Abstract
Nonalcoholic fatty liver disease (NAFLD) is becoming rapidly one of the most common indications for orthotopic liver transplantation in the world. Development of graft steatosis is a significant problem during the post-transplant course, which may happen as a recurrence of pre-existing disease or de novo NAFLD. There are different risk factors that might play a role in development of graft steatosis including post-transplant metabolic syndrome, immune-suppressive medications, genetics and others. There are few studies that assessed the effects of NAFLD on graft and patient survival; most of them were limited by the duration of follow up or by the number of patients. With this review article we will try to shed light on post-liver transplantation NAFLD, significance of the disease, how it develops, risk factors, clinical course and treatment options.
Keywords: Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Liver cirrhosis, Liver transplantation, Metabolic syndrome
Core tip: Nonalcoholic fatty liver disease (NAFLD) is projected to become the most common indication for liver transplantation in the United States by 2030 with high risk for disease recurrence after transplantation. De novo NAFLD may develop in patients transplanted for other indications due to the high prevalence of obesity and metabolic syndrome. The optimal management of NAFLD after liver transplantation requires future studies.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is seen worldwide and is the most common liver disorder in Western industrialized countries, where the major risk factors for NAFLD, central obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome are common[1]. There appear to be ethnic differences in the prevalence of NAFLD, with a higher prevalence of hepatic steatosis in Hispanics compared to Caucasians or African Americans[2].
NAFLD is characterized by fat accumulation within liver cells when no other etiologies for hepatic fat accumulation (e.g., heavy alcohol consumption) are present. NAFLD is known to progress to cirrhosis and is likely an important cause of cryptogenic cirrhosis[3]. The development of the NAFLD fibrosis scoring system and the realization that non-alcoholic steatohepatitis (NASH) can progress to cirrhosis (in 8 to 10 years) has dramatically decreased the diagnosis of cryptogenic cirrhosis[4].
NAFLD is recognized as a spectrum of closely related disorders that includes nonalcoholic fatty liver (NAFL) and NASH. In NAFL, hepatic steatosis is present without evidence of significant inflammation, whereas in NASH, hepatic steatosis is associated with hepatic inflammation and hepatocyte ballooning that may be histologically indistinguishable from alcoholic steatohepatitis[5]. Differentiation between simple steatosis and NASH in the management and prognosis of NAFLD is important, as the progression of NASH to cirrhosis is an indication for liver transplant.
Development of graft steatosis is a significant problem during the post-transplant course. The recurrence of steatosis is common in patients who undergo liver transplant for NAFLD[6]. In addition, a reasonable proportion of liver transplant recipients without steatosis prior to transplantation develop de novo fatty liver disease after transplant[7]. About 40% of the transplant recipients may develop steatosis within 3 to 4 years after transplant, indicating that post-transplant steatosis is common. Longer follow-up is needed, however, as NAFLD is typically a slowly progressive disease and more patients may develop steatohepatitis and fibrosis over a longer follow up period[8].
Although exact pathogenesis and underlying mechanism of graft steatosis is not fully understood, there is growing evidence that genetics in conjunction with development of metabolic syndrome or its components may play a role[9-11].
NASH CIRRHOSIS: THE MOST COMMON INDICATION FOR LIVER TRANSPLANT IN 2030?
In the United States, the prevalence of NAFLD has been rising over time, becoming an increasingly frequent indication for orthotopic liver transplantation (OLT)[12]. Approximately 10% of liver transplants performed in the United States are for NASH cirrhosis[2].
Between 1988 and 1994, the prevalence of NAFLD as an etiology for chronic liver disease was 47%[1], accounting for 5.5% of patients undergoing OLT. Those numbers increased to 63% and 9.8%, respectively, between 1999 and 2004. Astonishingly, between 2005-2008 NAFLD accounted for 75% of chronic liver disease and 11% of patients undergoing OLT, making it the third most common indication for OLT in the United States after hepatitis C virus (HCV) and alcohol[1]. With this increasing trend in the prevalence of NAFLD, coupled with expected improvements in HCV therapy, NAFLD may surpass HCV and alcohol as the most common indication for OLT in the near future.
One and 3-year survival for recipients undergoing liver transplantation for NASH were comparable to those for other indications (84% and 78%, vs 87% and 78% respectively). NASH as an indication for liver transplantation is independently predictive for renal dysfunction at any time after liver transplantation. The proportion of NASH recipients developing stage III chronic kidney injury compared with matched recipients for other indications is 31 vs 8% (P < 0.009)[13,14].
METABOLIC SYNDROME POST-TRANSPLANT: FERTILE SOIL FOR NAFLD
Metabolic syndrome is common among patients who have undergone liver transplantation. It is defined by a combination of hypertension, insulin resistance, dyslipidemia, and obesity. Liver transplant recipients may meet criteria for metabolic syndrome prior to OLT, in which case immunosuppressive medications exacerbate the problem. A study of 252 liver transplant patients found that 52% had metabolic syndrome following transplantation, compared with only 5% prior to transplantation[15]. This suggests that metabolic syndrome is a common problem post OLT.
Following transplantation, treatment with steroids or calcineurin inhibitors (CNI) predispose to weight gain. Obesity, insulin resistance, and diabetes are common metabolic complications in liver transplant recipients and are risk factors for increased morbidity and mortality[16]. Approximately one-third of patients will become obese following transplantation[17,18]. For example, in one study of 774 patients, mean body mass index increased from 24.8 kg/m2 at baseline to 27.0 kg/m2 at year one, to 28.1 kg/m2 at year two[19]. Another recent study of 492 patients showed that post-transplant recipients with steatosis at year 5, have BMI of 28.7 kg/m2 compared to 25.4 kg/m2 for those who didn’t develop steatosis.
