Abstract
Since 1963, when the first human liver transplantation (LT) was performed by Thomas Starzl, the world has witnessed 50 years of development in surgical techniques, immunosuppression, organ allocation, donor selection, and the indications and contraindications for LT. This has led to the mainstream, well-established procedure that has saved innumerable lives worldwide. Today, there are hundreds of liver transplant centres in over 80 countries. This review aims to describe the main aspects of LT regarding the progressive changes that have occurred over the years. We herein review historical aspects since the first experimental studies and the first attempts at human transplantation. We also provide an overview of immunosuppressive agents and their potential side effects, the evolution of the indications and contraindications of LT, the evolution of survival according to different time periods, and the evolution of methods of organ allocation.
Keywords: Liver transplantation, History, Survival, Indications, Organ allocation
Core tip: Liver transplantation is currently considered a life-saving procedure. Over the past 50 years, the world has witnessed evolving strategies in surgical techniques, immunosuppressive drugs, intensive pre- and post-operative care, and, the prevention of disease recurrence and has discussed policies of organ allocation. This review highlights some of these aspects regarding their historical evolution over the past 50 years.
INTRODUCTION
Liver transplantation (LT) celebrated its 50th anniversary in 2013, with the first human procedure being performed by Thomas Starzl in 1963. Since this time, many strategies have evolved worldwide regarding technical aspects, immunosuppressive agents, organ allocation, donor selection, indications and contraindications, prophylaxis of infection, and the prevention of recurrent diseases[1]. This review aims at describing some aspects of LT regarding the progressive changes that have occurred over the years.
HISTORICAL OVERVIEW
In 1952, in Milan, Italy, Vittorio Staudacher was the first to perform a LT in a large animal model, a canine species[2]. Two years later, Jack Cannon was credited with the first animal orthotopic LT[2]. Still in experimental models, in 1960, in Colorado, United States, Thomas Starlz reported his experience with almost 80 canine liver transplants, where the maximum survival was 20 d[3]. In the same year, Francis Moore also reported his experience with over 30 canine homotransplants in Boston, MA, United States[4]. These experimental experiences expanded the available knowledge on many issues such as venovenous bypass, organ preservation, tissue matching and immunosuppression.
In humans, solid organ transplantation began in 1954 with a successful kidney transplantation between identical twin brothers[5]. In 1963, Starzl et al[6] published the first three attempts at human LT, but it was not until 1967 that the procedure resulted in an extended survival. It was the case of a 19-mo-old girl with hepatocellular carcinoma, who died 13 mo after surgery for metastatic disease[7]. Roy Calne, in Cambridge, United Kingdom, joined Roger Williams in London, United Kingdom, in 1968, and reported 5 cases of liver transplant, detailing the technical difficulties encountered[8]. Thomas Starzl and Roy Calne were later honoured with the Lasker-DeBakey Clinical Medical Research Award in 2012 for these pioneer procedures.
The acceptance of the concept of brain death in the United States in 1968 was an additional landmark for LT development, which allowed donor organ preservation in ideal, physiologic conditions and resulted in better graft quality and survival[9,10].
The introduction of cyclosporine in the late 1970s as part of the immunosuppressive regimen in organ transplantation permitted less toxicity and the prevention of rejection and severe opportunistic infections when compared to azathioprine[11,12]. Later on, with the introduction of tacrolimus, the world observed further improvements in survival[13,14].
A series of 540 cases united by 4 different liver transplant units was presented at the 1983 NIH Conference, showing greater survival in these patients compared to those who did not undergo transplantation, permitting the establishment of LT as a beneficial procedure for patients with end-stage liver disease, as opposed to an experimental procedure[15].
In 1984, Bismuth et al[16] (France) reported the first left-lobe LT in a child, and in 1988, Pichlmayr performed the first split-LT in Hannover, Germany[17]. The following year, in Sao Paulo, Brazil, Silvano Raia described the first attempt at a living donor graft in a child[18], with a successful procedure performed by Strong et al[19] in Brisbane, Australia, in 1990. Since this time, the greatest experience with living donor grafts has been found in countries where cadaveric donation is not culturally accepted such as Japan and South Korea.
Since the nineties, the field of LT has witnessed a huge expansion of the number of institutions performing the procedure, and today, there are hundreds of liver transplant centres in over 80 countries. In addition, an increasing number of conditions associated with end-stage liver disease are now referred to LT. This has led to the scarcity of donor organs, thus obliging transplant coordinators worldwide to adopt evolving organ allocation strategies[20]. Immunosuppressive, technical, infection risk and intensive care management advances have made LT a long-lasting, efficient therapy for end-stage liver disease, hepatocellular carcinoma and other hepatic cancers. Advances in organ procurement, preservation, and allocation have accompanied these improvements. The indications have progressively expanded, and the contraindications have slowly changed as technical issues have evolved.
DEVELOPMENT OF SURGICAL TECHNIQUES
During these 50 years, many surgical techniques and strategies were reported, involving donor and recipient operations. The surgical techniques employed in the first experiments and in clinical trials are described and the following variations or evolutions are discussed: piggy-back vs conventional technique, split liver, living donor liver transplantation (LDLT), and domino liver transplantation (DLT).
First experiments and clinical trials
In 1955, Welch[21] described the insertion of a hepatic allograft in the right paravertebral gutter of dogs, without disturbing the native liver. The concept of liver replacement was first mentioned by Cannon[22] in 1956 (orthotopic transplantation). Formal research programmes for total hepatectomy and liver replacement in dogs were developed from 1958-1960[3,4].
These procedures consisted of removal of the native liver with the excision of the retrohepatic vena cava (conventional) and its replacement with donor liver containing a vena cava segment including the hepatic veins. Vena cava anastomoses above and below the liver were then performed. Portal vein, hepatic artery, and biliary tract anastomosis were performed with conventional methods.
In the first human liver trials by Starzl et al[6] in 1963, the various anastomoses were performed similarly to those in the dog experiments. In general, these procedures have remained the model for LT.
Piggy-back vs conventional techniques
LT may be performed by conventional or piggy-back techniques, using the caval anastomosis procedure. The conventional technique involves the resection and complete replacement of the retrohepatic vena cava. During the anhepatic phase, however, there is a substantial decrease in venous return, causing haemodynamic instability, metabolic alterations, and an overall reduction in renal flow. The safety of the operation has been improved by the use of a veno-venous bypass that permits decompression of the obstructed vena cava and splanchnic venous system[23-25].
The method of preserving the inferior vena cava was first described by Calne[8] and fully described and popularised by Tzakis[26] in the late 80s. Grafting is performed as in conventional techniques, except for outflow reconstruction, which is created between the graft suprahepatic vena cava and the anterior surface of the host vena cava (an orifice fashioned using the major hepatic veins). This technique, better known as a piggy-back, is currently the most widely used.
