Abstract
Helicobacter pylori (H. pylori) infects the human stomach during infancy and develops into chronic active inflammation. The majority of H. pylori tend to colonize within the mucous gel layer of the stomach. The stomach lacks its own immune function, thus innate immunity as the first line of defense is vital for specific immunity against H. pylori. We review recent discoveries in the pathophysiologic roles of toll-like receptors (TLRs), mainly TLR2 and TLR4, in H. pylori-induced inflammation. In addition, the TLR pathways activated by H. pylori-induced inflammation have been shown to be closely associated not only with gastric carcinogenesis, but also with formation of the tumor microenvironment through the production of pro-inflammatory cytokines, chemokines, and reactive oxygen species. Although the correlation between single nucleotide polymorphisms of TLRs and gastric cancer risk remains unclear, a recent study demonstrated that STAT3-driven up-regulation of TLR2 might promote gastric tumorigenesis independent of inflammation. Further research on the regulation of TLRs in H. pylori-associated gastric carcinogenesis will uncover diagnostic/predictive biomarkers and therapeutic targets for gastric cancer.
Keywords: Toll like receptors, Helicobacter pylori, Gastric cancer, Pathogen-associated molecular patterns, Damage-associated molecular patterns
Core tip: Toll-like receptors (TLRs) play important roles not only in the first line of defense against Helicobacter pylori (H. pylori), but also in gastric carcinogenesis. TLR signaling initiated by pathogen-associated molecular patterns of H. pylori consequently works to establish antigen-specific acquired immune responses. Simultaneously, damage-associated molecular patterns produced by chronic inflammation may contribute to gastric cancer development. A better understanding of TLRs will provide new insights into new diagnostic/predictive biomarkers and therapeutic targets for H. pylori-associated gastric cancer.
INTRODUCTION
Helicobacter pylori (H. pylori) is a gram-negative, spiral-shaped, microaerophilic bacteria which infects the human stomach during infancy. The majority of H. pylori tend to colonize within the mucous gel layer of the stomach and result in life-long inflammation in the human gastric mucosa[1-3]. A variety of gastric diseases, including peptic ulcers, gastric mucosa-associated lymphoid tissue lymphomas and gastric cancers, are strongly associated with H. pylori-induced gastritis[1,2].
A distinguishing characteristic of H. pylori-induced gastritis is chronic active inflammation, which consists of intraepithelial infiltration of neutrophils and acquired immune response by adaptive T helper type 1 lymphocytes[4]. The production of cytokine-associated gene A protein and vacuolating toxin are reported to be the major virulence factors of H. pylori, and harmful alterations can occur when the microbe directly interacts with the gastric epithelium[5-9]. These intra-bacterial virulent factors can evoke active inflammation to ward off the infection, but most H. pylori infections progress to chronic gastritis due to the specific immune response against H. pylori. In fact, not all H. pylori strains harbor these intra-bacterial factors, and less than 20% of the bacterial population binds to gastric epithelial cells[3]. Furthermore, the stomach lacks intraepithelial T lymphocytes and a coordinating lymph system[10]. These issues lead to the question of how the human stomach acquires adaptive immunity towards H. pylori?
All human beings have innate immunity, which serves as a first line of defense against foreign agents. This innate immunity is able to discriminate quickly between self and microbial non-self in a non-specific manner[11], and maintain mucosal homeostasis by recruiting immune cells and activating additional immune responses[12]. Thus, the innate immunity within gastric mucosa is vital to the establishment of adaptive immune responses against H. pylori. In this review, we aim to clarify recent discoveries in the role of pattern recognition systems in the innate immunity against H. pylori-related carcinogenesis.
TOLL-LIKE RECEPTORS IN INNATE IMMUNITY
Toll-like receptors (TLRs) are important pattern recognition receptors on cellular surfaces in the innate immune system[13]. TLRs are characterized by N-terminal leucine-rich repeats, a trans-membrane region and a cytoplasmic toll/interleukin (IL)-1R homology (TIR) domain. TLRs can sense structurally conserved molecules (pathogen-associated molecular patterns, PAMPs), such as lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid, lipoprotein, and unmethylated DNA with a CpG motif, from invading pathogens outside of the cell and in intracellular endosomes and lysosomes[14,15]. Although thirteen TLRs have been discovered in mammals, ten types of TLRs have been identified in humans. The intracellular signal propagation of human TLRs and their representative ligands are described in Figure 1[14-16].
