Abstract
AIM: To investigate the risk factors for liver-related mortality in chronic hepatitis C (CHC) patients.
METHODS: All deceased CHC inpatient data were collected from the Beijing 302 Hospital clinical database, which includes more than 8250 CHC inpatients during the period from 2002 to 2012. The controls were matched to cases by age (± 2 years), sex and date of hospital admission (within the same year). Potential risk factors were included for the evaluation, and odds ratios (OR) and 95%CI were estimated using univariate (unadjusted) and multivariate (adjusted OR, AOR) conditional logistic regression. All statistical tests were two-sided. P values < 0.05 were considered statistically significant.
RESULTS: Based on examinations of 144 CHC-related deceased cases and 576 controls, we found that antiviral therapy with interferon-α was associated with a 47% decrease in the risk of hepatic mortality (AOR = 0.53, 95%CI: 0.28-0.99, P = 0.048). Additionally, the initial diagnostic stage of the disease (AOR = 2.89, 95%CI: 1.83-4.56 and P < 0.001 for liver cirrhosis/AOR = 8.82, 95%CI: 3.99-19.53 and P < 0.001 for HCC compared with CHC), diabetes (AOR = 2.35, 95%CI: 1.40-3.95, P = 0.001), hypertension (AOR = 1.76, 95%CI: 1.09-2.82, P = 0.020), alcohol consumption (AOR = 1.73, 95%CI: 1.03-2.81, P = 0.037) and HBsAg positivity (AOR = 22.28, 95%CI: 5.58-89.07, P < 0.001) were associated with a significant increase in the risk of liver-related mortality in CHC patients.
CONCLUSION: This study indicates that interferon-α treatment, the stage at the initial diagnosis of the disease and comorbidities are all independent risk factors for liver-related mortality in CHC patients.
Keywords: Hepatitis C virus, Chronic hepatitis C, Risk factor, Mortality, Case control study
Core tip: Many previous studies have suggested that several complex factors have an important impact on hepatitis C virus-related mortality. However, the evaluation of such factors using a deceased case-living control study with a large number of patients has not been reported. The aim of the present study was to investigate the risk factors for liver-related mortality in chronic hepatitis C (CHC) patients using a deceased case-living control study design. This study indicates that interferon-α plus ribavirin treatment, the stage at the initial diagnosis and comorbidities are all independent risk factors for liver-related mortality in CHC patients.
INTRODUCTION
Hepatitis C virus (HCV) infection affects more than 160 million people worldwide and is associated with viral persistence, which progresses to chronic hepatitis C (CHC) in 80% of all cases[1,2]. HCV infection is now recognized as one of the main causes of liver cirrhosis, hepatocellular carcinoma (HCC) and death[1,2]. CHC progresses slowly but is accelerated in the presence of comorbidities such as alcohol consumption, diabetes or coinfection with other hepatotropic viruses[2]. However, whether these and other potential factors contribute to liver-related mortality in CHC patients is still unknown. Furthermore, CHC patients with the same age and gender but different survival status are commonly observed in the clinic, and few studies have addressed this phenomenon.
Many prior studies have suggested that some baseline parameters of CHC patients, such as age, albumin and disease stage, influence prognosis[3-5]. Several comorbidities, such as obesity, portal hypertension and bleeding esophageal varices, have been reported to contribute to disease progression[6-9]. Other studies have shown controversial conclusions that interferon-α therapy could or was unable to influence CHC-related HCC or mortality[10-17]. Furthermore, the response to antiviral therapy has been investigated for its impact on disease progression and prognosis in CHC patients[12,18,19]. However, several studies have not considered the relationship between CHC prognosis and the anti-hepatitis B core antibody (anti-HBc)[20,21], blood transfusion history and smoking[22].
Some of the above-mentioned results are logical, but it is difficult to come to definitive conclusions about other results due to varying study designs. Because it is ethically impossible to conduct a prospective study to evaluate risk factors and mortality without treatment, confirming these factors is difficult. We used our clinical database, which includes a large number of CHC inpatients, including many deceased CHC inpatients, to design a retrospective study to evaluate the association between several potential risk factors and liver-related mortality in CHC inpatients.
