Abstract
The worldwide increase in obesity has spurred numerous efforts to understand the regulation of eating behaviours and underlying brain mechanisms. These mechanisms can affordably be studied via neurobehavioural measures. Here, we systematically review these efforts, evaluating neurocognitive tests and personality questionnaires based on: a) consistent relationship with obesity and eating behaviour, and b) reliability. We also considered the measures’ potential to shed light on the brain mechanisms underlying these individual differences. Sixty-six neurocognitive tasks were examined. Less than 11%, mainly measures of executive functions and food motivation, yielded both replicated and reliable effects. Several different personality questionnaires were consistently related to BMI. However, further analysis found that many of these questionnaires relate closely to Conscientiousness, Extraversion and Neuroticism within the Five-Factor Model of personality. Both neurocognitive tests and personality questionnaires suggest that the critical neural systems related to individual differences in obesity are lateral prefrontal structures underpinning self-control and striatal regions implicated in food motivation. This review can guide selection of the highest yield neurobehavioural measures for future studies.
Keywords: Obesity, impulsivity, BMI, neuropsychology, executive functions, food motivation, Five-Factor Model, Big Five, personality, self-control, sensitivity to reward, reliability
1. Introduction
1.1. A Brain-Based Approach to Obesity Research
Health worsens as body-mass index (BMI, weight in kg/height in m2) increases (James, 2008), and throughout the world BMI continues to rise (Finucane et al., 2011). This alarming increase is likely due to many interacting factors, ranging from neurobiological mechanisms regulating our behaviour (Speliotes et al., 2010) to public policy, agricultural innovation, and business practices that have significantly lowered the cost and increased the availability of calorie dense food (Chandon and Wansink, 2010; Drewnowski, 2009; Lakdawalla and Philipson, 2002)
While the interaction between individual tendencies and a rapidly changing food environment seems to be critical in the increasing prevalence of obesity (Levitsky, 2005), not every individual is equally susceptible to these environmental pressures. How do individual differences in the ability to regulate food choices protect against weight gain in the modern environment of cheaper food and increased food consumption? While a variety of biological individual differences can conceivably be at play, those that relate to behaviour are likely to be of high interest and high impact: indeed, many of the current interventions to address the obesity epidemic are aimed at changing individual behaviour, notably through education and public health messages that exhort healthy choices and self-control.
We propose that a better understanding of the regulation of food choice and eating behaviour is crucial to explaining existing variability in BMI and increases in BMI, and may also be helpful in developing rational, tailored interventions to prevent or reverse weight gain, or at least in predicting who might benefit from a given intervention, whether that intervention relies on pharmacological, educational or social mechanisms. We argue that a brain-based view of these behaviours will allow mechanistic links between the growing body of knowledge about genetic and other biological determinants of BMI and the individual behaviours that lead to weight gain. There are several methods available to study the brain mechanisms underlying eating behaviours in humans. The tools of cognitive neuroscience are now being brought to bear on this question, with provocative results emerging from functional neuroimaging (Batterink et al., 2010; Killgore and Yurgelun-Todd, 2005; Martin et al., 2010; Stice et al., 2008, 2010; Stice, Yokum, Burger, et al., 2011; Wang et al., 2001), electrophysiology (Nijs, Muris, et al., 2010; Silva et al., 2002), non-invasive brain stimulation (Camus et al., 2009; Fregni et al., 2008; Uher et al., 2005) hormonal manipulations (Batterham et al., 2007; Farooqi et al., 2007; Malik et al., 2008), and genetics (Stice, Yokum, Zald, et al., 2011). While these approaches are useful for understanding the neural basis of food choice and other eating related behaviours in tightly focused experiments, they are unwieldy for use on the scale of the population level studies that are increasingly seen as necessary to fully understand the multivariate, multi-level determinants of the complex problem of obesity (Dubé et al., 2008).
1.2. Advantages of Neurobehavioural Measures
Neurobehavioural measures offer a potentially valuable intermediate tool: Such measures quantify a particular behaviour (i.e. psychological construct) in a way that can be linked to the brain, and are feasible for large-scale studies. There are two main types of neurobehavioural measures: neurocognitive measures, which are tasks, many with their origins in neuropsychology, that aim to measure specific cognitive-behavioural abilities, and personality questionnaires or scales that capture participants’ typical behaviour through (mainly self-reported) responses to behaviour-related questions. Commonly applied behavioural constructs in research on eating include self-control, impulsivity, executive control and sensitivity to reward, among others. Cognitive neuroscience research has begun to identify the neurobiological substrates of these constructs in general, and, more helpfully for our purposes, of specific measures of these constructs. As an example, self-control can be indexed by both a neurocognitive stop-signal task and a questionnaire measure of Conscientiousness. Both of these measures have been linked to maladaptive eating behaviours (Bogg and Roberts, 2004; Nederkoorn et al., 2010), and have been related to prefrontal structures (Aron and Poldrack, 2006; DeYoung et al., 2010). Thus, one or both of these measures, suitable for use in large-scale studies, might shed light on the role of prefrontal cortex in eating behaviours.
This paper aims to systematically review current knowledge regarding neurobehavioural measures in relation to obesity and eating behaviours. We set out to answer a practical question: Is there sufficient evidence to allow the confident selection of neurobehavioural measures, whether personality questionnaires or neurocognitive tasks, to characterize individual differences in BMI or ecologically-relevant eating behaviours in humans? Appropriate neurobehavioural measures must be both ecologically and conceptually valid: that is, 1) there must be a valid link between the measure and BMI or eating behaviours, and 2) the measure must be reliable i.e., reproducible and accurate. The existing literature will be reviewed in regards to these points, as well as considering whether the measures can be related to specific brain systems.
1.3. Eating Behaviours Related to Neurobehavioural Measures
Assuming suitable neurobehavioural measures are available, what eating behaviours should they be expected to predict? The most common correlative research designs involve measuring BMI concurrent with the administration of neurobehavioural measures and then comparing performance either between different weight groups or along a continuous BMI scale (e.g., Davis and Fox, 2008; Gunstad et al., 2007). Other related indexes of healthy weight have been used, such as waist-to-hip ratio or high waist circumference. Prospective studies involve measuring BMI at two time points and determining if a neurobehavioural measure is able to predict the change (e.g., Gunstad et al., 2010; Sutin et al., 2011). However, what BMI and other similar measures gain in public health relevance, convenience and reliability, they lose in behavioural specificity: Changes in BMI are the result of various factors accumulating over a period of time (Blundell and Cooling, 2000). Relevant neurobiological factors may not be discernible amongst the many other contributing variables. On the other hand, if a neurobiological factor is identified as relevant to a ‘big picture’ real world outcome such as BMI, particularly in a prospective longitudinal study, this would seem good evidence that it may be a high yield point of leverage in predicting risk or personalizing interventions.
Beyond BMI, a variety of more specific eating-related behaviours have also been examined. These offer more behavioural focus, making it more likely that a mechanistic link will be identified between neurobehavioural tasks and eating behaviours. On the other hand, their relevance to real world outcomes is uncertain. Examples include asking participants how much they plan to eat/avoid certain food products over a given time period and then measuring any discrepancy with actual recorded behaviour (planned-conducted behaviour; e.g., Hall et al., 2008; based on Thompson et al., 2004) or measuring how much participants eat in a particular context, such as in a bogus food tasting test (laboratory intake of food; e.g., Herman and Mack, 1975; Schachter et al., 1968). While more specific than BMI, these measures have not been positioned within a brain-based conceptual framework. In addition, studies using these approaches tend to use idiosyncratic tasks, making cross-study comparison more difficult.
1.4. Fragmentation of Neurobehavioural Evidence
Similarly to eating behaviours, there is great variety in the neurobehavioural measures that have been studied as correlates or predictors of eating behaviours. While constructs such as impulsivity or executive functions are considered important in obesity (e.g., Guerrieri et al., 2008; Smith et al., 2011), these are such broad categories that they provide little insight into the underlying mechanisms. More specific constructs would seem more informative, but the lack of an accepted, common set of measures and lack of communication between different research traditions has hindered progress. The nature of fragmentation is somewhat different in the two main types of neurobehavioural measures, and thus they will be addressed in different sections of this review.
1.4.1. Neurocognitive tasks
Neurocognitive tasks rarely correlate with each other which makes drawing conclusions about more specific constructs difficult. For instance, Hofman et al. (2009) found that stop-signal, affective inhibition and working memory tasks all relate to candy consumption but do not relate to each other. In a similar vein, neurocognitive self-control measures in general have an average correlation of 0.15 (Duckworth and Kern, 2011). A possible way to overcome this issue is to apply a domain-based approach, where tasks meant to capture a given construct, such as memory, language, or executive function, are clustered together to evaluate the domain’s feasibility in obesity research. Smith et al.(2011) applied a similar approach to highlight the general role of executive functions in obesity. Here, we attempt to evaluate all cognitive domains that have been tested in the context of maladaptive eating behaviours.
We conducted a systematic search combining terms referring to related neuropsychological domains and to BMI or laboratory measures of food intake. We then categorized the tasks by the primary cognitive domain that each is purported to measure. Whenever necessary, the tasks within a cognitive domain were further classified based on existing, empirically supported conceptual frameworks (Miyake et al., 2000; Oberauer et al., 2000) and neuropsychological expertise. This classification allowed identification of the domains most related to eating behaviours, enabling preliminary conclusions about domains where no single task has been frequently studied.
1.4.2. Personality questionnaires
In contrast to the heterogeneous variety of neurocognitive tests, questionnaire measures are more homogenous. The focus has been on a smaller number of constructs that are perceived to be relevant in obesity: Five-Factor Model personality dimensions, self-control, sensitivity to reward, impulsivity, and a handful of food-related personality constructs. Most of these constructs have established relationships with obesity and maladaptive eating behaviours (Bogg and Roberts, 2004; Bryant et al., 2008; Chalmers et al., 1990; de Ridder et al., 2011; Guerrieri et al., 2008; Herman and Polivy, 2008; Johnson et al., 2011; Lowe and Thomas, 2009; Macht, 2008; see later in this paper). While often each of these constructs has several measures, different measures of a single construct tend to correlate well (self-control questionnaires’ average r=0.50, Duckworth and Kern, 2011), which has enabled fruitful attempts to clarify which measures within a construct provide the best reliability and validity. This paper does not seek to double this work – rather we highlight the best measures identified so far in relation to BMI or eating behaviours.
Around a dozen personality constructs related to obesity and eating behaviours are actively applied in contemporary research. At this point one might ask: How different are these measures from each other? It seems highly unlikely that obesity would be so multifaceted that each of the highlighted constructs would represent an independent mechanism. Rather, it is quite probable that different measures ultimately rely on a common set of underlying processes that are named differently in different research traditions. For example, a recent review of self-control questionnaires showed that various self-control measures correlate with Neuroticism and Conscientiousness of the Five-Factor Model (McCrae and Löckenhoff, 2010). A similar analysis was conducted here to explore the possible overlap between measures deemed important for obesity. The Five-Factor Model (McCrae and Costa, 1987) was used as the baseline measure given its characterisation of personality as a whole, its established relationship with obesity(e.g., Sutin et al., 2011) and other eating-related behaviours (e.g., Mõttus et al., 2011, 2012), and the fact that most of the measures mentioned above have been correlated at least once with the Five-Factor Model.
1.5. Reliability
Reliability is an integral part of a measure’s validity, as it sets the upper limit to the potential correlation between a measure and an outcome. Despite its obvious importance, reliability has been of little concern for neurocognitive research; personality questionnaires have done much better in this regard. In this review, reliability will be reported for key measures.
1.6. Diverse Measures Tap Common Brain Mechanisms
Finally, neurocognitive measures and personality questionnaires capturing the same construct are still often considered as separate entities. For instance, in the domain of impulsivity recent reviews tend to focus on evidence from one of the approaches and are critical of the other, with neurocognitive research claiming that personality questionnaires are vulnerable to subjective bias (de Wit, 2009) and personality research claiming that neurocognitive research has problems with reliability (DeYoung, 2010a). While both of these criticisms are valid, both research traditions are taking steps to refine their measures(e.g., Parrott, 1991; Soto et al., 2008). Thus, evidence from sound measures from both measurement traditions is likely to offer relevant perspectives on the neurobiological underpinnings of a particular behaviour. Note that, while neurocognitive tests are often favoured in neurobehavioural research, the links between personality constructs and the brain are also becoming clearer (for overviews see Carnell et al., 2011; Davis and Panksepp, 2011; DeYoung and Gray, 2009; DeYoung, 2010b). Thus, obesity research is likely to benefit from evidence from both neurocognitive measures and personality questionnaires, perhaps by developing a more elaborated neurobiological framework through which to link neurobehavioural measures and outcomes of interest (e.g., Berthoud and Morrison, 2008; Carnell et al., 2011).
