Abstract
Converging evidence from humans and non-human animals indicates that the neurohypophysial hormone oxytocin (OT) evolved to serve a specialized function in social behavior in mammals. Although OT-based therapies are currently being evaluated as remedies for social deficits in neuropsychiatric disorders, precisely how OT regulates complex social processes remains largely unknown. Here we describe how a non-human primate model can be used to understand the mechanisms by which OT regulates social cognition and thereby inform its clinical application in humans. We focus primarily on recent advances in our understanding of OT-mediated social cognition in rhesus macaques (Macaca mulatta), supplemented by discussion of recent work in humans, other primates, and rodents. Together, these studies endorse the hypothesis that OT promotes social exploration both by amplifying social motivation and by attenuating social vigilance.
Introduction
Oxytocin (OT) is an evolutionarily conserved nonapeptide that mediates female sexual intercourse, parturition, lactation, as well as water regulation, and anxiolytic functions (Donaldson and Young, 2008). In highly social animals, these ancestral functions of OT have been co-opted to serve social functions, such as promoting maternal behavior (Champagne et al., 2001; Pedersen et al., 1982), fostering pair-bonding and affiliative behaviors (Cushing and Carter, 2000; Smith et al., 2010; Snowdon et al., 2010; Young and Wang, 2004), encouraging in-group bias (Dreu et al., 2010), reducing social vigilance (Ebitz et al., 2013; Heinrichs et al., 2003) and amplifying other-regarding behaviors (Barraza et al., 2011; Chang et al., 2012; van IJzendoorn et al., 2011). Although there appears to be a large range of OT-mediated effects, one might argue that some functions of OT may be common to most, if not all, of OT-mediated social cognition. The anxiolytic, approach-promoting, and tolerance-enhancing roles of OT (Amico et al., 2004; Averbeck, 2010; Heinrichs et al., 2003; Kemp and Guastella, 2010; Neumann et al., 2000a; Riem et al., 2011; Ring et al., 2006; Uvnäs-Moberg et al., 1994; Waldherr and Neumann, 2007; Yoshida et al., 2009; Young, 2002) may serve as foundational substrates that promote social exploration and interaction while, typically, suppressing social avoidance.
A large number of studies have been conducted to probe the role of OT in regulating social behavior in both healthy and pathological states (Bartz et al., 2011; De Dreu, 2012; Guastella et al., 2012; Heinrichs and Domes, 2008; Insel, 2010; MacDonald and Feifel, 2013; Meyer-Lindenberg et al., 2011). Nevertheless, the neural mechanisms through which OT regulates social behavior and cognition—particularly in humans—remain poorly understood. Standard noninvasive neuroscientific techniques, such as functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation (TMS), used to study human brain and cognition are limited in their capacity to reveal the neuronal and circuit mechanisms that mediate the regulation of social behavior and cognition by OT. Conversely, rodent models permit exquisitely fine dissection of these neural pathways but lack the behavioral complexity of human social function.
Compared to other animals, primates, including humans, are unique in that they show remarkably complex social behavior in a society typically made up of many individuals. Adapting to increasing social complexity may have played a major role in primate brain evolution (Dunbar and Shultz, 2007). For example, across primate species, social complexity, as measured by group size, strongly predicts forebrain volume (after correcting for body size) (Dunbar, 1998). Although rodents offer the opportunity for exploitation of powerful molecular genetic techniques, their social behavior is not very similar to the social behavior of humans. While molecular genetic techniques are only beginning to be developed for use in non-human primates (Diester et al., 2011; Sasaki et al., 2009), their social behavior is much more similar to the social behavior of humans.
Here we argue that a rhesus macaque model (Macaca mulatta) can effectively bridge this gap. Rhesus macaques are Old World monkeys that live in large, hierarchical, and mixed-sex social groups, that last shared a common ancestor with humans some 25 million years ago (Smuts, 1987). Critically, rhesus macaques display basic aspects of complex social behaviors that are typically considered ‘uniquely human’ (Frith and Frith, 2007; Saxe, 2006). These include social imitation (Ferrari et al., 2006; Subiaul, 2004), prosocial behaviors (Chang et al., 2011; Masserman et al., 1964), as well as understanding others’ perceptions (Flombaum and Santos, 2005; Santos et al., 2006). Mental capacities like these might be fundamental building blocks for empathy and theory of mind. Such similarities in social behaviors make rhesus macaques excellent models for studying neuropsychiatric conditions accompanied by complex social deficits, such as autism spectrum disorders (Watson and Platt, 2012). Although there are undoubtedly some differences between humans and rhesus monkeys (Byrne and Whiten, 1988), such as the strength of prosociality, rhesus macaques are outstanding models for studying the neural mechanisms underlying psychiatric disorders marked by social deficits. Due to their remarkably similar anatomy and physiology to humans, rhesus macaques have long served as the gold standard for electrophysiological, pharmacological, and lesion-based investigations into complex cognitive processes. In this review we highlight recent advances in understanding how OT influences social behavior in rhesus macaques, paving the way for future investigations into the neural mechanisms mediating these influences.