MATERIALS AND METHODS
The study protocol was approved by the Beijing 302 Hospital Research Ethics Committee. The committee waived the need for written informed consent from the participants due to the de-identified secondary data that were analyzed in this study.
Data source
The Beijing 302 Hospital, which provides diagnosis and treatment services for more than 650000 outpatients and 30000 inpatients with liver diseases annually, is the largest liver disease treatment center in China. Chronic hepatitis B and C, alcoholic liver disease, non-alcoholic liver disease, autoimmune liver disease and drug-related hepatitis are the most common diseases observed in this hospital. The clinical database includes the clinical history, related test results and prescription information for each patient. There were 8250 inpatients chronically infected with HCV between January 2002 and December 2012 in this database, which included cases of CHC, compensated liver cirrhosis, decompensated liver cirrhosis and HCC, as well as many cases of liver-related death. This dataset offers an opportunity to explore the risk factors for liver-related mortality in CHC patients.
Identification of cases and controls
The examined cases consisted of deceased inpatients with detailed clinical information and CHC diagnoses [International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10), Code B18.2] who were admitted between January 1, 2002 and December 25, 2012. Living inpatients who were admitted to the hospital with a diagnosis of CHC comprised the control group. We identified four control inpatients per deceased inpatient case. Control patients were matched to the cases by sex, year of birth (± 2 years) and index date (date of hospital admission). For controls, the index date was within the same year of the last index date of their matched case. One hundred and forty-four of the overall 155 deceased inpatient cases had sufficient clinical history and test information for inclusion in this study; thus, 576 matched controls were required (Figure 1).
Figure 1.
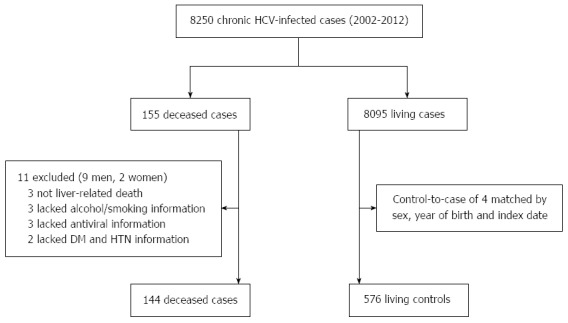
Derivation and definition of the study population. HCV: Hepatitis C virus; DM: Diabetes; HTN: Hypertension.
Potential factors
Factors that have the potential to contribute to both disease progression and mortality were considered risk factors in this study; these include the stage at which liver disease was diagnosed, hepatitis B surface antigen (HBsAg) status, anti-HBc status, anti-human immunodeficiency virus (HIV) antibody status, antiviral therapy, hypertension, diabetes, alcohol consumption, history of blood transfusion before diagnosis of CHC, smoking history and family history of viral hepatitis. Initial diagnostic stages were categorized into CHC, liver cirrhosis and HCC. Liver cirrhosis was diagnosed by liver biopsy or using a combination of at least two imaging tools (abdominal ultrasonography, angiography, computed tomography or magnetic resonance imaging) plus clinical evidence of manifestations. It may also been confirmed using one imaging method accompanied by complications such as esophageal varices, ascites and hepatic encephalopathy as well as abnormal laboratory results, liver functional and liver fibrosis tests. HCC was confirmed by liver histopathology, by at least two imaging tools or via one imaging diagnostic modality and a serum α-fetoprotein level of 400 ng/mL or higher. Antiviral therapy in this study was defined as patients who were treated with interferon-α and ribavirin for at least 12 wk, regardless of the viral or biochemical responses. The corresponding doses of interferon and ribavirin were calculated by the weight and tolerance of patients, but at least 3 million IU interferon-α was administered three times a week or 135/50 μg peg-interferon α-2a/α-2b was administered once a week, and at least 10.6 mg/kg of ribavirin was administered daily for interferon-treated patients. Alcohol consumption was defined as alcohol consumption at least 4 d per week for at least 5 years[7]. Smokers were defined as patients who had smoked cigarettes at least 4 d per week for at least 5 years[7]. Family history of viral hepatitis was defined as direct relatives who were chronically infected with hepatitis B virus (HBV) or HCV.