In sum, the current review seeks to unite the scattered knowledge from studies using both broad and narrow approaches to study the relationship between neurobehavioural measures and BMI or eating behaviours. The review will serve two goals: First, it will serve as a guide for researchers choosing measures for a comprehensive, reliable and informative test battery with the potential to predict obesity risk, suitable for use in adults. Second, we will review what these measures may reveal about the putative brain mechanisms contributing to obesity.
2. Neurocognitive Tasks
2.1. Search Strategy
On 18.11.11 a topic search in all databases was conducted at ISI Web of Knowledge pairing neurocognitive or psychological constructs with obesity and food words. The search included articles dating from 1985 to 2011 (see supplementary material for a list of keywords). The search was refined to exclude studies involving animals, children (< 18 years) and the elderly (> 60 years), and also to exclude work not related to psychology or obesity, resulting in total of 7,069 papers. Based on titles and abstracts we included papers that examined the relationship between a neurocognitive measure and one or more eating-related measurements: BMI, BMI change or laboratory food intake measures. Reference sections of the papers so-identified were also scanned to identify additional studies. We excluded results obtained from a particular clinical population, such as smokers or people with a psychiatric diagnosis (including eating disorders). For simplicity, we also excluded studies involving manipulations, such as fasting before testing or administration of alcohol, unless these papers also included measures under conditions of satiety or reported on non-clinical control groups. Only papers in English providing statistical data confirming the presence or absence of an effect were included. The final analysis was based on 65 papers including one review paper on implicit associations (Roefs et al., 2011). This review provided a thorough overview of implicit association tasks related to eating behaviours in a format suitable for our analysis. We extracted the neurocognitive measures from those 65 papers, excluding IQ measures and composite indexes encompassing several executive functions, as these are not intended to provide cognitive domain-specific information.
In these studies, obesity was usually defined as a BMI of 30 kg/m2 or above, overweight as a BMI between 25 – 30 kg/m2 and normal weight as a BMI between 18.5–25 kg/m2. Morbid obesity was usually defined by BMI of 35 or more. A few studies also employed high waist-to-hip ratio or high waist circumference as measures of adiposity, but the grouping criteria tended to vary from study to study.
2.2. Search Results
Our search identified 66 different neurocognitive tasks, 47% of which had been studied only once. The tasks that had been used more than once had been studied on the average 4.17 times. Significant relationships with BMI or food intake measures were reported for 61% of all measurements. Most often, better performance correlated with lower BMI or more adaptive performance on laboratory food intake tests. In three cases obese participants outperformed those of normal weight on a particular task. Most of the tasks used generic (i.e. non-food) stimuli. A smaller set of tasks used food-related stimuli to specifically probe food-related cognitive abilities.
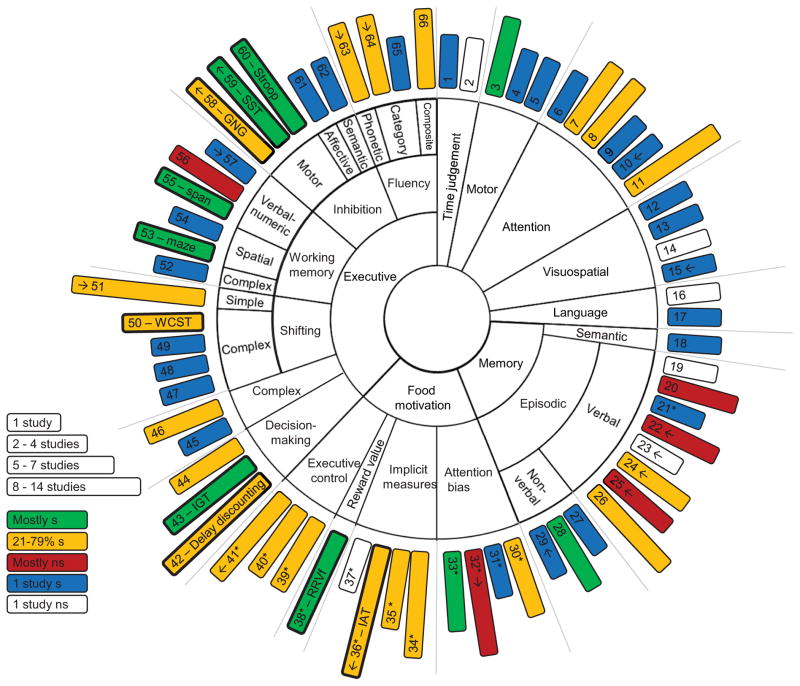
Many neurocognitive measures were developed in a clinical context, and they are usually related to each other within a framework of cognitive domains thought to rely on at least partly distinct brain systems (Lezak, 2004). It is worth noting that, to our knowledge there is only patchy empirical evidence for the validity of such conceptual frameworks in non-neurological populations. We relied on this evidence where it was available (Miyake et al., 2000; Oberauer et al., 2000), and otherwise categorized the tasks into different domains based on clinical neuropsychological convention. While the boundaries of some of these domains can be debated, they nonetheless offer a heuristic for roughly categorizing cognitive functions in a way that can be related to the brain. The final neuropsychological framework encompassed 66 tasks in 8 major domains – 21 in executive function, 2 in time judgement, 10 in (sustained/basic) attention, 4 in visuospatial, 3 in motor, 4 in language, 12 in memory and 9 in food motivation. The framework and the results are summarised in Figure 1, and all tasks and studies are listed in Table 1. Table 1 also summarises the BMI or food intake measures that have been tested with each particular task and if the significant findings have any constraints, such as being limited to particular populations. Several studies also reported significant interactions between measures of food motivation and executive function. Such findings are of high theoretical interest, and are also reported in Table 1.
Figure 1. Visual overview of neurocognitive measures and their possible links with obesity and weight-related appetitive behaviours.
The results of the systematic search are depicted in Figure 1. The major domains are positioned in the centre of the circle. Some of the domains are further broken into subdomains, when necessary. Each rectangle corresponds to a single neurobehavioural task. The numbers correspond to task numbers in Table 1. The length of the rectangle reflects the number of studies conducted with this task, and the colour reflects the overall outcome. Studies with replications have a separate colour scheme from studies with no replications (see legend). Asterisks indicate tasks that use food stimuli, as opposed to generic stimuli, and rectangles in bold indicate tasks that are discussed in more detail in this paper. Arrows indicate if task has been tested in a longitudional design.
* = task uses food stimuli; ↑↓= Outward arrow – task performance has been tested as a predictor of BMI change. Inward arrow – BMI change has been tested as a predictor of task performance; GNG= go/no go; IAT = Implicit Association Test; IGT = Iowa Gambling Task; maze = Austin Maze; ns = not significant; RRVf = Relative reinforcing value of food; s = significant; span = Computational span; SST = stop-signal test; WCST = Wisconsin Card Sorting Test
Table 1.
Overview of neurocognitive tests and their possible links with obesity and weight-related eating behaviours. The particular weight-related eating behaviours are introduced in the introduction. The first three designs are correlative (marked with small x) and last three are prospective (marked with large X).
| Nr | Measure | Studies | Weight-related eating behaviours | Comments | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| effect/no effect | Group comparison | Continuous BMI | Laboratory food intake | Planned-conducted | Score → BMI change | Score ← BMI change | Limits of papers showing effects; confounding results; interactions with other measures | ||
| Temporal judgment | |||||||||
| 1 | time judgment estimation | Etou et al. (1989) | x | NW vs morbidly obese, F only, small sample | |||||
| 2 | time judgment reproduction | /Etou et al. (1989) | x | ||||||
| Motor | |||||||||
| 3 | tap test | Etou et al. (1989), Stanek (2011)* | x | x | |||||
| 4 | transfer coordination test | Etou et al. (1989) | x | NW vs morbidly obese, F only, small sample | |||||
| 5 | traverse speed test | Etou et al. (1989) | x | ||||||
| Sustained/basic attention | |||||||||
| 6 | choice reaction time | Gunstad et al. (2007)* | x | ||||||
| 7 | D2 attention endurance test | Cserjési et al. (2009)/Loeber et al. (2011) | x | x | only F awaiting gastroplasty | ||||
| 8 | digit span forward | Stanek (2011)*/Cserjési et al. (2009), Gunstad et al. (2007*, 2010*) | x | x | |||||
| 9 | group embedded figure task | Roberts et al. (2007) | x | small sample, F only, edu not controlled | |||||
| 10 | selective attention test | Cournot et al. (2006)* | x | X | |||||
| 11 | Trail-Making-Test A (TMT A) | Chelune et al. (1986), Cserjési et al. (2009), Gunstad et al. (2007*, 2010*)/Boeka and Lokken (2008), Loeber et al. (2011), Ward et al. (2005)*, | x | In Gunstad et al (2010) faster performance is related to higher BMI and WC. | |||||
| Visuospatial | |||||||||
| 12 | block design tests | Nilsson and Nilsson (2009)* | x | results ns when excluding future health conditions | |||||
| 13 | card rotations test | Gunstad et al. (2010)* | x | x | higher WC linked to better performance | ||||
| 14 | Clock drawing test | /Fergenbaum et al. (2009) | x | edu not controlled | |||||
| 15 | Hooper Visual Organization Test | Wolf et al. (2007)* | x | X | |||||
| Language | |||||||||
| 16 | Boston naming test | /Gunstad et al. (2010)* | x | ||||||
| 17 | Hayling Sentence Completion task A | Cserjési et al. (2009) | x | only F awaiting gastroplasty | |||||
| Memory | |||||||||
| Semantic | |||||||||
| 18 | composite measure | Nilsson and Nilsson (2009)* | x | effect only for F | |||||
| Episodic | |||||||||
| 19 | Auditive verbal learning task | /Loeber et al (2011) | x | ||||||
| 20 | California Verbal Learning Tests | /Boeka and Lokken (2008), Gunstad et al. (2010)* | x | x | |||||
| 21 | explicit memory task | King (1991) | x | food stimuli; only F; restraint, edu, age not balanced | |||||
| 22 | Logical memory from Wechsler memory scale-III | /Boeka and Lokken (2008), Wolf et al. (2007)* | x | X | |||||
| 23 | paired associates (immediate & delayed) | /Wolf et al. (2007)* | x | X | |||||
| 24 | Rey Auditory Verbal Learning Test & adaptions | Cournot et al. (2006)*/Loeber et al. (2011), Ward et al. (2005)* | x | X | delayed better predictor than immediate (Cournot) | ||||
| 25 | short-term verbal memory, | /Conforto and Gershman (1985), Sabia et al. (2009)* | x | X | |||||
| 26 | recall-recognition composite; | Gunstad et al. (2006), Nilsson and Nilsson (2009)*/Gardner (1986), Pothos et al. (2009), Stanek (2011)* | x | x | |||||
| 27 | Benton retention test | Gunstad et al. (2010)* | x | ||||||
| 28 | Rey–Osterrieth Complex Figure | Boeka and Lokken (2008), Lokken et al. (2010)*, Roberts et al. (2007) | x | mostly NW vs morbidly OB | |||||
| 29 | Visual reproductions | Wolf et al. (2007)*/ | x | X | |||||
| Food motivation | |||||||||
| Attention bias | |||||||||
| 30 | free view of food picture pairs + eyetracker | Graham et al. (2011)*/Nijs et al. (2010) | x | only students | |||||
| 31 | visual oddball + eyetracker | Nummenmaa et al. (2011) | x | small sample, only NW students, no control variables | |||||
| 32 | visual probe | Nijs et al. (2010)/Calitri et al. (2010)*, Castellanos et al. (2009), Loeber et al. (2011), Pothos et al. (2009) | x | x | x | X | |||
| 33 | visual probe + eye tracker (vp+eye) | Castellanos et al. (2009), Werthmann et al. (2011) | x | x | eye-tracking data show opposing effects | ||||
| Implicit measures | |||||||||
| 34 | affective priming paradigm (APP) | Czyzewska and Graham (2008), 1 study in Roefs et al. (2011)*/3 studies in Roefs et al. (2011)* | x | x | |||||
| 35 | Extrinsic Affective Simon Task (EAST) | 1 study in Roefs et al. (2011)*/Pothos et al. (2009) | x | x | only students, few OW/OB participants | ||||
| 36 | Implicit Association Test (IAT) | Ayres et al. (2011), Churchill and Jessop (2011), Hofmann et al. (2008; 2009*), Nederkoorn et al. (2010)*, 5 studies in Roefs et al. (2011)*/4 studies in Roefs et al. (2011) | x | x | X | X | highly dependent on stimuli used; interactions with 55, 59, 61 and Emotional Eating Scale | ||
