Abstract
AIM: To determine the optimal initial treatment modality for acute superior mesenteric vein thrombosis (ASMVT) in patients with circumscribed peritonitis.
METHODS: A retrospective review was made of the Vascular Surgery Department’s medical records to identify adult patients (≥ 18 years old) presenting with circumscribed peritonitis and diagnosed with ASMVT by imaging or endoscopic examination. Patients were selected from the time period between October 2009 and October 2012 to assess the overall performance of a new first-line treatment policy implemented in May 2011 for patients with circumscribed peritonitis, which recommends transcatheter thrombolysis with local anticoagulation and endovascular mechanical thrombectomy. Of the 25 patients selected for study inclusion, 12 had undergone emergency surgical exploration (group 1) and 13 had undergone the initial catheter-directed thrombolysis (group 2). Data extracted from each patient’s records for statistical analyses included method of diagnosis, symptoms, etiology and risk factors, thrombus location, initial management, morbidity, mortality, duration and total cost of hospitalization (in Renminbi, RMB), secondary operation, total length of bowel resection, duration of and findings in follow-up, and death/survival.
RESULTS: The two treatment groups showed similar rates of morbidity, 30-d mortality, and 1-year survival, as well as similar demographic characteristics, etiology or risk factors, computed tomography characteristics, symptoms, findings of blood testing at admission, complications, secondary operations, and follow-up outcomes. In contrast, the patients who received the initial non-operative treatment of transcatheter thrombolysis had significantly shorter durations of admission to symptom elimination (group 1: 18.25 ± 7.69 d vs group 2: 7.23 ± 2.42 d) and hospital stay (43.00 ± 13.77 d vs 20.46 ± 6.59 d), and early enteral or oral nutrition restoration (20.50 ± 5.13 d vs 8.92 ± 1.89 d), as well as significantly less total length of bowel resection (170.83 ± 61.27 cm vs 29.23 ± 50.24 cm) and lower total cost (200020.4 ± 91505.62 RMB vs 72785.6 ± 21828.16 RMB) (P < 0.05 for all). Statistical analyses suggested that initial transcatheter thrombolysis is correlated with quicker resolution of the thrombus, earlier improvement of symptoms, stimulation of collateral vessel development, reversal of intestinal ischemia, receipt of localizing bowel resection to prevent short bowel syndrome, shorter hospitalization, and lower overall cost of treatment.
CONCLUSION: For ASMVT patients with circumscribed peritonitis, early diagnosis is key to survival, and non-operative transcatheter thrombolysis is feasible and effective as an initial treatment.
Keywords: Acute superior mesenteric venous thrombosis, Transcatheter thrombolysis, Initial management, Circumscribed peritonitis
Core tip: There is no consensus on the recommendation of initial management of acute superior mesenteric venous thrombosis (ASMVT) with moderate, circumscribed peritonitis and suspected bowel infarction. Signs of peritonitis have traditionally been considered an indicator for prompt surgery, but patients undergoing extensive bowel resection during an acute inflammatory phase frequently manifest short bowel syndrome. A non-operative treatment modality was recently developed to overcome this clinical challenge. Intravascular thrombolysis therapy has since been widely applied and favorable clinical outcomes have been reported. This study systematically investigates the outcome of transcatheter thrombolysis as a first-line treatment for ASMVT patients with circumscribed peritonitis.
INTRODUCTION
Acute superior mesenteric vein thrombosis (ASMVT) is a rare but potentially lethal abdominal condition, and accounts for 6% to 9% of all reported cases of acute mesenteric ischemia[1]. Intestinal gangrene, caused by mesenteric venous occlusion and treated by bowel resection, was first reported in 1895[2], but it was not until 1935 that mesenteric vein thrombosis (MVT) was described in detail and recognized as a distinct clinical entity[3]. To date, no distinctive symptom has been characterized, and the vague symptomological profile makes clinical diagnosis difficult. The most commonly reported clinical symptom is crampy abdominal pain, and MVT is suspected when treating physicians deem the pain to be out of proportion to other physical findings.
The advent of contrast-enhanced computed tomography (CT) and application for portal venography have remarkably improved the rate of early detection of ASMVT prior to laparotomy, with a reported sensitivity of greater than 90%[4]. The current consensus for clinical management of diagnosed ASMVT is prompt systemic anticoagulation, with imperative emergency laparotomy for cases with diffuse peritonitis or confirmed bowel perforation and/or transmural necrosis[5-7]. Cases of ASMVT with moderate, circumscribed peritonitis and suspected bowel infarction are clinically relatively common. No definitive initial management strategy has been established to address this complex condition, and conflicting data have been reported for the efficacies of prompt surgical exploration and non-operative treatment.
Signs of peritonitis have traditionally been considered as an indicator for prompt surgery[5,8]. However, the border between ischemic and viable bowel observable in exploratory laparotomy is obscure during the acute phase[9,10]. Moreover, extensive bowel resection carries a risk of short bowel syndrome (SBS), which is strongly associated with high 30-day mortality and low 5-year survival[11]. Non-operative treatment approaches, such as the recently developed intracatheter thrombolysis therapy, could achieve early recanalization of the superior mesenteric vein (SMV) to conserve as much of the bowel as possible; in general, these approaches have produced favorable clinical outcomes[12-17]. For example, compared to the mortality rates reported for surgical treatment (29%-38%), those for non-operative treatments are much lower (13%-19%)[14].
Recent retrospective studies have shown that performing initial non-surgical management with systemic anticoagulation delayed or precluded surgical treatment, thereby lowering the rates of SBS in these patients[8,17-20]. The non-surgical method of transcatheter thrombolysis therapy has been shown not only to effectively regress the ischemic lesion but also to consequently reduce the rates of cases requiring subsequent surgical treatment[13,21-24]. This study was designed to analyze and compare the features and outcomes of ASMVT cases with circumscribed peritonitis receiving prompt surgical treatment to cases given intravascular interventional therapy. The results will help to identify the optimal initial treatment strategy for this complex condition; in addition, this is the first study to investigate the clinical utility and safety of transcatheter thrombolysis as a first-line treatment for ASMVT with circumscribed peritonitis.
MATERIALS AND METHODS
Ethics statement
This retrospective observational study was designed according to the ethical principles outlined by the Declaration of Helsinki and approved by the ethics committee of Jinling Hospital.