Inhaling OT increases its concentration in the brain
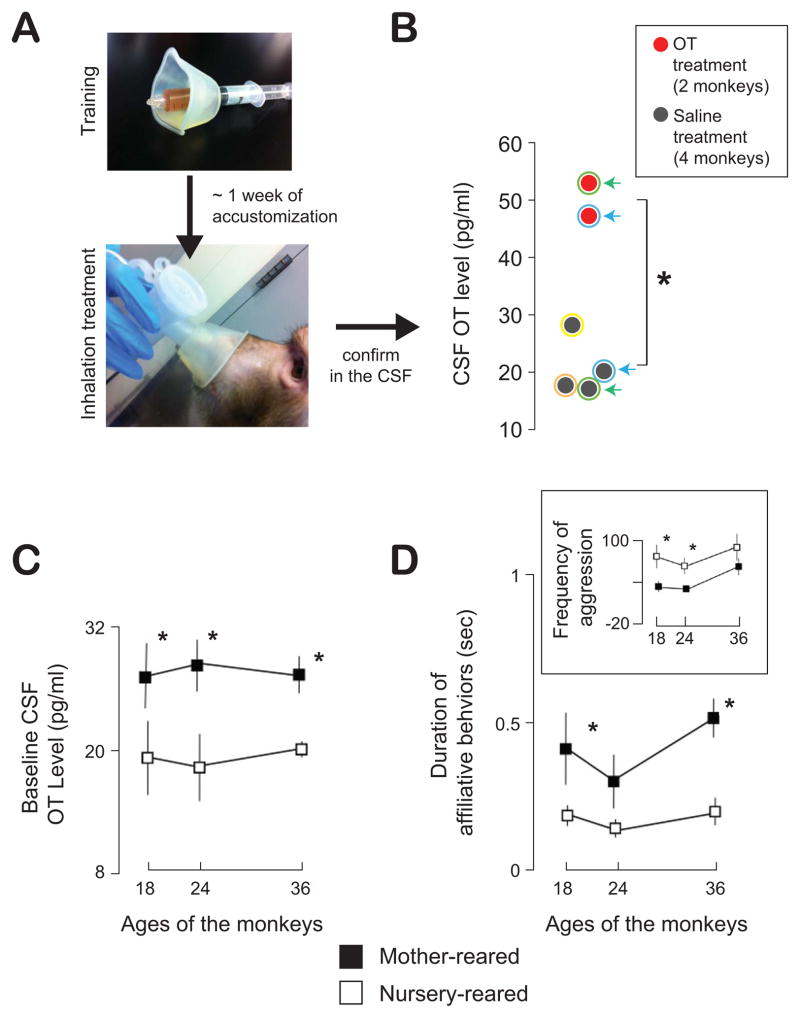
Numerous human studies have demonstrated that intranasally administered OT can modulate complex social cognition. One of the most exciting findings from recent OT studies is that the peptide appears to rescue some social deficits in individuals with psychopathological conditions (for a review, see: Insel, 2010; Meyer-Lindenberg et al., 2011). The clinical and basic science communities are currently working together to translate basic OT research into useful and safe OT therapies for social disorders (Miller, 2013). Nevertheless, whether or not intranasal administration of OT actually translocates the peptide into the central nervous system (CNS) remained unknown until recently. In humans, the closest demonstration was for arginine vasopressin (AVP), another neurohypophysial hormone with social functions closely related to OT and differing in only two amino acids. Intranasally administered AVP effectively increases CNS AVP concentration for a long duration (> 80 min.) in a dose-dependent manner (Born et al., 2002). More recently, data from rhesus macaques demonstrated that aerosolized OT using a nebulizer system (Fig. 1A) effectively reaches the brain. Using a pediatric nebulizer, we recently showed that inhaled oronasal administration of OT increases its concentration in the cerebrospinal fluid (CSF), measured at 30 min post-delivery (Chang et al., 2012) (Fig. 1B). A recent microdialysis study in rats and mice has demonstrated that nasal administration of OT increases levels in the central nervous system (sampled from the amygdala and hippocampus), peaking 30 – 60 minutes from the time of nasal delivery (Neumann et al., 2013). Subsequent work in primates from another laboratory reported that the inhaled administration of aerosolized OT effectively elevates OT levels in the CSF in rhesus macaques, but the application of intranasal spray, which has been the standard method in studies in humans, does not (Modi et al., in press).
Figure 1.
OT concentration in the cerebrospinal fluid (CSF) and social development in rhesus macaques. A. A nebulizer-based inhalation setup using a nebulizer (PARI Baby Nebulizer) in rhesus macaques. Notice that both nose and mouth are covered with the inhalation mask. Top, a training phase in which juice rewards are delivered through the nebulizer mask (about a week). Bottom, a treatment phase in which aerosolized OT or saline solutions are delivered to the monkeys. B. OT concentration in the CSF after inhaling 25 IU of OT (red) or saline (dark gray) in monkeys (5 minutes of nebulization at a 5 IU/min). The CSF samples were obtained from cervical punctures at 30 min post-delivery. Colored outlines on data points identify individual monkeys. Arrows with matching colors emphasize the CSF OT concentrations following saline (baseline) and OT inhalations within the same two monkeys. *, P < 0.05, Welch two-sample t test. C. Different levels of baseline CSF OT for mother-reared and nursery-reared male rhesus macaques across 18, 24, and 36 months of age. *, P <0.05, ANOVA. D. Enhanced duration of affiliative behavioral engagements (e.g., allogrooming and reciprocal male mounting) in mother-reared compared to nursery-reared monkeys. The inset shows reduced frequency of aggressive behaviors toward conspecifics for mother-reared compared to nursery-reared monkeys. *, P<0.05, ANOVA. [B adapted from: Chang, S.W.C., Barter, J.W., Ebitz, R.B., Watson, K.K., and Platt, M.L. (2012. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. 109, 959–964, Copyright (2012) National Academy of Sciences, U.S.A.; C, D adapted from: Winslow, J.T., Noble, P.L., Lyons, C.K., Sterk, S.M., and Insel, T.R. (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28, 910–918, Copyright (2003) Nature Publishing Group]
Anecdotally, monkeys readily accept and tolerate nebulization (e.g., training takes less than a week), a technique that is routinely used in babies and young children to administer drugs like albuterol to alleviate breathing difficulties, suggesting that this method may prove both effective and acceptable in young patients with neuropsychiatric disorders. This tolerability is particularly desired for an early OT intervention in young children or even infants. Further research will be needed to confirm whether the enhanced efficacy of oronasal nebulization of OT translates to humans. The outcome of such investigations will have a critical impact on how OT is administered to individuals in clinical settings as well as how peripheral versus central effects of OT on social cognition are understood.