| 37 | seman tic priming paradigm | /1 study from Roefs et al. (2011)* | x | ||||||
| Relative reinforcing value | |||||||||
| 38 | Relative reinforcing value of food (RRVf) | Epstein et al. (2007, 2010, 2011*), Giesen et al. (2010), Rollins et al. (2010), Saelens and Epstein (1996), | x | x | interaction with 42 | ||||
| Executive control | |||||||||
| 39 | food delay discounting | Rasmussen et al. (2010);/Manwaring et al. (2011)* | x | only %BF, only F students | |||||
| 40 | food probability discounting | Rasmussen et al 2010;/Manwaring, 2009* | x | only %BF, only F students | |||||
| 41 | food Stroop | Calitri et al. (2010)*, Phelan et al. (2011)*/Pothos et al. (2009), Nijs et al. (2010) | x | x | X | Scoring methods differ; different group types used | |||
| Executive | |||||||||
| Decision-making | |||||||||
| 42 | delay-discounting | Appelhans et al. (2011)*, Borghans and Golsteyn (2006)*, Ikeda et al. (2010), Rasmussen et al. (2010), Reimers et al. (2009), Rollins et al. (2010)*, Weller et al. (2008)/Borghans and Golsteyn (2006)*, Davis et al. (2010)*, Manwaring et al. (2011)*, Nederkoorn et al. (2006), Rasmussen et al. 2010, Yeomans et al. (2008) | x | x | effects for men manifest in population studies only; an interaction with 38 and Power of Food Scale. | ||||
| 43 | Iowa Gambling Task (IGT) | Brogan et al. (2010; 2010), Davis et al. (2004), Pignatti et al. (2006)/Davis et al. (2010) | x | x | differences in later IGT blocks only | ||||
| 44 | probability discounting | Rasmussen et al (2010)/Manwaring et al. (2011)* | x | only %BF, only F students | |||||
| Complex | |||||||||
| 45 | category test | Chelune et al. (1986) | x | no control variables; OB awaiting gastroplasty | |||||
| 46 | Tower tests | Wong and Mullan (2009)/Allan et al. (2010) | x | X | task versions differ | ||||
| Switching | |||||||||
| 47 | Brixton task | Roberts et al. (2007) | x | small sample, F only, edu not controlled | |||||
| 48 | CatBat task | Roberts et al. (2007) | x | ||||||
| 49 | haptic illusion task | Roberts et al. (2007) | x | ||||||
| 50 | Wisconsin Card Sorting Test (WCST) | Boeka and Lokken (2008), Lokken et al. (2010)*, Roberts et al. (2007)/Loeber et al. (2011) | x | mostly NW vs morbidly obese | |||||
| 51 | Trail-Making-Test B (TMT B) versions | Chelune et al. (1986), Cserjési et al. (2009)*, Fergenbaum et al. (2009), Gunstad et al. (2010)*, Roberts et al. (2007), Wolf et al. (2007)/Boeka and Lokken (2008), Gunstad et al. (2007)*, Loeber 2011, Ward et al. (2005) | x | x | X | scoring methods differ | |||
| Working memory/updating | |||||||||
| 52 | prospective memory test | Gunstad et al. (2010)* | x | ||||||
| 53 | Austin Maze | Gunstad et al. (2007)*, Stanek (2011)* | x | x | scoring methods differ | ||||
| 54 | span of visual memory, | Gunstad et al. (2007)* | x | ||||||
| 55 | computation span task (span) | Hofmann et al. (2008; 2009*), | x | only F students, no OW/OB; results only significant in an interaction with 36 | |||||
| 56 | digit span backwards | /Gunstad et al. (2010)*, Stanek (2011)* | x | ||||||
| 57 | digit symbol substitution tests | Cournot et al. (2006)* | x | X | |||||
| Inhibition | |||||||||
| 58 | go/nogo (GNG), | Hall et al. (2012*; 2008)/Allan et al. (2011), Loeber et al, (2011), Ratcliff (2010), Wong and Mullan (2009)* | x | X | X | scoring methods differ | |||
| 59 | Stop signal task (SST) versions | Guerriri et al. (2007), Hofmann et al., (2009)*, Houben (2011), Jansen et al. (2009)*, Nederkoorn et al. (2006, 2010*)/Guerriri, Nederkoorn and Jansen (2007) | x | x | X | only F, mostly students, edu not controlled for non- students, some results only significant in an interaction with 36 or Restraint Scale | |||
| 60 | Stroop test | Allan et al., (2010, 2011), Gunstad et al. (2007)*, Hall (2012)*/Phelan et al., (2011)* | x | x | X | ||||
| 61 | Affect regulation | Hofmann et al. (2009)* | x | only F students, no OW/OB; results only significant in an interaction with 36 | |||||
| 62 | Hayling Sentence Completion task B | Cserjési et al. 2009 | x | x | only F awaiting gastroplasty | ||||
| Fluency | |||||||||
| 63 | letter fluency tests | Boeka and Lokken (2008), Gunstad et al. (2010)*, Sabia et al. (2009)* | x | x | X | in Boeka and Lokken (2008) obese perform better than NW | |||
| 64 | animal naming tests | Sabia et al. (2009)*/Boeka and Lokken (2008) | x | X | |||||
| 65 | category fluency | Gunstad et al. (2010)* | x | x | |||||
| 66 | composite of category and phonetic fluency | Allan et al., 2010/Cserjési et al. (2009), Stanek (2011)* | x | x | only students, few M | ||||
Note. %BF = per cent body fat; edu = education; F = female; M = male; ns = not significant; NW = normal-weight; OB = obese; OW = overweight; WC = waist circumference;
Papers marked with an asterisk are notable for reporting on particularly large samples, or studies with particularly tight control of possible confounds.
In general, the most consistent effects were seen with executive function and food motivation tasks. Several papers also highlighted an interaction between the two. Other task domains were less consistently related to the outcomes of interest, or were infrequently studied. Hence, the following overview focuses on measures of executive function and food motivation.
2.2.1. Generic stimuli
2.2.1.1. Executive function
Executive function is an umbrella term for processes underlying flexible, goal-directed behaviour. This category has obvious face validity in relation to the control of food intake, and has been extensively studied in relation to BMI. One empirically supported framework proposes that executive functions can be subdivided into response inhibition, attention shifting, and manipulation in working memory (Miyake et al., 2000). Tasks tapping each of these subdimensions have been administered; those requiring the inhibition of prepotent responses, most commonly the stop signal or Stroop tasks, seem to have the most consistent relationship with BMI and eating behaviour, with at least 80% of measurements yielding a significant relationship (Figure 1 & Table 1: 59, 60). Performance on these tasks distinguish obese and non-obese subjects (Gunstad et al., 2007; Nederkoorn et al., 2006; but see Phelan et al., 2010), are associated with increased food intake (Guerrieri, Nederkoorn, Stankiewicz, et al., 2007; Hall, 2012; Hofmann, Friese, and Roefs, 2009; Houben, 2011; Jansen et al., 2009; but see Guerrieri, Nederkoorn, and Jansen, 2007), weight gain (Nederkoorn et al., 2010) and the gap between intended and actual food intake (Allan et al., 2010, 2011). In the case of the stop signal task, a few papers report a significant effect only through an interaction with a measure of food motivation (Hofmann, Friese, and Roefs, 2009; Nederkoorn et al., 2010) or with questionnaire measures of restraint – an eating style under cognitive control that is often undermined when self-control is undermined (Jansen et al., 2009). Go-no go tasks are thought to tap a similar construct, but their relationship with maladaptive eating behaviours has been mixed, with significant effects linked to go reaction time (Hall, 2012; Hall et al., 2008), rather than measures that would seem to capture response inhibition more directly (Figure 1 & Table 1: 58; Allan et al., 2011; Loeber et al., 2011; Ratcliff, 2010; Wong and Mullan, 2009). The possibly related construct of affective inhibition has also been reported to correlate with candy consumption in a laboratory eating test, albeit only through an interaction with a measure of food motivation (Figure 1 & Table 1: 61; Hofmann, Friese, and Roefs, 2009).
Although not a classical executive function, decision-making is also linked to the frontal lobes, and the tasks used to test it share some conceptual links with response inhibition. Paradigms tapping the ability to forego immediate rewards in favour of longer-term advantages show promise: The Iowa Gambling Task has shown a consistent relationship with BMI, although studies have compared subjects of normal weight only to those who are morbidly obese (Figure 1 & Table 1: 43; Davis, Levitan, et al., 2004; Pignatti et al., 2006; Brogan, Hevey, and Pignatti, 2010; Brogan, Hevey, O’Callaghan, et al., 2010; but see Davis et al., 2010). A more direct measure of the ability to wait for larger reward, the steepness of delay discounting, has yielded consistent results mainly in population-based survey approaches (Borghans and Golsteyn, 2006; Ikeda et al., 2010; Reimers et al., 2009). Some behavioural studies have established a direct link between delay discounting and eating behaviours, but the effects were limited to women and particular adiposity indexes (Figure 1 & Table 1: 42; Rasmussen et al., 2010; Weller et al., 2008; but see Davis et al., 2010; Manwaring et al., 2011; Rasmussen et al., 2010; Yeomans et al., 2008). Recent papers suggest that delay discounting might explain more variance in BMI through interactions with food motivation measures (Appelhans et al., 2011; Rollins et al., 2010). The few reports on probability discounting have also yielded conflicting findings (Figure 1 & Table 1: 44; Rasmussen et al., 2010; but see Manwaring et al., 2011). The amounts of money and delays or probabilities used in these tasks vary across studies, which might further contribute to the inconsistent results.
The results from measures of other executive function subdimensions are more complicated to interpret. Some working memory measures show an effect only in conjunction with food motivation tasks (Figure 1 & Table 1: 44; Hofmann et al., 2008; Hofmann, Friese, and Roefs, 2009). Other working memory tasks, such as the Austin Maze, seem more promising (Figure 1 & Table 1: 53; Gunstad et al., 2007; Stanek, 2011). Attention shifting tasks might have potential: the Wisconsin Card Sorting Task has mostly produced significant differences (Figure 1 & Table 1: 50; Boeka and Lokken, 2008; Lokken et al., 2010; Roberts et al., 2007), with the only null result arising from a study with a small sample size (Loeber et al., 2011). A more widely used, simpler shifting task (Trail-Making-Test B) has yielded more varied results (Figure 1 & Table 1: 51). More complex executive function and verbal fluency tasks have also yielded mixed results (Figure 1 & Table 1: 45–46, 63–66). One study found that phonologic verbal fluency was better in obese individuals than in those of normal weight (Boeka and Lokken, 2008).
In sum, the most promising subdomain in executive functions seems to be response inhibition. Complex shifting tasks and the Iowa Gambling Task might also provide useful information but more research is warranted. In most cases, delay discounting and working memory only seem to reliably relate to eating behaviours in conjunction with measures quantifying food motivation.
2.2.1.2. Neurocognitive tasks measuring other cognitive domains
Some non-executive neuropsychological measures have also been linked to BMI, notably simple motor tasks and non-verbal episodic memory tasks. Poorer performance on simple motor tasks may be due to physical factors, although cognitive explanations have also been offered (Figure 1 & Table 1: 3–5; Etou et al., 1989; Stanek, 2011). The Rey Complex Figure Task, intended as a measure of non-verbal memory, has been consistently related to BMI (Figure 1 & Table 1: 28). This is a complex task that may also rely on executive abilities (Shin et al., 2006). Other domains have fewer studies with mixed or mostly non-significant results (Figure 1 & Table 1: 1–2, 6–26).