Study design and patient selection
The medical records of Jinling Hospital’s Vascular Surgery Department were searched to identify patients with intraoperative or admitting diagnosis of acute mesenteric venous thrombosis according to the ICD-10 coding system during the 3-year period from October 2009 to October 2012. The 150 medical records retrieved were compiled into an anonymized (coded) database created using SPSS statistical software (SPSS Inc., Chicago, IL, United States). The patient data were then filtered to select for the following inclusion criteria: diagnosis of ASMVT by imaging examination [contrast-enhanced CT, magnetic resonance imaging (MRI), or digital subtraction angiography (DSA) portal venography] or exploratory laparotomy; receipt of initial treatment within 4 wk of symptom onset; adult patients aged between 18 and 80 years; signs of circumscribed peritonitis (abdominal guarding and/or rebound tenderness); and no history of splanchnic venous thrombosis. Patients were excluded according to: presence of hepatic or renal failure, malignancies or other serious systemic illnesses at admission; incomplete medical records or imaging data; follow-up participation for < 1 year; or allergy to anticoagulant or thrombolytic agents. In total, 125 patients were excluded from the study.
Twenty-five patients fit the criteria for study participation, including 12 who underwent emergency surgical exploration (group 1) and 13 who underwent initial catheter-directed thrombolysis (group 2). In agreement with accepted standards[7,25,26], our hospital considered peritonitis with suspected bowel infarction as an indicator for prompt surgery to treat ASMVT. The majority of surgically treated cases required extensive bowel resection and a high incidence of subsequent SBS was noted by the hospital staff. These administrative observations led to the implementation of a new policy in May 2011, which recommends transcatheter thrombolysis with local anticoagulation and endovascular mechanical thrombectomy in lieu of prompt laparotomy as the first-line treatment for patients with circumscribed peritonitis.
Each patient in this study was assessed for medical history, thrombus characteristics, initial management, morbidity, 30-d mortality, duration (in days) and total cost (in Renminbi, RMB) of hospitalization, secondary operation, total length (in cm) of bowel resection, duration (in days) of follow-up, and survival at 1-year.
Pre-treatment evaluation, clinical management, and follow-up
During the hospitalization period, in-patients underwent a physical examination at least twice daily, along with daily evaluation of white blood cell count and plasma C-reactive protein (CRP) and lactic acid levels. Systemic coagulation function was closely monitored by measuring the prothrombin time, activated partial thromboplastin time, international normalized ratio (INR), fibrinogen level, and antithrombin III level, and by thromboelastography. Thrombophilia screening was performed to determine the etiology. Pathological analysis of the resected bowel was performed to determine the proportion of ischemic to non-ischemic tissue.
All patients were treated with nasogastric suction (to reduce bowel movements and intraluminal pressure), broad-spectrum prophylactic antibiotics, and solutes (to maintain normal fluid, electrolyte and acid/base balance). In cases of poor microcirculation or low fluid volume, dextran was administered. In general, administration of vasopressors or somatostatin was discouraged to avoid exacerbation of the intestinal circulatory compromise. Immediately upon diagnosis, all patients were started on an anticoagulant regimen (5 mg/d oral fondaparinux sodium (GlaxoSmithKline, Greenford, United Kingdom) and 80 mL/d argatroban injection (TIPR Pharmaceutical Responsible Co., Ltd, Tianjing, China).
The transcatheter thrombolysis was administered directly via the percutaneous transhepatic or transjugular intrahepatic route, or indirectly via superior mesenteric artery access (SMA). The thrombolysis step was initiated by local delivery of an anticoagulation agent (100000 IU injection of urokinase; Nanjing Nanda Pharmaceutical Co., Ltd, Nanjing, China), after which a continuous infusion of urokinase (200000-300000 IU/d) with fondaparinux sodium (5 mg/d) plus argatroban (80 mL/d) was delivered through a 5-Fr multiple side-hole infusion catheter (Angiodynamics, Queensbury, NY, United States). In cases of secondary mesenteric arteriospasm, pavarin (Northeast Pharmaceutical Group Shenyang No. 1 Pharmaceutical Co., Ltd, Shenyang, China) was administered continuously via SMA (120 mg/d) until the condition resolved.
In cases with late stricture, bowel resection with primary anastomosis was carried out (Figure 1). In cases with bowel necrosis or perforation, a two-step resection, based on the “damage control surgery” concept[27], was carried out using jejunostomy plus ileostomy (Figure 1E), or an open abdomen technique was used with sequential resuscitation and organ function support in the intensive care unit. When thrombi were present within the trunk, intraoperative thrombectomy was performed with Fogarty catheters followed by an immediate injection of 4100 IU of low-molecular-weight heparin (Fraxiparine; GlaxoSmithKline) (Figure 1C). The definitive surgery of bowel anastomosis was scheduled to be performed following 3-6 mo of enteral nutrition therapy.
Figure 1.
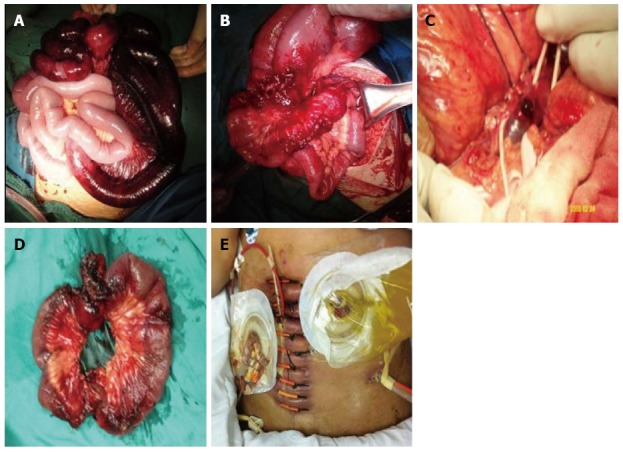
Various surgical procedures used to remove the thrombus in acute superior mesenteric vein thrombosis cases. Intraoperative images are presented from representative cases in groups 1 and 2. A: Extensive bowel ischemia during initial surgery (patient 2, group 1); B, C: Localized bowel necrosis and stricture during delayed operation after transcatheter thrombolysis (patient 3, group 2); D: Intraoperative thrombectomy (patient 3, group 1); E: Jejunostomy plus ileostomy with an open abdomen was carried out in a septic patient at a high risk for post-operative abdominal compartment syndrome and recurrence (patient 3, group 2).
Oral anticoagulation therapy (warfarin sodium; Jiufu Pharmaceutical Co., Ltd., Shanghai, China) was given as a 6-mo or permanent post-discharge regimen, respectively, for patients with known reversible factors or with idiopathic thromboembolism or thrombophilia. The patients on anticoagulation treatment were monitored by measuring the INR (target: 2-3 times the control value).