POST-TRANSPLANT NAFLD
Post-transplant NAFLD is a newly recognized disease. Although the risk factors, histological features and risk of progression have been extensively analyzed in native livers, it is unknown how much of this information can be applied to the post-transplant population. It is well known that proper control of NAFLD risk factors is not a requirement for transplant eligibility. Therefore, risk factors for NAFLD may persist or even worsen after transplantation[6]. The reported incidence of post-transplant NAFLD is variable and ranges between 25% to 60%. However, most of these studies are limited, by small sample size or limited follow-up[20-25]. Post-transplant NAFLD occurs either as recurrence of pre-transplant existing disease, or development of de novo NAFLD which was not present before transplant. Recurrent fatty liver disease was first documented in 1992 by Burke et al[26], while first case series of de novo NAFLD were reported in 2003 by Poordad et al[27] where four patients developed de novo NAFLD within 3 mo of transplant.
Development of post-transplant NAFLD depends on a combination of host and graft factors (Figure 1), which is why liver transplant has always been an interesting model to study the natural history and the determinants of post-transplant liver steatosis. As it still remains unclear which determinants are the main contributing factors for development of post-transplant steatosis.
Figure 1.
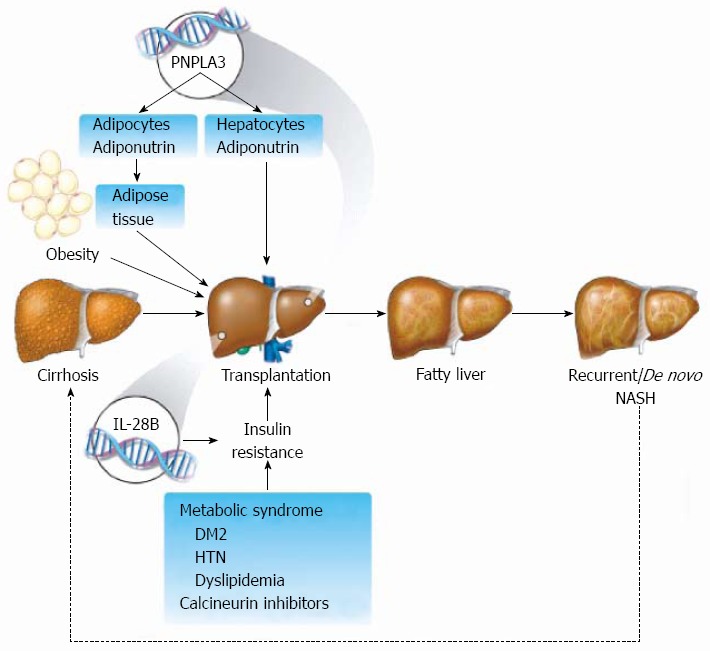
Development of post-transplant nonalcoholic fatty liver disease depends on a combination of host and graft factors. Schematic representation of potential mechanistic pathways that lead to development of post-transplant nonalcoholic fatty liver disease including genetic background, metabolic syndrome, insulin resistance and the effects of immunosuppressive medications. Recurrent of de novo nonalcoholic steatohepatitis (NASH) may eventually lead to the development of graft cirrhosis and the need for re-transplantation. IL28B: Interleukin 28B; PNPLA3: Patatin-like phospholipase domain-containing protein 3; DM: Diabetes mellitus; HTN: Hypertension.
According to most of the studies in native livers, none of the non-invasive methods, including serology testing[28], imaging techniques[29], and biomarker assays[30-32], can reliably distinguish steatosis from NASH; which means that histological assessment remains the gold standard for establishing a diagnosis and assessing the progression of NASH. Therefore, it is reasonable to assume the same for recurrent disease in the transplant recipient. The question of when to proceed to liver biopsy should be raised, given there is currently no documented protocol for post-transplant biopsies except in individuals transplanted for HCV.
Although liver biopsy is the only method of confirming the presence of NAFLD, its role in the diagnosis and management of post-transplant NAFLD and NASH is still evolving. Liver biopsies may be helpful in assessing disease stage and determining response to medical treatment, which may dictate changes in the immunosuppressive regimen, but are associated with morbidity and cost. We know that relying on alanine aminotransferase (ALT) is not helpful, as the entire histologic spectrum of NAFLD can be seen in individuals with normal ALT values. Furthermore, the histologic spectrum is not significantly different among patients with normal ALT in comparison to those with elevated ALT levels[33]. As NASH can recur and be severe, there may be a role for protocol-based liver biopsies. In some instances, a combination of normal ALT (< 40 IU/L) and ultrasound examination for steatosis has a 100% negative prediction for presence of NASH on liver biopsy. Charlton[34] recommend considering a liver biopsy in liver transplant recipients with a history of NASH who also have steatosis on ultrasound examination and/or persistently abnormal ALT, which cannot be explained by other causes.
Recurrent NAFLD
Increasing number of cases with recurrent NASH post OLT has been reported[6,24,25]. Factors that predict the presence of NAFLD in the pre-transplant setting include older age, increased BMI, and presence of diabetes mellitus[35]. More recently, studies have shown that genetics play an important role in NAFLD, with special consideration given to both patatin-like phospholipase domain-containing protein 3 (PNPLA3) polymorphism I148M and interleukin 28B (IL28B)[20,36-39]. These same factors may play a significant role in the development of NAFLD recurrence post-OLT.
Not surprisingly, at 1-year post OLT those individuals transplanted for NASH cirrhosis (60%) had the highest risk or developing steatosis, followed by alcoholic cirrhosis (15%), HCV (15%) and cholestatic diseases (5%)[23]. Recurrence of fibrosis is less frequent, with 5% of patients with NASH cirrhosis post OLT developing cirrhosis at 10-year follow-up[23]. A recent study assessing the frequency of steatosis recurrence in the transplanted graft over a 5-year period found that steatosis paralleled increase in body mass index[20]. A summary of studies that assessed for NAFLD recurrence is provided in Table 1.
Table 1.