2.2.2. Tasks using food stimuli
2.2.2.1.Food motivation tasks
Food motivation tasks are meant to measure how participants value a particular food in comparison to other food items or to non-food alternatives. It is hypothesised that a tendency to evaluate food as more rewarding correlates with obesity and maladaptive food intake. The current review identified three types of food motivation tasks that have been related to eating behaviours. The most uniform results have come from the relative reinforcing value of food task, which explicitly measures participants’ motivation to barpress for food in a gambling setting as compared to motivation to barpress for other reinforcers (Figure 1 & Table 1: 38; Epstein et al., 2007, 2010, 2011; Giesen et al., 2010; Rollins et al., 2010; Saelens and Epstein, 1996). Implicit food motivation seems best captured by the Implicit Associations Test (Figure 1 & Table 1: 36; see Roefs et al., 2011 for an overview). Here, participants are asked to indicate their liking or non-liking of different type of visual stimuli (e.g. food and non-food pictures) and their reaction time latencies are hypothesised to characterise differential automatic stimuli processing. The Implicit Association Test is highly dependent on the stimuli used. For instance, pictures are often more effective than words (Czyzewska et al., 2011). Finally, attention bias tasks have produced mixed results. In these tasks, participants view pairs of different types of food and non-food pictures or conduct a visual search for a target. Eating behaviours tend not to correlate with behavioural indexes on these tasks, but do correlate with eye-tracking measures which are perhaps revealing more ‘automatic’ influences (Figure 1 & Table 1: 30–33). However, eye-tracking results are conflicting, even in similar paradigms (compare Castellanos et al., 2009; Werthmann et al., 2011; see Nijs and Franken, 2012 for a more detailed review).
2.2.2.2. Food-related tasks measuring other cognitive domains
One explicit memory task has also used food-related stimuli (Figure 1 & Table 1: 21; King et al., 1991), finding that obese people remembered more weight- and food-related items than other items. However, these results should be treated with caution, since groups were sub-optimally matched.
2.2.3. Interactions between executive and food motivation tasks
Both relative reinforcing value of food and Implicit Associations Test performance have been reported to interact with several measures of executive function (Hofmann et al., 2008; Hofmann, Friese, and Roefs, 2009; Nederkoorn et al., 2010; Rollins et al., 2010) and also with the Emotional Eating Scale from the Dutch Eating Behaviours Questionnaire (Ayres et al., 2011). Studies finding an interaction have all reported a very similar pattern. Lower executive function combined with higher food motivation is more strongly related to maladaptive eating behaviours or BMI than any of these measures alone (Appelhans et al., 2011; Hofmann et al., 2008; Hofmann, Friese, and Roefs, 2009; Nederkoorn et al., 2010; Rollins et al., 2010). Such an interaction is often demonstrated with a ± 1SD split technique recommended by Aiken and West (1991) – in participants with an executive function score less than 1 SD below the mean, a food motivation score is successfully related to an eating behaviour or BMI, whereas the mean food motivation scores have no effects in participants with an executive function score greater than 1 SD above the mean. In simpler terms, if executive scores are low, food motivation becomes the key in explaining maladaptive eating behaviours. Or vice versa – executive functions only become important for eating behaviours if a person is highly interested in food. This interaction has been highlighted in several review papers (Appelhans, 2009; Hofmann, Friese, and Strack, 2009; van den Bos and de Ridder, 2006).
It is interesting to note that the nature of interactions remains similar across different type of executive tasks and food motivation measures. The food motivation tasks include relative reinforcing value of food (Rollins et al., 2010), Implicit Associations Test (Figure 1 & Table 1: 36, 38; Hofmann et al., 2008; Hofmann, Friese, and Roefs, 2009; Nederkoorn et al., 2010) and the Power of Food Scale (Appelhans et al., 2011); the executive measures include stop signal task, affect regulation, computational span and delay discounting (Figure 1 & Table 1: 42, 55, 59, 61). However, in other work the executive tasks themselves correlate poorly with each other (Duckworth and Kern, 2011; Hofmann, Friese, and Roefs, 2009). These poor correlations suggest that each of the executive functions has a separate effect on eating behaviours (Hofmann, Friese, and Roefs, 2009).
Some studies have also tried to combine food motivation and executive measures into a single task, resulting in tasks such as food Stroop, food delay discounting and food probability discounting. Food discounting, like money discounting, yields conflicting findings, with one study reporting differences between obese and normal weight participants (Figure 1 & Table 1: 39–40; Rasmussen et al., 2010) and another reporting no differences (Manwaring et al., 2011). Still, in Rasmussen et al. (2010), food discounting correlates better with obesity indexes than money discounting, suggesting that food-related executive control might be more relevant than general executive control in obesity. Food Stroop performance can also predict weight change (Figure 1 & Table 1: 41; Calitri et al., 2010) and differentiate weight loss maintainers from other groups (Phelan et al., 2010) but does not correlate with current BMI (Nijs, Franken, et al., 2010; Phelan et al., 2010; Pothos et al., 2009). Again, these inconsistent results might relate, in part, to the different indexes and task parameters used in different studies.
In sum, executive and food motivation tasks seem to provide the most consistent and promising effects, especially in their interaction. From these two domains the tasks most consistently related to various maladaptive eating behaviours are the stop signal task, Stroop task, go/no go, operation span, Austin maze, Wisconsin Card Sorting Test, Iowa Gambling Task, delay discounting, relative reinforcing value of food and Implicit Association Test (Figure 1 & Table 1: 36, 38, 42, 43, 50, 53, 55, 58, 59, 60).
2.3. Reliability
The neurobehavioural tasks identified as most promising were next scrutinised in terms of their measurement characteristics. While establishing a measure’s validity and reliability has been the gold standard in questionnaire development, reliability has been relatively neglected for many neuropsychological tasks, particularly those arising from the very recent cognitive neuroscience literature. Most studies applying questionnaires cite studies establishing the validity and reliability of a measure and also provide their own reliability scores. In contrast, most neuropsychological research seems to be concerned mainly with the validity of a measurement. Yet, a test cannot have high validity without high reliability – the reliability of a measure determines the maximum possible correlation between the measure and a given outcome.
Table 2 summarises the available reliability scores for the most promising neuropsychological tasks in two categories – test-retest reliability and internal consistency. Test-retest reliability measures the stability of a measure from one testing occasion to another. Poor test-retest reliability (<.70) significantly limits the usefulness of a task, notably this means that the results might only apply in that particular session, or might be prone to significant measurement error. Other potential sources of lower test-retest reliability include carry-over or learning effects, where improvement in a participant’s score is related to learning how to better perform the task, or different motivational states influencing level of engagement (Windle, 2012), rather than a true change in the underlying cognitive construct. While these issues can be somewhat alleviated with experiment designs, in general low test-retest reliability scores make a task poorly suited to studying longitudinal or intervention-related effects. Internal consistency reflects how consistently different parts of a test measure the same construct. Poor internal consistency (<.70) means that some parts of a test are not contributing to the intended outcome measure and might measure something else. However, very high internal consistency scores (>.90) are also not desired, as this may indicate undue narrowness or item redundancy (Streiner, 2003). Most tasks had just one reliability score available. For tasks with reliability tested more than once, a summary score or scores obtained from task designs most similar to designs used in eating behaviours research are presented.
Table 2.
Test-retest reliabilities and internal consistencies of neurocognitive tests related to obesity and weight-related eating behaviours
| Measure (nr in Table 1) | Test-retest reliability | Internal consistency | Studies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IAT (36) | .56c | .70–.90ab | Roefs et al.(2011) | ||||||
| RRVf (38) | .80c | n/a | Epstein et al. (2007) | ||||||
|
| |||||||||
| Delay discounting (42) | .60 – .90c depending on index and study setup | .89a | Hurst et al. (2010), Weatherly et al. (2011) | ||||||
| Iowa Gambling Task (43) | .08 – .47c | .21 – .54b | Cardoso et al. (2010), Gansler et al. (2011) | ||||||
|
| |||||||||
| Wisconsin Card Sorting Test (50) | Lineweaver et al. (1999), Paolo et al. (1996), Tate et al.(1998), Waldorf (2010) | ||||||||
| non-perseverative errors | .46c; .74e | .60b | |||||||
| perseverative errors | .64c; .72e | .53b | |||||||
|
| |||||||||
| Austin Maze (53) | n/a | Morrison and Gates (1988) | |||||||
| TTC trad | .79c | ||||||||
| TTC comp | .81c | ||||||||
| errors trad | .73c | ||||||||
| errors comp | .83c | ||||||||
| time trad | .75c | ||||||||
| time comp | .77c | ||||||||
| Computational span (55) | .77c | .76–.85a | Beckmann et al. (2007), Hofmann et al. (2008) | ||||||
| .79b | |||||||||
| CPT-II (similar to go/no go) (58) | .73–.95a | Schweiger et al. (2007), Soreni et al. (2009) | |||||||
| % OMM | .09*d | ||||||||
| % FP | .72*d | ||||||||
| HR RT | .76*d | ||||||||
| HR SE | .63*d | ||||||||
| Stop signal task (59) | Friedman et al. (2011), Soreni et al. (2009) | ||||||||
| SSRT | .72*d | .75*b | |||||||
| Stroop (single stimulus) (60) | Friedman et al. (2011), Siegrist (1995), Strauss (2005), | ||||||||
| incongruent – congruent | .46c | ||||||||
| incongruent – XXXX | .73c | .87–.88a; .91*b | |||||||
| RT to incongruent | .79–.87c | ||||||||
Note.
Cronbach alpha;
Split-half reliability;
correlation;
Intraclass Correlation Coefficient-mixed random effects model of absolute agreement;
Intraclass Correlation Coefficient - single-case approach;
= participants younger than 18;
% FP = commission errors; % OMM = omission errors; comp = computerised; CPT II = Conners’ Continuous Performance Test II; HR SE = standard error of hit rate reaction time; HR RT = hit rate reaction time; IAT = Implicit Associations Test; RRVf = Relative reinforcing value of food; RT = reaction time; SSRT = stop signal reaction time; trad = traditional; TTC = trials to completion. A value of .70 is the generally accepted minimum for both internal consistency and reliability and .90 the accepted maximum for internal consistency. Test-retest intervals ranged from 2 days to 2 years.
The available reliability scores for selected tasks are displayed in Table 2. Most common measures pass the criterion of .70 in both columns. However not all paradigms and indexes deliver equally reliable results, which may be important in choosing the particular measure in Stroop, go/no go and delay discounting tasks, for example. In addition, the go/no go index used by Hall (2012) and Hall et al. (2008) has not been tested for reliability. Two tasks, the Iowa Gambling Task and Wisconsin Card Sorting Test, perform relatively poorly according to both criteria. However, modifications of both tasks have been proposed that might improve their reliability (e.g., Gansler et al., 2011; Nyhus and Barceló, 2009; Rossell and David, 1997). Both tasks have a learning element that may explain the poor reliability; whether this is a problem depends on the research question. The Implicit Associations Test has not been tested for reliability with food stimuli. In general, the paradigm reports quite good internal consistency, but the average test-retest reliability does not meet the .70 criterion. This could mean that the implicit associations measured in this task are changing across time. Future obesity studies interested in this test should establish the reliability of their particular stimuli, especially as stimulus type is known to influence study outcomes (see Czyzewska et al., 2011; Roefs et al., 2011). Some of the reliability scores (especially for the stop-signal task) have been obtained from studies in children, and might be different in adult populations.
2.4. Neurocognitive tasks: Summary
Based on the available literature, it seems that the best tasks in terms of both consistent relationships with eating behaviours and reliable measurement characteristics are the stop-signal task, Austin Maze and certain measures from the Stroop task. These tasks are reliable and have each been related to various eating behaviours. Computational span and certain types of delay discounting are also reliable, but have shown the most robust relationships with BMI or eating behaviours through an interaction with a food motivation measure. Reaction time in the go/no go task also seems to provide consistent results, but the reliability of this index is unknown. The relative reinforcing value of food task seems to be the best measure of food motivation in terms of replicated relationships with BMI or eating behaviour, and reliability. The Implicit Associations Test has also performed quite well, but care must be taken in the choice of stimuli. In addition this measure is likely to have lower test-retest reliability, suggesting either that the measure could still be improved or that implicit attitudes are subject to quick change. The latter possibility would be a challenge for conceptual models of implicit food motivation as a relevant individual difference.