A major concern about the piggy-back technique is the risk of venous outflow obstruction related to the small calibre of the anastomosis or kinking of the venous suprahepatic segment. There are reports of early and late hepatic venous outflow blocks[27-29]. Gurusamy et al[30] published a systematic review evaluating the piggy-back technique for LT. Two randomised trials comparing the piggy-back method (n = 53) and the conventional method with veno-venous bypass (n = 53) were identified. There was no significant difference in post-operative mortality, primary graft non-function, vascular complications, renal failure, transfusion requirements, intensive therapy unit (ITU) stay, or hospital stay between the two groups. The warm ischaemia time was significantly shorter using the piggy-back method.
Split liver
Organ shortage is a major problem worldwide, and this scenario is more complex for small children. Alternative techniques have been developed to expand sources of grafts, including split LT and LDLT.
A split is defined as obtaining 2 grafts from a unique single deceased donor. The strategies for anatomical surgery of the liver described by Couinaud[31] and Bismuth[32] made this technique feasible. It was first described by Pichlmayr and colleagues in 1988[17]. Traditionally, the liver is split for an adult and a child (right trisegment graft/left lateral segment). The use of the split grafts for two adults is uncommon (full right graft /full left graft).
Emond et al[33] reported their preliminary experience in 1989. Nine whole livers were split to treat 18 patients (5 adults and 13 children) during a period of 10 mo. There was no difference in patient or graft survival, primary non-function, or arterial thrombosis when compared with whole organ transplantation in the same period. Biliary complications were more frequent in split grafts, occurring in 27% of cases compared to 4% in whole grafts.
Vagefi et al[34] reported their experience with 106 recipients (63 adults and 43 children) over a period of 7 years. In adults, the 1-, 5-, and 10-year overall patient survival rates were 93%, 77%, and 73%, respectively; the overall graft survival rates were 89%, 76%, and 65%, respectively. In children, the 1-, 5-, and 10-year overall patient survival rates were 84%, 75%, and 69%, respectively; the overall graft survival rates were 77%, 63%, and 57%, respectively. The main postoperative complications were biliary (29%) and vascular (11%).
Similar good results were reported in other series. Doyle et al[35] reported 53 split liver grafts from 1261 transplants (4.2%) over 7 years. The 1-, 5-, and 10-year patient and graft survival rates in adult recipients of split grafts were 95.5%, 89.5%, and 89.5%, respectively. Survival was similar to that of whole organ recipients (P = 0.15). In paediatric cases, the split 1-, 5-, and 10-year overall patient and graft survival rates were 96.7%, 80.0%, and 80.0% and 93.3%, 76.8, and 76.8%, respectively. Complications included retransplantation in 3 (10.0%) cases, bile leak in 5 (16.7%), hepatic arterial thrombosis in 2 (6.7%), bowel perforation in 2 (6.7%), and bleeding in 2 (6.7%). These results were equivalent in whole organ transplantation.
The results of split LT into 2 adults are more controversial. Lee et al[36] reported similar results compared to LDLT. The 3-mo and 1-, 3-, and 5-year survival rates for patients receiving right hemi liver grafts were 81.0%, 75.9%, 70.1%, and 70.1%, respectively, compared to 71.4%, 61.5%, 61.5%, and 61.5%, respectively, for patients receiving left hemi liver grafts (P = 0.457). On the other hand, Aseni et al[37] observed a high postoperative complication rate, with most of the complications being of biliary origin. A lower 5-year survival rate compared to that of recipients of a whole organ was observed (63.3% and 83.1%, respectively).
LDLT
Transplantation of a living donor liver was developed in the same context as the split liver. In 1989, in Sao Paulo, Brazil, Raia described the first attempt at a living donor graft in a child[18]. One year later, Strong et al[19] performed the first successful LDLT using a left lateral segment graft. In 1993, Hashikura et al[38] reported the successful use of the left lobe for an adult. In 1996, Lo performed the first successful transplantation of a right lobe in an adult recipient[39]. Finally, Lee reported the initial experience with the use of dual grafts in 2001[40].
Beyond its technical complexity, a major concern about LDLT is the morbidity and risk of death for the donors. It has been shown that donor right hepatectomy may carry a higher morbidity than that for the left lateral segment[41,42].
In a series of 200 donor right hepatectomies, Chan reported a morbidity of 20% and a mortality of 0.5%[43]. In a follow-up of 4111 living liver donors in the United States for a mean of 7.6 years, the risk of early death among the donors was 1.7 per 1000 donors, and mortality did not differ from that of healthy, matched individuals[44].
Late complaints are common in donors. In a series of right lobe donors, 53% reported symptoms, including intolerance to fatty meals and diarrhoea, gastroesophageal reflux, incisional discomfort, depression requiring hospitalisation and rib pain affecting lifestyle[45].
Kasahara et al[46] reported a cohort of more than 2200 paediatric patients who had undergone LDLT. The 1-, 5-, 10- and 20-year patient survival rates were 88.3%, 85.4%, 82.8% and 79.6%, respectively. Similar results were reported by other centres[34]. In a series of 891 adult recipients, the 2- and 5-year patient survival rates were 86.6 and 83.2%, respectively[47].
Beyond the risks to donors, there are some challenges related to the recipient’s surgery, including small for size syndrome, biliary complications, hepatic artery reconstruction, and optimisation of venous drainage.
Small for-size syndrome can be defined as dysfunction or failure of a “small” partial liver graft (graft to recipient weight ratio < 0.8%) during the first postoperative week after the exclusion of other causes[48]. The clinical presentation can involve jaundice, ascites, coagulopathy, and encephalopathy that can include irreversible organ non-function and patient death[49]. In general, treatment includes the reduction of portal flow and pressure to overcome graft hyperperfusion[49].
Biliary reconstruction has always been regarded as the “Achilles’ heel” of LT. Biliary reconstruction in LDLT is even more complex, due to the small size and multiple ducts[50]. The reported rates of biliary complications (strictures, leaks, and biloma) ranged from 16% to 67% in early series and 6.8% in more recent series with the use of a microscope[51-60].
In LDLT, the hepatic arterial system should be reconstructed using a branch of the hepatic artery and is technically demanding; the incidence of arterial complications is high. The reported incidence of hepatic artery thrombosis is 3.1%-22%, and that of hepatic artery stenosis is 4.8%-24.6%[61-68]. Anastomosis using surgical microscopy became a standard technique in this context[69].
The optimisation of venous drainage is an important issue. Venous congestion of segments V and VIII of the graft is frequently observed in right-lobe living donor liver transplants without middle hepatic vein drainage, and it can cause graft dysfunction and failure. Some series showed that inclusion of the middle hepatic vein was safe for donors and improved graft function[70].
DLT
Transplantation using grafts from patients with metabolic liver diseases such as familial amyloidotic polyneuropathy is denominated DLT. The Familial Amyloidotic Polyneuropathy World Transplant Register includes the experience of more than 1000 domino transplants performed in 21 countries until the end of 2011[71].