Statistical analysis
To compare the proportions, χ2 statistics were used. To compare the ages between the case and control groups, Mann-Whitney U tests were performed. A conditional logistic regression model was used to estimate the relative magnitude in relation to the potential factors mentioned above. The ORs and their 95%CIs were calculated using patients with no exposure as the reference. The Wald χ2 test for linear trends was performed by entering the diagnostic stage as a three-level ordinal variable (with the values 0-2) in the logistic regression model. Analyses were performed using the SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL, United States). All statistical tests were two-sided. P values < 0.05 were considered statistically significant.
RESULTS
Records for 144 deceased cases and 576 matched controls were included in the CHC mortality risk analysis. Figure 1 presents the derivation and definitions of the study population. Table 1 shows the distribution of the demographic characteristics and selected medical conditions of the HCV-related cases and controls. There was no significant difference between cases and controls for age or sex. Table 2 exhibits the primary death diagnosis and direct cause of death for the case group; HCC was the main diagnosis of the deceased CHC patients, followed by decompensated cirrhosis. The most common direct causes of death were hemorrhagic shock, hepatic encephalopathy and hepatorenal syndrome.
Table 1.
Demographic characteristics of hepatitis C virus-infected death cases and controls n (%)
| Variable | Cases (n = 144) | Controls (n = 576) | P value |
| Age (median) | 64 (31-85) | 64 (31-87) | 0.821 |
| Male | 105 (72.92) | 420 (72.92) | 1 |
| Female | 39 (27.08) | 156 (27.08) | 1 |
| Han nationality | 135 (93.75) | 544 (94.44) | NA |
| Anti-HIV antibody | 0 (0) | 0 (0) | NA |
| HCV RNA positive | 95 (65.97) | 444 (77.08) | NA |
| HCV RNA negative | 25 (17.36) | 121 (21.01) | NA |
| HCV RNA not determined | 24 (16.67) | 11 (1.91) | NA |
| Anti-HCV antibody positive | 144 (100) | 576 (100) | NA |
HCV: Hepatitis C virus; NA: Not applicable; HIV: Human immunoddficiency virus.
Table 2.
Main death diagnosis and direct causes of death in the case group
| Main death diagnosis | Number | Percentage | Direct death causes | Number | Percentage |
| Decompensated cirrhosis | 56 | 38.89% | Hemorrhagic shock | 60 | 41.67% |
| Hepatocellular carcinoma | 88 | 61.11% | Hepatic encephalopathy | 55 | 38.19% |
| Hepatorenal syndrome | 26 | 18.05% | |||
| Respiratory failure | 2 | 1.39% | |||
| Hepatopulmonary syndrome | 1 | 0.70% |
The relationship between the potential risk factors and CHC mortality is shown in Tables 3 and 4. Antiviral therapy with interferon-α, the initial diagnostic stage of disease, diabetes, hypertension, alcohol consumption, HBsAg positivity and anti-HBc positivity were all associated with an increased crude OR for CHC mortality. Adjustments for possible confounders (matching variables, antiviral therapy with interferon-α, initial diagnostic stage of disease, diabetes, hypertension, alcohol consumption, HBsAg positivity, HBcAb positivity, history of blood transfusion, smoking, and family history of viral hepatitis) only slightly altered the OR. Furthermore, anti-HBc positivity was removed from the adjusted model. In the final model, antiviral therapy was associated with a decreased risk of mortality (AOR = 0.53, 95%CI: 0.28-0.99), and HBsAg positivity (AOR = 22.28, 95%CI: 5.58-89.07), initial diagnostic stage of disease (χ2 for linear trend= 20.56, AOR = 2.89 and 95%CI: 1.83-4.56 for liver cirrhosis/χ2 for linear trend = 28.86, AOR = 8.82 and 95%CI: 3.99-19.53 for HCC when compared with CHC), diabetes (AOR = 2.35, 95%CI: 1.40-3.95), hypertension (AOR = 1.76, 95%CI: 1.09-2.82) and alcohol consumption (AOR = 1.73, 95%CI: 1.03-2.81) were independent risk factors for CHC mortality.
Table 3.