Figure 1.
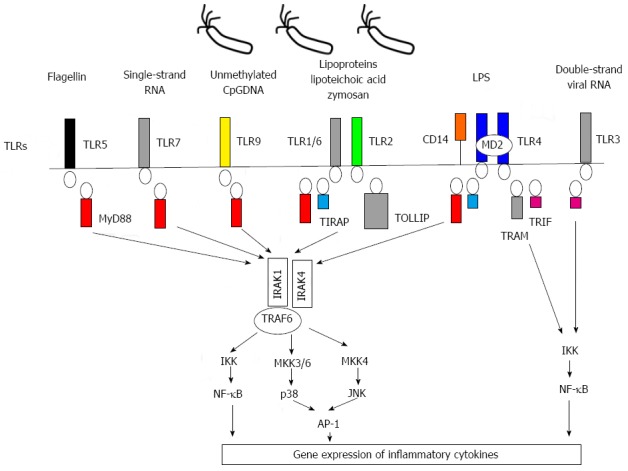
Intracellular signal propagation of human toll-like receptors and corresponding ligands in innate immunity. Toll-like receptors (TLRs) and ligands in human are revealed in the figure. Individual TLRs recognize specific (pathogen-associated molecular patterns, PAMPs) or damage-associated molecular patterns (DAMPs) of corresponding ligands. TLR signaling is propagated by activation of its cytoplasmic TIR (Toll/IL-1R) domain and cooperation with various adaptor molecules, such as myeloid differentiation factor 88 (MyD-88), toll-interleukin 1 receptor (TIR) domain-containing adapter protein (TIRAP), toll interacting protein (TOLLIP), (IRAK), (TRAF), TIR domain-containing adapter inducing interferon (IFN)-beta (TRIF), and TRIF-related adapter molecule (TRAM). TLR signaling consists of two distinct pathways, MyD-88-dependent and MyD-88-independent: (1) MyD-88-dependent pathway: The MyD88 dependent pathway is down-stream of TLR1, TLR2, TLR4, TLR5, TLR6, TLR7 and TLR9. This pathway leads to the production of proinflammatory cytokines and is triggered by the association of activated TIR domain and MyD-88, recruitment of IRAK1, IRAK4 and TRAF6 to the TLR-MyD-88 complex and consequent phosphorylation of IRAK1 and TRAF6. The signal is propagated by this phosphorylated adaptor molecular complex to the down-stream MAP kinases-AP1 and IKK complex-NF-κB; (2) MyD88-independent pathway: This is associated with the induction of INF-beta mediated by TLR3 or TLR4 activation. Intracellular signaling via the MyD-88 independent pathway is propagated by the action of TRIF and TRAM as adaptor molecules. The signal consequently activates the IKK pathway to produce INF-beta.
TLR ligands (TLRs bound to their specific ligand) transmit signals through myeloid differentiation primary response protein-88 (MyD88), TIR domain, and tumor necrosis receptor associated factor 6 (TRAF6). Downstream signaling pathways then activate phosphorylation of inhibitor of kappa B kinase (IKK) enzyme complex and mitogen activated protein (MAP) kinases. Eventually, nuclear factor-kappa B (NF-κB) activation translocates the nucleus for transcription of pro-inflammatory genes, such as inflammatory cytokines, type-I interferon (IFN), and chemokines in the inflammation[17-19]. Additionally, these signaling cascades can induce several co-stimulatory molecules, essential for initiation of adaptive immune responses in the host[20].
A new discovery is that TLRs are also able to recognize damage-associated molecular patterns (DAMPs) as endogenous ligands produced under stress conditions, such as heat-shock protein 60, extra domain A of fibronectin, hyaluronan fragments, chromatin-associated protein high-mobility group box 1, and neutrophil activating protein (NAP)[21-23]. The recognition of DAMPs accordingly directs the wide-ranging responsiveness of TLRs not only to foreign agents, but also to internal organisms denatured by inflammation.
ROLE OF TLRS IN INNATE IMMUNITY AGAINST H. PYLORI IN GASTRIC MUCOSA
Previous studies using an inflammatory cDNA array assay demonstrated strong involvement of TLRs, along with adhesion molecules, chemokines, and interleukins, in the mucosal response in H. pylori-associated gastritis[24].