Summary of studies that evaluated post-transplant nonalcoholic fatty liver disease and metabolic syndrome
| Ref. | Patient (n) | Follow up period | Outcomes post OLT n (%) | Metabolic syndrome post OLT |
| Kim et al[25] | 8 | 15 mo | 5/8 (63) → NAFL in 4 mo | BMI was higher in patients that developed steatosis |
| 1/8 (13) → NASH in 6 wk | Hyperlipidemia was observed in 5 patients who developed steatosis | |||
| No cirrhosis within the follow up period | ||||
| Contos et al[22] | 30 | > 4 yr | 4/27 (15) → NAFL in 6 mo | BMI and steroid use were higher in patients that developed steatosis |
| 1/27 (4) → NASH in 1 yr | DM prevalence was higher post OLT | |||
| 2/27 (7) → NASH in 3 yr | ||||
| 3/27 (11) → NASH in > 4 yr | ||||
| 1/30 (3) → cirrhosis, no time range was specified | ||||
| Charlton et al[57] | 31 | > 4 yr | 9/15 (60) → NAFL in 4 mo | MetS was observed more in the post-transplant setting |
| 2/15 (13) → NASH in 4 mo | No detailed reports on MetS components | |||
| 4/15 (27) → NASH in 1 yr | ||||
| 5/15 (33) → NASH in 2 yr | ||||
| 3/15 (20) → fibrosis < 1 yr | ||||
| 5/15 (33) → cirrhosis in > 4 yr | ||||
| Ayata et al[58] | 9 | 2 yr | Didn’t report steatosis or cirrhosis | No detailed report on post OLT metabolic syndrome |
| 2/9 (22) → NASH in 2 yr | ||||
| Seo et al[7] | 68 | > 4 yr | 12/68 (18) → NAFL, no time range was specified | MetS was higher in post OLT |
| BMI increased in 35% | ||||
| HTN developed in 69% | ||||
| 6/68 (9) → NASH, no time range was specified | DM was developed in 38% | |||
| Did not report cirrhosis | Hypertriglyceridemia in 25% | |||
| Malik et al[59] | 98 | Around 2 yr | 36/79 (45) → NAFL in 1 yr | MetS was higher post OLT and |
| (461 d) | BMI increased post OLT | |||
| 19/79 (24) → NASH in < 2 yr | HTN developed more frequently post OLT | |||
| DM was higher post OLT compared to pre-transplant period | ||||
| 14/79 (18) in < 2 yr | Hypertriglyceridemia was higher in post OLT setting | |||
| Bhagat et al[21] | 71 | 4 yr | Didn’t report steatosis | BMI increased in 17% |
| HTN developed in 35% | ||||
| 21/64 (33) → NASH in 6 mo | DM was developed in 16% | |||
| No cirrhosis in 4 yr | Hypertriglyceridemia in 18% | |||
| Yalamanchili et al[60] | 227 | 20 yr | 8.2% → NAFL in 1 yr | BMI increased post OLT and statistically significant compared to pre transplant BMI |
| 13.6% → in 2 yr | HTN developed in 52% | |||
| 25% → NAFL in 5 yr | DM developed in 36% | |||
| 33% → NAFL in 10 yr | ||||
| 6% → NASH 20 yr | ||||
| 5% → cirrhosis in 5 yr | ||||
| 10% → cirrhosis in 10 yr | ||||
| Finkenstedt et al[20] | 237 | 5 yr | 11.6% → NAFL in 1 yr | BMI was higher in patients with steatosis 28.7 compared to those without 25.4 (P = 0.005) |
| 27.5% → NAFL in 3 yr | ||||
| 32.6% → NAFL in 5 yr | DM was higher in patients who developed steatosis | |||
| No biopsies, no report on NASH | HTN development was not different between groups | |||
| No cirrhosis by imaging in 5 yr | Hypertriglyceridemia did not show difference |
BMI: Body mass index; MetS: Metabolic syndrome; DM: Diabetes mellitus; HTN: Hypertension; NAFL: Nonalcoholic fatty liver (steatosis); NASH: Nonalcoholic steatohepatitis; OLT: Orthotopic liver transplantation.
De novo NAFLD after OLT
Recurrence of NAFLD is common after OLT, but few studies have examined de novo NAFLD post OLT. Garcia et al[40] reported 4 cases of NASH post OLT with fatty infiltration observed within a mean of 21 mo and subsequent development of steatohepatitis and early fibrosis within a mean of 60 mo. Interestingly, early post-transplantation de novo NAFLD in the absence of predisposing factors, such as diabetes mellitus or obesity, has also been reported[27]. A retrospective study reported an 18% incidence of de novo NAFLD and 9% for de novo NASH after an average follow-up of 28 mo following liver transplantation. In this study, de novo NAFLD was associated with significant weight gain after liver transplantation. A 10% increase in BMI after OLT has been associated with a higher risk of developing NAFLD[7]. Zahmatkeshan et al[41], reported three cases of post-transplant NASH, all related to vascular complications. They concluded that vascular complications such as thrombosis of the portal system should be suspected in cases of early fatty infiltration of the liver after transplantation.
Although steatosis is not a predictor of survival following liver transplantation, cardiovascular morbidity is common in transplant recipients with NAFLD and cardiovascular events are a major cause of death during long-term follow-up[25,42].
GENETIC BACKGROUND IN NAFLD AFTER LIVER TRANSPLANTATION PNPLA3
Adiponutrin (ADPN) is a triacylglycerol lipase that mediates triglycerides hydrolysis in hepatocytes. ADPN is encoded in the PNPLA3 gene, and certain well-known polymorphisms within the gene are highly associated with development and progression of NAFLD - specifically, polymorphism at position 148 of PNPLA3 resulting in substitution of isoleucine (rs738409-C) with methionine (rs738409-M)[43]. This substitution reduces the hydrolytic activity of the enzyme to triglycerides[44]. The encoded ADPN protein, which appears to be membrane bound, may be involved in the balance of energy usage/storage in adipocytes, as the gene is also expressed in the adrenal glands and adipose tissue[45-47].
This PNPLA3 polymorphism was associated with higher liver fat content, with the strongest effect observed in Hispanics compared to European and African-American populations[43]. Hepatic triglycerides content is more than 70% higher in those with rs703409-GG genotype, than in those with rs703409-CC genotype. It is well established that those with rs738409-GG genotypes are more prone to develop advanced liver disease[48]. Besides its association with fatty liver, this PNPLA3 variant is also linked to both higher serum levels of ALT and an increased risk and severity of NAFLD disease[49,50]. The rs738409-G allele of PNPLA3 is considered an independent risk factor for progression of steatosis, fibrosis, cirrhosis and hepatocellular carcinoma in NAFLD[49].