A series centre analysis revealed that DLT recipients presented no difference in the rates of acute rejection, vascular complications, or biliary complications compared with deceased donor LTs and lower rates of biliary complication compared with LDLT[72].
The major disadvantage of DLT is the risk of transmitting the metabolic disease through the transplanted liver. Some series have reported a low incidence of approximately 3%, but the disease may have manifested earlier than theoretically expected in the domino recipients[73,74].
IMMUNOSUPPRESSION
Graft rejection, either acute, late acute, or chronic, is an immune-mediated disease. The risk of graft rejection is counterbalanced by the permanent use of immunosuppression, which has been a crucial concern in LT since the early 1960s. The use of efficient immunosuppression starting in the late 1970s represented a pivotal change in acute rejection and LT survival[75]. Today, mortality due to graft rejection is an uncommon event[76].
However, over time, the late complications of immunosuppression have been considered a major threat to LT morbidity. In addition to the risk of opportunistic viral, bacterial, and fungal infections, the continued use of immunosuppression may lead to degenerative and metabolic diseases as well as de novo malignancies[76]. A list of the most frequently prescribed immunosuppressive drugs and potential associated adverse events can be found in Table 1.
Table 1.
Most frequently prescribed immunosuppressive drugs and potential associated adverse events
| Drug class | Medications | Adverse events |
| Corticosteroids | Methyl prednisone | Bone disease, diabetes, hypertension, hypercholesterolemia, gastrointestinal disease |
| Prednisolone | ||
| Calcineurin inhibitors | Tacrolimus | Kidney disease, hypertension, hypercholesterolemia, diabetes (tacrolimus) |
| Cyclosporine | ||
| mTOR inhibitors | Sirolimus | Hypercholesterolemia, hypertension, pulmonary fibrosis, kidney disease |
| Everolimus | ||
| Anti-metabolites | Mycophenolate | Bone marrow suppression, gastrointestinal disease |
| Azathioprine | ||
| IL-2 receptor antibodies | Basiliximab | |
| Daclizumab | ||
| Polyclonal antibodies | Antithymocyte globulin |
IL: Interleukin.
The choice of the best immunosuppression regimen is an individual-based decision and should consider a combination of variables[76]: (1) Time since LT: after the first 90 d following LT, the necessity for immunosuppression is reduced, as the graft becomes somewhat tolerant to the recipient´s immune injury[76]. However, most recipients require lifelong immunosuppressive therapy. In a frequency that varies between 20% and 60% of recipients[77-80], a phenomenon called operational tolerance may occur, and graft rejection does not occur despite immunosuppression withdrawal. This is specific to LT compared to other organ transplants because livers have a unique microenvironment that promotes tolerance rather than immunity[81]. Several authors have recently discussed that the reduction or even discontinuation of immunosuppression may be planned and safely achieved in a percentage of recipients that varies between 41% and 62%[80,82-87]. This leads to the diminution of morbidity and mortality by preventing long-term adverse events and the occurrence of opportunistic diseases as well as the improvement of quality of life. Successful tolerance has been associated with a longer time period since LT[84,87-89], male gender[87] and lower lymphocyte reactivity, measured by the phytohaemagglutinin stimulation index[84]. Regarding recipient age, successful immunosuppression withdrawal has been achieved in higher percentages among paediatric patients[80], but older age at LT has also been associated with tolerance[87]. However, biomarkers have been studied to identify the select patients who could benefit from immunosuppression withdrawal. Some studies have shown that tolerant patients can be characterised by an enrichment in peripheral blood natural killer cell gene signatures along with an increased frequency of γδ T cells and regulatory T cells[89,90]. More recently, other genes have been found to possibly define tolerance, including an enrichment of iron-associated genes together with an anti-inflammatory gene set[88]; additionally, a 3-gene signature in peripheral blood leukocytes was reported in a paediatric population[91]. Apparently, liver tissue-derived biomarkers are more accurate than blood-related markers at predicting the success of drug withdrawal strategies. Currently, they constitute the most robust biomarkers of operational tolerance[88]. However, biomarker profiles need to be prospectively validated to identify these recipients before drug withdrawal[92,93]. Another issue is the induction of tolerance early following transplantation, and the link between this and gene signatures is not clear[45,47]. Additionally, the long-term histological consequences of immunosuppression withdrawal are also unclear; (2) Cause of end-stage liver disease. Disease recurrence may be affected by the type of immunosuppression. For example, in autoimmune hepatitis and primary biliary cirrhosis, the early reduction of anti-rejection drugs has been associated with increased recurrence rates[77,94-96]. However, excessive immunosuppression has been associated with increased HCV recurrence[97], and high exposure to calcineurin inhibitors during the first month post-LT, defined as a mean tacrolimus trough concentration > 10 ng/mL or a cyclosporine trough concentration > 300 ng/mL, is associated with an increased risk of HCC recurrence[98]; (3) History of rejection: recurrent or severe rejection history; (4) History or risk of cancer or infectious complications; and (5) Comorbidities and adverse drug events, including prior experiences with immunosuppressive drugs.
INDICATIONS AND CONTRAINDICATIONS
The indications for LT have changed over the years. Overall, LT is indicated for acute liver failure, chronic liver failure leading to cirrhosis, and inherited metabolic liver diseases. It is also indicated for hepatocellular carcinoma (HCC) and other hepatic cancers, including hepatoblastoma, epithelioid haemangioendothelioma, and hilar cholangiocarcinoma (CCA), in selected cases as well as some miscellaneous conditions. The main current indications for LT are listed in Table 2[99].
Table 2.
Current main indications for liver transplantation
| Category | Disease |
| Acute liver failure | Acute hepatitis A |
| Acute hepatitis B | |
| Drug/toxin hepatotoxicity | |
| Cirrhosis from chronic liver diseases | Chronic hepatitis C virus |
| Chronic hepatitis B virus | |
| Alcoholic liver disease | |
| Autoimmune hepatitis | |
| Cryptogenic liver disease | |
| Primary biliary cirrhosis and primary sclerosing cholangitis | |
| Secondary biliary cirrhosis | |
| Metabolic disorders | Alpha-1 antitrypsin deficiency |
| Hereditary haemochromatosis | |
| Wilson’s disease | |
| Glycogen-storage disorders | |
| Type 1 hyperoxaluria | |
| Familial homozygous hypercholesterolemia | |
| Malignancies | Primary hepatic cancer: hepatocellular carcinoma and cholangiocarcinoma |
| Metastatic: carcinoid tumours and islet cell tumours | |
| Miscellaneous | Polycystic liver disease |
| Budd-Chiari syndrome |
The Clichy and King’s College criteria are the two main scoring systems used to select patients in cases of acute liver failure[100,101]. Both models achieve high specificity but remain associated with limited negative predictive value.
Regarding HCC, initially, only patients with unresectable, large, or multinodular tumours or with other associated underlying liver dysfunction were selected for LT, resulting in low survival and high rates of recurrence[102]. The restrictive selection criteria widely known as the Milan criteria were first established by Mazzaferro et al[103] and significantly improved patient survival. Later, Yao et al[104] at the University of California, San Francisco, demonstrated that a set of less conservative criteria, known as the UCSF criteria, had similar outcomes.