Unadjusted univariate conditional logistic regression analysis of potential risk factors for chronic hepatitis C mortality n (%)
| Variable | Cases (n = 144) | Controls (n = 576) | UOR (95%CI) | B | P value |
| Initial diagnostic stage | |||||
| CHC | 55 (38.19) | 404 (70.14) | 1.00 (Reference) | NA | NA |
| LC | 60 (41.67) | 152 (26.39) | 3.03 (1.98-4.64) | 1.11 | 0.000 |
| HCC | 29 (20.14) | 20 (3.47) | 13.19 (6.41-27.12) | 2.58 | 0.000 |
| HBsAg negativity | 125 (86.81) | 570 (98.96) | 1.00 (Reference) | NA | NA |
| HBsAg positivity | 19 (13.19) | 6 (1.04) | 22.99 (6.77-78.04) | 3.14 | 0.000 |
| Without diabetes | 102 (70.83) | 464 (80.56) | 1.00 (Reference) | NA | NA |
| With diabetes | 42 (29.17) | 112 (19.44) | 1.75 (1.14-2.67) | 0.56 | 0.010 |
| Without antiviral therapy | 126 (88.89) | 392 (73.78) | 1.00 (Reference) | NA | NA |
| With antiviral therapy | 16 (11.11) | 151 (26.22) | 0.32 (0.18-0.57) | -1.14 | 0.000 |
| Without hypertension | 99 (68.75) | 452 (78.47) | 1.00 (Reference) | NA | NA |
| With hypertension | 45 (31.25) | 124 (21.53) | 1.65 (1.10-2.47) | 0.5 | 0.015 |
| Without alcohol consumption | 92 (63.89) | 419 (72.74) | 1.00 (Reference) | NA | NA |
| With alcohol consumption | 52 (36.11) | 157 (27.26) | 1.67 (1.08-2.59) | 0.51 | 0.021 |
| Anti-HBc negativity | 48 (33.33) | 263 (45.66) | 1.00 (Reference) | NA | NA |
| Anti-HBc positivity | 96 (66.67) | 313 (54.34) | 1.69 (1.15-2.48) | 0.52 | 0.008 |
| Without transfusion history | 123 (85.42) | 342 (59.37) | 1.00 (Reference) | NA | NA |
| With transfusion history | 21 (14.58) | 234 (40.63) | NA | NA | 0.060 |
| Non-smoker | 104 (72.22) | 444 (77.08) | 1.00 (Reference) | NA | NA |
| Ever smoker | 40 (27.78) | 132 (22.92) | NA | NA | 0.193 |
| Without family history of viral hepatitis | 131 (90.97) | 521 (90.45) | 1.00 (Reference) | NA | NA |
| Family history of viral hepatitis | 13 (9.03) | 55 (9.55) | NA | NA | 0.846 |
UOR: Unadjusted odds ratios; B: Regression coefficient; CHC: Chronic hepatitis C; LC: Liver cirrhosis; HCC: Hepatocellular carcinoma; NA: Not applicable; HBsAg: Hepatitis B surface antibody; Anti-HBc: Anti-hepatitis core antibody.
Table 4.
Adjusted multivariate conditional logistic regression analysis of potential risk factors for chronic hepatitis C mortality n (%)
| Variable | Cases (n = 144) | Controls (n = 576) | AOR (95%CI) | B | P value |
| Initial diagnostic stage | |||||
| CHC | 55 (38.19) | 404 (70.14) | 1.00 (Reference) | NA | NA |
| LC | 60 (41.67) | 152 (26.39) | 2.89 (1.83-4.56) | 1.06 | 0.000 |
| HCC | 29 (20.14) | 20 (3.47) | 8.82 (3.99-19.53) | 2.18 | 0.000 |
| HBsAg negativity | 125 (86.81) | 570 (98.96) | 1.00 (Reference) | NA | NA |
| HBsAg positivity | 19 (13.19) | 6 (1.04) | 22.28 (5.58-89.07) | 3.10 | 0.000 |
| Without diabetes | 102 (70.83) | 464 (80.56) | 1.00 (Reference) | NA | NA |
| With diabetes | 42 (29.17) | 112 (19.44) | 2.35 (1.40-3.95) | 0.86 | 0.001 |
| Without antiviral therapy | 126 (88.89) | 392 (73.78) | 1.00 (Reference) | NA | NA |
| With antiviral therapy | 16 (11.11) | 151 (26.22) | 0.53 (0.28-0.99) | -0.64 | 0.048 |
| Without hypertension | 99 (68.75) | 452 (78.47) | 1.00 (Reference) | NA | NA |
| With hypertension | 45 (31.25) | 124 (21.53) | 1.76 (1.09-2.82) | 0.56 | 0.020 |
| Without alcohol consumption | 92 (63.89) | 419 (72.74) | 1.00 (Reference) | NA | NA |
| With alcohol consumption | 52 (36.11) | 157 (27.26) | 1.70 (1.03-2.81) | 0.53 | 0.037 |
| Anti-HBc negativity | 48 (33.33) | 263 (45.66) | 1.00 (Reference) | NA | NA |
| Anti-HBc positivity | 96 (66.67) | 313 (54.34) | NA | NA | 0.946 |
AOR: Adjusted odds ratios; B: Regression coefficient; CHC: Chronic hepatitis C; LC: Liver cirrhosis; HCC: Hepatocellular carcinoma; NA: Not applicable; HBsAg: Hepatitis B surface antibody; Anti-HBc: Anti-hepatitis core antibody.