A recent study from Austria demonstrated that the frequency of the 148M allele is significantly higher in patients with end stage liver disease undergoing OLT than in the donor cohort[20]. The functional link between impaired triglyceride lipase activity and hepatic steatosis remains unresolved. Liver transplantation allows investigation into the effect of donor and recipient PNPLA3 genotype on determining the rate of hepatic fat accumulation. The same study demonstrated that the prevalence of hepatic steatosis increases over time in liver transplant recipients. In particular, steatosis five years after OLT was associated with the recipient but not with the donor PNPLA3 risk allele[20]. This suggests that the recipient genotype determines the rate of hepatic triglyceride accumulation regardless of adiponutrin activity in the donor liver. It was suggested by He et al[44], that reduced intracellular triglyceride breakdown is the primary cause for hepatocellular lipid accumulation in carriers of rs738409-G. However, recent data suggest that reduced PNPLA3 activity in extra-hepatic tissues may also be associated with hepatic fat accumulation. This hypothesis is supported by a recent finding that the recipient not the donor PNPLA3 genotype is associated with the development of obesity and diabetes mellitus after OLT[39]. Some studies have linked PNPLA3 polymorphisms with obesity following OLT. A greater increase was observed in BMI post-transplant associated with the G allele. Additionally, over 82% of patients with PNPLA3 GG polymorphism are obese (BMI > 30 kg/m2) at 3 years post-transplant. Non-obese patients with CC genotype were less likely to become obese in comparison to patients with CG or GG genotype post OLT[39,51].
There is also evidence of a significant link between PNPLA3 gene polymorphisms and development of diabetes/impaired fasting glucose in the liver transplant recipient[39]. The ability to identify high-risk patient before liver transplant may help physicians establish aggressive weight management plans in the perioperative and postoperative period.
IL28B
IL28B polymorphisms are strongly associated with response to treatment for HCV infection. IL28B acts on interferon-stimulated genes via the JAK-STAT pathway, which has been implicated in development of insulin resistance[52,53]. IL28B is associated with hepatic steatosis prevalence and severity in Caucasians with HCV genotype 1, suggesting differing genetic pathways to steatosis[54]. Although some suggestions have linked IL28B with post-transplant metabolic syndrome[55], the exact role of IL28B and its association with graft steatosis remains undetermined and requires further study.
It has been suggested that IL28B might play a significant role in post-transplant graft steatosis by promoting underlying risk factors for metabolic syndrome. Veldt et al[55], demonstrated that the risk of developing post-transplant DM is significantly increased in recipients carrying the TT polymorphism of the IL28B gene. However, there was no difference in overall graft survival according to recipient IL28B polymorphism. A study by Watt et al[39], did not find a strong association between IL28B genotype and post-transplant DM or obesity.
In summary, given the established association between PNPLA-3 and steatosis in NAFLD, alcoholic liver disease and chronic HCV, it is likely the overall effect of PNPLA-3 and the combination of PNPLA-3 and IL28B on diabetes/IFG in the liver transplant population is significantly underestimated. These findings indicate that patients who are known to have PNPLA3 polymorphisms should undergo aggressive control of other risk factors associated with development of NAFLD, especially obesity and insulin resistance, as well as the other components of the metabolic syndrome. As genetic screening continues to become more relevant in the clinical setting, it may be appropriate to carry out PNPLA3 genotyping on OLT-recipients in order to identify those who may be at risk of early progression of NAFLD[20,39,52]. However, further research into PNPLA3 polymorphisms and other genetic components of NAFLD should be carried out.
THE EFFECT OF GRAFT STEATOSIS ON TRANSPLANT OUTCOME AND SURVIVAL
Graft function
Post-transplant steatosis is a common occurrence, and due to the slowly progressive nature of NAFLD, more post-transplant patients are likely to progress to steatohepatitis[8]. Graft survival may be significantly altered by the fibrosis and cirrhosis associated with progression of NASH, and thus it is important to recognize risk factors and extend post-transplant follow up to accommodate for the lengthy progression of NAFLD. Pre-transplant BMI has been identified as a risk factor associated with development of graft steatosis, emphasizing the need for weight management in the transplant recipient, both before and after surgery[8]. There are some indications that graft steatosis may be more aggressive in its progression to NASH than in the pre-transplant setting, and this was indeed the case in one study[7] where 9% of patients developed NASH within 12 mo. However, the long-term implications of post OLT NAFLD remains unclear. A recent review by Patil et al[56], analyzed the survival data from seven post OLT NAFLD recurrence studies[21,22,25,57-60], and determined that cirrhosis is a relatively infrequent event in this subset of patients. Studies from Kim et al[25], Malik et al[59], Contos et al[22] and Bhagat et al[21] documented no cases of cirrhosis at 4 year follow-up, in a total of 168 patients. These studies suggest that cirrhosis is relatively uncommon in individuals who develop NAFLD post OLT, at least within the follow-up timeframe.
Cardiovascular risk
As improved surgical techniques and advances in medical management lead to an increase in the number of long-term survivors post-OLT, post OLT cardiovascular (CV) events has become the leading cause of non-graft-related deaths in OLT patients[42]. In a recent study[61] the cumulative risk of CV events post OLT was reported to be 4.5% and 10.1%, at 1 and 3-year respectively. Interestingly, this 1- and 3-year cumulative risk was significantly higher in patients undergoing transplantation for NASH (15.3% and 19.3%) as compared to all other etiologies[61]. The risk of CV events after OLT is likely due to the increased prevalence of metabolic syndrome in patients having undergone a liver transplant. The prevalence of metabolic syndrome in patients after liver transplantation has been reported to be between and 44% and 58%[18,62]. The high prevalence of CV events and metabolic syndrome post-transplant is important to recognize for long-term management of this special population. Cardiovascular health and its potential consequences must be emphasized, and the physician should identify those patients who are at an elevated risk of experiencing a cardiovascular event.