According to the European LT Registry, in the past, cancers constituted almost half of all the indications, and currently, this indication accounts for approximately 15%[105]. Transplantation for primary biliary cirrhosis has also decreased over time, as opposed to the increasing number of indications for alcoholic and hepatitis C cirrhosis, in Europe as well as in the United States[106].
Over time, the contraindications for LT have also evolved. One example of the change in strategy is hepatitis B. Before the availability of antivirals and hepatitis B immunoglobulin, there was poor survival of patients infected with hepatitis B due to the uncontrolled viral replication. This led most centres to abandon transplantation for this indication for several years. Nonetheless, currently, transplant survival for these patients presents excellent survival.
It was also believed that HIV infection constituted an absolute contraindication in the past; however, with the advent of highly active antiviral therapy and the evolving knowledge of drug interactions, these patients are regarded as potential candidates[107].
Advanced age is another relative contraindication in which we have observed changing patterns over the years. In the nineties, the population of patients over 60 years old accounted for approximately 10% of all transplanted patients, whereas currently, they constitute almost 20% of the procedures[105]. As there is no universally accepted age limit for considering transplantation, centres have dealt with this issue on a case-by-case basis, according to the physiological and functional status of the individual.
Portal vein thrombosis was also initially considered an absolute contraindication. However, long after the first report of LT in this context[108], operative strategies such as simple thrombectomy, extra-anatomic venous graft, arterialisation of the portal vein, and cavoportal hemitransposition may now be employed.
The absolute contraindications have hardly changed over time and constitute circumstances in which short- and/or long-term survival is compromised. The current absolute and relative contraindications are listed in Table 3[99,109].
Table 3.
Current absolute and relative contraindications in liver transplantation
| Absolute contraindications |
| Active extrahepatic malignancy |
| Hepatic malignancy with macrovascular or diffuse tumour invasion |
| Uncontrolled infection, except infection of the hepatobiliary system |
| Active substance or alcohol abuse |
| Severe comorbid conditions |
| Noncompliance or insufficient motivation |
| Technical impediment |
| Brain death |
| Relative contraindications |
| Advanced age |
| HIV infection |
| Cholangiocarcinoma |
| Portal vein thrombosis |
| Psychosocial problems |
HIV: Human immunodeficiency virus.
ORGAN ALLOCATION
Reasonable and impartial allocation criteria were not an issue during the first year of LT, as organs had a very short viability period and transplants were restricted to candidate recipients who were lucky enough to be managed at the same institution as the deceased donor[10].
However, the acceptance of brain death criteria in several countries improved donor organ preservation and quality, making it possible to allocate donated livers at distant sites. At the same time, an increasing number of conditions associated with end-stage liver disease have inflated transplant waiting lines, making it essential to develop a fair and structured organ distribution system[10,110].
Similar to renal transplantation, the first listing systems were based solely on waiting time. In the 1990s in the United States, the listing criteria were substituted by government-regulated policies, which later established priority for candidate recipients based on disease severity, according to a medical status system. In 1998, the US Department of Health and Human Services (DHHS) defined the principles of allocation policies and procedures to guide the Organ Procurement and Transplant Network (OPTN). From 1996 to 1999, the more objective Child-Turcotte-Pugh score was also incorporated, but disparities in waiting lines across regions were mounting. For many years, the modified Child-Turcotte-Pugh score was the main tool for estimating survival without transplant and was originally developed to predict the survival rates of patients undergoing portosystemic shunt surgery[111].
Lastly, the Model for End-stage Liver Disease (MELD) was adopted for recipient ranking in February 2002 in the United States[10,110]. For paediatric patients, a similar scoring system (Pediatric End-Stage Liver Disease - PELD) was created.
Several studies have analysed the impact of the use of the MELD/PELD score[110,112,113] and have demonstrated both a reduced waiting list mortality and unchanged patient and graft survival, despite the fact that transplants are performed in patients with worse clinical conditions[10]. Figure 1 illustrates the history of organ allocation policies in liver transplant in the United States.
Figure 1.
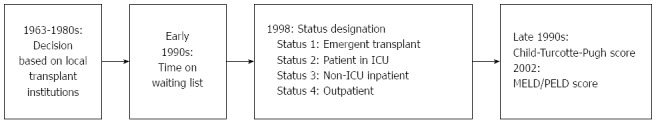
History of organ allocation policies in liver transplant in the United States. ICU: Intensive care unit; MELD: Model for End-stage Liver Disease; PELD: Pediatric End-Stage Liver Disease.
In Europe, there are no uniform rules for organ allocation. Organ procurement organisations for different countries include Eurotransplant (participating countries include Germany, The Netherlands, Belgium, Luxembourg, Austria, Slovenia, and Croatia), United Kingdom Transplant, Organización Nacional de Trasplantes in Spain, Scandiatransplant (Sweden, Finland, Norway, Denmark, and Iceland), North Italian transplant, and Agence de la Biomédecine (previously Établissement français des Greffes) in France. Although the majority of organs are allocated and transplanted within each organisation, there is some degree of collaboration among them. Within Eurotransplant and in France, allocation is patient based, but in Spain, Scandiatransplant and United Kingdom Transplant, it is centre based.
In Eurotransplant, the allocation can be categorised based on two time periods. From 2000 until 2006, recipient selection was based on a scoring system that considered the degree of medical urgency, donor weight, ABO blood group, waiting list time, and donor region[114]. Due to an increasing waiting list and positive experience with the use of MELD/PELD scores in the United States, this allocation system was implemented in December 2006. In France, grafts were allocated to a transplant centre by rotation, except for emergencies, until 2007. Within each centre, patients generally underwent transplantation according to their waiting time. In March 2007, due to high rates of mortality in some geographic areas, the French Liver Allocation Score (FLAS) was adopted. This score considers the MELD score as well as other conditions such as HCC and retransplantation indication, which are not necessarily associated with high MELD scores[115].
In the United Kingdom, an analysis of factors predicting transplant list mortality in more than 1000 patients in the waiting list identified 4 independent predictive factors, resulting in the development and validation of the UKELD score, which includes sodium in addition to the MELD score factors (bilirubin, INR of prothrombin time and serum creatinine)[116].
In July 2005, Argentina was the first country after the United States to adopt the MELD score system[117]. Initially, liver allocation was based on the location of care and time on the waiting list, with 2 categories (emergency and non-emergency patients).
In Brazil, from 1997 until 2007, liver allocation was based on the chronological order of registration on the waiting list and was substituted by the MELD/PELD scores in 2007[118].
SURVIVAL
The two main objectives of LT are to prolong survival and improve quality of life.