H. pylori is a gram-negative bacteria; therefore, H. pylori-derived LPS is considered a direct stimulator of TLR4-mediated innate immunity (Figure 2). Previous immunohistochemical studies revealed that the expression of TLR4 in H. pylori gastritis was higher than that in uninfected gastric mucosa. The increased expression of TLR4 at the apical site of gastric epithelial cells was also characteristic of H. pylori-infected epithelium in comparison to the basolateral site of non-inflamed gastric epithelial cells[25-29].
Figure 2.

Role of toll-like receptors in Helicobacter pylori induced innate immunity. Toll-like receptors (TLRs) can sense structurally conserved molecules (pathogen-associated molecular patterns, PAMPs) as well as damage-associated molecular patterns (DAMPs) produced under stress conditions. These recognition systems direct the wide-ranging responsiveness of TLRs not only to foreign agents, but also to internal organisms denatured by inflammation.
H. pylori-LPS initiates the NF-κB pathway through ligation with TLR4 and then activates the promoter of pro-inflammatory cytokines, such as the IL-8 pathway[26-30]. H. pylori-LPS has also been reported to promote gastric cancer through the TLR4 pathway with suppressive effects on the anti-tumor reaction of mononuclear cells[31].
However, the participation of TLR4 in gastric innate immunity against H. pylori infection is still controversial. Some studies demonstrated that H. pylori-LPS-induced production of inflammatory cytokines in gastric epithelial cells or human macrophages was independent of TLR4[32-34]. Contrary to expectations, H. pylori-LPS is a weak stimulus of the immune response in comparison to LPS from other gram-negative bacteria owing to the unique structure of lipid core A of H. pylori-LPS[35,36].
PARTICIPATION OF TLR2 IN H. PYLORI - INDUCED INNATE IMMUNITY
TLR2 can recognize a variety of PAMPs from gram-positive bacteria and mycobacteria[14,15], but TLR2 is believed to ligate different structures of LPS recognized by TLR4[14]. A comprehensive microarray study revealed that TLR2-transfected HEK-293 cells stimulated by H. pylori up-regulated the expression of 28 key genes of inflammatory mediators through the TLR2 pathway[37]. Smith et al[38] showed that TLR2 of gastric epithelial cells initiated the Tribbles 3-NF-κB signaling pathway by stimulation of H. pylori-LPS. NAP has also been reported to work as a ligand of TLR2 via DAMP recognition[39]. Therefore, TLR2 is considered an important participant in the formation of gastric innate immunity against H. pylori[37-42].
COOPERATION BETWEEN TLR4 AND TLR2 IN H. PYLORI - INDUCED INNATE IMMUNITY
Previously, we reported that H. pylori-LPS was able to augment TLR2 expression through TLR4 signaling, which was propagated through the extracellular-signal-regulated kinase (ERK)-NF-κB pathway in human gastric epithelial cells[19]. TLR2 activation by H. pylori-LPS was also shown to enhance the expression of TLR4 via the MAP/ERK 1/2 kinases pathway during proliferation of gastric epithelial cells[43]. Yet, other studies have reported that TLR2 and TLR4 work in isolation. Lepper et al[44] demonstrated that, although H. pylori LPS induced an inflammatory response through TLR2, H. pylori-LPS from some strains possibly antagonized TLR4. Obonyo et al[45] showed that TLR2 induced proinflammatory cytokines, IL-1 and IL-6, in the presence of H. pylori, whereas TLR4 induced immune regulatory cytokines IL-10 and IL-12.
Due to differences in the study model, cell context, H. pylori strains, and purity of H. pylori-LPS, study results to date have been inconclusive regarding the responsiveness of TLR2 and TLR4 in H. pylori-infected gastric mucosa[46]. However, the cooperation between TLR2 and TLR4 is advantageous to enhance and establish innate and acquired immunity. In the low acidic environment of H. pylori-infected atrophic gastric mucosa, the interaction of TLR2 and TLR4 increases the susceptibility to a variety of H. pylori virulence determinants and concomitant infection with gram-positive bacteria from salivary or dietary contamination[19,47].
OTHER TLRS RELATED TO H. PYLORI INFECTION
In addition to TLR2 and TLR4, other TLRs are related to H. pylori infection. H. pylori possess multiple flagella. H. pylori-flagellin A (FlaA) is a component protein of flagella, which is recognized by flagellin receptor TLR5. In general, TLR5 signaling is known to induce IL-8 secretion via p38 MAP kinase signaling initiated by recognition of a bacterial flagellin[48-50]. However, some studies showed that FlaA was a very weak activator of IL-8 induction[51,52].