At a domain level, the most useful measures of executive functions capture response inhibition, working memory and, to a lesser extent, decision-making. While attention shifting has shown some promising results, the Wisconsin Card Sorting Test unfortunately suffers from low reliability and the Trail-Making-Test B has not shown consistent relationships with BMI. Future studies interested in shifting might want to test this subdomain more thoroughly with more reliable tasks. Verbal fluency and complex executive function subdomains have also not shown any consistent effects.
In the food motivation domain, the current analysis has highlighted quite reliable and consistent measures within the subdomains of reward value and implicit associations. The executive control subdomain that combines executive tasks and food stimuli still needs more attention – food Stroop might eventually be considered, once the field agrees upon common indexes and establishes reliability scores. The attention bias subdomain is inconsistent, at least on the level of commonly measured behavioural indexes. While the subdomain’s consistency might eventually be improved by eye-tracking measures, this requires devices that add complexity.
In sum, the current analysis substantially narrows the “menu” for selecting neurocognitive measures that may relate to eating behaviours. Out of the 66 tasks reported in the literature, fewer than 11% provide consistent and reliable effects. The most consistent results arise from tasks capturing executive functions and food motivation, and within these two domains a few tasks stand out both for replication of effects, and for adequate measurement properties. We take up the question of how these tasks relate to the brain in a later section. Other cognitive domains seem to be less related to BMI or eating behaviours, although they have also been less studied. Future work would benefit from clear hypotheses as to how these domains might influence eating behaviours.
3. Personality Questionnaires
Personality research in relation to eating behaviours can be divided into three major approaches: relating various eating behaviours with personality scales that capture a) general, b) specific, or c) eating-related aspects of human personality. The use of general personality scales provides a framework for understanding eating behaviour in a wider context, as this enables comparing personality profiles related to eating behaviours to personality profiles related to other behaviours of interest. Specific measures of personality are preferred when researchers have a particular interest in a single construct, such as impulsivity, self-control or sensitivity to reward. Eating-related measures are explicitly designed to capture different personality types within the eating domain.
3.1. General Measures of Personality
The most widely used taxonomy of personality traits viewed as a whole is the Five-Factor Model. Several other personality models exist, but they share the same underlying structure as the Five-Factor Model (e.g., Markon et al., 2005). In this model, a variety of specific personality traits are understood as aspects of five broad domains/factors (or blends of two or more of those domains): Neuroticism, Extraversion, Openness/Intellect, Agreeableness and Conscientiousness. Neuroticism reflects sensitivity to punishment and negative affect, Extraversion captures sensitivity to reward and positive affect towards the social and material world, Openness/Intellect reflects cognitive and perceptual flexibility and exploration, Agreeableness characterizes altruism as opposed to exploitation of others, and finally Conscientiousness reflects top-down control over impulses that facilitates goal-directed behaviour. Each of these domains can be further divided into several intercorrelated subdomains/subfacets. For instance, Neuroticism comprises subdomains labelled N1: Anxiety, N2: Angry Hostility, N3: Depression, N4: Self-Consciousness, N5: Impulsiveness, and N6: Vulnerability. While the tools measuring at this level of detail are often considerably longer, this added detail may be important in relation to eating behaviours. The most widespread complete measure of personality is the Revised NEO Personality Inventory (NEO-PI-R; Costa and McCrae, 1992); there is also a public domain alternative – International Personality Item Pool version of NEO (IPIP-NEO; Goldberg et al., 2006).
Large-scale studies with samples of several thousand people have found that obese people tend to score high in aspects of Neuroticism and low on aspects of Conscientiousness, as well as showing aspects of high Extraversion and low Agreeableness (e.g., Brummett et al., 2006; Sutin et al., 2011; Terracciano et al., 2009). A closer look at the subdomains found to be important by both Sutin et al.(2011) and Terraciano et al. (2009) reveals a more precise characterisation –compared to healthy weight participants, obese persons tend to be more volatile, unable to resist temptation (N5: Impulsiveness), are assertive/wanting (high E3: Assertiveness, low E4: Activity) and score lower on self-control (low C2: Order, low C5: Self-Discipline). The most crucial subdomain seems to be N5: Impulsiveness, with persons scoring in the top 10% of N5: Impulsiveness being on average 11kg heavier than persons scoring in the bottom 10%. C2: Order is the next in line, with high scorers weighing 4.5 kg less than low scorers (Sutin et al., 2011). The combination of high Neuroticism and low Conscientiousness is known to be associated with self-control problems in general, especially in the midst of difficulties or frustration (Costa and Piedmont, 2003). Higher scores in E3: Assertiveness seem to be related to an additional seeking/wanting approach to rewards, including food. Longitudinal weight gain is also predicted by high Neuroticism (N5: Impulsivity), with other relevant subdomains including risk-taking (high E5: Excitement-Seeking) and low Agreeableness characterised by cynicism and competitiveness (low A1: Trust, low A4: Compliance)(Sutin et al., 2011). The combination of high Neuroticism and Extraversion is also associated with risky behaviour, as such persons seem to combine poor emotional regulation with high reward responsiveness. The emergence of Agreeableness as a correlate of obesity is in accordance with previous results demonstrating that higher hostility is associated with greater likelihood to continue eating when satiated (van den Bree et al., 2006), and with research suggesting that social support is important for maintaining a healthy weight (e.g., Wing and Jeffery, 1999). Thus, people scoring low on Agreeableness, who are less able to develop or maintain social relationships, may have an additional risk factor for becoming obese. Earlier work on eating behaviours with other personality scales has also shown the importance of impulsivity and conscientiousness more generally as obesity correlates (Bogg and Roberts, 2004; Chalmers et al., 1990; Rydén et al., 2003, 2004). However, the Five-Factor Model with its subdimensions has provided more fine-grained insight into potential behavioural mechanisms for the observed relationships.
Openness/Intellect is the only personality dimension not directly related to obesity or weight gain. However, Openness/Intellect has recently been highlighted as being most strongly related to consuming healthy Mediterranean dietary items and avoiding traditional dietary items in North Americans and Europeans (Brummett et al., 2008; Goldberg and Strycker, 2002; Mõttus et al., 2011, 2012). Thus, adherence to novel, healthier diets may be more likely in\those who are more intellectually open and curious. Interventions may benefit from being tailored to this aspect of personality (Mõttus et al., 2011, 2012).
3.2. Specific Personality Questionnaires
More specific personality constructs relevant in food research are impulsivity, self-control and sensitivity to reward. Each of these constructs has emerged from different backgrounds, and several measures exist for each. Impulsivity, a tendency to act without sufficient forethought, has been mostly related to eating behaviours via the broad personality assessments highlighted above, but some evidence also exists for impulsivity-specific questionnaires as correlates of obesity (Guerrieri, Nederkoorn, and Jansen, 2007; Churchill and Jessop, 2011; Larsen et al., 2012; Strimas et al., 2008; but see Loeber et al., 2011; Weller et al., 2008). The most comprehensive measure is the UPPS Impulsive Behaviour Scale, developed from a factor analysis of the eight most commonly used impulsivity questionnaires. This scale is able to distinguish four dimensions of impulsivity - Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation-Seeking (Whiteside and Lynam, 2001). Higher scores in Urgency and lack of Perseverance have been related to the inability to stick to the intention of avoiding snacks (Churchill and Jessop, 2011). UPPS is designed to match particular sub-dimensions of the Five-Factor Model (N5, C6, C5 and E5, respectively), which suggests that scores on these personality subdimensions can be used as proxies for impulsivity assessment (Miller et al., 2003).
Other research has focused on an opposing construct called self-control, a capacity to change dominant response tendencies and regulate oneself. A recent meta-analysis demonstrated that self-control has repeatedly been related to weight and eating behaviours (de Ridder et al., 2011). The same review also identified the Self-Control Scale (Tangney et al., 2004) as delivering the most coherent results.
Another construct repeatedly related to eating behaviours is sensitivity to reward, which measures responsiveness to reward cues and reinforcing behaviours (Davis and Fox, 2008; Davis et al., 2007; Davis, Strachan, et al., 2004; Franken and Muris, 2005; Pagoto et al., 2006). Recent results suggest that reward sensitivity’s relationship to BMI might in fact be curvilinear, with lean and overtly obese individuals manifesting less sensitivity to reward than overweight persons (Davis and Fox, 2008). The best measure of sensitivity to reward is the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (Torrubia et al., 2001) which has demonstrated superior validity over other commonly used sensitivity to reward scales (Caseras et al., 2003; Smillie and Jackson, 2005).
3.3. Specific Eating-Related Questionnaires
At least five different food-related personality constructs have emerged from several decades of research: restraint, disinhibition, susceptibility to hunger, emotional eating and external eating. Recent reviews highlight that all of these constructs have repeatedly been related to overweight and eating behaviours (Bryant et al., 2008; Herman and Polivy, 2008; Johnson et al., 2011; Lowe and Thomas, 2009; Macht and Simons, 2011; Macht, 2008; Williamson et al., 2007). A higher restraint score is related to overeating tendencies in normal weight individuals, whereas in overweight persons, a higher restraint score might in fact be a protective factor (Johnson et al., 2011). Two instruments are most often used to capture these constructs: the Three-Factor Eating Questionnaire (Eating Inventory) (Cappelleri et al., 2009; Karlsson et al., 2000; Stunkard and Messick, 1985) and the Dutch Eating Behaviours Questionnaire (van Strien et al., 1986). Some have proposed to reduce the number of constructs. For instance, Karlsson et al (2000) have proposed that hunger and disinhibition be merged in their revised version of the Three-Factor Eating Questionnaire. Similarly, other researchers suggest that external and emotional eating reflect the same construct (Heaven et al., 2001; Jansen et al., 2011; but see van Strien et al., 2012; Volkow et al., 2003).
Recent shorter food motivation measures may even further reduce the number of constructs. Most notably, the Power of Food Scale reports .54 – .66 correlations with all the above-mentioned food constructs, apart from restraint (.30) (Lowe et al., 2009), which instead characterises food avoidance. Questionnaire measures of the relative reinforcing value of food have alson been developed (Epstein et al., 2010; Goldfield et al., 2005) but they have not been compared to other eating-related questionnaires. Another interesting approach is the food dimension of the Domain-specific Impulsivity Scale (Tsukayama et al., 2012). This scale seeks to combine into one measure both general impulsivity and food motivation. In sum, all these new measures provide quicker assessments of eating behaviour tendencies, and at least the Power of Food Scale seems to account for a considerable amount of the variance measured with more detailed scales. As there have not been any comparative studies among food motivation measures, the best scale cannot be established.
3.4. Convergence Across Constructs
To explore the possible overlap between previously highlighted constructs we mapped them to the Five-Factor Model, as in McCrae and Löckenhoff (2010). Most of the above-mentioned questionnaires have been correlated with a measure of the Five-Factor Model, apart from the newer food motivation measures such as Power of Food Scale, questionnaire measures of relative reinforcing value of food, and the food dimension of the Domain-specific Impulsivity Scale. As illustrated in Table 3, all specific personality questionnaires and eating-related questionnaires that could be evaluated have quite similar Five-Factor Model profiles. Such convergence explains why, through different routes, all of these constructs have eventually been found relevant in food research – based on Five-Factor Model profiles all these measures seem to reflect a very similar concept jointly constructed from Neuroticism and Conscientiousness, accompanied by subdimensions of Extraversion and Agreeableness. Not surprisingly, this same combination has emerged in Five-Factor Model studies of obesity (Sutin et al., 2011; Terracciano et al., 2009).
Table 3.