In the 1970s, the overall 1-year survival was approximately 30%, and the majority of patients died from rejection and/or infection[119]. Currently, 10-year survival rates may exceed 70% in many indications. The indications with better survival are generally primary biliary cirrhosis and autoimmune cirrhosis, whereas malignant liver tumours and hepatitis C may have worse outcomes due to high rates of recurrence[120]. Despite improved survival rates, LT recipients have an estimated loss of 7 years compared with an age- and sex-matched general population, with increasing differences for recipients at younger age[121].
Factors that may influence survival rates depend on donor, recipient, perioperative, and post-operative characteristics[122]. Donor parameters that may result in poorer outcomes include advanced age, high BMI, length of hospitalisation, use of vasopressors, and the presence of infection. Recipient parameters include urgent indication, renal dysfunction, age, mechanical ventilation requirement, hepatitis C, poor nutritional status, and the presence of infection. Perioperative factors include cold and warm ischaemia time, blood product requirements, and surgical difficulties. Finally, postoperative factors include primary non-function, renal dysfunction, centre experience, need for mechanical ventilation, and prolonged stay in an intensive care unit.
The majority of deaths and retransplantations occur soon after LT. The causes may vary, according to time after LT. Infection and primary non-function predominate in the early period, with perioperative factors accounting for nearly 60% of deaths in the first post-LT year[76,120]. After this initial period, de novo malignancies and other comorbidities such as cardiovascular disorders prevail alongside the recurrence of pretransplant diseases such as hepatitis C and autoimmune disease.
Long-term survival also results in higher morbidity due to prolonged immunosuppression as a result of the reactivation of prior infections, newly acquired infections, metabolic disorders (hypertension, diabetes, dyslipidemia, obesity), and de novo hepatic or extrahepatic malignancies, including posttransplant lymphoproliferative diseases (PTLDs)[76]. Cardiovascular failure and renal failure are the leading nonhepatic causes of morbidity and mortality in the long term[76].
In post-transplant care, LT institutions must include close follow-up to reduce cardiovascular risk, to prevent infections, monitor for cancer, and prevent and provide early treatment for recurrent diseases[76]. These measures impact both the survival and quality of life of these recipients.
CONCLUSION
LT has seen enormous progress over the last 50 years and can currently be considered a true life-saving procedure for patients presenting with liver failure. The main aspects that have evolved since the first series are surgical techniques and methods, the balance of immunosuppressive therapy, the prevention of infectious and non-infectious complications, and organ allocation. Much remains to be refined, such as the prevention of complications that are associated with long-term immunosuppression, organ allocation with the aim of improved survival, and the worldwide problem of organ shortage.
ACKNOWLEDGMENTS
Edson Abdala has received fees as a travel grant and for serving as a speaker for MSD, as a travel grant from Pfizer, and for serving as a speaker for Novartis.
Footnotes
P- Reviewers: Conchon S, Wang DS S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10:434–440. doi: 10.1038/nrgastro.2013.88. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, De Carlis LG, Mihaylov PV, Gridelli B, Fassati LR, Starzl TE. The first report of orthotopic liver transplantation in the Western world. Am J Transplant. 2012;12:1385–1387. doi: 10.1111/j.1600-6143.2012.04026.x. [DOI] [PubMed] [Google Scholar]
- 3.Starlz TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 4.Moore FD, Wheele HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374–387. [PMC free article] [PubMed] [Google Scholar]
- 5.Murray JE, Tilney NL, Wilson RE. Renal transplantation: a twenty-five year experience. Ann Surg. 1976;184:565–573. doi: 10.1097/00000658-197611000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calne RY, Williams R. Liver transplantation in man. I. Observations on technique and organization in five cases. Br Med J. 1968;4:535–540. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landmark article Aug 5, 1968: A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1984;252:677–679. [PubMed] [Google Scholar]
- 10.Merion RM, Sharma P, Mathur AK, Schaubel DE. Evidence-based development of liver allocation: a review. Transpl Int. 2011;24:965–972. doi: 10.1111/j.1432-2277.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 11.Calne RY, White DJ, Rolles K, Smith DP, Herbertson BM. Prolonged survival of pig orthotopic heart grafts treated with cyclosporin A. Lancet. 1978;1:1183–1185. doi: 10.1016/s0140-6736(78)90971-6. [DOI] [PubMed] [Google Scholar]
- 12.Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE. The long reach of liver transplantation. Nat Med. 2012;18:1489–1492. doi: 10.1038/nm.2927. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health Consensus Development Conference on Liver Transplantation. Sponsored by the National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases and the National Institutes of Health Office of Medical Applications of Research. Hepatology. 1984;4:1S–110S. [PubMed] [Google Scholar]
- 16.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 17.Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation] Langenbecks Arch Chir. 1988;373:127–130. [PubMed] [Google Scholar]
- 18.Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet. 1989;2:497. doi: 10.1016/s0140-6736(89)92101-6. [DOI] [PubMed] [Google Scholar]
- 19.Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. doi: 10.1056/NEJM199005243222106. [DOI] [PubMed] [Google Scholar]
- 20.Sass DA, Reich DJ. Liver transplantation in the 21st century: expanding the donor options. Gastroenterol Clin North Am. 2011;40:641–658. doi: 10.1016/j.gtc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Welch CS. A note on transplantation of the whole liver in dogs. Transpl Bull. 1955;2:54–55. [Google Scholar]
- 22.Cannon JA. Brief report. Transpl Bull. 1956;3:7. [Google Scholar]
- 23.Denmark SW, Shaw BW Jr, Starzl TE, Griffith BP. Veno-venous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum. 1983;34:380–382. [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw BW, Martin DJ, Marquez JM, Kang YG, Bugbee AC, Iwatsuki S, Griffith BP, Hardesty RL, Bahnson HT, Starzl TE. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith BP, Shaw BW, Hardesty RL, Iwatsuki S, Bahnson HT, Starzl TE. Veno-venous bypass without systemic anticoagulation for transplantation of the human liver. Surg Gynecol Obstet. 1985;160:270–272. [PMC free article] [PubMed] [Google Scholar]