TLR9 has been shown to recognize H. pylori unmethylated CpG DNA, resulting in the production of type- I IFN[53]. Rad et al[54] showed that TLR9 stimulated by H. pylori induced proinflammatory cytokines, such as IL-6 and IL-12. Moreover, according to immunohistochemical studies, the apical and basolateral expressions of TLR5 and TLR9 identified in non-inflamed gastric epithelium dynamically acquired a basolateral distribution in H. pylori-infected mucosa[25,50].
RELATIONSHIPS BETWEEN TLRS AND GASTRIC CARCINOGENESIS
H. pylori was classified as a group I definite carcinogen in 1994 by the International Agency for Research on Cancer[55]. The inflammation-cancer association plays an important role in formation of the tumor microenvironment, characterized by infiltration of tumor-associated macrophages, activation of fibroblasts, epithelial cell remodeling, and angiogenesis[56]. The TLR pathways activated by H. pylori-induced inflammation may be implicated not only in gastric carcinogenesis, but also in formation of the tumor microenvironment through the production of pro-inflammatory cytokines, chemokines, and reactive oxygen species.
The degree of expression of TLR2, TLR4, and TLR5 gradually increases from intact gastric epithelium, to metaplasia, to dysplasia, and adenocarcinoma[49]. In addition, their localization shifts diffusely and homogeneously into the cytoplasm in gastric cancer cells, as compared to that in H. pylori-infected gastric mucosa[49]. In late-stage gastric carcinogenesis, their intense expressions in cancerous cells are likely to activate pro-oncogenic processes through prolonged stimulation of TLRs[57].
Recently, Tye et al[58] revealed that STAT3-driven up-regulation of TLR2 promoted gastric carcinogenesis using the gp130F/F preclinical mouse model, which would lead to gastritis and gastric cancer through activation of STAT3 even in a non-infectious condition. STAT3 signaling is initiated by the action of the IL-6 cytokine family, the production of which is facilitated by the interaction between TLR2 and H. pylori. As a result, TLR2 and IL-6/STAT3 signaling possibly form a positive feedback loop in H. pylori-infected gastric mucosa[59,60]. STAT3 was also reported to correlate with lymph node metastasis and immortality of gastric cancer cells[61]. Therefore, a TLR2/IL-6/STAT3 loop might play an important role in gastric carcinogenesis.
Other studies have shown that up-regulation of DAMPs may augment TLR4-mediated immune and non-immune signaling in the development of colitis-associated colon cancer[59]. Additional roles of DAMPs-activated TLR pathways probably contribute to gastric carcinogenesis caused by H. pylori infection.
GENE POLYMORPHISMS OF TLRS IN GASTRIC CARCINOGENESIS
H. pylori is a major cause of gastric cancer, but only 1%-2% of H. pylori-infected individuals develops gastric cancer[62]. Recently a meta-analysis of large population-based cohort studies in Europe revealed an association between a variation of TLR1 genetic loci (4p14) and H. pylori seroprevalence[63]. The study was conducted in White populations, therefore some sample bias should be considered in different races. However, the polymorphisms of TLRs might play an important role in modulation of the direction and magnitude of the host response against infection. This might also encourage a possible link between genetic polymorphisms of TLRs and H. pylori-associated carcinogenesis. Recent studies revealed the presence of single-nucleotide polymorphisms (SNPs) of TLRs and their relationships with gastric cancer development (Table 1). However, the implications of SNPs in genetic function have not been fully clarified.
Table 1.