Although effective, non-invasive, and easy to administer, it is virtually impossible to determine the precise amount delivered to the brain using intranasal or inhaled administration. During the intranasal or inhalation process, some amount of OT is bound to be absorbed peripherally or simply leak out. This limitation of intranasal and inhalation delivery may limit the attractiveness of OT therapy if it requires a precise dose to be effective and safe. Future studies in animals should focus on estimating, as accurately and reliably as possible, the amount of OT delivered versus not delivered to the brain following intranasal or inhalation administration.
OT is critical for normal social development in rhesus macaques
Like humans (Hinde, 1974), the development of social behavior in infant rhesus monkeys depends critically on how they are raised. Infant monkeys raised away from their mothers later show a number of social deficits and stress-related abnormalities (Champoux et al., 1992; Harlow et al., 1965; Sackett, 1984; Suomi, 1991; Winslow et al., 2003). Based on the importance of OT in mother-infant relationships and bonding-related behaviors, such as mutual gaze, vocalizations, and affiliative touching (Feldman et al., 2007; Francis et al., 2000; Galbally et al., 2011; Pedersen, 1997; Riem et al., 2011; Strathearn et al., 2009), it seems likely that the peptide plays a crucial role in the early development of social behavior. Indeed, OT seems to shape the social behavior of both mothers and their infants. Evidence suggests that individual differences in maternal behaviors (e.g., attachment styles) are linked to oxytocinergic systems in reward-related brain regions, such as ventral striatum and amygdala (Francis et al., 2000; Strathearn et al., 2009). In rhesus macaques, baseline CSF OT levels, but not CSF AVP levels, are significantly reduced in males raised in a nursery compared to peers raised by their mothers (Winslow et al., 2003) (Fig. 1C), confirming a specialized role of OT in early social development. Furthermore, mother-reared monkeys display substantially more affiliative behaviors (e.g., allogrooming and reciprocal male mounting behaviors) (Fig. 1D) and less aggressive behaviors (Fig. 1D inset) toward other individuals, suggesting a link between CNS OT levels and affiliative social tendencies developed later in their lives (18 – 36 months of age) (Winslow et al., 2003). Similarly, human children who are neglected by their parents immediately after birth also show significantly reduced urinary OT levels later on (on average age of 4.5 years old) (Wismer-Fries et al., 2005). It remains to be determined whether urinary OT levels are directly associated with behaviors mediated by the central oxytonergic system. Overall, one needs to be cautious in interpreting these results since some variables other than early developmental style could be driving the changes in either central or peripheral OT levels. Furthermore, it is likely that the hypothalamic–pituitary–adrenal (HPA) system may contribute to the effects of different rearing conditions, resulting in changes in both social behavior and OT function. Nevertheless, OT levels (present either in the central or periphery system) are correlated with long-lasting social behaviors shaped from the very first encounters between infants and mothers. These findings strongly endorse the initiation of OT-based therapies as early as possible (using a tolerable nebulizer method) after detection of a neuropsychiatric disorder with social deficits, such as autism.
OT amplifies intrinsic social motivation in rhesus macaques
Much of our social interactions are driven by reinforcement, both direct rewards we receive ourselves and the reward we experience when good things happen to others (i.e., vicarious reinforcement (Bandura et al., 1963; Berber, 1962)). How might OT in the CNS shape both self and vicarious reinforcement during social decisions? We recently developed a social reward-allocation paradigm in rhesus macaques (Chang et al., 2011), in which an actor monkey makes a series of decisions to deliver juice rewards to himself, to a recipient monkey present in the room, to both simultaneously, or to no one. When given a choice between rewarding self and rewarding self and the other monkey together at no additional cost, actor monkeys prefer to deliver juice rewards to themselves only, displaying an antisocial preference (Chang et al., 2011). Moreover, not surprisingly, when given a choice between rewarding themselves only and the recipients only, the actor monkeys prefer to deliver juice almost exclusively to themselves (Chang et al., 2012, 2013a). On the other hand, when choosing between rewarding the other monkey and rewarding no one, the same actor monkeys prefer to deliver juice rewards to the recipient monkey, demonstrating an other-regarding preference that in humans would be considered prosocial (Chang et al., 2011, 2012, 2013a).
Oronasal nebulization of OT amplifies these self-regarding and other-regarding preferences in rhesus macaques (Chang et al., 2012). When choosing between allocating juice rewards to a recipient monkey and no one at all, exogenous OT enhances the baseline prosocial bias (Fig. 2A). Amplified prosocial preference is accompanied by an increase in gaze shifts to the recipient monkeys’ face region following OT (Fig. 2B). Critically, the OT-treated actors do not increase such gaze shifts to the recipient monkeys when the juice rewards are delivered to the recipients by the experimenter (i.e., cued condition) (Chang et al., 2012). These OT-mediated gaze patterns during active decision-making suggest a link between reinforcing action and observation of the rewarding experience of the recipient monkeys. Such OT-mediated increases in attention to social stimuli like eyes and faces have been well documented in healthy humans as well as those with autism spectrum disorders (e.g., Andari et al., 2010; Guastella et al., 2008).
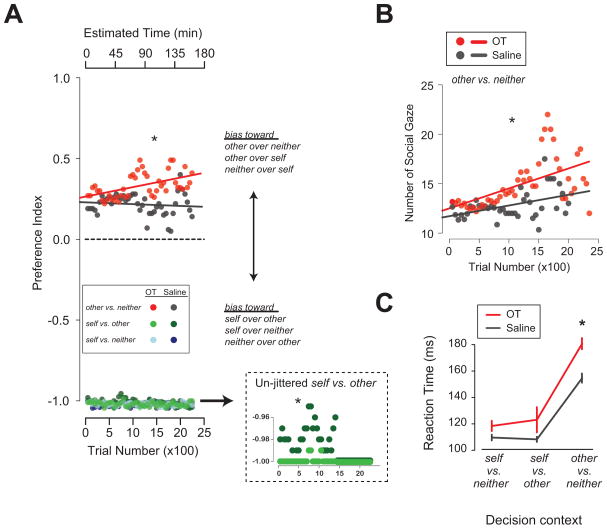
Figure 2.