MANAGEMENT OF NAFLD IN THE OLT RECIPIENT
The goal of management with NAFLD in the OLT recipient is primarily to prevent its progression to NASH, as is the case with pre-OLT NAFLD. Much of what is known about the management of NAFLD is within the non-transplant population, with data regarding management in post-transplant patients remaining limited. Pharmacologic therapy, mitigation of components of metabolic syndrome, alterations in immunosuppressive regimens, and bariatric surgery are all involved in the management of NAFLD in the post-transplant setting.
Pharmacologic therapy
None of the pharmacological therapies for NAFLD have been extensively studied in the post-transplant setting. Currently, vitamin E and pentoxifylline (PTX) are being used as therapy for NAFLD. Vitamin E has antioxidant effects and has been shown to replete glutathione stores and reduce oxidative stress in a mouse model of NASH[63]. The PIVENS protocol, which was studied in a large randomized control trial carried out by Sanyal et al[64], showed that treatment with vitamin E improved the histological features of NASH in a cohort of 247 patients when compared to pioglitazone or placebo.
PTX, a phosphodiesterase inhibitor, has been shown to have immunomodulatory effects by decreasing tumor necrosis factor-alpha (TNFα) transcription[65]. Randomized placebo control trials have shown PTX to be a well-tolerated treatment that can improve histological features of NASH, possibly through decreasing lipid oxidation[66,67]. Serum levels of ALT, aspartate aminotransferase, in addition to TNFα levels were decreased in several other clinical trials[68], with nausea being the only reported adverse outcome. While these results are encouraging for treatment of NASH in the pre-transplant setting, the immunomodulatory effects of PTX have not been studied in the post-transplant setting, and thus caution should be exercised until further data is available.
The use of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) as a potential therapy for NAFLD is currently being explored. The use of ACE-inhibitors was associated with a reduced risk of developing de novo heaptatic steatosis in a study by Seo et al[7] While data from human studies remain limited, there have been several studies[69-73] that demonstrate a therapeutic benefit of ARBs in patients with HCV as well as patients with NAFLD. The administration of ARBs led to an improved fibrosis score[71], down-regulation of hepatic expression of fibrogenic genes[69], improvements in insulin resistance, serum levels of ALT[70] and plasma transforming growth factor-1[72]. Both ACE inhibitors and ARBs may improve insulin sensitivity and hepatic steatosis by increasing circulating adiponectin and hepatic adipoR2 levels, in addition to reducing pro-inflammatory cytokine levels.
While these results seem to indicate the use of RAAS-inhibitors as a therapy for NAFLD and NASH, there is limited data on the subject and the studies remain contradictory, as well as a need for more randomized control trials on the effects of ACE inhibitors and ARBs[74]. Indeed, there is evidence that the use of ACE inhibitors in liver transplant recipients may exacerbate calcineurin inhibitor-induced hyperkalemia, and therefore caution should be exercised[34].
Other pharmacological therapies for NAFLD are currently being explored. Obeticholic acid (OCA) has shown promise as a potential therapy in NAFLD. OCA is a derivative of chenodeoxycholic acid, the natural agonist of the farnesoid X receptor, which is a nuclear hormone receptor that regulates glucose and lipid metabolism[75]. A recent double-blind, placebo-controlled clinical trial by Mudaliar et al[75], showed that daily OCA over a period of 6 wk increased insulin sensitivity and reduced markers of liver inflammation fibrosis in patients with NAFLD and type II diabetes mellitus. Additionally, studies investigating the role of simtuzumab, a monoclonal antibody with immunomodulatory effects, as a treatment for fibrosis and cirrhosis secondary to NASH are currently underway.
Management of metabolic syndrome
As discussed earlier, metabolic syndrome is a common occurrence in the post-transplant setting. Prevention and management of hypertension, dyslipidemia, insulin resistance, and obesity are critical to preventing the progression of NAFLD to NASH as well as reducing the patient’s risk of experiencing cardiovascular events.
In non-transplant patients, BMI and metabolic syndrome are highly associated with the development of NAFLD[76,77], and based on limited data this association appears to extend to the post-transplant population as well[8]. The first-line therapy for obese patients in need of weight reduction prior to liver transplant is a multidisciplinary approach centered on behavioral management and restricting dietary intake. A recent study from Ryan et al[78], concluded that the Mediterranean diet led to a reduction in liver steatosis and improved insulin sensitivity in an insulin-resistant population with NAFLD. Other options for obese NAFLD patients beyond dietary and lifestyle changes include pharmacotherapy and bariatric surgery. It is also worth noting that the use of thiazolidinediones in NASH patients has been associated with weight gain[34].
Approximately 65%-70% of liver transplant recipients develop hypertension following transplantation[79]. The cause of hypertension is multifactorial but is mostly related to the use of calcineurin inhibitors (e.g., cyclosporine or tacrolimus) and glucocorticoids[80]. The hyperlipidemia observed in liver transplant recipients is mostly related to the side effects of glucocorticoids, cyclosporine, and tacrolimus, however tacrolimus appears to have a less prominent effect on lipids than cyclosporine, and there is some evidence that conversion from cyclosporine to tacrolimus can improve lipid profiles in liver transplant patients[81]. Sirolimus is a potent immunosuppressive drug that is used by more liver transplant programs as part of CNI-sparing regimens; however, dyslipidemia is a well-recognized side effect of sirolimus therapy which may contribute to the development of metabolic syndrome.
Many risk factors predisposing patients to insulin resistance are commonplace in the post-transplant setting, including sedentary lifestyle, hepatic denervation, and immunosuppression[82]. Immunosuppression may be partly responsible for the prevalence of diabetes in post-transplant patients, as prednisone, tacrolimus, and cyclosporine have been shown to be diabetogenic[83]. The insulin-sensitizing agent metformin has been studied extensively in the setting of NASH, and its use has no significant effect on liver histology[84]. While the literature contains varying results of metformin’s impact on aminotransferases and hepatic insulin sensitivity[85-87], a recent meta-analysis concluded that lifestyle intervention and metformin for 6-12 mo did not improve aminotransferases or liver histology[88].