Associations between polymorphisms in toll-like receptor genes and risk of gastric cancer
| TLR | SNP | SNP number | Population | Case | Control | OR | 95%CI | Genotyping | Ref. |
| TLR2 | 196 to 174 del | Chinese | 69 | 212 | 1.3 | 0.87-1.95 | Allele specific PCR | [70] | |
| Brazilian | 200 | 240 | 2.32 | 1.56-3.46 | Allele specific PCR | [65] | |||
| Chinese | 248 | 496 | 0.71 | 0.56-0.89 | DHPLC | [74] | |||
| Japanese | 583 | 1097 | 1.08 | 0.93-1.26 | PCR-CTPP | [72] | |||
| Japanese | 289 | 455 | 1.34 | 1.07-1.67 | Allele specific PCR | [73] | |||
| TLR4 | Asp299Gly | rs4986790 | Brazilian | 174 | 225 | 2.68 | 1.24-5.81 | PCR-RFLP | [65] |
| Chinese | 60 | 162 | 0.15 | 0.02-1.15 | PCR-RFLP | [66] | |||
| Italian | 171 | 151 | 1.05 | 0.46-2.37 | PCR-RFLP | [67] | |||
| Polish | 312 | 419 | 2.5 | 1.6-4.0 | PCR-RFLP | [68] | |||
| United States | 211 | 184 | 2.1 | 1.1-4.2 | PCR-RFLP | [68] | |||
| Mexican | 78 | 236 | 1.07 | 0.41-2.77 | PCR-RFLP | [69] | |||
| AspThr399ll3 | rs4986791 | Brazilian | 174 | 225 | 1.97 | 0.69-5.57 | PCR-RFLP | [65] | |
| Italian | 171 | 151 | 3.9 | 1.30-11.72 | PCR-RFLP | [67] | |||
| Mexican | 78 | 236 | 0.23 | 0.03-1.76 | PCR-RFLP | [69] | |||
| 3725 G>C | rs11536889 | Chinese | 68 | 203 | 1.89 | 1.23-2.92 | Mass spectrometry | [70] | |
| Germany, Lithuania, Latvia | 113 | 238 | 1.03 | 0.62-1.71 | PCR-CTPP | [71] | |||
| Japanese | 583 | 1056 | 0.99 | 0.84-1.16 | PCR-CTPP | [72] | |||
| TLR5 | 889 T>C | rs5744174 | Chinese | 248 | 496 | 1.43 | 1.03-1.97 | DHPLC | [74] |
| TLR9 | 1486 T>C | Chinese | 314 | 314 | 1.49 | 1.07-2.07 | PCR-RFLP | [75] | |
| 1237 T>C | Polish | 312 | 419 | 0.9 | 0.6-1.3 | PCR-RFLP | [76] | ||
| United States | 211 | 184 | 0.6 | 0.4-1.0 | PCR-RFLP | [76] |
TLRs: Toll-like receptors; PCR: Polymerase chain reaction; SNPs: Single-nucleotide polymorphisms.
The TLR4 gene is located in chromosome 9q32-q33 and contains 4 exons. TLR4 Asp299Gly (rs4986790) and Thr399Ile (rs4986791) are located in the coding sequence in amino acid substitutions associated with the TLR4 extracellular domain[64]. TLR4 Asp299Gly polymorphism can functionally diminish the binding affinity of bacterial ligands, resulting in a diminished response to H. pylori-LPS. These alterations might contribute to prolonged infection, subsequent chronic inflammation, and carcinogenesis. However, there are discrepancies in the association between TLR4 Asp299Gly[65-69] or TLR4 Thr399Ile[65,67,69] polymorphism and gastric cancer incidence, i.e., a Chinese case-control study showed a positive association between TLR4 3725G/C polymorphism and gastric cancer risk[70], but the association was not confirmed by Caucasian or Japanese case-control studies[71,72].
TLR2 is located in the long arm of chromosome 4 comprising two 5’ non-coding exons followed by a third coding exon. A TLR2 -196 to -174del polymorphism by a 22-bp deletion can affect the promoter activity of TLR2. A Brazilian study and a Japanese study both revealed a positive relationship between TLR2 -196 to -174del polymorphism and the development of gastric cancer[69,73], but a Chinese study revealed a negative relationship[74], and another study carried out in Japan showed no significant association[72].
Neither SNPs of TLR5 (889T/C[74]) nor TLR9 (1486T/C[75], 1237T/C[75,76]) is associated with gastric cancer incidence. Studies of the association between SNPs of TLRs and gastric cancer incidence are still limited and conflicting. A recent study by de Oliveira et al[65] demonstrates that a combination of polymorphisms of cytokines and TLRs strongly indicates a higher risk of developing gastric cancer. Therefore, further studies are warranted to assess the pathophysiologic significance of SNPs of TLRs.
