the critical evaluation study committee. Alzheimer’s & Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18:639–657. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychological Methods. 2009;14:150–164. doi: 10.1037/a0015566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Deary IJ, Whalley LJ. Openness to experience and activity engagement facilitate the maintenance of verbal ability in older adults. Psychology and Aging. 2012;27:849–854. doi: 10.1037/a0029066. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Ioanndis JPA. Why most published research findings are false. PLoS Medicine, 2, Issue. 2005;8:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajodia A, Borders A. Memory predicts changes in depressive symptoms in older adults: A bidirectional longitudinal analysis. Journal of Gerontology: Psychological Sciences. 2011;66:571–581. doi: 10.1093/geronb/gbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylha M, Volpato S, Guralnik JM. Self rated health showed a graded association with frequently used biomarkers in a large population sample. Journal of Clinical Epidemiology. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. NY: Guilford Press; 2005. [Google Scholar]
- Kohler S, van Boxtel MPJ, van Os J, Thomas AJ, O’Brien JT, Jolles J, Verhey FRJ, Allardyce J. Depressive symptoms and cognitive decline in community-dwelling older adults. Journal of American Geriatric Society. 2010;58:873–879. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology. 2003;60:82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychology and Aging. 2009;24:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Lindwall M, Cimino CR, Gibbons LE, Mitchell MB, Benitez A, Brown CL, Kennison RF, Shirk SD, Atri A, Robitaille A, MacDonald SWS, Zelinski EM, Willis SL, Schaie KW, Johansson B, Praetorius M, Dixon RA, Mungas DM, Hofer SM, Piccinin AM. Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. Journal of Aging Research. 2012 doi: 10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Ronnlund M, Wahlin A, Backman L, Nyberg L, Nilsson LG. The extent of stability and change in episodic and semantic memory in old age: Demographic predictors of level and change. Journal of Gerontology: Psychological Sciences. 2004;59B:130–134. doi: 10.1093/geronb/59.3.p130. [DOI] [PubMed] [Google Scholar]
- Martin M, Park DC. The Martin and Park Environmental Demands (MPED) questionnaire: Psychometric properties of a brief instrument to measure self-reported environmental demands. Aging Clinical and Experimental Research. 2003;15:77–82. doi: 10.1007/BF03324483. [DOI] [PubMed] [Google Scholar]
- Mascherek A, Zimprich D. Correlated change in memory complaints and memory performance across 12 years. Psychology and Aging. 2011;26:884–889. doi: 10.1037/a0023156. [DOI] [PubMed] [Google Scholar]
- Meijer WA, van Boxtel MPJ, van Gerven PWM, van Hooren SAH, Jolles J. Interaction effects of education and health status on cognitive change: A 6-year follow-up of the Maastricht Aging Study. Aging & Mental Health. 2009;13:521–529. doi: 10.1080/13607860902860821. [DOI] [PubMed] [Google Scholar]
- Miller DI, Taler V, Davidson PSR, Messier C. Measuring the impact of exercise on cognitive aging: Methodological issues. Neurobiology of Aging. 2012;33:622.329–622.e43. doi: 10.1016/j.neurobiolaging.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, Kennison RF, Shirk SD, Atri A, Robitaille A, MacDonald SWS, Lindwall M, Zelinski EM, Willis SL, Schaie KW, Johansson B, Dixon RA, Mungas DM, Hofer SM, Piccinin AM. Cognitively stimulating activities: Effects of cognition across four studies with up to 21 years of longitudinal data. Journal of Aging Research. 2012:1–12. doi: 10.1155/2012/461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EL, Barefoot JC, Avlund K. Do depressive traits and hostility predict age-related decline in general intelligence? Journal of Aging Research. 2012:1–9. doi: 10.1155/2012/973121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self rated health status as a health measure: The predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. Journal of Clinical Epidemiology. 1997;50:517–528. doi: 10.1016/s0895-4356(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: A cross-sectional and longitudinal examiniation. Journal of Gerontology: Psychological Sciences. 2005;60B:113–120. doi: 10.1093/geronb/60.3.p113. [DOI] [PubMed] [Google Scholar]
- Parisi JM, Gross AL, Rebok GW, Saczynski JS, Crowe M, Cook SE, Langbaum JBS, Sartori A, Unverzagt FW. Modeling change in memory performance and memory perceptions: Findings from the ACTIVE study. Psychology and Aging. 2011;26:518–524. doi: 10.1037/a0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ronnlund M, Nilsson LG. Adult life-span patterns in WAIS-R Block Design performance: Cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence. 2006;34:63–78. [Google Scholar]
- Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the use it or lose it hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The paradox of cognitive change. Journal of Clinical and Experimental Neuropsychology. 2010;32:622–629. doi: 10.1080/13803390903401310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Does the direction and magnitude of cognitive change depend on initial level of ability? Intelligence. 2012;40:352–361. doi: 10.1016/j.intell.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Evaluating the correspondence of different cognitive batteries. Assessment. doi: 10.1177/1073191113486690. (in press-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selectivity of attrition in longitudinal studies of cognitive functioning. Journal of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbt046. (in press-b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging. 2002;17:548–557. doi: 10.1037//0882-7974.17.4.548. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM. Contextual analysis of fluid intelligence. Intelligence. 2008;36:464–486. doi: 10.1016/j.intell.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Tucker-Drob EM. Implications of short-term retest effects for the interpretation of longitudinal change. Neuropsychology. 2008;22:800–811. doi: 10.1037/a0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sharp ES, Reynolds CA, Pedersen NL, Gatz M. Cognitive engagement and cognitive aging: Is openness protective? Psychology and Aging. 2010;25:60–73. doi: 10.1037/a0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RJ. Limits to the measurement of habitual physical activity by questionnaires. British Journal of Sports Medicine. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Gueguen A, Martikainen P, Ferrie J, Marmot M, Shipley M. Self-rated health and mortality: Short- and long-term associations in the Whitehall II study. Psychosomatic Medicine. 2007;69:138–143. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Annals of Neurology. 2011;70:296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ. Tracking cognition-health changes from 55 to 95 years of age. Journals of Gerontology: Psychological Sciences. 2011;66B:153–161. doi: 10.1093/geronb/gbq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26:144–155. doi: 10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine. 2010;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Soubelet A, Salthouse TA. The role of activity engagement in the relations between Openness/Intellect and cognition. Personality and Individual Differences. 2010;8:896–901. doi: 10.1016/j.paid.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubelet A, Salthouse TA. Personality-cognition relations across adulthood. Developmental Psychology. 2011;47:303–310. doi: 10.1037/a0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual of the state-trait anxiety inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sternang O, Jonsson B, Wahlin A, Nyberg L, Nilsson LG. Examination of the common cause account in a population-based longitudinal study with narrow age cohort design. Gerontology. 2010;56:553–563. doi: 10.1159/000279754. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: A longitudinal examination of age-associated declines in reasoning and processing speed. Developmental Psychology. 2009;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Kommer TN, Comijs HC, Aartsen MJ, Huisman M, Deeg DJH, Beekman ATF. Depression and cognition: How do they interrelate in old age? American Journal of Geriatic Psychiatry. 2013;21:398–410. doi: 10.1016/j.jagp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, van Gerven PWM, van Voxtel MPJ, van der Elst W, Jolles J. No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht Aging Study. Psychology and Aging. 2008;23:119–130. doi: 10.1037/0882-7974.23.1.119. [DOI] [PubMed] [Google Scholar]
- Van Hooren SAH, Valentijn SAM, Bosma H, Ponds RWHM, van Boxtel MPJ, Jolles J. Relation between health status and cognitive functioning: A 6-year follow-up of the Maastricht Aging Study. Journal of Gerontology: Psychological Sciences. 2005;60B:57–60. doi: 10.1093/geronb/60.1.p57. [DOI] [PubMed] [Google Scholar]
- Von Oertzen T, Hertzog C, Lindenberger U, Ghisletta P. The effect of multiple indicators on the power to detect inter-individual differences in change. British Journal of Mathematical and Social Psychology. 2010;63:627–646. doi: 10.1348/000711010X486633. [DOI] [PubMed] [Google Scholar]
- Von Stumm S, Ackerman PL. Investment and intellect: A review and meta-analysis. Psychological Bulletin. 2013;139:841–869. doi: 10.1037/a0030746. [DOI] [PubMed] [Google Scholar]
- Wahlin A, Maitland SB, Backman L, Dixon RA. Interrelations between subjective health and episodic memory cnange in Swedish and Canadian samples of older adults. International Journal of Aging and Human Development. 2003;57:21–35. doi: 10.2190/9VAA-KMYV-U2HU-PVAW. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-IV: Administration and scoring manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioral Assessment of the Dysexecutive Syndrome. Bury St. Edmund, UK: Thames Valley Test Company; 1996. [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SWS, Manly JJ. Journal of the International Neuropsychological Society. 2011;17:1–8. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]