- 26.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stieber AC. One surgeon’s experience with the piggyback versus the standard technique in orthotopic liver transplantation: is one better than the other? Hepatogastroenterology. 1995;42:403–405. [PubMed] [Google Scholar]
- 28.Parrilla P, Sánchez-Bueno F, Figueras J, Jaurrieta E, Mir J, Margarit C, Lázaro J, Herrera L, Gomez-Fleitas M, Varo E, et al. Analysis of the complications of the piggy-back technique in 1112 liver transplants. Transplant Proc. 1999;31:2388–2389. doi: 10.1016/s0041-1345(99)00394-2. [DOI] [PubMed] [Google Scholar]
- 29.Brostoff JM, Bhati CS, Syn WK. Late venous outflow obstruction after liver transplant: The ‘piggy-back’ syndrome. Eur J Intern Med. 2008;19:374–376. doi: 10.1016/j.ejim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Gurusamy KS, Pamecha V, Davidson BR. Piggy-back graft for liver transplantation. Cochrane Database Syst Rev. 2011;(1):CD008258. doi: 10.1002/14651858.CD008258.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Couinaud C. Le foie: etudes anatomiques et chirurgicales. 1. Paris: Masson et Cie; 1957. [Google Scholar]
- 32.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 33.Emond JC, Whitington PF, Thistlethwaite JR, Cherqui D, Alonso EA, Woodle IS, Vogelbach P, Busse-Henry SM, Zucker AR, Broelsch CE. Transplantation of two patients with one liver. Analysis of a preliminary experience with ‘split-liver’ grafting. Ann Surg. 1990;212:14–22. doi: 10.1097/00000658-199007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco, experience from 1993 to 2010. Arch Surg. 2011;146:1052–1059. doi: 10.1001/archsurg.2011.218. [DOI] [PubMed] [Google Scholar]
- 35.Doyle MB, Maynard E, Lin Y, Vachharajani N, Shenoy S, Anderson C, Earl M, Lowell JA, Chapman WC. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013;217:102–112; discussion 113-114. doi: 10.1016/j.jamcollsurg.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Lee WC, Chan KM, Chou HS, Wu TJ, Lee CF, Soong RS, Wu TH, Lee CS. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann Surg. 2013;258:306–311. doi: 10.1097/SLA.0b013e3182754b8e. [DOI] [PubMed] [Google Scholar]
- 37.Aseni P, De Feo TM, De Carlis L, Valente U, Colledan M, Cillo U, Rossi G, Mazzaferro V, Donataccio M, De Fazio N, et al. A prospective policy development to increase split-liver transplantation for 2 adult recipients: results of a 12-year multicenter collaborative study. Ann Surg. 2014;259:157–165. doi: 10.1097/SLA.0b013e31827da6c9. [DOI] [PubMed] [Google Scholar]
- 38.Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, Kiyosawa K, Ichida T. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233–1234. doi: 10.1016/s0140-6736(94)92450-3. [DOI] [PubMed] [Google Scholar]
- 39.Lo CM, Fan ST, Liu CL, Lo RJ, Lau GK, Wei WI, Li JH, Ng IO, Wong J. Extending the limit on the size of adult recipient in living donor liver transplantation using extended right lobe graft. Transplantation. 1997;63:1524–1528. doi: 10.1097/00007890-199705270-00027. [DOI] [PubMed] [Google Scholar]
- 40.Lee SG, Hwang S, Park KM, Kim KH, Ahn CS, Lee YJ, Cheon JY, Joo SH, Moon DB, Joo CW, et al. Seventeen adult-to-adult living donor liver transplantations using dual grafts. Transplant Proc. 2001;33:3461–3463. doi: 10.1016/s0041-1345(01)02491-5. [DOI] [PubMed] [Google Scholar]
- 41.Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, Tanaka K, Monden M. Operative morbidity of living liver donors in Japan. Lancet. 2003;362:687–690. doi: 10.1016/S0140-6736(03)14230-4. [DOI] [PubMed] [Google Scholar]
- 42.Kwon KH, Kim YW, Kim SI, Kim KS, Lee WJ, Choi JS. Postoperative liver regeneration and complication in live liver donor after partial hepatectomy for living donor liver transplantation. Yonsei Med J. 2003;44:1069–1077. doi: 10.3349/ymj.2003.44.6.1069. [DOI] [PubMed] [Google Scholar]
- 43.Chan SC, Fan ST, Lo CM, Liu CL, Wong J. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg. 2007;245:110–117. doi: 10.1097/01.sla.0000225085.82193.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muzaale AD, Dagher NN, Montgomery RA, Taranto SE, McBride MA, Segev DL. Estimates of early death, acute liver failure, and long-term mortality among live liver donors. Gastroenterology. 2012;142:273–280. doi: 10.1053/j.gastro.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulos GC, Radtke A, Molmenti EP, Schroeder T, Baba HA, Frilling A, Broelsch CE, Malagó M. Long-term follow-up after right hepatectomy for adult living donation and attitudes toward the procedure. Ann Surg. 2011;254:694–700; discussion 700-701. doi: 10.1097/SLA.0b013e31823594ae. [DOI] [PubMed] [Google Scholar]
- 46.Kasahara M, Umeshita K, Inomata Y, Uemoto S. Long-term outcomes of pediatric living donor liver transplantation in Japan: an analysis of more than 2200 cases listed in the registry of the Japanese Liver Transplantation Society. Am J Transplant. 2013;13:1830–1839. doi: 10.1111/ajt.12276. [DOI] [PubMed] [Google Scholar]
- 47.Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, Ahn CS, Moon DB, Hwang GS, Kim KM, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920–927. doi: 10.1002/lt.20734. [DOI] [PubMed] [Google Scholar]
- 48.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 49.Serenari M, Cescon M, Cucchetti A, Pinna AD. Liver function impairment in liver transplantation and after extended hepatectomy. World J Gastroenterol. 2013;19:7922–7929. doi: 10.3748/wjg.v19.i44.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CL, Concejero AM, Lin TS, Lin YH, Chiang YC, Wang CC, Wang SH, Lin CC, Liu YW, Yong CC. Outcome of routine use of microsurgical biliary reconstruction in pediatric living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2013;20:492–497. doi: 10.1007/s00534-013-0609-z. [DOI] [PubMed] [Google Scholar]
- 51.Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, Kiuchi T, Uemoto S, Inomata Y, Tanaka K. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235–240. doi: 10.1097/00000658-200208000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726–732. doi: 10.1097/01.tp.0000116604.89083.2f. [DOI] [PubMed] [Google Scholar]
- 53.Fan ST, Lo CM, Liu CL, Tso WK, Wong J. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676–683. doi: 10.1097/00000658-200211000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, Kiuchi T, Yazumi S, Shibata T, Tanaka K. Biliary reconstruction in right lobe living-donor liver transplantation: Comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559–566. doi: 10.1097/01.sla.0000206419.65678.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scatton O, Meunier B, Cherqui D, Boillot O, Sauvanet A, Boudjema K, Launois B, Fagniez PL, Belghiti J, Wolff P, et al. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432–437. doi: 10.1097/00000658-200103000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Icoz G, Kilic M, Zeytunlu M, Celebi A, Ersoz G, Killi R, Memis A, Karasu Z, Yuzer Y, Tokat Y. Biliary reconstructions and complications encountered in 50 consecutive right-lobe living donor liver transplantations. Liver Transpl. 2003;9:575–580. doi: 10.1053/jlts.2003.50129. [DOI] [PubMed] [Google Scholar]