Follow-up assessment included clinical and imaging examinations, starting 1 mo post-treatment, then every 2 mo over the next 6 mo and thereafter every 3 mo. The clinical examination included clinician observations and patient reports of symptoms and signs, laboratory testing of routine blood parameters, hepatic and renal function markers and coagulation. The imaging examination included contrast-enhanced CT and ultrasonography of the portal vein and SMV. The rates of 30-d and 1-year mortality and recurrence were calculated.
Statistical analysis
All statistical analyses were carried out using the SPSS statistical software suite v14.0 (SPSS Inc.). Means ± SD of continuous variables were calculated if the values were normally distributed, otherwise median and interquartile ranges (IQR; 25th percentile to 75th percentile) were calculated. Differences between two groups were assessed by t test or Mann-Whitney U test (for normally and non-normally distributed continuous variables, respectively) or Fisher’s exact test (for categorical variables), with a P value < 0.05 set as the threshold for significance. Kaplan-Meier estimates were used to assess survival, and the log-rank test was used to test for differences in survival between groups.
RESULTS
Clinical characteristics and etiology/risk factors
The study population of 25 ASMVT patients with circumscribed peritonitis was composed of 10 women and 15 men, with a mean age of 45.08 ± 11.74 years. Among the total ASMVT study population, nine patients tested positive in thrombophilia screening; the etiologies are listed in Table 1. High-risk factors for ASMVT were found for each patient in their medical history (including on-going comorbidities) and sub-group analysis of thrombophilic and non-thrombophilic patients showed no statistically significant differences between the two (Table 1).
Table 1.
Clinical characteristics and etiology/risk factors of the acute superior mesenteric vein thrombosis patient cohort n (%)
| Variable | Overall | Group 1 | Group 2 | P value |
| (n = 25) | (n = 12) | (n = 13) | ||
| Age (yr)1 | 45.08 ± 11.74 | 46.69 ± 12.69 | 43.33 ± 10.88 | 0.48 |
| Sex | ||||
| Male | 15 (60.0) | 9 (75.0) | 6 (46.2) | 0.23 |
| Female | 10 (40.0) | 3 (25.0) | 7 (53.9) | |
| Primary | ||||
| Protein C deficiency | 2 (8.0) | 1 (8.3) | 1 (7.7) | 1.00 |
| Protein S deficiency | 2 (8.0) | 0 (0.0) | 2 (15.4) | 0.48 |
| Antithrombin IIIdeficiency | 2 (8.0) | 1 (8.3) | 1 (7.7) | 1.00 |
| Factor V Leiden disorder | 1 (4.0) | 1 (8.3) | 0 (0.0) | 0.48 |
| Antiphospholipid syndrome | 2 (8.0) | 1 (8.3) | 1 (7.7) | 1.00 |
| No thrombophilia | 16 (64.0) | 8 (66.7) | 8 (61.5) | 1.00 |
| Secondary | ||||
| History of splenectomy | 6 (24.0) | 4 (33.3) | 2 (15.4) | 0.38 |
| Other earlier abdominal surgery | 6 (24.0) | 4 (33.3) | 2 (15.4) | 0.38 |
| Prior DVT/PE | 3 (12.0) | 1 (8.3) | 2 (15.4) | 1.00 |
| Cerebral infarction | 4 (16.0) | 3 (25.0) | 1 (7.7) | 0.32 |
| Pancreatitis | 2 (8.0) | 1 (8.3) | 1 (7.7) | 1.00 |
| Inflammatory bowel disease | 1 (4.0) | 1 (8.3) | 0 (0.0) | 0.48 |
| Oral contraceptive use | 3 (12.0) | 1 (8.3) | 2 (15.4) | 1.00 |
| Pregnancy | 3 (12.0) | 2 (16.7) | 1 (7.7) | 0.59 |
| Alcohol abuse | 4 (16.0) | 2 (16.7) | 2 (15.4) | 1.00 |
| Malignancy | 3 (12.0) | 1 (8.3) | 2 (15.4) | 1.00 |
| Cirrhosis/portal hypertension | 8 (32.0) | 3 (25.0) | 5 (38.5) | 0.67 |
| Bilharzial cirrhosis | 2 (8.0) | 1 (8.3) | 1 (7.7) | 1.00 |
| Non-bilharzial cirrhosis | 6 (24.0) | 2 (16.7) | 4 (30.8) | 0.65 |
| Diabetes mellitus | 1 (4.0) | 1 (8.3) | 0 (0.0) | 0.48 |
| Hypertension | 1 (4.0) | 0 (0.0) | 1 (7.7) | 1.00 |
Analysis was performed by t test or Fisher’s exact test. DVT: Deep vein thrombosis; PE: Pulmonary embolism.
Means of diagnosis among the two treatment-type groups were distinct in that group 1 mainly occurred by exploratory laparotomy (n = 10) with the remaining 2 patients diagnosed by CT portal venography, in contrast to the majority of patients in group 2 who were primarily diagnosed by CT portal venography (n = 12) with the remaining 1 patient diagnosed by MRI portal venography. The most common presenting symptoms were abdominal pain and distension; however, sub-group analysis of groups 1 and 2 showed no significant differences for any of the presenting symptoms. In contrast, group 1 did show a significantly shorter duration of symptoms before admission (6.25 ± 2.09 d vs group 2: 9.07 ± 2.40 d, P < 0.05). For the total ASMVT study group, the most common clinical signs at hospital admission were decreased bowel sounds and ascites, and pyrexia was infrequent; all but one patient presented with an elevated CRP level (75.50 ± 75.59 mg/L; normal value: < 8 mg/L).
Imaging characteristics
According to results from enhanced helical CT portal venography scanning for the total ASMVT study group, thrombi were most common in the SMV + PV (portal vein); however, the thrombus location profiles of groups 1 and 2 were not significantly different. All of the ASMVT-related features detected by CT upon admission were remarkably improved following receipt of the thrombolysis therapy (Figure 2C, D, F and G). Two of the patients in group 2 with CT-detected thrombi also underwent MRI portal venography (Figure 2A and B). The extent of thrombolysis achieved by catheter-directed therapy of SMV was assessed using DSA and color duplex ultrasonography (Figure 2E and H).
Figure 2.