CONCLUSION
In this review, we have provided a summary of novel TLR-related research. The role of TLRs in gastric innate immune responses against H. pylori should be carefully interpreted with close attention to differences in study models, such as cell-contexts, strains of H. pylori, and ethnicity of study subjects. The role of TLRs in gastric carcinogenesis remains unclear, since there is a possibility that the dual role of TLR signaling in carcinogenesis might act as a double-edged sword, i.e., tumor progression and suppression dependent on the cellular origin or the types of TLRs. However, TLR-associated innate immunity is essential in first-line defense and the initiation of acquired immunity against H. pylori infection. The pathophysiologic roles of TLRs in gastric cancer development, including their SNPs, remain unknown. Both PAMPs derived from H. pylori and DAMPs from H. pylori-induced inflammation may be implicated in the various processes of gastric cancer development. In future, TLR targeting therapy might provide a foresight of gastric cancer treatment. Further studies are warranted to assess the association between H. pylori infection and gastric cancer development.
Footnotes
P- Reviewers: Lee YY, Lin YH, Vorobjova T S- Editor: Zhai HH L- Editor: Webster JR E- Editor: Liu XM
References
- 1.Hofman P, Waidner B, Hofman V, Bereswill S, Brest P, Kist M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2004;9 Suppl 1:15–22. doi: 10.1111/j.1083-4389.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 2.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 3.Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, Dixon MF. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31:134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Zauco NA, Torres J, Pérez-Figueroa GE, Álvarez-Arellano L, Camorlinga-Ponce M, Gómez A, Giono-Cerezo S, Maldonado-Bernal C. Impact of cagPAI and T4SS on the inflammatory response of human neutrophils to Helicobacter pylori infection. PLoS One. 2013;8:e64623. doi: 10.1371/journal.pone.0064623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P. New strategies for the prevention of gastric cancer: Helicobacter pylori and genetic susceptibility. J Surg Oncol. 2005;90:134–138; discussion 138. doi: 10.1002/jso.20216. [DOI] [PubMed] [Google Scholar]
- 7.Konturek SJ, Starzynska T, Konturek PC, Karczewska E, Marlicz K, Lawniczak M, Jaroszewicz-Heigelman H, Bielanski W, Hartwich A, Ziemniak A, et al. Helicobacter pylori and CagA status, serum gastrin, interleukin-8 and gastric acid secretion in gastric cancer. Scand J Gastroenterol. 2002;37:891–898. doi: 10.1080/003655202760230838. [DOI] [PubMed] [Google Scholar]
- 8.Nedrud JG, Blanchard SS, Czinn SJ. Helicobacter pylori inflammation and immunity. Helicobacter. 2002;7 Suppl 1:24–29. doi: 10.1046/j.1523-5378.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 9.Rassow J. Helicobacter pylori vacuolating toxin A and apoptosis. Cell Commun Signal. 2011;9:26. doi: 10.1186/1478-811X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries PN, Griebel PJ. Mucosal dendritic cell diversity in the gastrointestinal tract. Cell Tissue Res. 2011;343:33–41. doi: 10.1007/s00441-010-1030-4. [DOI] [PubMed] [Google Scholar]
- 11.Patel MK, Trombly MI, Kurt-Jones EA. Innate immune responses to Helicobacter pylori infection: an overview. Methods Mol Biol. 2012;921:205–207. doi: 10.1007/978-1-62703-005-2_23. [DOI] [PubMed] [Google Scholar]
- 12.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–1193. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GK, Edfeldt K. Toll to be paid at the gateway to the vessel wall. Arterioscler Thromb Vasc Biol. 2005;25:1085–1087. doi: 10.1161/01.ATV.0000168894.43759.47. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 15.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 16.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479–486. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Shaykhiev R, Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol. 2007;82:1–15. doi: 10.1189/jlb.0207096. [DOI] [PubMed] [Google Scholar]
- 19.Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T, Yoshimura T. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004–G1012. doi: 10.1152/ajpgi.00096.2007. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 23.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Wen S, Felley CP, Bouzourene H, Reimers M, Michetti P, Pan-Hammarström Q. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. J Immunol. 2004;172:2595–2606. doi: 10.4049/jimmunol.172.4.2595. [DOI] [PubMed] [Google Scholar]
- 25.Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Müller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–526. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856–44864. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- 27.Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, Rokutan K. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J Med Invest. 2001;48:190–197. [PubMed] [Google Scholar]