OT promotes social motivation in rhesus macaques. A. Choice preference index following inhaled OT (red) or saline (gray) administration for rewards delivered to: other (recipient monkey) versus (vs.) neither, self (actor) vs. other, and self vs. neither in the social reward-allocation task. Data points from self vs. other and self vs. neither are jittered on the left plot for visibility. The inset shows unjittered data from self vs. other trials. *, P < 0.05, Welch two-sample t test. B. Number of gaze shifts made to the recipient monkey after reward delivery over the course of each session on other vs. neither trials. *, P < 0.05, Welch two-sample t test. C. OT selectively increases the decision deliberation time in the other vs. neither context (choosing to deliver juice rewards to other or no one) in which actor rhesus monkeys show a preference for delivering juice rewards to another monkey. *, P < 0.05, Welch two-sample t test. [A – C adapted from: Chang, S.W.C., Barter, J.W., Ebitz, R.B., Watson, K.K., and Platt, M.L. (2012). Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. 109, 959–964, Copyright (2012) National Academy of Sciences, U.S.A.]
Furthermore, inhaled OT significantly increases reaction times when the actor monkeys choose between donating juice rewards to the recipient monkey and no one (Chang et al., 2012) (Fig. 2C), inviting the possibility that OT promotes prosocial choices by increasing internal deliberative processing in rhesus macaques. Such increased deliberation processes might be necessary for enhancing prosocial behaviors in highly despotic rhesus macaques, compared to humans who seem to spontaneously prefer, and thus show faster reactions times for, prosocial decisions (Rand et al., 2012). These results suggest that OT enhances vicariously reinforcing actions by possibly coupling reinforcement and social observation.
By contrast, when choosing between delivering juice rewards to themselves and to the recipients, inhaled OT amplifies the self-regarding preference (i.e., delivering juice to only themselves over only the recipients), essentially eliminating the small number of prosocial choices in this competitive context (Fig. 2A). Therefore, as in humans (Bartz et al., 2011), OT seems to elicit context-specific social behaviors in rhesus macaques. OT-mediated enhancement of social preferences in rhesus macaques is consistent with the effects of OT manipulation on prosocial choices in pair-bonding marmosets (Callithrix penicillata). In that study, treatment with an OT receptor antagonist effectively eliminated species-typical food sharing behavior between paired male and female marmosets (Smith et al., 2010). Taken together, these observations are consistent with the hypothesis that OT regulates the gain of pre-existing social preferences rather than changing their fundamental character.
OT relaxes social vigilance, thereby permitting social exploration in rhesus macaques
One way to promote social interactions is by modulating the social state of an animal in order to encourage social exploration. To investigate the role of OT in modulating the social state in rhesus macaques, our group has recently investigated social vigilance behavior in male monkeys following OT inhalation (Ebitz et al., 2013). When monkeys choose whether to acquire different types of visual information about the local social context (viewing different social images), OT selectively reduces species-typical tendencies to view the faces of dominant monkeys, a threatening but highly informative stimulus (Fig. 3A). Inhaled OT also eliminates the privileged processing for dominant faces over other images, effectively slowing monkeys down when making this particular decision (Fig. 3B). Moreover, OT substantially attenuates species-typical distraction by the peripheral flash of images of unfamiliar monkey faces, indexed by a reduction in gaze deflection towards them (Fig. 3C). These findings endorse the idea that OT helps regulate species-typical social vigilance. Reducing social vigilance state in turn could free up cognitive resources and promote social exploration (Ebitz et al., 2013).
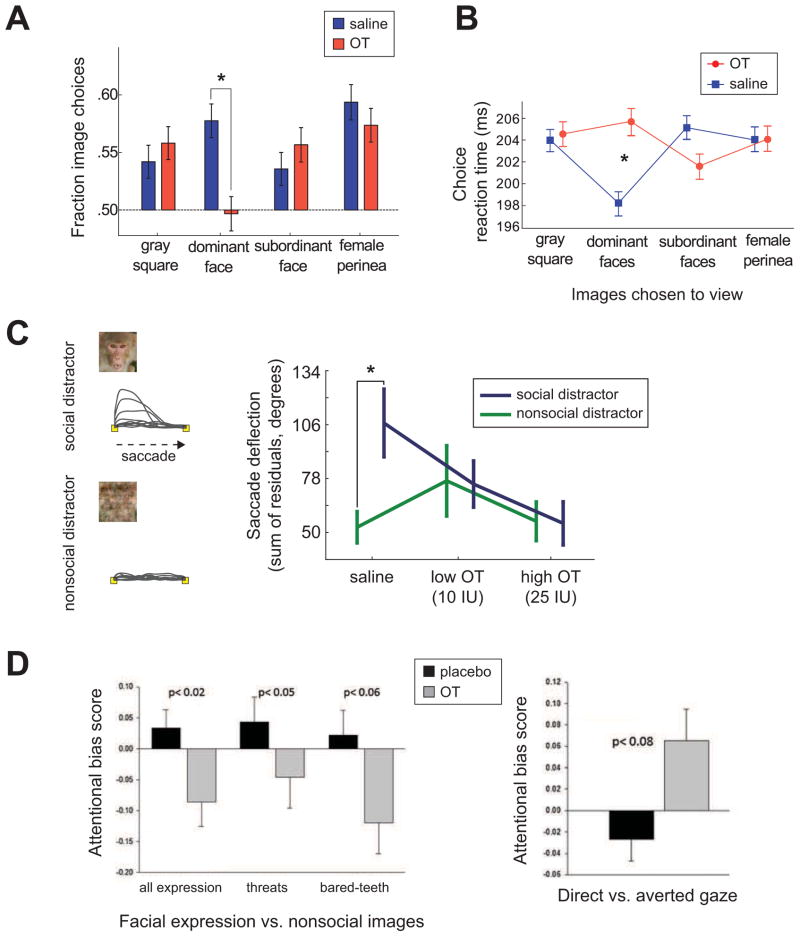
Figure 3.