Classification of specific personality eating-related questionnaires in terms of the Five-Factor Model of personality traits.
| Measure | Study | Domain/Factor |
||||
|---|---|---|---|---|---|---|
| N | E | O | A | C | ||
| UPPS Impulsivity factors | Whiteside and Lynam (2001) | Urgency (.58) | Sensation Seeking (.48) |
Perseverance
(.80) Premeditation (.63) Low Urgency (−.54) |
||
| Self-control Scale | Tangney et al. (2004) | Low self-control (−.50) | Self-control (.29) | Self-control (.54) | ||
| SR from SPSRQ | Mitchell et al. (2007) | Sensitivity to reward (.13) | Sensitivity to reward (.38) | Low sensitivity to reward(−.34) | Low sensitivity to reward (−.17) | |
| TFEQ | Provencher et al. (2008) |
Disinhibition (.37) Hunger (.29) |
Low disinhibition (−.29) Low hunger (−.29) |
Low hunger (− .27) | Restraint (.16) Low disinhibition (−.21) Low hunger (−.25) |
|
| DEBQ | Elfhag et al. (2008) | Low restraint
(−.18) Emotional eating (.48) External eating (.36) |
Restraint (.15) Low emotional eating (−.14) |
Restraint (.13) | Restraint (.22) Low emotional eating (−.26) Low external eating (−.17) |
|
Note: Scales with correlations or factor loadings greater than .30 are given in bold. DEBQ = Dutch Eating Behaviour Questionnaire; SPSRQ = Sensitivity to Punishment and Sensitivity to Reward Questionnaire; SR = Sensitivity to Reward; TFEQ = Three-Factor Eating Questionnaire; UPPS = UPPS Impulsivity scale.
3.5. Reliability
Almost all of these personality instruments provide good internal consistency and test-retest reliability (Table 4). The test-retest reliability of the UPPS Impulsive Behaviour Scale and longer versions of IPIP-NEO have not yet been established, but given these measures’ strong relationships with the Five-Factor Model (e.g., Whiteside and Lynam, 2001), reliability is likely to be acceptable. Test-retest reliability has also not yet been established for the Domain-specific Impulsivity Scale for food. The questionnaire measures of relative reinforcing value of food do not have established reliability, so these scales should be used with caution. Some caution is also required for a few subdimensions of the Revised NEO Personality Inventory as they have slightly lower internal consistency. In addition, several food personality dimensions have notably high internal consistencies, indicating that these dimensions might be too narrow or have redundant items (Streiner, 2003).
Table 4.
Test-retest reliabilities and internal consistencies of personality measures related to obesity and weight-related eating behaviours
| Measure | Test-retest reliability | Internal consistency | Studies |
|---|---|---|---|
| IPIP-NEO, MINI-IPIP | Donnelan et al. (2006) (“International Personality Item Pool,” 2012) | ||
| MINI-IPIP, Domains | .72 – .89c | .78 – .91a | |
| IPIP-NEO, Domains | n/a | .81 – .86a | |
| IPIP-NEO, Subdomains | n/a | .71 – .88a | |
| NEO-PI-R | McCrae et al. (2010) | ||
| Domains | .87 – .92f | .86 – .92a | |
| Subdomains | .72 – .88f | .56 – .81a | |
|
| |||
| Self-Control Scale | .89c | .89a | Tangney et al. (2004) |
| SPSRQ | Torrubia et al. (2001) | ||
| Sensitivity to Reward | .61–.87c | .77a | |
| UPPS | n/a | .83 – .89a | Whiteside et al. (2005) |
|
| |||
| DEBQ | Calitri et al. (2010) | ||
| Restrained Eating | .81c | .95a | van Strien et al. (1986) |
| Emotional Eating (13 items) | .79c | .94a | |
| Emotional Eating (9 items) | .93a | ||
| Emotional Eating (4 items) | .86a | ||
| External Eating | .81c | .80a | |
| DSIS - food | n/a | .85a | Tsukayama et al. (2012) |
| Qm of RRVf | n/a | n/a | |
| Power of Food Scale | .77c | .91a | Lowe et al. (2009) |
| TFEQ (Eating Inventory) | Stunkard and Messick (1985) | ||
| Restraint | .81–.93c | .93a | |
| Disinhibition | .80–.86c | .91a | |
| Hunger | .75–.83c | .85a | |
| TFEQ-R18v2 | Cappelleri et al. (2009) | ||
| Cognitive restraint | n/a | .78a | |
| Uncontrolled Eating (Disinhibition + Hunger) | .89a | ||
| Emotional Eating | .94a | ||
Note.
Cronbach alpha;
correlation;
Average correlation from several measurements
DEBQ = Dutch Eating Behavior Questionnaire; DSIS = Domain-specific Impulsivity Scale; IPIP = International Personality Item Pool; NEO-PI-R = NEO Personality Inventory-revised; Qm of RRVf = questionnaire measures of relative reinforcing value of food; SPSRQ = Sensitivity to Punishment and Sensitivity to Reward Questionnaire; TFEQ = Three-Factor Eating Questionnaire. A value of .70 is the generally accepted minimum for both internal consistency and reliability; .90 should be the accepted maximum for internal consistency. Test-retest intervals ranged from three weeks to three years.
3.6. Personality Scales: Summary
In sum, different approaches to personality have each highlighted their own constructs that are consistently related to obesity and eating behaviours. In terms of psychometrics, the majority of the measures with reliability data available are highly reliable (reliability indexes above .70). Thus, the preference for a particular questionnaire should be determined by other factors, such as the trade-off between level of detail and number of items, and comparability with other research. General personality measures provide more detailed insights and comparability with research from other domains, but are considerably longer if applied at full length. Specific personality measures are quicker and maintain comparability but lose the detailed insight into potential mechanisms offered by a full personality assessment. For instance, the Sensitivity to Reward scale from the Sensitivity to Reward and Sensitivity to Punishment Questionnaire seems to tap quite well the personality subdimensions deemed important for obesity (Mitchell et al., 2007; Sutin et al., 2011). However, this questionnaire does not allow analysis of the potential modifying effects of differences in the personality domains captured in aggregate as sensitivity to reward. At first glance, the moderately long specific eating-related personality measures might seem to have the benefit of an extremely detailed characterisation of various eating-related behaviours. However, some researchers have suggested that this level of detail is unnecessary as several of these eating-related constructs are highly interrelated. Restraint still remains independent of other measures, but the rest might loosely be labelled as measuring “food motivation”. These claims are supported by Table 3, where most eating-related measures all share a quite similar Five-Factor Model profile, whereas restraint correlations are in the opposite direction. Still, more evidence is needed to support these claims – to our knowledge no study has applied all the main specific eating-related personality measures at full length to the same population. As food personality research is still missing the integrative approach that Whiteside and Lynam (2001) applied to the multidimensionality of impulsivity, the set of relevant food constructs remains to be authoritatively established.
4. Brain Mechanisms
Several brain-based models of eating behaviour have been proposed, which vary in their level of detail and main focus (e.g., Berthoud and Morrison, 2008; Carnell et al., 2011). Most suggest at least three central mechanisms in the control of eating: 1) a hypothalamic system sensitive to homeostatic signals, through which the organism matches food intake with energy requirements, 2) a striatal and limbic emotion/memory system sensitive to current and past reward experiences, through which the organism regulates food motivation, and 3) a cortical executive system that allows the pursuit of more abstract goals, through which the organism tries to match its current and future nutritional needs with requirements and affordances from the surrounding environment, and to take into account longer term goals, such as health, in the control of eating behaviour. While, the role of hypothalamic and emotion/memory systems in feeding have been mapped out quite extensively through animal models (e.g., Berthoud and Morrison, 2008), the mechanisms of human executive control over eating are less clear. As our search strategy for neurocognitive tasks excluded studies manipulating hunger, the current analysis will focus on the executive and emotion/memory systems, relying on the neurobehavioural measures found relevant in the analysis above. Evidence from neurocognitive measures and personality questionnaires will be used interchangeably as conceptually similar measures tend to relate to similar brain structures. While neurocognitive tasks in many cases arose from the clinical-neuropsychological tradition and so are more easily related to brain systems (Lezak, 2004), recent neuroimaging evidence suggests that each of the Big Five personality traits can also be related to the function of mostly non-overlapping brain regions (DeYoung et al., 2010), as assessed by anatomical and functional magnetic resonance imaging (MRI). For example, individual variations in cortical gray matter in specific brain regions has been related to variations in each of these personality traits.
4.1. Executive Control
Several neurocognitive tasks and questionnaire constructs have been related to fronto-cortical regions. Work in patients with focal frontal lobe damage, as well as functional neuroimaging in healthy subjects, suggests distinct, at most partly overlapping frontal-subcortical substrates for Stroop, stop-signal, working memory, and decision-making abilities (Aron and Poldrack, 2006; Fellows and Farah, 2005; Nee et al., 2007; Tsuchida and Fellows, 2009, Submitted). Higher Conscientiousness has been associated with greater volumes in the lateral prefrontal cortex (LPFC) (DeYoung et al., 2010), an area associated with long-range planning, goal setting, monitoring, and executive control. It is reasonable to posit that this brain region supports aspects of self-control. Considerable evidence suggests that activation of the lateral PFC in functional MRI experiments represents engagement of self-control mechanisms (reviewed in Dagher, 2012). Asking subjects to focus on the health aspects of visually displayed foods (Hare et al., 2011) or to downregulate their appetitive response to tasty foods (Hollmann et al., 2012) activated lateral PFC. In the latter study, the signal change in LPFC correlated with a measure of dietary restraint taken from Strunkard’s Three-Factor Eating Questionnaire.
4.2. Emotion/Memory System
In support of the behavioural evidence linking obesity to an enhanced sensitivity to the rewarding properties of food, functional MRI studies consistently demonstrate increased neural reactivity to food stimuli in limbic brain regions involved in reward and motivation in obese or at-risk individuals (Dagher 2012). These regions include the orbitofrontal cortex, insula, striatum, and medial temporal lobe, and their activity likely reflects the incentive value of food stimuli to the individual. In addition, this cue-induced neural response also correlates with an individual’s score on Carver and White’s (Carver and White, 1994) behavioural activation scale (Beaver et al., 2006), a measure of sensitivity to reward. Sensitivity to reward is related to both Neuroticism and Extraversion (Mitchell et al., 2007; Torrubia et al., 2001) and indeed, these traits have also been linked to the same reward-related areas. Extraversion correlates with greater functional connectivity of limbic reward areas at rest (Adelstein et al., 2011) and the trait has been related to the volume of the medial orbitofrontal cortex (DeYoung et al., 2010; Rauch et al., 2005). Activity in the medial orbitofrontal cortex reflects the current reward value of food stimuli (Plassmann et al., 2007). Thus, Extraversion appears to correlate with an enhanced responsivity of brain regions involved in motivated behaviours. Neuroticism also is associated with reward-related regions’ greater reactivity to novel stimuli, but also to aversive stimuli (reviewed in DeYoung, 2010b). In addition, Neuroticism has been related to poor connectivity between reward structures and the anterior cingulate cortex (ACC), as well as other prefrontal regions (Cremers et al., 2010), suggesting weaker top-down control relying on prefrontal and limbic interconnections.
Similar activation patterns of reward-related areas arise from food-specific personality research (reviewed in Carnell et al., 2011). In particular, higher scores in external eating are related to heightened modulation of ventral striatum by the amygdala, higher connectivity between striatal regions and motor areas and a decreased modulation of amygdala and ventral striatum by the ACC. Higher scores in disinhibited eating are again related to higher activation in areas involved with reward/motivation (medial PFC, midbrain and insula) paired with lesser ACC responses to visual food vs. non food cues and lesser dorsolateral PFC activation. Higher emotional eating scores have been related to higher dopaminergic striatal responses to gustatory and olfactory cues(Volkow et al., 2003) and greater activation in parahippocampal gyrus, ventral pallidum, thalamus and ACC in anticipating and/or consuming milkshakes (Bohon et al., 2009; reviewed in Carnell et al., 2011).
4.3. Brain Mechanisms: Summary
In sum, the executive and self-control related neurocognitive tasks and personality questionnaires have all been related to prefrontal regions, whereas various measures of sensitivity to food or novel stimuli have been associated with reward-related limbic areas, including medial temporal lobe, striatum, and orbitofrontal cortex. This accumulating evidence suggests that obesity is linked to higher reactivity of reward circuitry, lower activity in lateral prefrontal regions and weaker connectivity between reward circuitry and prefrontal regions. This model is consistent with the interaction patterns highlighted in the neurocognitive section, where an executive measure score moderates the effect of food motivation. It seems that people with lower reward circuitry activity are less vulnerable to obesity and people with higher reward circuitry activity benefit from the moderating role of prefrontal regions. The specific contribution of prefrontal regions is still unclear as highlighted by the independent effect of different executive measures (e.g., Hofmann, Friese, and Roefs, 2009). Future studies will need to investigate the precise frontal mechanisms contributing to self-control.