Immunosuppression
As discussed in the previous section, immunosuppression following OLT is associated with increased risk of developing hypertension, insulin resistance, dyslipidemia, and weight gain. Immunosuppression is known to have a profound effect on hypertension in the post-transplant setting[89]. Tacrolimus and cyclosporine are associated with an increased risk of hypertension. Evidence has shown that switching an immunosuppressive regimen from cyclosporine to tacrolimus can lower mean systolic blood pressure, however tacrolimus is associated with an increased risk of post-transplant diabetes mellitus[90-93]. Glucocorticoids, cyclosporine, tacrolimus, and weight gain also predispose to the development of diabetes following liver transplantation. Around 5%-30% of recipients develop de novo diabetes[17,39,94-96]. A meta-analysis that included 16 studies with 3813 liver transplant recipients found that the risk of developing de novo insulin-requiring diabetes was significantly higher among patients treated with tacrolimus compared with those treated with cyclosporine[97].
Bariatric surgery
Bariatric surgery remains an option for patients who have undergone liver transplantation and are unable to sustain weight reduction. Sustained weight reduction is difficult to achieve in the post-transplant patient. A recent study[98] analyzed the effectiveness of a multidisciplinary protocol for obese patients requiring OLT, including a noninvasive pre-transplant weight loss program, and a combined OLT plus sleeve gastrectomy for obese patients who failed to lose weight prior to OLT. This method was used successfully in three patients and resulted in effective weight loss and was associated with fewer post-OLT metabolic complications. While further follow-up is needed, this novel strategy may provide an alternative means of weight management in the post-transplant setting. It is important to note that there have been reports[99] of postoperative hepatic decompensation occurring in patients with NASH having undergone bariatric surgery. This rare occurrence developed due to the rapid mobilization of peripheral triglycerides, which exacerbates hepatic steatosis resulting in rapidly progressive cholestasis and encephalopathy.
Data concerning bariatric surgery as a pre-transplant procedure in patients with liver cirrhosis is limited. Shimizu et al[100] concluded that laparoscopic roux-en-y gastric bypass and laparoscopic sleeve gastrectomy could be performed in select patients with cirrhosis with improvement in obesity and obesity-related co-morbidities and without prohibitive complication rates. Additionally, it should be noted that NASH was not associated with an increased risk for postoperative complications following bariatric surgery[101].
CONCLUSION
NAFLD prevalence is increasing rapidly with the potential of becoming the most common indication in the near future. Graft steatosis is a common problem following liver transplant but its impact on long term outcomes in not well understood. Hepatologists and transplant surgeons should be aware of the risk of NAFLD recurrence and de novo NAFLD. The need for routine liver biopsy following transplant remains controversial. Careful pre-operative evaluation for patients with pre-existing risk factors for NAFLD should be taken with special attention for those risk factors in post-operative course. The role of bariatric surgery and pharmacotherapy in the post-transplant NAFLD and metabolic syndrome is to be determined.
Footnotes
P- Reviewers: Kikuchi L, Kubota K, Oliveira CPMS S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530.e1; quiz e60. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Desai HG. Cryptogenic cirrhosis: a vanishing entity. J Assoc Physicians India. 2009;57:751–754, 759. [PubMed] [Google Scholar]
- 5.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684–689. doi: 10.1097/TP.0b013e31820b6b84. [DOI] [PubMed] [Google Scholar]
- 7.Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844–847. doi: 10.1002/lt.20932. [DOI] [PubMed] [Google Scholar]
- 8.Lim LG, Cheng CL, Wee A, Lim SG, Lee YM, Sutedja DS, Da Costa M, Prabhakaran K, Wai CT. Prevalence and clinical associations of posttransplant fatty liver disease. Liver Int. 2007;27:76–80. doi: 10.1111/j.1478-3231.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 9.Brandman D, Pingitore A, Lai JC, Roberts JP, Ferrell L, Bass NM, Terrault NA. Hepatic steatosis at 1 year is an additional predictor of subsequent fibrosis severity in liver transplant recipients with recurrent hepatitis C virus. Liver Transpl. 2011;17:1380–1386. doi: 10.1002/lt.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010;105:613–620. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- 11.Watt KD, Dierkhising RA, Fan C, Krishnan A, Tillmann HL, Goldstein DB, Heimbach J, Charlton M. Recipient but not Donor PNPLA3 and IL28B Genotype are Important Determinants of Posttransplant Diabetes and Obesity. Hepatology. 2013;56:193A. [Google Scholar]
- 12.Malik SM, deVera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 13.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, Paris S, Gunson BK, Bramhall SB, Mutimer DJ, et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl. 2011;17:1292–1298. doi: 10.1002/lt.22382. [DOI] [PubMed] [Google Scholar]
- 15.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 16.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995;60:1057–1060. [PubMed] [Google Scholar]
- 18.Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648–1654. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 19.Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285–296. doi: 10.1002/lt.500040402. [DOI] [PubMed] [Google Scholar]
- 20.Finkenstedt A, Auer C, Glodny B, Posch U, Steitzer H, Lanzer G, Pratschke J, Biebl M, Steurer M, Graziadei I, et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol. 2013;11:1667–1672. doi: 10.1016/j.cgh.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 22.Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 23.Maor-Kendler Y, Batts KP, Burgart LJ, Wiesner RH, Krom RA, Rosen CB, Charlton MR. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000;70:292–297. doi: 10.1097/00007890-200007270-00009. [DOI] [PubMed] [Google Scholar]
