Abstract
Neuroinflammatory signaling pathways in the CNS are of current interest as potential pharmacotherapy targets for alcohol dependence. In this study, we examined the ability of ibudilast, a non-selective phosphodiesterase inhibitor, to reduce alcohol drinking and relapse in alcohol-preferring P rats, high-alcohol drinking HAD1 rats, and in mice made dependent on alcohol through cycles of alcohol vapor exposure. When administered twice daily, ibudilast reduced alcohol drinking in rats by approximately 50% and reduced drinking by alcohol dependent mice at doses which had no effect in non-dependent mice. These findings support the viability of ibudilast as a possible treatment for alcohol dependence.
Keywords: Alcohol, alcohol dependence, alcohol preference, alcoholism, AV-411, ethanol, ibudilast, MN-166, phosphodiesterase, neuroimmune
Pathways engaging neuroinflammatory signaling in the CNS are of current interest as potential pharmacotherapy targets for alcohol dependence (Blednov et al. 2012; Litten et al. 2012). Indeed, neuroimmune modulation via microglial and astroglial cells may contribute to stress-induced drug reinstatement (Frank et al., 2011). Inhibitors of type-4 phosphodiesterase (PDE) are known for their anti-inflammatory effects in a variety of inflammatory cells, including glia. For instance, ibudilast (also known as MN-166 or AV-411), a non-selective PDE inhibitor, crosses the blood–brain barrier and suppresses TNF-alpha production or release as well as astrocyte and microglial activation (Wakita et al., 2003; Ledeboer et al., 2007). It currently is used in Japan for asthma and cerebrovascular disorders and is being developed in the United States for progressive multiple sclerosis, neuropathic pain, methamphetamine addiction and opiate addiction (Rolan et al., 2009). Here we report that ibudilast significantly reduces alcohol consumption in three different rodent models of high alcohol consumption.
We examined the ability of ibudilast (from MediciNova, but coded with unknown identity to investigators) to decrease voluntary ethanol consumption during a 2h, two-bottle choice (15% ethanol vs. water) test session, under blind testing conditions, in selectively-bred alcohol-preferring (P) and high-alcohol-drinking (HAD1) rats, and in a mouse model of ethanol dependence in which mice received repeated cycles of chronic intermittent ethanol (CIE) exposure (see Litten et al., 2012). Each model is characterized by elevated alcohol intake believed to result from biological mechanisms relevant to human alcohol dependence (Egli, 2005). As such, these models are sensitive to clinically effective drugs such as naltrexone and topiramate, whereas clinically ineffective medications such as quetiapine and levatiracetam do not reduce ethanol drinking selectively in these models (unpublished data).
Adult male P and HAD1 rats were assigned randomly to receive one of 4 ibudilast doses (0, 3, 6 or 9 mg/kg; n= 8/dose) with the groups balanced according to average 2h/day ethanol (15% v/v) intake. The doses were selected based on prior animal efficacy studies and to approximate and bracket human clinical PK parameters in MN-166 clinical development (Ledeboer et al., 2007, Rolan et al., 2008 and 2009). Water was concurrently available and Mazola® corn oil served as the drug vehicle. In the Maintenance Test phase, rats were injected subcutaneously (2 ml/kg; sc.) 60 min before each ethanol test session and again 8h later for 4 consecutive days. Following the Maintenance Test, recovery of ethanol drinking was evaluated for 2 weeks with no treatments given. Rats then were deprived of ethanol for 2 weeks and the effects of ibudilast on ethanol drinking were examined under the same conditions as the Maintenance Test Phase when ethanol was re-introduced for 5 consecutive days (i.e., the Relapse Test phase). Each animal received the same dose during both tests. Following this, recovery of ethanol drinking was evaluated for 2 weeks with no treatments administered.