OT regulates social vigilance in rhesus macaques. A. Inhaled OT selectively reduces monkeys’ species-typical choices to view dominant face images in a task in which monkeys choose to view the images of dominant high status monkeys, subordinate low status monkeys, female perinea, or gray square (Deaner et al., 2005). *, P < 0.05, Tukey LSD. B. Accompanied by the reduction in monkeys’ species-typical choices to view dominant face images (Fig. 3A), OT selectively eliminates typical reaction time facilitation of choosing to view dominant monkey faces. *, P < 0.05, post hoc LSD. C. Left, Examples of saccade residuals from trials with social distractors (upper) and nonsocial distractors (lower) presented at a neutral location relative to a target. Right, OT selectively reduces the typical saccade interferences (measured by the magnitude of deflections) to social distractors. *, P < 0.01, Bonferroni-corrected post doc Tukey LSD. D. Left, Attentional bias scores for facial expression stimuli categories after inhaled OT or placebo. Right, attentional bias scores for direct versus averted gaze trials after inhaled OT or placebo. OT reduces attention to emotional face stimuli (left), whereas it shows a trend of enhancing attention to facial stimuli with direct gaze (right). [A – C adapted from: Ebitz, R.B., Watson, K.K., and Platt, M.L. (2013). Oxytocin blunts social vigilance in the rhesus macaque. Proc. Natl. Acad. Sci. 110, 11630–11635, Copyright (2013) National Academy of Sciences, U.S.A.; D adapted from: Parr, L.A., Modi, M., Siebert, E., and Young, L.J. (2013). Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology 38, 1748–1756, Copyright (2013) Elsevier]
Consistent with the role of OT in reducing social vigilance state, another recent study in rhesus macaques reported that inhaled OT selectively reduces attention to emotional facial expressions while enhancing attention to faces with direct gaze (Parr et al., 2013) (Fig. 3D), which is a threatening gesture in macaques. Moreover, OT delivered intranasally to squirrel monkeys (Suimiri sciureus) attenuates stress responses by lowering adrenocorticotropic hormone (ACTH) (i.e., corticotropin) levels following 90 min of social isolation (Parker et al., 2005). Such OT-mediated reduction in ACTH levels suggests that OT regulates social stress by acting through the hypothalamic-pituitary-adrenal (HPA) axis. Taken together, these findings suggest that OT may facilitate social interactions by lowering social vigilance and reducing social stress (Carter, 1998; Chang et al., 2013b; Ebitz et al., 2013; Neumann et al., 2000b; Uvnäs-Moberg et al., 1994).
Following intranasal OT (Syntocinon spray, Novartis), humans spend more time visually inspecting the eye region of faces compared to placebo (i.e., all inactive ingredients except for the peptide) (Gamer et al., 2010; Guastella et al., 2008). Moreover, intranasal OT improves test scores for the Reading the Mind in the Eyes Test (RMET) (Domes et al., 2007), which requires participants to infer mental states from photographs of eyes (Baron-Cohen et al., 2001). Intranasal OT also increases attention to the eyes in individuals with social deficits. Individuals with autism, for example, spend more time attending to the eye region of faces following intranasal OT compared to placebo (Andari et al., 2010). These results in humans may at first appear contradictory to the social vigilance results from rhesus monkeys (Ebitz et al., 2013; Parr et al., 2013). Although exogenous OT reduces social vigilance to social threats in rhesus macaques (Fig. 3A), it increases the amount of time rhesus spend looking at faces and eyes, as in humans (see Discussion in Ebitz et al., 2013). Moreover, when deciding whether or not to allocate juice rewards to another monkey present in the room, inhaled OT increases gaze to the face of the recipient monkey (Fig. 2B) (Chang et al., 2012). Based on these results, we conjecture that reduced vigilance might play a permissive role in social approach behavior, favoring increased attention to faces and eyes and subsequent enhancements in social cognition. Further studies will be necessary to test the idea that OT plays a permissive, rather than promotional, role in social behavior in rhesus macaques, and perhaps humans as well.
Increasing social exploration should have obvious consequences for forming and maintaining social bonds. Research in non-human primates as well as in humans (Bartz et al., 2011; De Dreu, 2012; Guastella et al., 2012; Heinrichs and Domes, 2008; Insel, 2010; MacDonald and Feifel, 2013; Meyer-Lindenberg et al., 2011) suggests that OT might be an important component of the neuroendocrinological regulation of social relationships. In non-human animals, social relationships can be studied by examining naturally-occurring social bonds (Brent et al., in press, 2013). A recent study in wild chimpanzees (Pan troglodytes) measured urinary OT levels after grooming bouts with different partners (Crockford et al., 2013). urinary OT levels after grooming behaviors predicted the strength of social bonds among the partners, and, surprisingly, this effect was not explained by genetic relatedness. Therefore, the action of OT in social processing seems to critically depend on the history of social interactions between two individuals. Future studies examining how prior social experience influences neural circuits regulated by OT should reveal important new insights into the neural basis of social relationships.