DISCUSSION
In this case-control based study, the use of interferon and ribavirin for at least 12 wk was associated with a 47% decrease in liver-related mortality among CHC patients. We also found that there was a significant trend toward an increasing liver-related mortality in patients with HBsAg positivity, diabetes, hypertension, alcohol consumption and initial diagnosis at an advanced stage after controlling for potential confounders.
Chronic HCV infected cases are associated with higher mortality rates compared to non-infected individuals[23-26], which suggests that those with chronic HCV infection are at a higher risk of death and should be closely monitored. Previous studies have suggested that gender and age may influence the prognosis of CHC[3,15,27,28]; thus, the controls in the present study were matched by chronic HCV infection, gender, age and date of hospital admission to control for confounders. These variables were excluded from the statistic models. Patients with an early diagnosis of chronic HCV infection may have a better outcome, and a previous study showed that CHC-related cirrhosis increased the rate of mortality nearly four-fold compared to CHC patients without cirrhosis[15]. In our study, this rate was 2.89, while patients with HCC had a liver-related mortality 8.82 times higher than CHC patients after adjusting for confounders.
Chronic HCV infection does not have a significant impact on all-cause mortality in the first decade of infection[29]; however, liver disease progression is accelerated in the presence of cofactors such as HBV infection[2]. HBsAg positivity for longer than 6 mo is the most important indicator of chronic HBV infection, and anti-HBc positivity indicates past exposure to HBV. Previous studies have suggested that anti-HBc is not related to the prognosis of HCV infection[20,21]. In the present study, HBsAg and anti-HBc were associated with an increased rate of mortality in an unadjusted univariate analysis model; however, after adjusting for the confounders, only HBsAg remained an independent risk factor for CHC mortality. Additionally, chronic alcohol consumption in the presence of obesity and viral hepatitis could damage the liver[30]. Several studies have indicated that obesity and alcohol synergistically increase the risk of HCC and death[7,31-33]. In addition, smoking is always considered a risk factor for disease progression and poor prognosis[8,34,35], although controversial results have been reported[22]. In the present study, we found that alcohol consumption is associated with an increased risk of mortality in both univariate and multivariate analyses. In contrast, smoking was not related to mortality in either analysis. This result indicates that abstinence from habitual alcohol drinking is more directly beneficial for liver-related outcomes. Two studies have shown that diabetes was relevant to the mortality of CHC patients[6,36], and this relationship was confirmed in the present study. Furthermore, there are no data, to our knowledge, concerning the association between hypertension and the risk of HCV-related death. We found that hypertension is an independent risk factor for the increase of liver-related CHC mortality.
Antiviral therapy with interferon-α and ribavirin has been the standard of care for CHC, and among those who achieve sustained virologic response, 99% permanently remain HCV RNA-negative[37]. Many studies have suggested that interferon therapy is associated with decreased mortality, even in patients with cirrhosis[11,12,14,18,19]. There have also been reports demonstrating that the rate of progression to HCC was lowered two-fold following treatment with at least 3 × 106 IU interferon three times a week for 3 mo, regardless of the biological and virologic responses[38]. In contrast, a different study indicated that interferon-α did not affect the survival of patients with CHC[13]. However, most of these studies included very few deceased patients. In the present study, we included 144 deceased CHC inpatients for evaluation and found that patients treated with combination therapy had increased survival. To our knowledge, using this case-control study method, these are the first data showing that patients treated with interferon for at least 12 wk have reduced mortality.