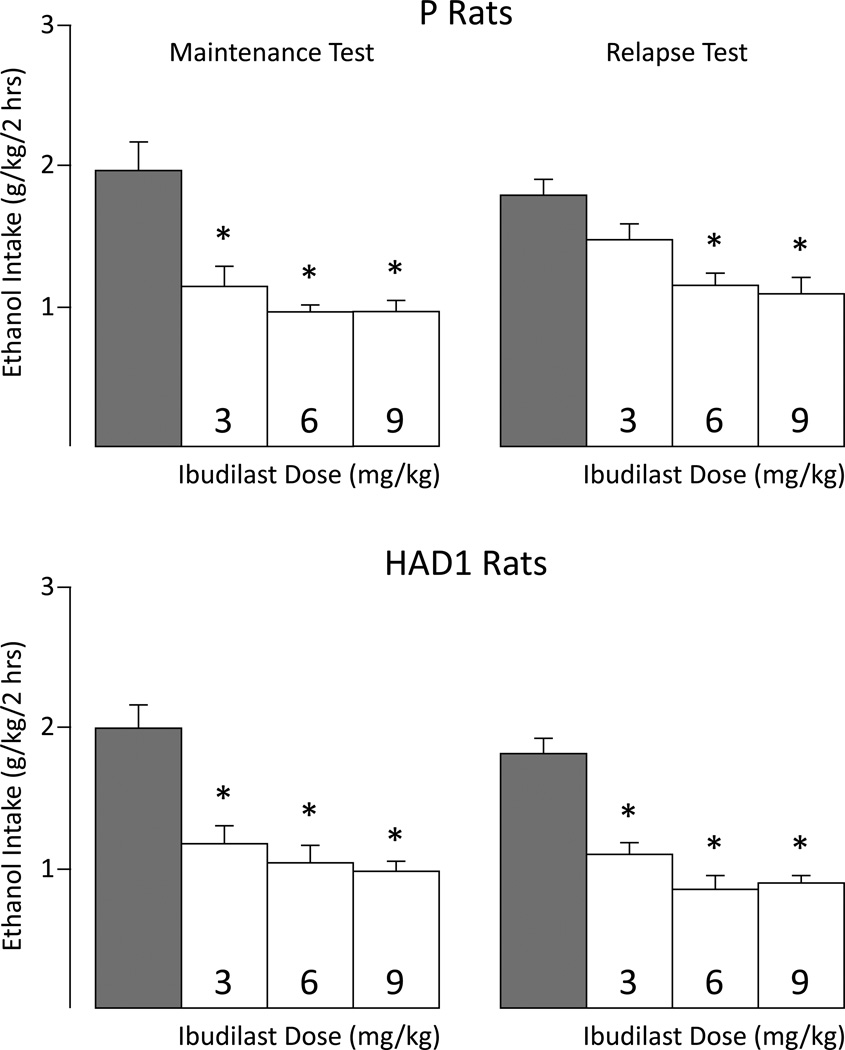
In the Maintenance Test, ibudilast reduced ethanol intake by approximately 50% in both P and HAD1 rats (Figure 1, left panel). Separate 2-way mixed ANOVAs revealed significant main effects of Dose for P [F(3,28) = 12.42, p < 0.001] and HAD1 [F(3,28) = 14.94, p < 0.001] rats. All doses reduced ethanol drinking over the 4-day test-phase relative to vehicle-injected controls (p’s ≤ 0.001). Ethanol drinking levels recovered in all groups on the first day following the test period (data not shown).
Figure 1.
Mean ethanol intake for P (upper panels) and HAD1 (lower panels) rats during the maintenance (left side of panel) and relapse (right side of panel) test-phases. * indicates the respective dose differed significantly from vehicle (p< 0.05). Grey bar indicates vehicle value.
Likewise, ibudilast reduced ethanol drinking in P and HAD1 rats by about 50% during the 5-day Relapse Test phase (Figure 1, right panel). Separate 2-way mixed ANOVAs revealed significant main effects of Dose for P rats [F(3,28) = 8.48, p < 0.001], with the 6 and 9 mg/kg doses significantly reducing ethanol drinking relative to vehicle controls (p < 0.05), and for HAD1 rats [F(3,28) = 25.80, p < 0.001], with all doses significantly reducing ethanol intake compared to controls (p < 0.05). Significant Dose×Day interactions in P [F(12,112) = 3.25, p < 0.001] and HAD1 [F(12,112) = 2.09, p = 0.023] lines indicated that the greatest effect of ibudilast occurred on the first day of administration (Figure S1). When ibudilast treatment was discontinued following the Relapse Test, ethanol drinking levels recovered by the second day.
In a separate study, adult male C57BL/6J mice were trained to drink ethanol in a 2h/day free-choice (15% v/v ethanol vs. water) drinking procedure and separated into dependent (EtOH) and nondependent (CTL) groups (N= 37–38/group). EtOH mice were exposed to chronic intermittent ethanol (CIE) vapor exposure (16h/day×4 days) using a well-established dependence procedure (Becker and Lopez, 2004; Griffin et al., 2009). Following a 72h forced abstinence period, mice were given 2h/day ethanol access for a 5-day test period. This pattern of weekly CIE exposures followed by 5-day test periods was repeated for 9 cycles. CTL mice were treated similarly, but were exposed to air in the inhalation chambers. All mice received injections (sc.) of the vehicle at 9- and 1-hr prior to the start of daily drinking sessions during Test Cycles 4, 5 and 6 to acclimate the animals to handling/injections. EtOH and CTL groups then were separated further into treatment conditions, with animals receiving one of 4 ibudilast doses (0, 3, 6 or 12 mg/kg) during Test Cycles 7 and 8 (n= 9–10/group). During Test Cycle 9, EtOH and CTL mice received one of 4 doses of ibudilast (0, 6, 12, 18 mg/kg) using the same treatment regimen. For Test Cycle 9, mice previously treated with the 3 mg/kg dose now were treated with the 18 mg/kg dose. One CTL mouse previously treated with 12 mg/kg ibudilast died for unknown reasons during Test Cycle 9 and, consequently, its data were removed from analyses.