OT receptor distribution in the brain
Current knowledge regarding the distribution of OT receptors in the mammalian brain is derived primarily from rodents, though there have been some studies in humans and monkeys. In rats, some of the regions with high densities of OT receptor include the olfactory nucleus, hypothalamic regions, and the central amygdala (Tribollet and Barberis, 1996; Tribollet et al., 1992). Importantly, varying levels of OT receptors were detected across many functional subsystems, such as the limbic, basal ganglia, cortical, brainstem and the spinal cord (Tribollet et al., 1992). Autoradiographic studies have demonstrated that the distributions of OT receptors differ greatly between monogamous prairie voles (Microtus ochrogaster) and polygamous montane voles (Microtus montanus), in a manner consistent with differences in social bonding between these species (Insel and Shapiro, 1992; Young and Wang, 2004). Compared to montane voles, OT receptors in prairie voles are densely distributed in prelimbic areas, including a region analogous to the primate anterior cingulate gyrus, the nucleus accumbens, the bed nucleus of stria terminalis, and the lateral amygdala (Insel and Shapiro, 1992; Young et al., 1996). In human and non-human primates, immunoreactive studies have yielded mixed results with respect to finding OT receptors in the same areas as in rodents (Caffé et al., 1989; Jenkins et al., 1984; Sofroniew et al., 1981; Wang et al., 1997). Similar to findings in rats (Audigier and Barberis, 1985; Tribollet et al., 1992), OT immunoreactive cells were found in the hypothalamus, the bed nucleus of the stria terminalis, and the medial amygdala in common marmosets (Callithrix jacchus) (Wang et al., 1997), the new world primates that pair-bond similar to prairie voles. Nevertheless, studies in human and non-human primates have also found some notable differences in OT receptor distributions compared to rodents (Boccia et al., 2013; Caffé et al., 1989; Jenkins et al., 1984; Loup et al., 1991). A recent immunohistochemical study in postmortem human brains localized OT receptors in the central and basolateral amygdala, medial preoptic area, hypothalamus, anterior cingulate cortex, and ventrolateral septum, among others (Boccia et al., 2013). In contrast to rat brains, the authors did not localize OT receptors in the hippocampus, supraoptic nucleus, and nucleus accumbens, among others, in their human brain tissues (Boccia et al., 2013). Clearly, resolving the distribution and binding affinities of OT receptors in human and non-human primate brains is a high priority and will require development of new ligands for OT receptors. Despite differences between species and these methodological limitations, it is interesting to note that some regions appear to express OT receptors across species, notably the amygdala. In the next section, we review current understanding of the role of the amygdala in OT-mediated social cognition.
Amygdala and context-specific OT-mediated modulations
The amygdala, which possesses an unusually high density of OT receptors (Francis et al., 2000; Insel and Shapiro, 1992; Young et al., 1996), may be a key site through which OT influences social behavior. The specialized functions of the amygdala in social cognition has been extensively reported in healthy humans and amygdala-damaged human patients (for a review, Adolphs, 2010). Experimental lesions to the amygdala in rhesus macaques lead to stereotyped social deficits, such as reduced aggression and increased submission (Amaral, 2002; Dicks et al., 1969; Kling, 1972; Kluver and Bucy, 1939; Meunier et al., 1999; Rosvold et al., 1954). Furthermore, neonatal bilateral amygdala lesions in rhesus macaques result in blunted affective expressions in adulthood toward both positive and negative stimuli (Bliss-Moreau et al., 2011). Moreover, neurons in the primate amygdala signal motivational values and is causally implicated in reward-guided learning (Baxter and Murray, 2002; Paton et al., 2006). These findings place the amygdala as an ideal anatomical substrate for the OT-mediated processing of motivation and vigilance state for guiding social behavior.
To date, most of the OT research examining the role of the amygdala in social cognition has focused on, broadly speaking, social state modulations. Human functional neuroimaging studies have shown that inhaling OT reduces BOLD (blood-oxygen-level dependent) activation in the amygdala when viewing or associating aversive social stimuli (Kirsch, 2005; Petrovic et al., 2008), during trust formation (Baumgartner et al., 2008), in response to pain (Singer et al., 2008), and in response to infant crying (Riem et al., 2011). Furthermore, OT inhalation reduces amygdala activation when viewing fearful faces but increases amygdala activation when viewing happy faces (Gamer et al., 2010). These findings suggest that OT modulates context-specific processing associated with both negative and positive valence in the amygdala, consistent with the encoding of both positive and negative motivational values by the primate amygdala neurons (Belova et al., 2007; Paton et al., 2006). For mediating fear-related responses, it has been verified in rats that oxytocinergic neurons regulate autonomic fear responses through a specialized circuit via the central amygdala (Huber, 2005; Viviani et al., 2011). This OT-mediated autonomic circuit might be also utilized for regulating social vigilance. Whether there are parallel oxytocinergic circuits for social motivational processing in the amygdala (e.g., in the basolateral subdivisions) remains unknown. Taken together, these findings suggest that OT may promote social exploration through modulations in socioemotional processing within the amygdala and its interconnected limbic circuitry.
Interestingly, one study on human participants found that the BOLD signals in the amygdala in women, unlike in men, actually increase when viewing fearful faces (Domes et al., 2010). One possible mechanism for this gender-specific effect of OT in amygdala activations is the interactions between oxytocin receptors and gonadal steroid hormones (Domes et al., 2010). It has been shown that estradiol promotes oxytocin receptor binding by increasing the oxytocin receptor concentrations, whereas progesterone inhibits oxytocin receptor by repressing its synthesis (Choleris et al., 2008; Gimpl et al., 2002; Nissenson et al., 1978). Interactions between OT and gonadal steroids have also been reported in monkeys as well. In male squirrel monkeys, ventricular injection of OT enhances sexual and aggressive behaviors toward a familiar female monkey in dominant males who have high testosterone levels, but enhances affiliative behaviors like touching and huddling in subordinate males who have low testosterone levels (Winslow and Insel, 1991). Such gender-specific effects of OT have been documented numerously in prairie voles (Microtus ochrogaster), and are verified to be centrally-mediated based on ventricular peptide manipulation experiments. For instance, the context-specific role of OT in pair-bonding and mate-guarding aggression is more critical to female voles, whereas the role of AVP in these two types of behaviors is more critical to male voles (Bales and Carter, 2003; Cushing and Carter, 2000; Insel and Hulihan, 1995; Winslow et al., 1993; Young and Wang, 2004). Further research is required to understand how and where in the brain OT system interacts with sex hormones to differentially influence social cognition in men and women. These interactions may govern the type of social states that is encouraged by OT in a context-specific fashion.