- 28.Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496–3502. doi: 10.1128/IAI.71.6.3496-3502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, Miyaoka Y, Kazumori H, Ishimura N, Amano Y, et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol. 2004;173:1406–1416. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun. 2001;69:4382–4389. doi: 10.1128/IAI.69.7.4382-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chochi K, Ichikura T, Kinoshita M, Majima T, Shinomiya N, Tsujimoto H, Kawabata T, Sugasawa H, Ono S, Seki S, et al. Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin Cancer Res. 2008;14:2909–2917. doi: 10.1158/1078-0432.CCR-07-4467. [DOI] [PubMed] [Google Scholar]
- 32.Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–32560. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 33.Bäckhed F, Rokbi B, Torstensson E, Zhao Y, Nilsson C, Seguin D, Normark S, Buchan AM, Richter-Dahlfors A. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J Infect Dis. 2003;187:829–836. doi: 10.1086/367896. [DOI] [PubMed] [Google Scholar]
- 34.Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda Y, Ogawa T, Kashihara W, Oikawa M, Shimoyama T, Hayashi T, Tamura T, Kusumoto S. Chemical structure of lipid A from Helicobacter pylori strain 206-1 lipopolysaccharide. J Biochem. 1997;121:1129–1133. doi: 10.1093/oxfordjournals.jbchem.a021705. [DOI] [PubMed] [Google Scholar]
- 36.Moran AP. Cell surface characteristics of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995;10:271–280. doi: 10.1111/j.1574-695X.1995.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 37.Ding SZ, Torok AM, Smith MF, Goldberg JB. Toll-like receptor 2-mediated gene expression in epithelial cells during Helicobacter pylori infection. Helicobacter. 2005;10:193–204. doi: 10.1111/j.1523-5378.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Moran AP, Duggan SP, Ahmed SE, Mohamed AS, Windle HJ, O’Neill LA, Kelleher DP. Tribbles 3: a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharide. J Immunol. 2011;186:2462–2471. doi: 10.4049/jimmunol.1000864. [DOI] [PubMed] [Google Scholar]
- 39.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D’Elios MM, Del Prete G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–6454. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol. 2000;165:5767–5772. doi: 10.4049/jimmunol.165.10.5767. [DOI] [PubMed] [Google Scholar]
- 42.Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. 2005;175:8242–8252. doi: 10.4049/jimmunol.175.12.8242. [DOI] [PubMed] [Google Scholar]
- 43.Yokota S, Okabayashi T, Rehli M, Fujii N, Amano K. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect Immun. 2010;78:468–476. doi: 10.1128/IAI.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepper PM, Triantafilou M, Schumann C, Schneider EM, Triantafilou K. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell Microbiol. 2005;7:519–528. doi: 10.1111/j.1462-5822.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- 45.Obonyo M, Sabet M, Cole SP, Ebmeyer J, Uematsu S, Akira S, Guiney DG. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect Immun. 2007;75:2408–2414. doi: 10.1128/IAI.01794-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 47.Kodama K, Sumii K, Kawano M, Kido T, Nojima K, Sumii M, Haruma K, Yoshihara M, Chayama K. Gastric juice nitrite and vitamin C in patients with gastric cancer and atrophic gastritis: is low acidity solely responsible for cancer risk? Eur J Gastroenterol Hepatol. 2003;15:987–993. doi: 10.1097/00042737-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Torok AM, Bouton AH, Goldberg JB. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect Immun. 2005;73:1523–1531. doi: 10.1128/IAI.73.3.1523-1531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmausser B, Andrulis M, Endrich S, Müller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–185. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Lee SK, Stack A, Katzowitsch E, Aizawa SI, Suerbaum S, Josenhans C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003;5:1345–1356. doi: 10.1016/j.micinf.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis. 2004;189:1914–1920. doi: 10.1086/386289. [DOI] [PubMed] [Google Scholar]
- 53.Otani K, Tanigawa T, Watanabe T, Nadatani Y, Sogawa M, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y, et al. Toll-like receptor 9 signaling has anti-inflammatory effects on the early phase of Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun. 2012;426:342–349. doi: 10.1016/j.bbrc.2012.08.080. [DOI] [PubMed] [Google Scholar]