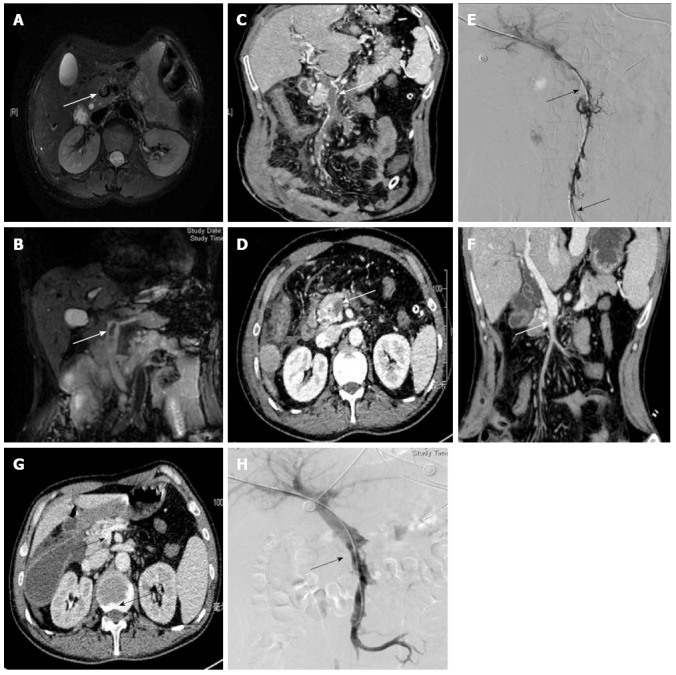
Portal venography results from different modalities used at admission and after thrombolysis. A, B: Transverse and coronal reformation of magnetic resonance imaging portal venography showed hypointensity in the superior mesenteric venous (SMV) (white arrow) at admission; C, D, F, G: Transverse and coronal reformation on three-dimensional helical computed tomography (CT) portal venography showed a filling defect and refilling in the SMV (white arrow) at admission and after thrombolysis; contrast enhanced CT image showed bowel dilatation, bowel wall thickening, and peritoneal effusion secondary to acute superior mesenteric vein thrombosis; E, H: Portal venography in digital subtraction angiography showed a filling defect and refilling of contrast medium in the trunk of the SMV at admission and after thrombolysis.
The CT image taken at admission was used to estimate the length of bowel affected by ischemia and compared to the length of resected bowel. As shown in Table 2, the thrombolysis therapy led to significantly shorter sections of bowel requiring resection.
Table 2.
Procedure and outcome of transcatheter thrombolysis therapy in group 2 n (%)
| Patient No. | Route | Duration of thrombolysis (d) | Lysis degree | Percentage of ischemic bowel at admission (estimated length in cm) | Length of resection (cm) | P value |
| 1 | SMA | 13 | Complete | 10 (50) | 0 | |
| 2 | PT | 14 | Partial | 20 (100) | 0 | |
| 3 | PT | 12 | Complete | 10 (50) | 0 | |
| 4 | TI | 15 | Complete | 15 (75) | 0 | |
| 5 | TI + SMA | 13 | Partial | 30 (150) | 80 | |
| 6 | TI + SMA | 12 | Partial | 20 (100) | 50 | |
| 7 | PT | 10 | Complete | 10 (50) | 0 | |
| 8 | TI + SMA | 11 | Complete | 20 (100) | 0 | |
| 9 | TI + SMA | 12 | Partial | 50 (250) | 150 | |
| 10 | SMA | 15 | Partial | 40 (200) | 100 | |
| 11 | PT + SMA | 12 | Complete | 15 (75) | 0 | |
| 12 | SMA | 13 | Complete | 15 (75) | 0 | |
| 13 | TI | 14 | Partial | 25 (125) | 0 | |
| Average | 12.77 ± 1.48 | 100 (range: 62.5-137.5) | 0 (range: 0-65) | 0.002 |
SMA: Superior mesenteric artery access; PT: Percutaneous transhepatic route; TI: Transjugular intrahepatic route.
Treatment course
All patients in this study were diagnosed in less than one week. After resuscitation and receipt of initial anticoagulation and supportive care, patients in group 1 underwent extensive bowel resection surgery (mean resected length: 170.83 ± 61.27 cm) within 12 h of admission (Figure 1A). Eight of the patients in group 1 received post-operative systemic anticoagulation, and four of the patients underwent transcatheter thrombolysis with local anticoagulation 3-6 d after the surgery. Two patients necessitated a second-look laparotomy due to suspicion of perforation and stricture, respectively (Table 3).
Table 3.
Treatment outcomes of acute superior mesenteric vein thrombosis patients n (%)
| Outcome | Overall (n = 25) | Group 1 (n = 12) | Group 2 (n = 13) | P value |
| Morbidity | ||||
| Bleeding | 4 (16.0) | 1 (8.3) | 3 (23.1) | 0.590 |
| Wound infection | 3 (12.0) | 3 (25.0) | 0 (0.0) | 0.100 |
| Acute kidney injury | 4 (16.0) | 2 (16.7) | 2 (15.4) | 1.000 |
| Short bowel syndrome | 5 (20.0) | 5 (41.7) | 0 (0.0) | 0.020 |
| Secondary portal hypertension | 1 (4.0) | 1 (8.3) | 0 (0.0) | 0.480 |
| Cavernous transformation of the portal vein | 2 (8.0) | 2 (16.7) | 0 (0.0) | 0.220 |
| Delayed or secondary operation | 6 (24.0) | 2 (16.7) | 4 (30.8) | 0.650 |
| Bowel transmural necrosis | 2 (8.0) | 0 (0.0) | 2 (15.4) | 0.480 |
| Bowel perforation | 1 (4.0) | 1 (8.3) | 0 (0.0) | 0.480 |
| Bowel stricture | 3 (12.0) | 1 (8.3) | 2 (15.4) | 1.000 |
| Laparoscopic surgery | 4 (16.0) | 0 (0.0) | 4 (30.8) | 0.100 |
| Length of resection (cm)2 | 100 (range: 0, 155) | 155 (105, 200) | 0 (0, 65) | < 0.001 |
| Time from treatment to symptom elimination1 | 12.52 ± 7.85 | 18.25 ± 7.69 | 7.23 ± 2.42 | < 0.001 |
| Time from admission to enteral or oral nutrition (d)1 | 14.48 ± 6.98 | 20.50 ± 5.13 | 8.92 ± 1.89 | < 0.001 |
| Duration of hospitalization in (d)1 | 31.28 ± 15.51 | 43.00 ± 13.77 | 20.46 ± 6.59 | < 0.001 |
| Total cost in RNB1 | 133895.20 ± 90996.47 | 200020.40 ± 91505.62 | 72785.60 ± 21828.16 | 0.001 |
| < 30-d mortality | 24 (96.0) | 11 (91.7) | 0 (0.0) | 0.480 |
| 1-yr survival | 21 (84.0) | 9 (75.0) | 12 (92.3) | 0.320 |
Analysis was performed by t test;
Mann-Whitney U test; or Fisher’s exact test.