Caveats in developing OT therapy for social disorders
Without doubt, more research is necessary to fully and safely develop and implement OT treatment protocols for social deficits. In particular, determining the efficacy and safety of chronic OT treatment is a top priority because the majority of OT studies to date have focused on establishing the effects of acute OT on specific social functions. Unfortunately, chronic studies are practically difficult and sometimes ethically questionable in human subjects. For these reasons, we believe that non-human primates provide suitable models for monitoring the long-term safety and efficacy of repeated OT administration, both behaviorally and physiologically. Using non-human primates, researchers can administer OT at a regular interval (e.g., daily or weekly) in the same subjects over a long time period (e.g., months or years) to estimate the efficacy and safety of repeated OT treatments in humans. It is also feasible to record neuronal activity or measure hemodynamic responses regularly over time in such studies in order to evaluate efficacy and safety at the neurophysiological level. Furthermore, it is also feasible to conduct other physiological measurements every month to assess inflammatory processes, including urine and blood analysis to measure cytokine and C-reactive protein levels.
Another line of future research should focus on the potential side effects of repeated exogenous OT exposure. In some cases, OT has been shown to evoke antisocial behaviors, such as envy (Shamay-Tsoory et al., 2009), negative out-group bias (De Dreu et al., 2011), negative social perception in individuals with anxious attachment (Bartz et al., 2010), declined social interactions (Bales and Carter, 2003), as well as aggressive behaviors, such as mate-guarding aggression displayed by female voles toward other females (Bales and Carter, 2003). These important studies support the idea that the effects of OT depend critically on behavioral context (Bartz et al., 2011). Future studies thus need to determine the neurobiological mechanisms responsible for antisocial or aggressive behaviors elicited by OT. Neuronal recording studies in non-human primates designed to evoke their natural prosocial or antisocial behaviors following OT infusion will provide important insights into the underlying neural mechanisms, which can be refined by additional studies using molecular genetic techniques in rodents. It is also important to recognize the possibility that OT may acutely promote positive social behavior but may later promote negative social behaviors after repeated or long-term administration.
Concluding remarks
A rhesus macaque model of OT research has a clear translational value for understanding and treating neuropsychiatric disorders with social deficits. Most importantly, it can provide a unique opportunity for investigating how OT mediates human-like social cognition at both the neural circuit level and the level of the whole animal. The social behaviors of rhesus macaques are remarkably similar to those of humans (Maestripieri, 2007; Smuts, 1987). Arguably, most of the rudimentary forms of complex human cognition are present in these animals. Coupled with complex social behaviors, the amenability to single-unit recording and pharmacological perturbations permits scientists to directly examine the neural mechanisms mediating OT influences on social cognition. Furthermore, rhesus macaques are also tolerant to controlled experiments with an extremely high number of repeated trials (~1000 trials per day) allowing precise quantifications of behaviors as well as how these behaviors are related to underlying neuronal activity, which by nature is often highly variable. The efficacy of the nebulized inhalation method for delivering OT to the CNS in alert rhesus macaques prior to experimental tasks (Chang et al., 2012; Modi et al., in press) makes it easier to compare neurophysiological results from animal studies with behavioral or neuroimaging results obtained from both healthy people and those with neuropsychiatric disorders. Finally, non-human primates will be valuable for monitoring the efficacy and safety of repeated OT administrations continuously for months and even years at the behavioral as well as neuronal level in a controlled setting.
Acknowledgments
This work was supported by K99-MH099093 (S.W.C.C.) and R01-MH086712 (M.L.P. and S.W.C.C.) from National Institute for Mental Health, and W81XWH-11-1-0584 from Department of Defense (M.L.P. and S.W.C.C.).
Footnotes
Author Contributions
S.W.C.C and M.L.P. wrote the paper.