The relative influence of routes of infection on the prognosis of liver disease remains controversial[22,39-42]. Additionally, whether transfusion-associated HCV and a family history of viral hepatitis are associated with a higher risk of mortality than other routes is largely unknown. The results of the present study indicate that neither the blood transfusion history nor a family history of viral hepatitis are associated with an increased risk of mortality, as indicated by both univariate and multivariate analyses.
Several limitations of the present study should be noted. First, we did not have access to information on the socioeconomic status of the subjects. It is difficult to investigate the real economic status in many patients, although a previous study showed that it impacts the prognosis of CHC patients[43]. Second, although we involved many potential factors in the statistical analysis, a number of other possible confounding variables, such as body mass index, were not included in our model because of the potential interaction with alcohol. Third, not all direct causes of death of the deceased patients were obtained from postmortem examinations due to a lack of family permission. Fourth, anti-HIV antibody status was initially included in this study; however, all cases and controls were negative for anti-HIV antibodies, and evaluating this factor was thus not possible. Fifth, HCV RNA was not determined in 16.67% (24) of cases and 1.91% (11) of controls at their last index date because HCV RNA in the case group was collected from the last admission of cases, which included some cases with bleeding varices and hepatic encephalopathy, and nearly all of the patients died shortly after this time point. At this urgent point, HCV RNA is unable to guide the treatment options; thus, HCV RNA was not determined in these 24 cases. Sixth, accurately measuring the cumulative intake of tobacco smoke and alcohol was not possible, which makes further stratification and analysis difficult. Seventh, because the initial diagnostic stage is a potential risk factors involved in assessment, it is improper to consider this a matched factor, which results in disproportionate stages between the two groups. Finally, as with any observational study, residual confounding by unmeasured factors that are different between cases and controls is possible. However, the confounding effect of medical attention could be corrected for by hospitalization, and all of the subjects in this study were inpatients.
In summary, our study demonstrates that the initial diagnostic stage of disease and comorbidities, including HBsAg seropositive status, alcohol consumption, diabetes and hypertension, are independent risk factors for liver-related mortality, whereas antiviral therapy decreases the risk of liver-related mortality in CHC patients. To our knowledge, this study is the first to investigate the risk of CHC mortality using a deceased case-living control study design and the first to indicate that hypertension may be a risk and antiviral therapy for a period of at least 12 wk may be beneficial for liver-related CHC mortality. We suggest that physicians should consider the above-mentioned conditions during disease evaluation.
COMMENTS
Background
Previous studies have indicated that some risk factors have an important impact on chronic hepatitis C (CHC) mortality. However, an evaluation of potential factors using a deceased case-living control study with a large number of patients has not been reported.
Research frontiers
In the field of hepatitis C virus (HCV)-related mortality, the research hotspot is to identify the independent risk factors to evaluate patients’ clinical prognosis. Factors that have the potential to contribute to both disease progression and mortality were studied to identify the independent risk factors in this manuscript.
Related publications
This is the first case control study designed to investigate the risk factors for CHC mortality.
Innovations and breakthroughs
This study is the first deceased case-living control study to investigate the risk of CHC mortality and the first to indicate that hypertension might be a risk factor for CHC mortality; other risk factors are also demonstrated in this manuscript.
Applications
The authors suggested that physicians should consider the initial diagnostic stage of disease, antiviral therapy and comorbidities such as HBsAg seropositive status, alcohol consumption, diabetes and hypertension in survival evaluations of CHC patients.
Peer review
The authors examined a large number of cases and controls from the hospital clinical database to investigate the independent risk or beneficial factors for the CHC mortality and arrived at some valuable conclusions.