- 54.Rad R, Ballhorn W, Voland P, Eisenächer K, Mages J, Rad L, Ferstl R, Lang R, Wagner H, Schmid RM, et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 55.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 56.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 57.Pimentel-Nunes P, Gonçalves N, Boal-Carvalho I, Afonso L, Lopes P, Roncon-Albuquerque R, Henrique R, Moreira-Dias L, Leite-Moreira AF, Dinis-Ribeiro M. Helicobacter pylori induces increased expression of Toll-like receptors and decreased Toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesis. Helicobacter. 2013;18:22–32. doi: 10.1111/hel.12008. [DOI] [PubMed] [Google Scholar]
- 58.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Tye H, Jenkins BJ. Tying the knot between cytokine and toll-like receptor signaling in gastrointestinal tract cancers. Cancer Sci. 2013;104:1139–1145. doi: 10.1111/cas.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010;16:5380–5387. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 63.Mayerle J, den Hoed CM, Schurmann C, Stolk L, Homuth G, Peters MJ, Capelle LG, Zimmermann K, Rivadeneira F, Gruska S, et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013;309:1912–1920. doi: 10.1001/jama.2013.4350. [DOI] [PubMed] [Google Scholar]
- 64.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 65.de Oliveira JG, Silva AE. Polymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian population. World J Gastroenterol. 2012;18:1235–1242. doi: 10.3748/wjg.v18.i11.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt HM, Ha DM, Taylor EF, Kovach Z, Goh KL, Fock KM, Barrett JH, Forman D, Mitchell H. Variation in human genetic polymorphisms, their association with Helicobacter pylori acquisition and gastric cancer in a multi-ethnic country. J Gastroenterol Hepatol. 2011;26:1725–1732. doi: 10.1111/j.1440-1746.2011.06799.x. [DOI] [PubMed] [Google Scholar]
- 67.Santini D, Angeletti S, Ruzzo A, Dicuonzo G, Galluzzo S, Vincenzi B, Calvieri A, Pizzagalli F, Graziano N, Ferraro E, et al. Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypes. Clin Exp Immunol. 2008;154:360–364. doi: 10.1111/j.1365-2249.2008.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, Vaughan TL, McColl KE, Lissowska J, Zatonski W, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–912. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 69.Garza-Gonzalez E, Bosques-Padilla FJ, Mendoza-Ibarra SI, Flores-Gutierrez JP, Maldonado-Garza HJ, Perez-Perez GI. Assessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the development of distal gastric cancer. BMC Cancer. 2007;7:70. doi: 10.1186/1471-2407-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castaño-Rodríguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. The role of TLR2, TLR4 and CD14 genetic polymorphisms in gastric carcinogenesis: a case-control study and meta-analysis. PLoS One. 2013;8:e60327. doi: 10.1371/journal.pone.0060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kupcinskas J, Wex T, Bornschein J, Selgrad M, Leja M, Juozaityte E, Kiudelis G, Jonaitis L, Malfertheiner P. Lack of association between gene polymorphisms of Angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med Genet. 2011;12:112. doi: 10.1186/1471-2350-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hishida A, Matsuo K, Goto Y, Mitsuda Y, Hiraki A, Naito M, Wakai K, Tajima K, Hamajima N. Toll-like receptor 4 +3725 G/C polymorphism, Helicobacter pylori seropositivity, and the risk of gastric atrophy and gastric cancer in Japanese. Helicobacter. 2009;14:47–53. doi: 10.1111/j.1523-5378.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 73.Tahara T, Arisawa T, Wang F, Shibata T, Nakamura M, Sakata M, Hirata I, Nakano H. Toll-like receptor 2 -196 to 174del polymorphism influences the susceptibility of Japanese people to gastric cancer. Cancer Sci. 2007;98:1790–1794. doi: 10.1111/j.1349-7006.2007.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng HM, Pan KF, Zhang Y, Zhang L, Ma JL, Zhou T, Su HJ, Li WQ, Li JY, Gerhard M, et al. Genetic variants of toll-like receptor 2 and 5, helicobacter pylori infection, and risk of gastric cancer and its precursors in a chinese population. Cancer Epidemiol Biomarkers Prev. 2011;20:2594–2602. doi: 10.1158/1055-9965.EPI-11-0702. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Xue L, Yang Y, Xu L, Zhang G. TLR9 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. PLoS One. 2013;8:e65731. doi: 10.1371/journal.pone.0065731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hold GL, Rabkin CS, Gammon MD, Berry SH, Smith MG, Lissowska J, Risch HA, Chow WH, Mowat NA, Vaughan TL, et al. CD14-159C/T and TLR9-1237T/C polymorphisms are not associated with gastric cancer risk in Caucasian populations. Eur J Cancer Prev. 2009;18:117–119. doi: 10.1097/CEJ.0b013e3283101292. [DOI] [PMC free article] [PubMed] [Google Scholar]