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and Stress Responses in Female Oxytocin Deficient Mice. J Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audigier S, Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO J. 1985;4:1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci. 2010;107:9033–9034. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bandura A, Ross D, Ross SA. VICARIOUS REINFORCEMENT AND IMITATIVE LEARNING. J Abnorm Psychol. 1963;67:601–607. doi: 10.1037/h0045550. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Barraza JA, McCullough ME, Ahmadi S, Zak PJ. Oxytocin infusion increases charitable donations regardless of monetary resources. Horm Behav. 2011;60:148–151. doi: 10.1016/j.yhbeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci. 2010;107:21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation Modulates Neural Responses to Pleasant and Aversive Stimuli in Primate Amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berber SM. Conditioning through vicarious instigation. Psychol Rev. 1962;69:450–466. [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Brent LJN, Chang SWC, Gariépy J-F, Platt ML. Neuroethology of friendship. Year Cogn Neurosci in press. [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Skene JHP, Platt ML. Genetic origins of social networks in rhesus macaques. Sci Reports. 2013;3 doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne RW, Whiten A. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford; New York: Clarendon Press ; Oxford University Press; 1988. [Google Scholar]
- Caffé AR, Van Ryen PC, Van Der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux M, Byrne E, DeLizio R, Suomi SJ. Motherless mothers revisited: Rhesus maternal behavior and rearing history. Primates. 1992;33:251–255. [Google Scholar]
- Chang SWC, Winecoff AA, Platt ML. Vicarious Reinforcement in Rhesus Macaques (Macaca Mulatta) Front Neurosci. 2011;5 doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Gariépy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013a;16:243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Neuroethology of primate social behavior. Proc Natl Acad Sci U S A. 2013b;110(Suppl 2):10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc R Soc B Biol Sci. 2013;280 doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys Pay Per View: Adaptive Valuation of Social Images by Rhesus Macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Dicks D, Myers RE, Kling A. Uncus and amiygdala lesions: effects on social behavior in the free-ranging rhesus monkey. Science. 1969;165:69–71. doi: 10.1126/science.165.3888.69. [DOI] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin Improves “Mind-Reading” in Humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dreu CKWD, Greer LL, Handgraaf MJJ, Shalvi S, Kleef GAV, Baas M, Velden FST, Dijk EV, Feith SWW. The Neuropeptide Oxytocin Regulates Parochial Altruism in Intergroup Conflict Among Humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm Behav. 2012;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci U S A. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI. The social brain hypothesis. Brain. 1998;9:10. [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the Social Brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proc Natl Acad Sci. 2013;110:11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enger H, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a Neuroendocrinological Foundation of Human Affiliation: Plasma Oxytocin Levels Across Pregnancy and the Postpartum Period Predict Mother-Infant Bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal Imitation in Rhesus Macaques. PLoS Biol. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flombaum JI, Santos LR. Rhesus monkeys attribute perceptions to others. Curr Biol CB. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social Cognition in Humans. Curr Biol. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, van IJzendoorn M, Permezel M. The Role of Oxytocin in Mother-Infant Relations: A Systematic Review of Human Studies: Harv. Rev Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Wiegand V, Burger K, Fahrenholz F. Cholesterol and steroid hormones: modulators of oxytocin receptor function. Prog Brain Res. 2002;139:43–55. doi: 10.1016/s0079-6123(02)39006-x. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin Increases Gaze to the Eye Region of Human Faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Graustella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54:90. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Progress in Brain Research. Elsevier; 2008. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans; pp. 337–350. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Biological bases of human social behaviour. New York: McGraw-Hill; 1974. [Google Scholar]
- Huber D. Vasopressin and Oxytocin Excite Distinct Neuronal Populations in the Central Amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Huffmeijer R, Alink LRA, Bakermans-Kranenburg MJ, Tops M. The Impact of Oxytocin Administration on Charitable Donating is Moderated by Experiences of Parental Love-Withdrawal. Front Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JS, Ang VTY, Hawthorn J, Rossor MN, Iversen LL. Vasopressin, oxytocin and neurophysins in the human brain and spinal cord. Brain Res. 1984;291:111–117. doi: 10.1016/0006-8993(84)90656-5. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. Oxytocin: prosocial behavior, social salience, or approach-related behavior? Biol Psychiatry. 2010;67:e33. doi: 10.1016/j.biopsych.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Kirsch P. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A. The Neurobiology of the Amygdala. Springer US; 1972. Effects of Amygdalectomy on Social-Affective Behavior in Non-Human Primates; pp. 511–536. [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–000. [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Macachiavellian intelligence: how rhesus macaques and humans have conquered the world. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Masserman JH, Wechkin S, Terris W. “ALTRUISTIC” BEHAVIOR IN RHESUS MONKEYS. Am J Psychiatry. 1964;121:584–585. doi: 10.1176/ajp.121.6.584. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miller G. The Promise and Perils of Oxytocin. Science. 2013;339:267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases plasma and cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2014.02.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000a;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000b;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nissenson R, Flouret G, Hechter O. Opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc Natl Acad Sci. 1978;75:2044–2048. doi: 10.1073/pnas.75.4.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38:1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DG, Greene JD, Nowak MA. Spontaneous giving and calculated greed. Nature. 2012;489:427–430. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MAS, Vermeiren RRJM, van IJzendoorn MH, Rombouts SARB. Oxytocin Modulates Amygdala, Insula, and Inferior Frontal Gyrus Responses to Infant Crying: A Randomized Controlled Trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Sackett GP. A nonhuman primate model of risk for deviant development. Am J Ment Defic. 1984;88:469–476. [PubMed] [Google Scholar]
- Santos LR, Nissen AG, Ferrugia JA. Rhesus monkeys, Macaca mulatta, know what others can and cannot hear. Anim Behav. 2006;71:1175–1181. [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal Administration of Oxytocin Increases Envy and Schadenfreude (Gloating) Biol Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, Petrovic P, Silani G, Heinrichs M, Dolan RJ. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8:781–791. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts BB. Primate societies. Chicago: University of Chicago Press; 1987. [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Weindl A, Schrell U, Wetzstein R. Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta Histochem Suppl. 1981;24:79–95. [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subiaul F. Cognitive Imitation in Rhesus Macaques. Science. 2004;305:407–410. doi: 10.1126/science.1099136. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Child Environ Adult Dis. 1991:171–188. doi: 10.1002/9780470514047.ch11. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C. Vasopressin and Oxytocin Receptors in the Central Nervous System. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin Receptors in the Central Nervous System. Ann N Y Acad Sci. 1992;652:29–38. doi: 10.1111/j.1749-6632.1992.tb34343.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin Selectively Gates Fear Responses Through Distinct Outputs from the Central Amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synap New York N. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Watson KK, Platt ML. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord. 2012;4:21. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci. 1991;11:2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wismer-Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence That Oxytocin Exerts Anxiolytic Effects via Oxytocin Receptor Expressed in Serotonergic Neurons in Mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. Species Differences in Central Oxytocin Receptor Gene Expression: Comparative Analysis of Promoter Sequences. J Neuroendocrinol. 1996;8:777–783. doi: 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]