- 24.Molloy RM, Komorowski R, Varma RR. Recurrent nonalcoholic steatohepatitis and cirrhosis after liver transplantation. Liver Transpl Surg. 1997;3:177–178. doi: 10.1002/lt.500030212. [DOI] [PubMed] [Google Scholar]
- 25.Kim WR, Poterucha JJ, Porayko MK, Dickson ER, Steers JL, Wiesner RH. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation. 1996;62:1802–1805. doi: 10.1097/00007890-199612270-00021. [DOI] [PubMed] [Google Scholar]
- 26.Burke GW, Cirocco R, Hensley G, Ranjan D, Reddy R, Jeffers L, Schiff E, Miller J. Liver transplantation for cirrhosis following jejuno-ileal bypass--regional cytokine differences associated with pathological changes in the transplant liver. Transplantation. 1992;54:374–377. [PubMed] [Google Scholar]
- 27.Poordad F, Gish R, Wakil A, Garcia-Kennedy R, Martin P, Yao FY. De novo non-alcoholic fatty liver disease following orthotopic liver transplantation. Am J Transplant. 2003;3:1413–1417. doi: 10.1046/j.1600-6143.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- 28.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 29.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 30.Das SK, Balakrishnan V. Role of cytokines in the pathogenesis of non-alcoholic Fatty liver disease. Indian J Clin Biochem. 2011;26:202–209. doi: 10.1007/s12291-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carazo A, León J, Casado J, Gila A, Delgado S, Martín A, Sanjuan L, Caballero T, Muñoz JA, Quiles R, et al. Hepatic expression of adiponectin receptors increases with non-alcoholic fatty liver disease progression in morbid obesity in correlation with glutathione peroxidase 1. Obes Surg. 2011;21:492–500. doi: 10.1007/s11695-010-0353-2. [DOI] [PubMed] [Google Scholar]
- 32.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 34.Charlton M. Evolving aspects of liver transplantation for nonalcoholic steatohepatitis. Curr Opin Organ Transplant. 2013;18:251–258. doi: 10.1097/MOT.0b013e3283615d30. [DOI] [PubMed] [Google Scholar]
- 35.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 36.Peng XE, Wu YL, Lin SW, Lu QQ, Hu ZJ, Lin X. Genetic variants in PNPLA3 and risk of non-alcoholic fatty liver disease in a Han Chinese population. PLoS One. 2012;7:e50256. doi: 10.1371/journal.pone.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petta S, Grimaudo S, Cammà C, Cabibi D, Di Marco V, Licata G, Pipitone RM, Craxì A. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J Hepatol. 2012;56:1356–1362. doi: 10.1016/j.jhep.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Valenti L, Alisi A, Nobili V. I148M PNPLA3 variant and progressive liver disease: A new paradigm in hepatology. Hepatology. 2012:Epub 2012 Feb 6. doi: 10.1002/hep.25634. [DOI] [PubMed] [Google Scholar]
- 39.Watt KD, Dierkhising R, Fan C, Heimbach JK, Tillman H, Goldstein D, Thompson A, Krishnan A, Charlton MR. Investigation of PNPLA3 and IL28B genotypes on diabetes and obesity after liver transplantation: insight into mechanisms of disease. Am J Transplant. 2013;13:2450–2457. doi: 10.1111/ajt.12355. [DOI] [PubMed] [Google Scholar]
- 40.Garcia RF, Morales E, Garcia CE, Saksena S, Hübscher SG, Elias E. Recurrent and de novo non-alcoholic steatohepatitis following orthotopic liver transplantation. Arq Gastroenterol. 2001;38:247–253. doi: 10.1590/s0004-28032001000400007. [DOI] [PubMed] [Google Scholar]
- 41.Zahmatkeshan MBG, Eshraghian A, Nikeghbalian S, Bahador A, Salahi H, Malek-Hosseini SA. De novo Fatty Liver Due to Vascular Complications After Liver Transplantation. Transplantation Proceedings. Elsevier Inc. 2011;43:615–617. doi: 10.1016/j.transproceed.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Pruthi J, Medkiff KA, Esrason KT, Donovan JA, Yoshida EM, Erb SR, Steinbrecher UP, Fong TL. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811–815. doi: 10.1053/jlts.2001.27084. [DOI] [PubMed] [Google Scholar]
- 43.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50 Suppl:S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- 47.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 48.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 49.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romeo S, Sentinelli F, Dash S, Yeo GS, Savage DB, Leonetti F, Capoccia D, Incani M, Maglio C, Iacovino M, et al. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes (Lond) 2010;34:190–194. doi: 10.1038/ijo.2009.216. [DOI] [PubMed] [Google Scholar]
- 52.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129.e18. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F, et al. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009–1015. doi: 10.1002/hep.21782. [DOI] [PubMed] [Google Scholar]
- 54.Clark PJ, Thompson AJ, Zhu Q, Vock DM, Zhu M, Patel K, Harrison SA, Naggie S, Ge D, Tillmann HL, et al. The association of genetic variants with hepatic steatosis in patients with genotype 1 chronic hepatitis C infection. Dig Dis Sci. 2012;57:2213–2221. doi: 10.1007/s10620-012-2171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldt BJ, Duarte-Rojo A, Thompson AJ, Watt KD, Heimbach JK, Tillmann HL, Goldstein DD, McHutchison JG, Charlton MR. Recipient IL28B polymorphism is an important independent predictor of posttransplant diabetes mellitus in liver transplant patients with chronic hepatitis C. Am J Transplant. 2012;12:737–744. doi: 10.1111/j.1600-6143.2011.03843.x. [DOI] [PubMed] [Google Scholar]
- 56.Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl. 2012;18:1147–1153. doi: 10.1002/lt.23499. [DOI] [PubMed] [Google Scholar]
- 57.Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 58.Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol. 2002;33:1098–1104. doi: 10.1053/hupa.2002.129419. [DOI] [PubMed] [Google Scholar]
- 59.Malik SM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 60.Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 61.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370–375. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 62.Hanouneh IA, Feldstein AE, McCullough AJ, Miller C, Aucejo F, Yerian L, Lopez R, Zein NN. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14:1287–1293. doi: 10.1002/lt.21524. [DOI] [PubMed] [Google Scholar]
- 63.Phung N, Pera N, Farrell G, Leclercq I, Hou JY, George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int J Mol Med. 2009;24:171–180. doi: 10.3892/ijmm_00000220. [DOI] [PubMed] [Google Scholar]