Footnotes
Supported by National Natural Science Foundation of China, No. 81302593, No. 81271848 and No. 81101589; the Grant of Beijing Nova Program of China, No. Z121107002512071; the National Key Basic Research Program of China, No. 2009CB522507; and the National Grand Program on Key Infectious Disease, No. 2009ZX10005-017 and No. 2012ZX10002007
P- Reviewers: Al Olaby R, Chen J, Liu DW, Sutti S S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Grebely J, Raffa JD, Lai C, Kerr T, Fischer B, Krajden M, Dore GJ, Tyndall MW. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J Viral Hepat. 2011;18:32–41. doi: 10.1111/j.1365-2893.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 4.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311–1316. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- 5.Nagao Y, Sata M. Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol J. 2010;7:375. doi: 10.1186/1743-422X-7-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordanino C, Ceretto S, Bo S, Smedile A, Ciancio A, Bugianesi E, Pellicano R, Fagoonee S, Versino E, Costa G, et al. Type 2 diabetes mellitus and chronic hepatitis C: which is worse? Results of a long-term retrospective cohort study. Dig Liver Dis. 2012;44:406–412. doi: 10.1016/j.dld.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, Trinchet JC, Beaugrand M, Chevret S. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–136. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, Borzio M, Redaelli A, Chiesa A, Silini EM, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147–1158. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 10.Akuta N, Suzuki F, Suzuki Y, Sezaki H, Hosaka T, Someya T, Kobayashi M, Saitoh S, Arase Y, Ikeda K, et al. Long-term follow-up of interferon monotherapy in 454 consecutive naive patients infected with hepatitis C virus: multi-course interferon therapy may reduce the risk of hepatocellular carcinoma and increase survival. Scand J Gastroenterol. 2005;40:688–696. doi: 10.1080/00365520510015467. [DOI] [PubMed] [Google Scholar]
- 11.Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105–114. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Imai Y, Kasahara A, Tanaka H, Okanoue T, Hiramatsu N, Tsubouchi H, Yoshioka K, Kawata S, Tanaka E, Hino K, et al. Interferon therapy for aged patients with chronic hepatitis C: improved survival in patients exhibiting a biochemical response. J Gastroenterol. 2004;39:1069–1077. doi: 10.1007/s00535-004-1448-0. [DOI] [PubMed] [Google Scholar]
- 13.Gramenzi A, Andreone P, Fiorino S, Cammà C, Giunta M, Magalotti D, Cursaro C, Calabrese C, Arienti V, Rossi C, et al. Impact of interferon therapy on the natural history of hepatitis C virus related cirrhosis. Gut. 2001;48:843–848. doi: 10.1136/gut.48.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka H, Tsukuma H, Kasahara A, Hayashi N, Yoshihara H, Masuzawa M, Kanda T, Kashiwagi T, Inoue A, Kato M, et al. Effect of interferon therapy on the incidence of hepatocellular carcinoma and mortality of patients with chronic hepatitis C: a retrospective cohort study of 738 patients. Int J Cancer. 2000;87:741–749. [PubMed] [Google Scholar]
- 15.Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 16.Benvegnù L, Chemello L, Noventa F, Fattovich G, Pontisso P, Alberti A. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer. 1998;83:901–909. doi: 10.1002/(sici)1097-0142(19980901)83:5<901::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Arase Y, Murashima N, Chayama K, Kumada H. Long-term interferon therapy for 1 year or longer reduces the hepatocellular carcinogenesis rate in patients with liver cirrhosis caused by hepatitis C virus: a pilot study. J Gastroenterol Hepatol. 2001;16:406–415. doi: 10.1046/j.1440-1746.2001.02450.x. [DOI] [PubMed] [Google Scholar]
- 18.Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Long-term cohort study of chronic hepatitis C according to interferon efficacy. J Gastroenterol Hepatol. 2012;27:291–299. doi: 10.1111/j.1440-1746.2011.06871.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, Sheen IS, Chang WY, Lee CM, Liaw YF. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–994. [PubMed] [Google Scholar]