The features of the initial transcatheter thrombolysis given to each patient in group 2 are summarized in Table 2. Complete lysis was achieved in most (11/13) cases; however, 4 of those patients required delayed bowel resection to treat localized bowel infarction (n = 2) and secondary stricture (n = 2). In addition, 4 patients underwent diagnostic laparoscopy to assess bowel viability and bowel necrosis or potential perforation during the thrombolysis treatment course (Table 3).
Outcome, complications and findings during follow-up
As shown in Table 3, group 2 had a significantly lower rate of SBS and significantly better treatment-related characteristics, including length of resection, days from treatment to symptom elimination, days from admission to enteral or oral nutrition, length of hospitalization, and total cost. However, there was no significant difference between groups 1 and 2 in the rates of 30-d mortality and 1-year survival. One patient in group 1 died of septic shock during hospitalization.
Group 2 had a shorter mean duration of follow-up (28.3 ± 13.5 mo vs 19.0 ± 4.8 mo). During the follow-up, 3 patients in group 1 developed complications, including secondary portal hypertension (n = 1) and cavernous transformation of the portal vein (n = 2); no patients in group 2 developed complications during the follow-up. In addition, a total of 3 patients in group 1 died during the follow-up period, and the causes included acute myocardial infarction (at follow-up month 2), massive variceal hemorrhage (at follow-up month 7), and clear-cell carcinoma of kidney (at follow-up month 8). One patient in group 2 died of multiple organ dysfunction syndrome (at follow-up month 9). No patient in either group experienced thrombosis recurrence.
DISCUSSION
In recent years, the ASMVT incidence has increased worldwide; taking Sweden as an example, the incidence of 2.0 per 100000 patient-years reported between 1970 and 1982 rose to 2.7 per 100000 patient-years between 2000 and 2006[28]. This increase in diagnosis may merely reflect the improved diagnostic capabilities and widespread application of high-resolution CT venography[4,19]. DSA portal venography, the gold-standard of MVT, is reserved for equivocal cases on non-invasive imaging and used in conjunction with catheter-directed thrombolysis[18].
The etiological profile of ASMVT has remained steady during this period of increased incidence, with the two main causes being primary hypercoagulable states (i.e., factor V Leiden gene mutation, hyperhomocysteinemia, hyperfibrinogenemia, paroxysmal, nocturnal hemoglobinuria, antithrombin III deficiency, protein C/S deficiency, antiphospholipid or anticardiolipin antibody syndrome, collagen vascular disease, and dysfibrinogenemia) and local inflammation factors (i.e., cirrhosis, portal hypertension, abdominal trauma, post-splenectomy, malignancy, inflammatory state, pregnancy, and oral contraceptive use)[8,29]. Not surprisingly, the number of primary or idiopathic ASMVT cases has continued to decrease as the ability to diagnose inherited hypercoagulable states has continued to improve[6].
Advances in the diagnostic technologies and attainment of a better understanding of the predisposing factors for this disease have promoted physicians’ abilities to recognize cases in early stages and to initiate treatment when non-operative approaches may be feasible[8,18]. Absence of transmural bowel necrosis is the prerequisite to initiate non-operative treatment for ASMVT. Peritoneal signs have traditionally been considered as an indication for surgical exploration[30]. However, cases have been reported of patients without peritonitis that require emergency surgery during anticoagulation therapy for mucosal necrosis (instead of transmural necrosis) as well as for delayed bowel perforation secondary to transmural necrosis[14,17]. Further research is necessary to understand the potential new indications for emergency surgical exploration, such as specific CT findings and measurements of gastric intramural pH measurement or serum markers, because peritonitis may not strictly correlate with bowel infarction. In fact, some investigators have already suggested new criteria for prompt surgery, including bowel-wall thickness and enhancement on the arterial phase of CT[14,31]. In our experience, patients with severe, diffuse peritonitis, particularly those featuring pungent smell, fresh appearance and bloody ascites, should be treated by laparotomy as soon as possible; in contrast, initial operation should be avoided for patients with moderate, localized peritonitis, if recanalization could be achieved in a short period.
Historically, the initial management of ASMVT has mainly relied upon early surgical exploration[32]. However, no guideline of optimal management has been developed and no consensus has been reached for the complex cases of suspected bowel infarction in particular. Early anticoagulation to prevent thrombus propagation is a mainstay to avoid resection of the macroscopically infarcted (but potentially reversible) bowel because non-transmural infarction is a common clinical observation (in 83% of resected specimens of early surgery)[8,17,18,33]. Nevertheless, for ASMVT patients with circumscribed peritonitis or extensive thrombi, conservative management of systemic anticoagulation requires a prolonged treatment period and hospitalization yet is associated with a low likelihood of rapid thrombus removal and high risk of hemorrhage[5]. Additionally, secondary mesenteric arteriolar spasm frequently occurs with delayed transmural infarction[1,14]. Moreover, the safety and efficacy of systemic anticoagulation in portomesenteric venous thrombosis patients with cirrhosis or acute pancreatitis remains controversial[34,35].
In this study, catheter-directed thrombolysis was used as the initial treatment for ASMVT patients with circumscribed peritonitis and favorable outcomes were achieved. At our institute, interventional route selection involves panel discussion by a medical team made up of interventionalists, radiologists and gastrointestinal surgeons, who consider the thrombus extent and location (according to CT imaging) and the patient’s vascular and general conditions. Percutaneous transhepatic access is technically easier under the guidance of ultrasound or X-ray, and is most effective in removal of larger thrombi within the trunk of SMV[22,36-38]. Since this approach traverses the hepatic capsule and the thrombolysis plus anticoagulation are initiated sequentially, then postinterventional embolization of the tract is required to prevent intraperitoneal or subcapsular hepatic hemorrhage[39,40]. For patients with ascites or coagulation disorder, a transjugular intrahepatic approach is safer since it avoids traversing the hepatic capsule and allows for additional therapies to be implemented, such as venoplasty and stent placement for elastic recoil or stenosis[23,24,41,42].
Despite the technical difficulty and potential for inducing hepatic function injury or intra-abdominal bleeding[23], this route is most often applied at our institute. Indirect thrombolytic therapy via SMA is technically more simple and efficient in resolving thrombi within capillaries and venules[21,43]. Furthermore, pavarin infusion into SMA is effective for relieving mesenteric arteriospasm secondary to venous engorgement[1]. However, this approach may result in lytic agents being diverted through patent branches or collaterals, ultimately prolonging the thrombolysis[13,44]. Techniques of mechanical thrombectomy (e.g., stent implantation, angioplasty, transjugular intrahepatic portosystemic shunt creation, and aspiration thrombectomy), which augment the ability of rapid thrombus removal and recanalization, have evolved as a significant step in thrombolysis[37,45,46].