- 64.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diehl AM. Tumor necrosis factor and its potential role in insulin resistance and nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:619–638, x. doi: 10.1016/j.cld.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–1299. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. doi: 10.1186/1476-511X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colmenero J, Bataller R, Sancho-Bru P, Domínguez M, Moreno M, Forns X, Bruguera M, Arroyo V, Brenner DA, Ginès P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G726–G734. doi: 10.1152/ajpgi.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Enjoji M, Kotoh K, Kato M, Higuchi N, Kohjima M, Nakashima M, Nakamuta M. Therapeutic effect of ARBs on insulin resistance and liver injury in patients with NAFLD and chronic hepatitis C: a pilot study. Int J Mol Med. 2008;22:521–527. [PubMed] [Google Scholar]
- 71.Sookoian S, Fernández MA, Castaño G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol. 2005;11:7560–7563. doi: 10.3748/wjg.v11.i48.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Kamada Y, Sakuta S. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology. 2002;36:1022. doi: 10.1053/jhep.2002.32679. [DOI] [PubMed] [Google Scholar]
- 73.Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond) 2007;113:109–118. doi: 10.1042/CS20070026. [DOI] [PubMed] [Google Scholar]
- 74.Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327–331. doi: 10.4254/wjh.v4.i12.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 76.Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001;7:S13–S21. doi: 10.1053/jlts.2001.29167. [DOI] [PubMed] [Google Scholar]
- 77.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O’Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, Emre S, Fishbein TM, Guy SR, Schwartz ME, et al. Long-term medical complications in patients surviving & gt; or = 5 years after liver transplant. Transplantation. 2000;69:781–789. doi: 10.1097/00007890-200003150-00018. [DOI] [PubMed] [Google Scholar]
- 80.Textor SC, Canzanello VJ, Taler SJ, Schwartz L, Augustine J. Hypertension after liver transplantation. Liver Transpl Surg. 1995;1:20–28. [PubMed] [Google Scholar]
- 81.Roy A, Kneteman N, Lilly L, Marotta P, Peltekian K, Scudamore C, Tchervenkov J. Tacrolimus as intervention in the treatment of hyperlipidemia after liver transplant. Transplantation. 2006;82:494–500. doi: 10.1097/01.tp.0000231711.82193.41. [DOI] [PubMed] [Google Scholar]
- 82.Luzi L, Perseghin G, Regalia E, Sereni LP, Battezzati A, Baratti D, Bianchi E, Terruzzi I, Hilden H, Groop LC, et al. Metabolic effects of liver transplantation in cirrhotic patients. J Clin Invest. 1997;99:692–700. doi: 10.1172/JCI119213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. J Endocrinol Invest. 2000;23:482–490. doi: 10.1007/BF03343761. [DOI] [PubMed] [Google Scholar]
- 84.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, Coban S, Erden E, Soykan I, Emral R, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:200–208. doi: 10.1111/j.1365-2036.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- 87.Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 88.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 89.Kallwitz ER. Metabolic syndrome after liver transplantation: preventable illness or common consequence? World J Gastroenterol. 2012;18:3627–3634. doi: 10.3748/wjg.v18.i28.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neal DA, Gimson AE, Gibbs P, Alexander GJ. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transpl. 2001;7:533–539. doi: 10.1053/jlts.2001.24637. [DOI] [PubMed] [Google Scholar]
- 91.Canzanello VJ, Schwartz L, Taler SJ, Textor SC, Wiesner RH, Porayko MK, Krom RA. Evolution of cardiovascular risk after liver transplantation: a comparison of cyclosporine A and tacrolimus (FK506) Liver Transpl Surg. 1997;3:1–9. doi: 10.1002/lt.500030101. [DOI] [PubMed] [Google Scholar]
- 92.Stegall MD, Wachs ME, Everson G, Steinberg T, Bilir B, Shrestha R, Karrer F, Kam I. Prednisone withdrawal 14 days after liver transplantation with mycophenolate: a prospective trial of cyclosporine and tacrolimus. Transplantation. 1997;64:1755–1760. doi: 10.1097/00007890-199712270-00023. [DOI] [PubMed] [Google Scholar]
- 93.Williams R, Neuhaus P, Bismuth H, McMaster P, Pichlmayr R, Calne R, Otto G, Groth C. Two-year data from the European multicentre tacrolimus (FK506) liver study. Transpl Int. 1996;9 Suppl 1:S144–S150. doi: 10.1007/978-3-662-00818-8_36. [DOI] [PubMed] [Google Scholar]
- 94.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583–595. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 95.Navasa M, Bustamante J, Marroni C, González E, Andreu H, Esmatjes E, García-Valdecasas JC, Grande L, Cirera I, Rimola A, et al. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996;25:64–71. doi: 10.1016/s0168-8278(96)80329-1. [DOI] [PubMed] [Google Scholar]
- 96.Saab S, Shpaner A, Zhao Y, Brito I, Durazo F, Han S, Farmer DG, Ghobrial RM, Yersiz H, Goldstein LI, et al. Prevalence and risk factors for diabetes mellitus in moderate term survivors of liver transplantation. Am J Transplant. 2006;6:1890–1895. doi: 10.1111/j.1600-6143.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 97.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;(4):CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, Hay JE, Wiesner RH, Sanchez W, Rosen CB, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363–368. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 99.Cotler SJ, Vitello JM, Guzman G, Testa G, Benedetti E, Layden TJ. Hepatic decompensation after gastric bypass surgery for severe obesity. Dig Dis Sci. 2004;49:1563–1568. doi: 10.1023/b:ddas.0000043364.75898.c8. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu H, Phuong V, Maia M, Kroh M, Chand B, Schauer PR, Brethauer SA. Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis. 2013;9:1–6. doi: 10.1016/j.soard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 101.Weingarten TN, Swain JM, Kendrick ML, Charlton MR, Schroeder BJ, Lee RE, Narr BJ, Ribeiro TC, Schroeder DR, Sprung J. Nonalcoholic steatohepatitis (NASH) does not increase complications after laparoscopic bariatric surgery. Obes Surg. 2011;21:1714–1720. doi: 10.1007/s11695-011-0521-z. [DOI] [PubMed] [Google Scholar]