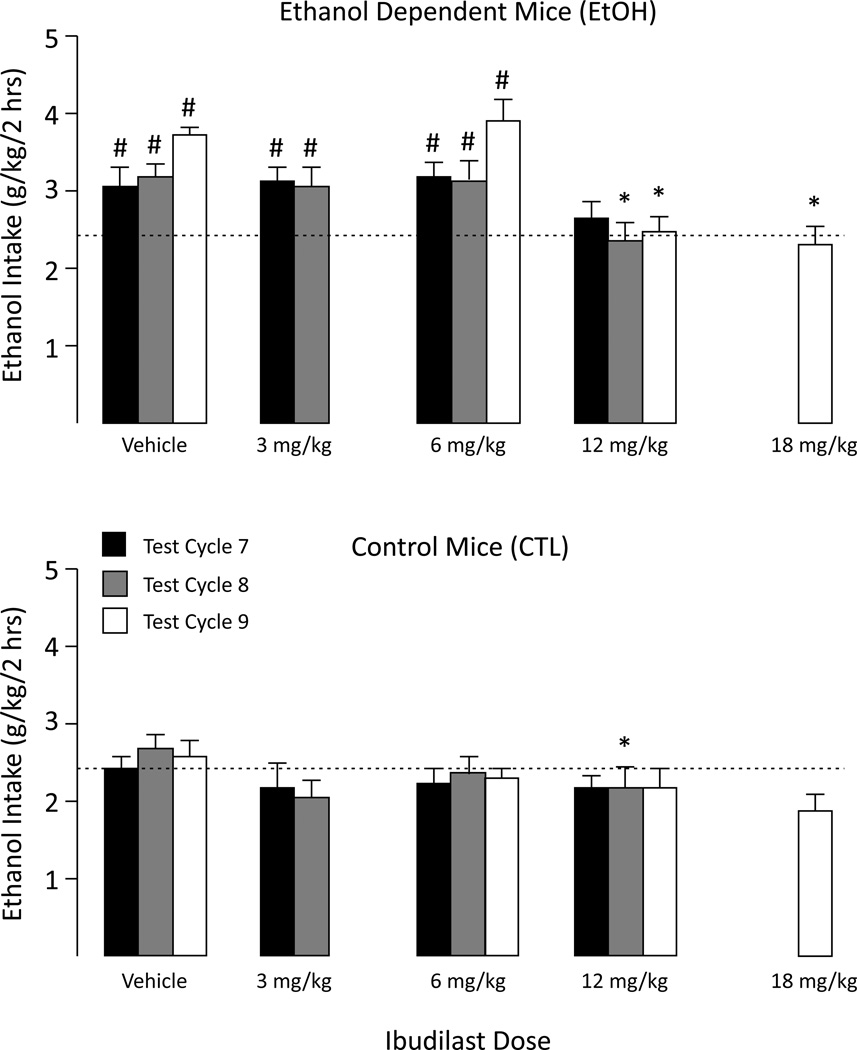
Ethanol intake escalated over successive CIE exposure cycles whereas ethanol consumption in CTL mice remained relatively stable throughout the study. That is, EtOH mice consumed significantly more ethanol than CTL mice starting at Test Cycle 6 (main effect of Group [F(1,67)=35.84, p<0.001), and this effect persisted during Test Cycle 7 [F(1,67)=21.80, p<0.001], Test Cycle 8 [F(1,67)=12.52, p<0.001], and Test Cycle 9 [F(1,66)=32.55, p<0.001]. In EtOH mice, ibudilast’s ability to reduce ethanol consumption appeared to increase with repeated testing cycles (and drug treatments). During Test Cycle 7 there was a trend for the highest dose of ibudilast (12 mg/kg) to reduce drinking in EtOH mice, but this effect did not achieve statistical significance. However, during Test Cycle 8, a significant main effect of Dose [F(3,67)=3.36, p<0.05] indicated that the 12 mg/kg dose significantly reduced ethanol intake in both EtOH and CTL groups relative to their respective vehicle conditions (p’s<0.05).