- 20.Jinjuvadia R, Liangpunsakul S, Antaki F. Past exposure to hepatitis B: a risk factor for increase in mortality? J Clin Gastroenterol. 2014;48:267–271. doi: 10.1097/MCG.0b013e3182972254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsubouchi N, Uto H, Kumagai K, Sasaki F, Kanmura S, Numata M, Moriuchi A, Oketani M, Ido A, Hayashi K, et al. Impact of antibody to hepatitis B core antigen on the clinical course of hepatitis C virus carriers in a hyperendemic area in Japan: A community-based cohort study. Hepatol Res. 2013;43:1130–1138. doi: 10.1111/hepr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyong SJ, Tsukuma H, Hiyama T. Case-control study of hepatocellular carcinoma among Koreans living in Osaka, Japan. Jpn J Cancer Res. 1994;85:674–679. doi: 10.1111/j.1349-7006.1994.tb02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 24.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omland LH, Jepsen P, Krarup H, Schønning K, Lind B, Kromann-Andersen H, Homburg KM, Christensen PB, Sørensen HT, Obel N. Increased mortality among persons infected with hepatitis C virus. Clin Gastroenterol Hepatol. 2011;9:71–78. doi: 10.1016/j.cgh.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Neal KR, Ramsay S, Thomson BJ, Irving WL. Excess mortality rates in a cohort of patients infected with the hepatitis C virus: a prospective study. Gut. 2007;56:1098–1104. doi: 10.1136/gut.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 28.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 29.Harris HE, Ramsay ME, Andrews N, Eldridge KP. Clinical course of hepatitis C virus during the first decade of infection: cohort study. BMJ. 2002;324:450–453. doi: 10.1136/bmj.324.7335.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakhari S. Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J Gastroenterol Hepatol. 2013;28 Suppl 1:18–25. doi: 10.1111/jgh.12207. [DOI] [PubMed] [Google Scholar]
- 31.Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007;31:285–292. doi: 10.1111/j.1530-0277.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Martino V, Crouzet J, Hillon P, Thévenot T, Minello A, Monnet E. Long-term outcome of chronic hepatitis C in a population-based cohort and impact of antiviral therapy: a propensity-adjusted analysis. J Viral Hepat. 2011;18:493–505. doi: 10.1111/j.1365-2893.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- 33.Makimoto K, Higuchi S. Alcohol consumption as a major risk factor for the rise in liver cancer mortality rates in Japanese men. Int J Epidemiol. 1999;28:30–34. doi: 10.1093/ije/28.1.30. [DOI] [PubMed] [Google Scholar]
- 34.Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 35.Fujita Y, Shibata A, Ogimoto I, Kurozawa Y, Nose T, Yoshimura T, Suzuki H, Iwai N, Sakata R, Ichikawa S, et al. The effect of interaction between hepatitis C virus and cigarette smoking on the risk of hepatocellular carcinoma. Br J Cancer. 2006;94:737–739. doi: 10.1038/sj.bjc.6602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kita Y, Mizukoshi E, Takamura T, Sakurai M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Impact of diabetes mellitus on prognosis of patients infected with hepatitis C virus. Metabolism. 2007;56:1682–1688. doi: 10.1016/j.metabol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, Abergel A, Pessôa MG, Lin A, Tietz A, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–1601. doi: 10.1053/j.gastro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 38.International Interferon-alpha Hepatocellular Carcinoma Study Group. Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. Lancet. 1998;351:1535–1539. [PubMed] [Google Scholar]
- 39.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, Seeff LB. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134:120–124. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 40.Thein HH, Yi Q, Heathcote EJ, Krahn MD. Prognosis of hepatitis C virus-infected Canadian post-transfusion compensation claimant cohort. J Viral Hepat. 2009;16:802–813. doi: 10.1111/j.1365-2893.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 41.Fujino Y, Mizoue T, Tokui N, Yoshimura T. A prospective study of blood transfusion history and liver cancer in a high-endemic area of Japan. Transfus Med. 2002;12:297–302. doi: 10.1046/j.1365-3148.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- 42.Krahn M, Wong JB, Heathcote J, Scully L, Seeff L. Estimating the prognosis of hepatitis C patients infected by transfusion in Canada between 1986 and 1990. Med Decis Making. 2004;24:20–29. doi: 10.1177/0272989X03261568. [DOI] [PubMed] [Google Scholar]
- 43.Omland LH, Osler M, Jepsen P, Krarup H, Weis N, Christensen PB, Roed C, Sørensen HT, Obel N. Socioeconomic status in HCV infected patients - risk and prognosis. Clin Epidemiol. 2013;5:163–172. doi: 10.2147/CLEP.S43926. [DOI] [PMC free article] [PubMed] [Google Scholar]