It is critical to monitor a patient’s response to therapy during the initial thrombolysis treatment. Urgent surgery remains the only option to treat irreversible mesenteric venous ischemia that develops in response to either bowel gangrene or perforation and late stricture after SMV recanalization. Postoperative recurrent thrombosis, which frequently develops at the anastomosis site, is catastrophic due to the secondary extended bowel resection and extra risk associated with the re-operation procedure[47,48]. The liberal application of a “second-look” laparotomy, which a recent study indicated as positive in only 23.5% of cases[20], has resulted in an unacceptable amount of cases put at unnecessary risk[49,50]. Therefore, the two-stepped operation modality based on the “damage control surgery” concept has been employed at our institute[51,52]. In our experience, laparoscopy is a safe, effective and minimally invasive tool to assess bowel viability for ASMVT, either in early surgical exploration during thrombolysis or as a post-operative second-look strategy; this approach has been reported to prevent unnecessary laparotomies when noninvasive investigations fail to detect the degree of bowel ischemia[53,54].
Both anticoagulation and thrombolysis predispose a patient to pathological hemorrhage. Close monitoring of systemic coagulation by daily testing using thromboelastography was used in this study, and only 1 case of transient bleeding at the puncture site and 1 case of gastrointestinal bleeding occurred in the study cohort. Extrahepatic portal hypertension and cavernous transformation of the portal vein might have been prevented, as no patients developed these two long-term complications; other studies of transcatheter thrombolysis for ASMVT reported similar results during the follow-up[13,21-24].
Some limitations to the current study design may have impacted our findings and should be considered when interpreting our results. Mainly, the inherent traits of a retrospective observational study and the single-institution series are of concern. On account of the rareness of ASMVT, the number of cases in our cohort was small. Although the thrombus location in each patient was classified, stratification analysis by precise location was not performed, and this may be a variable that could impact the treatment outcome. During the study period, a detailed algorithm of treatment had not been established at our institute. In addition, long-term follow-up outcomes are not available yet. Randomized prospective trials are underway to provide higher quality evidence in the future.
In summary, initial transcatheter thrombolysis for ASMVT is feasible and efficient when early diagnosis is established by CT portal venography. Though initial surgical and non-operative management produced similar rates of morbidity, mortality, and overall survival in this small-scale study, initial catheter-directed thrombolysis is beneficial in avoiding extensive resection of potentially reversible bowel to prevent SBS. In addition, the thrombus was resolved with abundant collateral vessel development and rapid improvement in symptomology. Patients were able to undergo earlier enteral or oral nutrition and experienced a shortened hospitalization with less cost. New indicators of prompt surgery, augmenting the signs of peritonitis alone, are necessary and should be addressed by future studies.
COMMENTS
Background
Acute superior mesenteric vein thrombosis (ASMVT) is a rare but potentially lethal abdominal condition. Recent widespread use of contrast-enhanced computed tomography portal venography has facilitated early detection of ASMVT prior to laparotomy, thereby reducing the high mortality rate of cases. Currently, the consensus for management is prompt initiation of systemic anticoagulants upon diagnosis, which has been demonstrated as more effective for patients without peritonitis; moreover, emergency laparotomy is imperative for cases with diffuse peritonitis or other definitive evidence of bowel perforation and transmural necrosis. However, ASMVT with moderate, circumscribed peritonitis and suspected bowel infarction is frequently encountered by treating physicians. The optimal approach for initial management remains to be established with reports of outcomes from prompt surgical exploration and non-operative treatment modalities showing inconsistent findings.
Research frontiers
Endovascular therapies, such as transcatheter thrombolysis via percutaneous transhepatic, transjugular intrahepatic, superior mesenteric arterial route or surgically placed mesenteric vein catheter, mechanical thrombectomy and other endovascular manipulations, alone or as part of a hybrid procedure, have yielded favorable clinical outcomes and are emerging as a key method for revascularization in ASMVT.
Innovations and breakthroughs
Clinical signs of peritonitis are often considered an indicator for prompt surgery. Unfortunately, the border between ischemic and viable bowel is not clear when the disease is in the acute phase; moreover, surgeons must be careful not to perform too extensive resection of the bowel as there is a high risk for inducing short bowel syndrome, which is strongly associated with high 30-d mortality and low 5-year survival. Non-operative treatment modalities, especially the recently developed intravascular thrombolysis therapy, can achieve early recanalization of the superior mesenteric vein and conserve as much of the ischemic bowel as possible; as such, these procedures have gained popularity as a treatment for ASMVT and the reported clinical outcomes have been largely favorable. The study described herein represents the first reported investigation of the efficacy and safety of non-operative transcatheter thrombolysis-based management, compared with exploratory surgery, as a first-line treatment using a cohort of ASMVT patients.
Applications
The results suggest that ASMVT patients with circumscribed peritonitis benefit from initial transcatheter thrombolysis by achieving rapid resolution of their thrombi, early improvement of their symptoms, stimulated development of collateral vessels, reversal of intestinal ischemia, and better localization of the necessary bowel length for resection to prevent short bowel syndrome; ultimately, use of this non-surgical procedure as a first-line treatment shortens the patient’s hospitalization and decreased the monetary cost.
Terminology
Mesenteric vein thrombosis is an uncommon yet potentially life-threatening abdominal disease, which is established during hypercoagulable states and local-abdominal processes and leads to impaired venous return, bowel-wall edema, impaired microvascular perfusion, bowel distention, and consequent infarction of the involved intestinal segments; the most common vessels involved include the superior mesenteric vein, the portal vein, and the inferior mesenteric vein. Transcatheter thrombolysis is a non-surgical medical procedure used to treat vascular diseases, such as mesenteric vein thrombosis, which can be delivered directly (via the percutaneous transhepatic and transjugular intrahepatic route) or indirectly (via superior mesenteric artery access).
Peer review
The authors present their experience with transcutaneous catheter-directed thrombolysis as a treatment for mesenteric vein thrombosis. The data of patient outcome, as compared to that of the traditional exploratory surgery, provide novel insights that will be of particular interest to treating physicians as a consensus of clinical management is currently lacking for this life-threatening disease. This retrospective clinical study was well-designed and the results indicate that initial transcatheter thrombolysis for ASMVT is a feasible and efficient approach for treating patients when an early diagnosis is established by contrast-enhanced computed tomography of the portal vein phase.