- 57.Dulundu E, Sugawara Y, Sano K, Kishi Y, Akamatsu N, Kaneko J, Imamura H, Kokudo N, Makuuchi M. Duct-to-duct biliary reconstruction in adult living-donor liver transplantation. Transplantation. 2004;78:574–579. doi: 10.1097/01.tp.0000128912.09581.46. [DOI] [PubMed] [Google Scholar]
- 58.Kawachi S, Shimazu M, Wakabayashi G, Hoshino K, Tanabe M, Yoshida M, Morikawa Y, Kitajima M. Biliary complications in adult living donor liver transplantation with duct-to-duct hepaticocholedochostomy or Roux-en-Y hepaticojejunostomy biliary reconstruction. Surgery. 2002;132:48–56. doi: 10.1067/msy.2002.125314. [DOI] [PubMed] [Google Scholar]
- 59.Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, Schwartz ME, Miller C. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842–1848. doi: 10.1097/01.tp.0000123077.78702.0c. [DOI] [PubMed] [Google Scholar]
- 60.Lee KW, Joh JW, Kim SJ, Choi SH, Heo JS, Lee HH, Park JW, Lee SK. High hilar dissection: new technique to reduce biliary complication in living donor liver transplantation. Liver Transpl. 2004;10:1158–1162. doi: 10.1002/lt.20230. [DOI] [PubMed] [Google Scholar]
- 61.Sheiner PA, Varma CV, Guarrera JV, Cooper J, Garatti M, Emre S, Guy SR, Schwartz ME, Miller CM. Selective revascularization of hepatic artery thromboses after liver transplantation improves patient and graft survival. Transplantation. 1997;64:1295–1299. doi: 10.1097/00007890-199711150-00011. [DOI] [PubMed] [Google Scholar]
- 62.Millis JM, Cronin DC, Brady LM, Newell KA, Woodle ES, Bruce DS, Thistlethwaite JR, Broelsch CE. Primary living-donor liver transplantation at the University of Chicago: technical aspects of the first 104 recipients. Ann Surg. 2000;232:104–111. doi: 10.1097/00000658-200007000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikegami T, Hashikura Y, Nakazawa Y, Urata K, Mita A, Ohno Y, Terada M, Miyagawa S, Kushima H, Kondoh S. Risk factors contributing to hepatic artery thrombosis following living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:105–109. doi: 10.1007/s00534-005-1015-y. [DOI] [PubMed] [Google Scholar]
- 64.Abbasoglu O, Levy MF, Vodapally MS, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Hepatic artery stenosis after liver transplantation--incidence, presentation, treatment, and long term outcome. Transplantation. 1997;63:250–255. doi: 10.1097/00007890-199701270-00013. [DOI] [PubMed] [Google Scholar]
- 65.Kim SJ, Yoon YC, Park JH, Oh DY, Yoo YK, Kim DG. Hepatic artery reconstruction and successful management of its complications in living donor liver transplantation using a right lobe. Clin Transplant. 2011;25:929–938. doi: 10.1111/j.1399-0012.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 66.Park YS, Kim KW, Lee SJ, Lee J, Jung DH, Song GW, Ha TY, Moon DB, Kim KH, Ahn CS, et al. Hepatic arterial stenosis assessed with doppler US after liver transplantation: frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011;260:884–891. doi: 10.1148/radiol.11102257. [DOI] [PubMed] [Google Scholar]
- 67.Sugawara Y, Tamura S, Kaneko J, Iida T, Mihara M, Makuuchi M, Koshima I, Kokudo N. Single artery reconstruction in left liver transplantation. Surgery. 2011;149:841–845. doi: 10.1016/j.surg.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Marubashi S, Kobayashi S, Wada H, Kawamoto K, Eguchi H, Doki Y, Mori M, Nagano H. Hepatic artery reconstruction in living donor liver transplantation: risk factor analysis of complication and a role of MDCT scan for detecting anastomotic stricture. World J Surg. 2013;37:2671–2677. doi: 10.1007/s00268-013-2188-1. [DOI] [PubMed] [Google Scholar]
- 69.Wei WI, Lam LK, Ng RW, Liu CL, Lo CM, Fan ST, Wong J. Microvascular reconstruction of the hepatic artery in live donor liver transplantation: experience across a decade. Arch Surg. 2004;139:304–307. doi: 10.1001/archsurg.139.3.304. [DOI] [PubMed] [Google Scholar]
- 70.Zhang S, Dong Z, Zhang M, Xia Q, Liu D, Zhang JJ. Right lobe living-donor liver transplantation with or without middle hepatic vein: a meta-analysis. Transplant Proc. 2011;43:3773–3779. doi: 10.1016/j.transproceed.2011.08.100. [DOI] [PubMed] [Google Scholar]
- 71.The Familial Amyloidotic Polyneuropathy World Transplant Registry and The Domino Liver Transplant Registry. Accessed January 2014. Available from: http://www.fapwtr.org/ram_domino.htm.
- 72.Sebagh M, Yilmaz F, Karam V, Falissard B, Ichaï P, Roche B, Castaing D, Guettier C, Samuel D, Azoulay D. Cadaveric full-size liver transplantation and the graft alternatives in adults: a comparative study from a single centre. J Hepatol. 2006;44:118–125. doi: 10.1016/j.jhep.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 73.Adams D, Lacroix C, Antonini T, Lozeron P, Denier C, Kreib AM, Epelbaum S, Blandin F, Karam V, Azoulay D, et al. Symptomatic and proven de novo amyloid polyneuropathy in familial amyloid polyneuropathy domino liver recipients. Amyloid. 2011;18 Suppl 1:174–177. doi: 10.3109/13506129.2011.574354065. [DOI] [PubMed] [Google Scholar]
- 74.Antonini TM, Lozeron P, Lacroix C, Mincheva Z, Durrbach A, Slama M, Vibert E, Samuel D, Adams D. Reversibility of acquired amyloid polyneuropathy after liver retransplantation. Am J Transplant. 2013;13:2734–2738. doi: 10.1111/ajt.12378. [DOI] [PubMed] [Google Scholar]
- 75.Dienstag JL, Cosimi AB. Liver transplantation--a vision realized. N Engl J Med. 2012;367:1483–1485. doi: 10.1056/NEJMp1210159. [DOI] [PubMed] [Google Scholar]
- 76.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 77.Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, Williams R. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926–933. doi: 10.1002/hep.510270406. [DOI] [PubMed] [Google Scholar]
- 79.Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 80.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 81.Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140:51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation - still champions or threatened by serious competitors? Liver Int. 2013;33:656–665. doi: 10.1111/liv.12133. [DOI] [PubMed] [Google Scholar]
- 83.Levitsky J. Immunosuppression withdrawal following liver transplantation: the older, the wiser… but maybe too late. Hepatology. 2013;58:1529–1532. doi: 10.1002/hep.26576. [DOI] [PubMed] [Google Scholar]
- 84.de la Garza RG, Sarobe P, Merino J, Lasarte JJ, D’Avola D, Belsue V, Delgado JA, Silva L, Iñarrairaegui M, Sangro B, et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transpl. 2013;19:937–944. doi: 10.1002/lt.23686. [DOI] [PubMed] [Google Scholar]
- 85.Levitsky J. Operational tolerance: past lessons and future prospects. Liver Transpl. 2011;17:222–232. doi: 10.1002/lt.22265. [DOI] [PubMed] [Google Scholar]