The analysis of neuroimaging data will benefit from being grounded in robust and validated neurobiological models of eating behaviour. It is encouraging that there is some overlap in the putative brain mechanisms underlying relevant Five-Factor Model dimensions and eating-related personality measures and also neurocognitive measures. The convergence in the brain structures related to relevant questionnaires and relevant neurocognitive measures is also encouraging in the light of notoriously low intercorrelations between self-control questionnaires and neurocognitive measures of executive function more generally (average r=.11, Duckworth and Kern, 2011). Current evidence suggests that eating-related constructs rely on common brain structures, notably lateral frontal cortex in the case of self-control and executive function.
5. General Discussion
This review has summarised the current literature relating BMI, change in BMI or eating behaviours with neurobehavioural measures in otherwise healthy adults. Amongst neurocognitive measures, those sensitive to executive function and food motivation provide the most robust and reliable associations. Of the 66 tasks reviewed, only a few dependent measures from particular tasks both provide consistent results and have reliable psychometrics. The most robust and reliable executive function tasks are the Stop Signal Task and Stroop task measuring inhibitory control, computational span task and Austin Maze task measuring working memory, and delay discounting measuring one aspect of decision-making. The food motivation measures of highest yield seem to be the Relative Reinforcing Value of food task and Implicit Association Test. Interestingly, the most promising neurocognitive approach related to eating behaviours seems to be the combination of an executive and a food motivation measure.
Several other neurocognitive tasks were somewhat promising but were excluded for various reasons. First, many tasks have been studied only once, and so require replication. Other tasks seemed less conceptually informative, measuring either overly simple (tap test) or overly complex neuropsychological constructs (Rey-Osterrieth Complex Figure test). Poor psychometric reliability scores make some of the other, more consistently related single-domain measures difficult to recommend.
Several personality questionnaires measuring self-control, reward sensitivity and food motivation-related constructs seem to both relate to eating behaviours and have reliable psychometrics. The multiplicity of potential measures raises the possibility of overlapping constructs, supported by our analysis showing considerable overlap between the questionnaires based on their correlation with Big Five dimensions. Thus, the variety of different personality constructs related to eating behaviours may not be as great as the diversity of construct names suggests. Using the the Five-Factor Model as a framework, the mechanisms contributing to increased likelihood of obesity are increased reward seeking behaviour (high Extraversion), more reactivity to novel and aversive stimuli (high Neuroticism) and poor top-down control (low Conscientiousness), combined with poorer social abilities (low Agreeableness). Many other questionnaires characterise a combination of these dimensions and thus provide a quick and reliable estimation for the risk of obesity and maladaptive eating behaviours. However, general personality measures such as sensitivity to reward and impulsivity questionnaires provide opportunities to link results with studies of self-regulation in other domains, and Five-Factor Model measures additionally support more detailed analysis.
Both personality questionnaires and neurocognitive tests seem to point to common brain mechanisms underlying maladaptive eating behaviours. Apart from Agreeableness, there is considerable conceptual overlap between personality and neurocognitive research, pointing to a combination of increased food motivation (roughly equivalent to a combination of high Extraversion and Neuroticism) and weak executive control (low Conscientiousness). While the neurocognitive and personality measures do not seem to correlate well with each other, the neuroscience evidence suggest that measures tapping similar constructs implicate similar brain structures – the key structures underlying Conscientiousness and executive control seem to be prefrontal regions, whereas the key structures for Extraversion, Neuroticism and food motivation seem to be the reward circuitry and its connectivity with prefrontal structures.
While work needs to be done to confirm these mechanisms, the results provide a preliminary set of tools for a researcher interested in taking potential brain mechanisms into account when designing population-level studies to fully understand the multivariate, multi-level determinants of obesity. While large-scale neuroimaging studies become quite expensive, the neurocognitive and personality tools highlighted here provide an affordable way to shed light on how environmental variables, such as fast food restaurant density, interact with brain-level mechanisms of food motivation and self-control (e.g., Paquet et al., 2010). Of course, the level of resolution is currently very coarse – future work might include neurobehavioural measures that tap into more specific brain mechanisms.
Still, existing work has not addressed the overlap between neuropsychological tasks and questionnaires: would one or the other approach be sufficient to characterize individual differences in key aspects of BMI or eating related behaviours? From a measurement perspective, are these approaches capturing the same constructs? The impulsivity literature has wrestled with this dilemma for decades (e.g., Gerbing et al., 1987), as the correlation between neurocognitive and personality measures of impulsivity is low. In eating research, only a few attempts have been made to combine different types of impulsivity measures in a single study and the results have been conflicting, with one study showing effects for both questionnaires and neurocognitive measures, but others showing effects for only one of them (Guerrieri, Nederkoorn, Stankiewicz, et al., 2007; Guerrieri, Nederkoorn, and Jansen, 2007; Nederkoorn et al., 2006). If any of these measures are to be applied in large-scale studies or to guide interventions at the population level, further steps will be required to minimize test burden and optimize feasibility.
While the aforementioned neurocognitive tasks have quite robust cross-sectional relationships with eating behaviours, the longitudinal predictive power, important for establishing the direction of causality, is less clear. Only a handful of studies report the tasks’ ability to predict changes in BMI or eating behaviours and these results have yet to be replicated. Moreover, weight change itself has been reported to have subtle effects on performance on executive and memory tasks, possibly mediated by the effects of obesity-associated comorbidities (diabetes, lipid disorders, mental health issues) on brain function (Siervo et al., 2011; Smith et al., 2011). These findings raise the possibility of a bidirectional relationship between BMI and task performance, which should be controlled for in future studies.
The current results also highlight the widespread use of BMI in quantifying eating behaviours. While being easily measurable and of obvious health importance, BMI is presumably the result of a myriad of interactions throughout a person’s life (Dubé et al., 2008). Indeed, it is remarkable that single or interacting neurobehavioural measures have a detectable relationship with BMI. A better understanding of the path by which such measures and higher BMI are related will be vital, and will require more specific eating-related outcome measures, longitudinal and interventional designs. A few specific eating behaviours have been identified in this review, but many more are possible (e.g., Mesas et al., 2012).
One limitation of this review is the exclusion of studies that manipulated hunger. The hypothalamic homeostatic system has been shown to interact with hedonic and cognitive factors in several experiments (Batterham et al., 2007; Farooqi et al., 2007; Malik et al., 2008) and hunger state should be controlled for in any food-related study. However, the research on the moderating role of hunger and satiety was too vast to be covered in the current paper. The role of the homeostatic system in relation to neurobehavioural measures and eating behaviour in general deserves a systematic review on its own.
Another important issue not covered in this review is whether these measures are applicable in children. It will be important to establish this, to allow early identification of the risk of obesity at the point where it can be effectively prevented, i.e. in early childhood. Parent, teacher, and self reported self-control in childhood has been related to several health outcomes decades later, supporting the plausibility of this approach (e.g., Moffitt et al., 2011). However, it is quite likely that the tasks identified as highest yield in the current analysis need modifications before they can be applied to children (e.g., Baron and Banaji, 2006). Also, task performance is likely to change across the life span (Bedard et al., 2002), presumably owing to developmental changes in the brain (Jolles et al., 2011; Velanova et al., 2009) so norms must be established for each age group. More importantly, the mechanisms important in self-regulation of eating behaviours may well differ at different stages of development based on both social/environmental and neural mechanisms.
Despite all the work that still needs to be done, the current results provide an important intermediate step –we have highlighted the few key neurocognitive and personality measures that consistently and reliably can be related to BMI and maladaptive eating behaviours. Accumulating neuroimaging and lesion-based evidence suggests that these measures can also be linked to particular systems in the brain, and thus they provide a suitable and convenient alternative to neuroimaging for larger scale studies. Future work with measures that can be more tightly linked to particular brain mechanisms may be useful in advancing our understanding of the brain mechanisms related to obesity.
Supplementary Material
Highlights.
Individual neurobehavioral differences may predispose to obesity
The evidence relating specific behavioral measures and eating or BMI is reviewed
Certain executive function and food motivation tasks are reliable and predictive
Many relevant questionnaire measures tap similar personality factors
We identify measures indexing key behavioral differences predisposing to higher BMI
Acknowledgments
This study was supported by the Social Sciences and Humanities Research Council of Canada and the Canadian Foundation for Innovation.
The authors would like to thank Ami Tsuchida for help with categorising neurocognitive tasks, Matthias Doucerain and Margit Kõiv for their help with designing Figure 1, and Jüri Allik for helpful comments on an earlier version of this manuscript.