Footnotes
Supported by Grants from the National Science Foundation of China, No. 81300278; the Natural Science Foundation of Jiangsu Province, No. BK20130697; and the Jiangsu Provincial Special Program of Medical Science, No. BL2012006
P- Reviewers: Ciccone MM, Lazo-Langner A S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Singal AK, Kamath PS, Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc. 2013;88:285–294. doi: 10.1016/j.mayocp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Elliot JW. II. The Operative Relief of Gangrene of Intestine Due to Occlusion of the Mesenteric Vessels. Ann Surg. 1895;21:9–23. doi: 10.1097/00000658-189521060-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren S, Eberhardt TP. Mesenteric venous thrombosis. Surg Obstet Gynecol. 1935;61:102–120. [Google Scholar]
- 4.Morasch MD, Ebaugh JL, Chiou AC, Matsumura JS, Pearce WH, Yao JS. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg. 2001;34:680–684. doi: 10.1067/mva.2001.116965. [DOI] [PubMed] [Google Scholar]
- 5.Bergqvist D, Svensson PJ. Treatment of mesenteric vein thrombosis. Semin Vasc Surg. 2010;23:65–68. doi: 10.1053/j.semvascsurg.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407–418. doi: 10.1177/1358863X10379673. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–1688. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 8.Joh JH, Kim DI. Mesenteric and portal vein thrombosis: treated with early initiation of anticoagulation. Eur J Vasc Endovasc Surg. 2005;29:204–208. doi: 10.1016/j.ejvs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Grisham A, Lohr J, Guenther JM, Engel AM. Deciphering mesenteric venous thrombosis: imaging and treatment. Vasc Endovascular Surg. 2005;39:473–479. doi: 10.1177/153857440503900603. [DOI] [PubMed] [Google Scholar]
- 10.Hefny AF, Ahmed I, Branicki FJ, Ramadan K, Czechowski J, Abu-Zidan FM. Management of mesenteric vascular occlusion. Singapore Med J. 2008;49:316–319. [PubMed] [Google Scholar]
- 11.Abu-Daff S, Abu-Daff N, Al-Shahed M. Mesenteric venous thrombosis and factors associated with mortality: a statistical analysis with five-year follow-up. J Gastrointest Surg. 2009;13:1245–1250. doi: 10.1007/s11605-009-0833-7. [DOI] [PubMed] [Google Scholar]
- 12.Henao EA, Bohannon WT, Silva MB. Treatment of portal venous thrombosis with selective superior mesenteric artery infusion of recombinant tissue plasminogen activator. J Vasc Surg. 2003;38:1411–1415. doi: 10.1016/s0741-5214(03)01052-8. [DOI] [PubMed] [Google Scholar]
- 13.Hollingshead M, Burke CT, Mauro MA, Weeks SM, Dixon RG, Jaques PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651–661. doi: 10.1097/01.RVI.0000156265.79960.86. [DOI] [PubMed] [Google Scholar]
- 14.Brunaud L, Antunes L, Collinet-Adler S, Marchal F, Ayav A, Bresler L, Boissel P. Acute mesenteric venous thrombosis: case for nonoperative management. J Vasc Surg. 2001;34:673–679. doi: 10.1067/mva.2001.117331. [DOI] [PubMed] [Google Scholar]
- 15.Semiz-Oysu A, Keussen I, Cwikiel W. Interventional radiological management of prehepatic obstruction of [corrected] the splanchnic venous system. Cardiovasc Intervent Radiol. 2007;30:688–695. doi: 10.1007/s00270-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 16.Espeel B, Gérard C, Mansvelt B, Bertrand C, Vermonden J. [Extensive mesenteric venous thrombosis treatment by regional thrombolysis] Ann Fr Anesth Reanim. 2005;24:274–277. doi: 10.1016/j.annfar.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Cenedese A, Monneuse O, Gruner L, Tissot E, Mennesson N, Barth X. Initial management of extensive mesenteric venous thrombosis: retrospective study of nine cases. World J Surg. 2009;33:2203–2208. doi: 10.1007/s00268-009-0168-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Duan ZQ, Song QB, Luo YW, Xin SJ, Zhang Q. Acute mesenteric venous thrombosis: a better outcome achieved through improved imaging techniques and a changed policy of clinical management. Eur J Vasc Endovasc Surg. 2004;28:329–334. doi: 10.1016/j.ejvs.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Alvi AR, Khan S, Niazi SK, Ghulam M, Bibi S. Acute mesenteric venous thrombosis: improved outcome with early diagnosis and prompt anticoagulation therapy. Int J Surg. 2009;7:210–213. doi: 10.1016/j.ijsu.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Al Salamah SM. Acute mesenteric venous thrombosis: management controversies. JK-Practitioner. 2004;11:242–247. [Google Scholar]
- 21.Wang MQ, Guo LP, Lin HY, Liu FY, Duan F, Wang ZJ. Transradial approach for transcatheter selective superior mesenteric artery urokinase infusion therapy in patients with acute extensive portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol. 2010;33:80–89. doi: 10.1007/s00270-009-9777-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Patra A, Khan J, Arepally A, Streiff MB. Transhepatic catheter-directed thrombectomy and thrombolysis of acute superior mesenteric venous thrombosis. J Vasc Interv Radiol. 2005;16:1685–1691. doi: 10.1097/01.RVI.0000182156.71059.B7. [DOI] [PubMed] [Google Scholar]
- 23.Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Acute symptomatic mesenteric venous thrombosis: treatment by catheter-directed thrombolysis with transjugular intrahepatic route. Abdom Imaging. 2011;36:390–398. doi: 10.1007/s00261-010-9637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang MQ, Lin HY, Guo LP, Liu FY, Duan F, Wang ZJ. Acute extensive portal and mesenteric venous thrombosis after splenectomy: treated by interventional thrombolysis with transjugular approach. World J Gastroenterol. 2009;15:3038–3045. doi: 10.3748/wjg.15.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarapurkar DN, Patel ND, Jatania J. Primary mesenteric venous thrombosis: a study from western India. Indian J Gastroenterol. 2007;26:113–117. [PubMed] [Google Scholar]