- 86.Barbier L, Garcia S, Cros J, Borentain P, Botta-Fridlund D, Paradis V, Le Treut YP, Hardwigsen J. Assessment of chronic rejection in liver graft recipients receiving immunosuppression with low-dose calcineurin inhibitors. J Hepatol. 2013;59:1223–1230. doi: 10.1016/j.jhep.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 87.Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martínez-Llordella M, López M, Angelico R, Bohne F, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 88.Bohne F, Martínez-Llordella M, Lozano JJ, Miquel R, Benítez C, Londoño MC, Manzia TM, Angelico R, Swinkels DW, Tjalsma H, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122:368–382. doi: 10.1172/JCI59411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martínez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, Lerut J, Latinne D, Margarit C, Bilbao I, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Wozniak LJ, Rodder S, Heish S, Talisetti A, Wang Q, Esquivel C, Cox K, Chen R, McDiarmid SV, et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am J Transplant. 2012;12:1218–1228. doi: 10.1111/j.1600-6143.2011.03928.x. [DOI] [PubMed] [Google Scholar]
- 92.Gökmen R, Hernandez-Fuentes MP. Biomarkers of tolerance. Curr Opin Organ Transplant. 2013;18:416–420. doi: 10.1097/MOT.0b013e3283636fd5. [DOI] [PubMed] [Google Scholar]
- 93.McCaughan GW, Bowen DG, Bertolino P. Operational tolerance in liver transplantation: shall we predict or promote? Liver Transpl. 2013;19:933–936. doi: 10.1002/lt.23719. [DOI] [PubMed] [Google Scholar]
- 94.Prados E, Cuervas-Mons V, de la Mata M, Fraga E, Rimola A, Prieto M, Clemente G, Vicente E, Casanovas T, Fabrega E. Outcome of autoimmune hepatitis after liver transplantation. Transplantation. 1998;66:1645–1650. doi: 10.1097/00007890-199812270-00013. [DOI] [PubMed] [Google Scholar]
- 95.Sempoux C, Horsmans Y, Lerut J, Rahier J, Geubel A. Acute lobular hepatitis as the first manifestation of recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver. 1997;17:311–315. doi: 10.1111/j.1600-0676.1997.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 96.Czaja AJ. The immunoreactive propensity of autoimmune hepatitis: is It corticosteroid-dependent after liver transplantation? Liver Transpl Surg. 1999;5:460–463. doi: 10.1002/lt.500050512. [DOI] [PubMed] [Google Scholar]
- 97.García-Reyne A, Lumbreras C, Fernández I, Colina F, Abradelo M, Magan P, San-Juan R, Manrique A, López-Medrano F, Fuertes A, et al. Influence of antiviral therapy in the long-term outcome of recurrent hepatitis C virus infection following liver transplantation. Transpl Infect Dis. 2013;15:405–415. doi: 10.1111/tid.12097. [DOI] [PubMed] [Google Scholar]
- 98.Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O’Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193–1199. doi: 10.1016/j.jhep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed A, Keeffe EB. Current Indications and Contraindications for Liver Transplantation. Clin Liver Dis. 2007;11:227–47. doi: 10.1016/j.cld.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 100.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 101.Bernuau J, Goudeau A, Poynard T, Dubois F, Lesage G, Yvonnet B, Degott C, Bezeaud A, Rueff B, Benhamou JP. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6:648–651. doi: 10.1002/hep.1840060417. [DOI] [PubMed] [Google Scholar]
- 102.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 104.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 105.Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3–18. doi: 10.1055/s-0029-1192052. [DOI] [PubMed] [Google Scholar]
- 106.United Network for Organ Sharing. Accessed September 2013. Available from: http://www.unos.org/data/about/viewDataReports.
- 107.Samuel D, Duclos Vallee JC, Teicher E, Vittecoq D. Liver transplantation in patients with HIV infection. J Hepatol. 2003;39:3–6. doi: 10.1016/s0168-8278(03)00213-7. [DOI] [PubMed] [Google Scholar]
- 108.Shaw BW, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66–68. [PMC free article] [PubMed] [Google Scholar]
- 109.Francoz C, Belghiti J, Durand F. Indications of liver transplantation in patients with complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:175–190. doi: 10.1016/j.bpg.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Freeman RB. A decade of model for end-stage liver disease: lessons learned and need for re-evaluation of allocation policies. Curr Opin Organ Transplant. 2012;17:211–215. doi: 10.1097/MOT.0b013e3283534dde. [DOI] [PubMed] [Google Scholar]
- 111.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 112.Magder LS, Regev A, Mindikoglu AL. Comparison of seven liver allocation models with respect to lives saved among patients on the liver transplant waiting list. Transpl Int. 2012;25:409–415. doi: 10.1111/j.1432-2277.2012.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanwal F, Dulai GS, Spiegel BM, Yee HF, Gralnek IM. A comparison of liver transplantation outcomes in the pre- vs. post-MELD eras. Aliment Pharmacol Ther. 2005;21:169–177. doi: 10.1111/j.1365-2036.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- 114.Mehrabi A, Fonouni H, Müller SA, Schmidt J. Current concepts in transplant surgery: liver transplantation today. Langenbecks Arch Surg. 2008;393:245–260. doi: 10.1007/s00423-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 115.Francoz C, Belghiti J, Castaing D, Chazouillères O, Duclos-Vallèe J-C, Duvoux C, Lerut J, Le Treut Y-P, Moreau R, Mandot A, et al. Model for end-stage liver disease exceptions in the context of the french model for end-stage liver disease score–based liver allocation system. Liver Transpl. 2011;17:1137–1151. doi: 10.1002/lt.22363. [DOI] [PubMed] [Google Scholar]
- 116.Barber K, Madden S, Allen J, Collett D, Neuberger J, Gimson A. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92:469–476. doi: 10.1097/TP.0b013e318225db4d. [DOI] [PubMed] [Google Scholar]
- 117.McCormack L, Gadano A, Lendoire J, Imventarza O, Andriani O, Gil O, Toselli L, Bisigniano L, de Santibañes E. Model for end-stage liver disease-based allocation system for liver transplantation in Argentina: does it work outside the United States? HPB (Oxford) 2010;12:456–464. doi: 10.1111/j.1477-2574.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferraz-Neto BH. Meld score, step forward to justice of liver graft allocation in Brazil. Arq Gastroenterol. 2007;44:187–188. doi: 10.1590/s0004-28032007000300001. [DOI] [PubMed] [Google Scholar]
- 119.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Picache R, Husberg BS, Halgrimson CG, Schroter G. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 120.Åberg F, Isoniemi H, Höckerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- 121.Barber K, Blackwell J, Collett D, Neuberger J. Life expectancy of adult liver allograft recipients in the UK. Gut. 2007;56:279–282. doi: 10.1136/gut.2006.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saidi RF. Current status of liver transplantation. Arch Iran Med. 2012;15:772–776. [PubMed] [Google Scholar]