Results from Test Cycle 9 indicated that both the 12 and 18 mg/kg ibudilast doses significantly reduced ethanol intake in EtOH, but not CTL, mice (Group×Dose interaction: [F(3,66)=4.50, p<0.01]). Pair-wise comparisons indicated that EtOH mice treated with vehicle or 6 mg/kg ibudilast consumed significantly more ethanol than the respective CTL-treated groups (p’s<0.05). In contrast, there were no significant differences in ethanol intake between the EtOH and CTL groups treated with 12 or 18 mg/kg ibudilast; i.e., ibudilast reduced ethanol drinking in dependent (EtOH) mice to nondependent control levels (Figure 2). At all doses, ibudilast’s effects were greatest when first administered and then diminished over each 5-day test period (Figure S2).
Figure 2.
Mean ethanol intake for Chronic Intermittent Ethanol (EtOH) exposed mice and control (CTL) mice during Test Cycles 7, 8, 9. For comparison purposes, the horizontal dashed line indicates the mean ethanol intake by vehicle-treated CTL mice during test cycle 7. * indicates significantly less ethanol intake compared to vehicle-treated mice (p< 0.05). # indicates significantly greater intake than CTL group (p< 0.05).
The present study revealed that ibudilast reduced ethanol intake in three different rodent models of alcohol-dependence. Enhanced or selective reduction of alcohol drinking in EtOH (dependent) mice to CTL (nondependent) levels as observed in Test Cycle 9 (Figure 2) may indicate that the drug is targeting processes underlying the development and/or expression of alcohol dependence in particular (Rimondini et al. 2002). Such effects have been observed for clinically effective drugs such as acamprosate as well as for CRF1, NK1, and kappa opioid receptor antagonists (Heilig et al. 2010). It is unlikely that decreased ethanol intake observed in this study resulted from a general suppression of ingestive behavior. For example, ibudilast reduced ethanol drinking in EtOH mice at doses that did not affect drinking in CTL mice. We did not record water intake in mice because fluid intake during the 2h sessions is, for the most part, limited to ethanol, with water intake being negligible (unpublished observations). In addition, there were no systematic changes in water intake for P or HAD1 rats during the 2h test sessions following ibudilast administration except for occasional statistically significant increases (see Tables S1 and S2). Nevertheless, ibudilast, especially at the 9 mg/kg dose, produced transitory reductions in 24h food, but not water, intake in P and HAD1 rats. This effect diminished over 5 days and did not result in reduced body weight. Thus, reduced ethanol drinking following ibudilast administration appears to be independent of this effect.
Consistent with our study, other groups have demonstrated that the type-4 PDE inhibitor, rolipram, decreased alcohol intake in mice and rats (Hu et al. 2011; Wen et al., 2012). Ibudilast’s other known molecular target, aside from PDE’s-3,4,10,11 (Gibson et al., 2006) is macrophage migration inhibitory factor (MIF) which has been shown in model systems to contribute to neuroinflammatory and neurodegenerative conditions (Cho et al., 2010; Kithcart et al., 2010). Given the possible presence of these conditions in many chronic alcoholics (see Crews et al. 2011, 2013; Qin and Crews, 2012), future studies should examine whether treatment with this compound ameliorates some of the deleterious consequences of chronic alcoholism. Additional preclinical studies delineating the precise mechanisms through which ibudilast reduces alcohol drinking in these animal models are warranted, as are studies in additional animal models. The present results suggest initial efficacy studies in alcohol dependent patients are justified to confirm the possible utility of PDE inhibitors, such as ibudilast, in treating alcohol dependence.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health contracts HHSN267200700037C and HHSN267200700038C from the National Institute on Alcohol Abuse and Alcoholism. We wish to thank Malath Makhay for her expert project support and critical reading of the manuscript.
Footnotes
Author Contributions
RLB, ME, KWJ and HCB were responsible for the study design. RLB, MFL, KMF, and HCB collected the data, performed data analysis and interpreted the data. RLB, ME, CC, KWJ and HCB interpreted findings and drafted the manuscript. All authors critically reviewed content and approved the final version of the manuscript.
References
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci USA. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25(Suppl 1):S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases voluntary ethanol drinking and ethanol concentration in the nucleus accumbens. Psychopharmacology. 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology. 2011;218:331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kithcart AP, Cox GM, Sielecki T, Short A, Pruitt J, Papenfuss T, Shawler T, Gienapp I, Satoskar AR, Whitacre CC. A small-molecule inhibitor of macrophage migration inhibitory factor for the treatment of inflammatory disease. FASEB J. 2010;24:4459–4466. doi: 10.1096/fj.10-162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rolan P, Gibbons JA, He L, Chang E, Jones D, Gross MI, Davidson JB, Sanftner LM, Johnson KW. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66:792–801. doi: 10.1111/j.1365-2125.2008.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M. Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res. 2003;992:53–59. doi: 10.1016/j.brainres.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.