- 26.Endean ED, Barnes SL, Kwolek CJ, Minion DJ, Schwarcz TH, Mentzer RM. Surgical management of thrombotic acute intestinal ischemia. Ann Surg. 2001;233:801–808. doi: 10.1097/00000658-200106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chovanes J, Cannon JW, Nunez TC. The evolution of damage control surgery. Surg Clin North Am. 2012;92:859–75, vii-viii. doi: 10.1016/j.suc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245–1251. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 29.Okoli A. Additional information on mesenteric venous thrombosis. Mayo Clin Proc. 2013;88:902–903. doi: 10.1016/j.mayocp.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Hassan HA, Raufman JP. Mesenteric venous thrombosis. South Med J. 1999;92:558–562. doi: 10.1097/00007611-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kim HK, Chun JM, Huh S. Anticoagulation and delayed bowel resection in the management of mesenteric venous thrombosis. World J Gastroenterol. 2013;19:5025–5028. doi: 10.3748/wjg.v19.i30.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grendell JH, Ockner RK. Mesenteric venous thrombosis. Gastroenterology. 1982;82:358–372. [PubMed] [Google Scholar]
- 33.Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17–27. doi: 10.1002/bjs.4459. [DOI] [PubMed] [Google Scholar]
- 34.Congly SE, Lee SS. Portal vein thrombosis: should anticoagulation be used? Curr Gastroenterol Rep. 2013;15:306. doi: 10.1007/s11894-012-0306-0. [DOI] [PubMed] [Google Scholar]
- 35.Park WS, Kim HI, Jeon BJ, Kim SH, Lee SO. Should anticoagulants be administered for portal vein thrombosis associated with acute pancreatitis? World J Gastroenterol. 2012;18:6168–6171. doi: 10.3748/wjg.v18.i42.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Choi L, Lin PH, Dardik A, Eraso A, Lumsden AB. Percutaneous transhepatic thrombectomy and pharmacologic thrombolysis of mesenteric venous thrombosis. Vascular. 2007;15:41–45. doi: 10.2310/6670.2007.00013. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Kuroki K, Yanaga K. Percutaneous transhepatic mechanical thrombectomy for acute mesenteric venous thrombosis. J Endovasc Ther. 2005;12:508–511. doi: 10.1583/04-1335MR.1. [DOI] [PubMed] [Google Scholar]
- 38.Lopera JE, Correa G, Brazzini A, Ustunsoz B, Patel S, Janchai A, Castaneda-Zuniga W. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg. 2002;36:1058–1061. doi: 10.1067/mva.2002.127526. [DOI] [PubMed] [Google Scholar]
- 39.Ozkan U, Oğuzkurt L, Tercan F, Tokmak N. Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol. 2006;12:105–107. [PubMed] [Google Scholar]
- 40.Goldberg MF, Kim HS. Treatment of acute superior mesenteric vein thrombosis with percutaneous techniques. AJR Am J Roentgenol. 2003;181:1305–1307. doi: 10.2214/ajr.181.5.1811305. [DOI] [PubMed] [Google Scholar]
- 41.Ferro C, Rossi UG, Bovio G, Dahamane M, Centanaro M. Transjugular intrahepatic portosystemic shunt, mechanical aspiration thrombectomy, and direct thrombolysis in the treatment of acute portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol. 2007;30:1070–1074. doi: 10.1007/s00270-007-9137-z. [DOI] [PubMed] [Google Scholar]
- 42.Sze DY, O’Sullivan GJ, Johnson DL, Dake MD. Mesenteric and portal venous thrombosis treated by transjugular mechanical thrombolysis. AJR Am J Roentgenol. 2000;175:732–734. doi: 10.2214/ajr.175.3.1750732. [DOI] [PubMed] [Google Scholar]
- 43.Safieddine N, Mamazza J, Common A, Prabhudesai V. Splenic and superior mesenteric artery thrombolytic infusion therapy for acute portal and mesenteric vein thrombosis. Can J Surg. 2007;50:68–69. [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H, Kim TH, Oh HJ, Kim JI, Park SH, Han JY, Kim JK, Choi KY, Chung IS, Lee SH, et al. [Portal and superior mesenteric venous thrombosis treated with urokinase infusion via superior mesenteric artery] Korean J Gastroenterol. 2006;48:46–50. [PubMed] [Google Scholar]
- 45.Nakayama S, Murashima N, Isobe Y. Superior mesenteric venous thrombosis treated by direct aspiration thrombectomy. Hepatogastroenterology. 2008;55:367–370. [PubMed] [Google Scholar]
- 46.Rossi C, Zambruni A, Ansaloni F, Casadei A, Morelli C, Bernardi M, Trevisani F. Combined mechanical and pharmacologic thrombolysis for portal vein thrombosis in liver-graft recipients and in candidates for liver transplantation. Transplantation. 2004;78:938–940. doi: 10.1097/01.tp.0000137104.38602.9f. [DOI] [PubMed] [Google Scholar]
- 47.Clavien PA, Dürig M, Harder F. Venous mesenteric infarction: a particular entity. Br J Surg. 1988;75:252–255. doi: 10.1002/bjs.1800750322. [DOI] [PubMed] [Google Scholar]
- 48.Rhee RY, Gloviczki P, Mendonca CT, Petterson TM, Serry RD, Sarr MG, Johnson CM, Bower TC, Hallett JW, Cherry KJ. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20:688–697. doi: 10.1016/s0741-5214(94)70155-5. [DOI] [PubMed] [Google Scholar]
- 49.Alam MK, El-Kayali A, Mohammed AA, Galul RT. Superior mesenteric venous thrombosis: Riyadh Central Hospital experience. Kuwait Med J. 2001;33:18–21. [Google Scholar]
- 50.Rhee RY, Gloviczki P. Mesenteric venous thrombosis. Surg Clin North Am. 1997;77:327–338. doi: 10.1016/s0039-6109(05)70552-1. [DOI] [PubMed] [Google Scholar]
- 51.Freeman AJ, Graham JC. Damage control surgery and angiography in cases of acute mesenteric ischaemia. ANZ J Surg. 2005;75:308–314. doi: 10.1111/j.1445-2197.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 52.Gong JF, Zhu WM, Wu XJ, Li N, Li JS. [Damage control surgery for acute mesenteric ischemia] Zhonghua Wei Chang Wai Ke Zazhi. 2010;13:22–25. [PubMed] [Google Scholar]
- 53.Sauerland S, Agresta F, Bergamaschi R, Borzellino G, Budzynski A, Champault G, Fingerhut A, Isla A, Johansson M, Lundorff P, et al. Laparoscopy for abdominal emergencies: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2006;20:14–29. doi: 10.1007/s00464-005-0564-0. [DOI] [PubMed] [Google Scholar]
- 54.Yanar F, Agcaoglu O, Sarici IS, Sivrikoz E, Ucar A, Yanar H, Aksoy M, Kurtoglu M. Local thrombolytic therapy in acute mesenteric ischemia. World J Emerg Surg. 2013;8:8. doi: 10.1186/1749-7922-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