Footnotes
6. Conflict of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelstein JS, Shehzad Z, Mennes M, Deyoung CG, Zuo XN, Kelly C, Margulies DS, Bloomfield A, Gray JR, Castellanos FX, Milham MP. Personality is reflected in the brain’s intrinsic functional architecture. PLoS ONE. 2011;6:e27633. doi: 10.1371/journal.pone.0027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; Newbury Park, Calif: 1991. [Google Scholar]
- Allan JL, Johnston M, Campbell N. Unintentional eating. What determines goal-incongruent chocolate consumption? Appetite. 2010;54:422–425. doi: 10.1016/j.appet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Allan JL, Johnston M, Campbell N. Missed by an inch or a mile? Predicting the size of intention–behaviour gap from measures of executive control. Psychol & Health. 2011;26:635–650. doi: 10.1080/08870441003681307. [DOI] [PubMed] [Google Scholar]
- Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obes. 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obes. 2011;19:2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006;26:2424– 2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres K, Prestwich A, Conner M, Smith P. Emotional eating moderates the relationship between implicit measures of attitudes and chocolate consumption. Eur J Pers. 2011;25:317–325. [Google Scholar]
- Baron AS, Banaji MR. The development of implicit attitudes. Evidence of race evaluations from ages 6 and 10 and adulthood. Psychol Sci. 2006;17:53–58. doi: 10.1111/j.1467-9280.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SCR. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann B, Holling H, Kuhn JT. Reliability of verbal–numerical working memory tasks. Pers Individ Dif. 2007;43:703–714. [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The Brain, Appetite, and Obesity. Annual Review of Psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices and activity. Br J Nutr. 2000;83(Suppl 1):S33–38. doi: 10.1017/s0007114500000933. [DOI] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol. 2008;23:467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychol Bull. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Bohon C, Stice E, Spoor S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. International Journal of Eating Disorders. 2009;42:210–221. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghans L, Golsteyn BHH. Time discounting and the body mass index: Evidence from the Netherlands. Econ Hum Biol. 2006;4:39–61. doi: 10.1016/j.ehb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, O’Callaghan G, Yoder R, O’Shea D. Impaired decision making among morbidly obese adults. J Psychosom Res. 2010;70:189–196. doi: 10.1016/j.jpsychores.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, Pignatti R. Anorexia, bulimia, and obesity: Shared decision making deficits on the Iowa Gambling Task (IGT) J Int Neuropsychol Soc. 2010;16:711–715. doi: 10.1017/S1355617710000354. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Williams RB, Barefoot JC, Costa PT, Siegler IC. NEO personality domains and gender predict levels and trends in body mass index over 14 years during midlife. J Res Pers. 2006;40:222–236. [Google Scholar]
- Brummett BH, Siegler IC, Day RS, Costa PT. Personality as a predictor of dietary quality in spouses during midlife. Behav Med. 2008;34:5–10. doi: 10.3200/BMED.34.1.5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant E, King N, Blundell J. Disinhibition: Its effects on appetite and weight regulation. Obes Rev. 2008;9:409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Calitri R, Pothos EM, Tapper K, Brunstrom JM, Rogers PJ. Cognitive biases to healthy and unhealthy food words predict change in BMI. Obes. 2010;18:2282–2287. doi: 10.1038/oby.2010.78. [DOI] [PubMed] [Google Scholar]
- Camus M, Halelamien N, Plassmann H, Shimojo S, O’Doherty J, Camerer C, Rangel A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur J Neurosci. 2009;30:1980–1988. doi: 10.1111/j.1460-9568.2009.06991.x. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Lowe MR, Karlsson J. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: Results from a large diverse sample of obese and non-obese participants. Int J Obes. 2009;33:611–620. doi: 10.1038/ijo.2009.74. [DOI] [PubMed] [Google Scholar]
- de Cardoso CO, Carvalho JCN, Cotrena C, di Bakos DGS, Kristensen CH, Fonseca RP. Estudo de fidedignidade do instrumento neuropsicológico Iowa Gambling Task [Reliability study of the neuropsychological test Iowa Gambling Task] J Bras Psiquiatr. 2010;59:279–285. [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner C, Geliebter A. Neuroimaging and obesity: Current knowledge and future directions. Obes Rev. 2011;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Caseras X, Āvila C, Torrubia R. The measurement of individual differences in behavioural inhibition and behavioural activation systems: A comparison of personality scales. Pers Individ Dif. 2003;34:999–1013. [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: Evidence for altered reward system function. Int J Obes. 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Chalmers DK, Bowyer CA, Olenick NL. Problem drinking and obesity: A comparison in personality patterns and life-style. Substance Use & Misuse. 1990;25:803–817. doi: 10.3109/10826089009056219. [DOI] [PubMed] [Google Scholar]
- Chandon P, Wansink B. Is food marketing making us fat? A multi-disciplinary review. Found Trends Mark. 2010;5:113–196. [Google Scholar]
- Chelune GJ, Ortega D, Linton JC, Boustany MM. Personality and cognitive findings among patients electing gastroplasty for morbid obesity. Int J Eat Disord. 1986;5:701–712. [Google Scholar]
- Churchill S, Jessop DC. Reflective and non-reflective antecedents of health-related behaviour: Exploring the relative contributions of impulsivity and implicit self-control to the prediction of dietary behaviour. Br J Health Psychol. 2011;16:257–272. doi: 10.1348/135910710X498688. [DOI] [PubMed] [Google Scholar]
- Conforto RM, Gershman L. Cognitive processing differences between obese and nonobese subjects. Addict Behav. 1985;10:83–85. doi: 10.1016/0306-4603(85)90056-5. [DOI] [PubMed] [Google Scholar]
- Costa P, Piedmont R. Multivariate assessment: NEO-PI R profiles of Madeline G. Paradigms of Personality Assessment. 2003:262–280. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEOFFI) – professional manual. Psychological Assessment Resources, Inc; Odessa, FL: 1992. [Google Scholar]
- Cournot M, Marquie J, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJA, Veltman DJ, Roelofs K. Neuroticism modulates amygdala–prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963– 970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Cserjési R, Luminet O, Poncelet AS, Lénárd L. Altered executive function in obesity. Exploration of the role of affective states on cognitive abilities. Appetite. 2009;52:535–539. doi: 10.1016/j.appet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Czyzewska M, Graham R. Implicit and explicit attitudes to high-and low-calorie food in females with different BMI status. Eat Behav. 2008;9:303–312. doi: 10.1016/j.eatbeh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Czyzewska M, Graham R, Ceballos NA. Explicit and implicit attitudes to food. In: Preedy VR, Watson RR, Martin CR, editors. Handbook of Behavior, Food and Nutrition. Springer New York; New York, NY: 2011. pp. 673–692. [Google Scholar]
- Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab. 2012;23:250–260. doi: 10.1016/j.tem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Davis C, Fox J. Sensitivity to reward and body mass index (BMI): Evidence for a nonlinear relationship. Appetite. 2008;50:43–49. doi: 10.1016/j.appet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: A risk model for obesity. Obes Res. 2004;12:929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54:208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: A model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Davis KL, Panksepp J. The brain’s emotional foundations of human personality and the affective neuroscience personality scales. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- de Ridder DTD, Lensvelt-Mulders G, Finkenauer C, Stok FM, Baumeister RF. Taking stock of self-control: A meta-analysis of how trait self-control relates to a wide range of behaviors. Pers Soc Psychol Rev. 2011;16:76–99. doi: 10.1177/1088868311418749. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG. Handbook of Self-regulation: Research, Theory, and Applications. Guilford Press; New York: 2010a. Impulsivity as a personality trait; pp. 485–502. [Google Scholar]
- DeYoung CG. Personality neuroscience and the biology of traits. Soc Personal Psychol Compass. 2010b;4:1165–1180. [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: Explaining individual differences in affect, behaviour and cognition. In: Corr PJ, Matthews G, editors. The Cambridge Handbook of Personality Psychology. Cambridge University Press; New York, NY, US: 2009. pp. 323–346. [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci. 2010;21:820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan MB, Oswald FL, Baird BM, Lucas RE. The Mini-IPIP Scales: Tiny-yet-effective measures of the Big Five Factors of Personality. Psychol Assess. 2006;18:192–203. doi: 10.1037/1040-3590.18.2.192. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Obesity, diets, and social inequalities. Nutr Rev. 2009;67(Suppl 1):S36–39. doi: 10.1111/j.1753-4887.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- Dubé L, Bechara A, Böckenholt U, Ansari A, Dagher A, Daniel M, DeSarbo WS, Fellows LK, Hammond RA, Huang TTK, Huettel S, Kestens Y, Knäuper B, Kooreman P, Moore DS, Smidts A. Towards a brain-to-society systems model of individual choice. Mark Lett. 2008;19:323–336. [Google Scholar]
- Duckworth AL, Kern ML. A meta-analysis of the convergent validity of self-control measures. J Res Pers. 2011;45:259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfhag K, Morey LC. Personality traits and eating behavior in the obese: poor self-control in emotional and external eating but personality assets in restrained eating. Eat Behav. 2008;9:285–293. doi: 10.1016/j.eatbeh.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr. 2011;94:12. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Roba LG. A questionnaire approach to measuring the relative reinforcing efficacy of snack foods. Eat Behav. 2010;11:67–73. doi: 10.1016/j.eatbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D_ receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etou H, Sakata T, Fujimoto K, Kurata K, Terada K, Fukagawa K, Ookuma K, Miller RE. Characteristics of psychomotor performance and time cognition in moderately obese patients. Physiol Behav. 1989;45:985–988. doi: 10.1016/0031-9384(89)90225-4. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fergenbaum JH, Bruce S, Lou W, Hanley AJG, Greenwood C, Young TK. Obesity and lowered cognitive performance in a Canadian First Nations population. Obes. 2009;17:1957–1963. doi: 10.1038/oby.2009.161. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51:34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Robinson JL, Hewitt JK. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Dev Psychol. 2011;47:1410–1430. doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler DA, Jerram MW, Vannorsdall TD, Schretlen DJ. Comparing alternative metrics to assess performance on the Iowa Gambling Task. J Clin Exp Neuropsychol. 2011;33:1040–1048. doi: 10.1080/13803395.2011.596820. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Sandoval Y, Reyes B. Signal-detection analysis of recognition memory of obese subjects. Percept Mot Skills. 1986;63:227–234. doi: 10.2466/pms.1986.63.1.227. [DOI] [PubMed] [Google Scholar]
- Gerbing DW, Ahadi SA, Patton JH. Toward a conceptualization of impulsivity: Components across the behavioral and self-report domains. Multivariate Behav Res. 1987;22:357–379. doi: 10.1207/s15327906mbr2203_6. [DOI] [PubMed] [Google Scholar]
- Giesen JCAH, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: The association of BMI and snack reinforcement. Obes. 2010;18:966–970. doi: 10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, Gough HG. The international personality item pool and the future of public-domain personality measures. J Res Pers. 2006;40:84–96. [Google Scholar]
- Goldberg LR, Strycker LA. Personality traits and eating habits: The assessment of food preferences in a large community sample. Pers Individ Dif. 2002;32:49–65. [Google Scholar]
- Goldfield GS, Epstein LH, Davidson M, Saad F. Validation of a questionnaire measure of the relative reinforcing value of food. Eat Behav. 2005;6:283–292. doi: 10.1016/j.eatbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Graham R, Hoover A, Ceballos NA, Komogortsev O. Body mass index moderates gaze orienting biases and pupil diameter to high and low calorie food images. Appetite. 2011;56:577–586. doi: 10.1016/j.appet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007;48:119–122. doi: 10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. The effect of an impulsive personality on overeating and obesity: Current state of affairs. Psychol Top. 2008;17:265–286. [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: Results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. Obesity is associated with memory deficits in young and middle-aged adults. Eat Weight Disord. 2006;11:e15–19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hall PA. Executive control resources and frequency of fatty food consumption: findings from an age-stratified community sample. Health Psychol. 2012;31:235–241. doi: 10.1037/a0025407. [DOI] [PubMed] [Google Scholar]
- Hall PA, Fong GT, Epp LJ, Elias LJ. Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychol Health. 2008;23:309–326. doi: 10.1080/14768320701212099. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaven PCL, Mulligan K, Merrilees R, Woods T, Fairooz Y. Neuroticism and conscientiousness as predictors of emotional, external, and restrained eating behaviors. Int J Eat Disord. 2001;30:161–166. doi: 10.1002/eat.1068. [DOI] [PubMed] [Google Scholar]
- Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975;43:647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. External cues in the control of food intake in humans: the sensory-normative distinction. Physiol Behav. 2008;94:722–728. doi: 10.1016/j.physbeh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Roefs A. Three ways to resist temptation: The independent contributions of executive attention, inhibitory control, and affect regulation to the impulse control of eating behavior. J Exp Soc Psychol. 2009;45:431–435. [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspect Psychol Sci. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Gschwendner T, Friese M, Wiers RW, Schmitt M. Working memory capacity and self-regulatory behavior: toward an individual differences perspective on behavior determination by automatic versus controlled processes. J Pers Soc Psychol. 2008;95:962–977. doi: 10.1037/a0012705. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A. Neural correlates of the volitional regulation of the desire for food. Int J Obes. 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Houben K. Overcoming the urge to splurge: Influencing eating behavior by manipulating inhibitory control. J Behav Ther Exp Psychiatry. 2011;42:384–388. doi: 10.1016/j.jbtep.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Hurst RM, Kepley HO, McCalla MK, Livermore MK. Internal consistency and discriminant validity of a delay-discounting task with an adult self-reported ADHD sample. J Atten Disord. 2010;15:412–422. doi: 10.1177/1087054710365993. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kang MI, Ohtake F. Hyperbolic discounting, the sign effect, and the body mass index. J Health Econ. 2010;29:268–284. doi: 10.1016/j.jhealeco.2010.01.002. [DOI] [PubMed] [Google Scholar]
- International Personality Item Pool [WWW Document] International Personality Item Pool: A Scientific Collaboratory for the Development of Advanced Measures of Personality Traits and Other Individual Differences. 2012 URL http://ipip.ori.org/
- James WPT. The epidemiology of obesity: The size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Jansen A, Nederkoorn C, Roefs A, Bongers P, Teugels T, Havermans R. The proof of the pudding is in the eating: Is the DEBQ-external eating scale a valid measure of external eating? Int J Eat Disord. 2011;44:164–168. doi: 10.1002/eat.20799. [DOI] [PubMed] [Google Scholar]
- Jansen A, Nederkoorn C, van Baak L, Keirse C, Guerrieri R, Havermans R. High-restrained eaters only overeat when they are also impulsive. Behav Res Ther. 2009;47:105–110. doi: 10.1016/j.brat.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Johnson F, Pratt M, Wardle J. Dietary restraint and self-regulation in eating behavior. Int J Obes. 2011;36:665–674. doi: 10.1038/ijo.2011.156. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Kleibeuker SW, Rombouts SARB, Crone EA. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Dev Sci. 2011;14:713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Persson LO, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24:1715–1725. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005;16:859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- King GA, Polivy J, Herman CP. Cognitive aspects of dietary restraint: Effects on person memory. Int J Eat Disord. 1991;10:313–321. [Google Scholar]
- Lakdawalla D, Philipson TJ. The growth of obesity and technological change: A theoretical and empirical examination. 2002 doi: 10.1016/j.ehb.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JK, Hermans RCJ, Engels RCME. Food intake in response to food-cue exposure. Examining the influence of duration of the cue exposure and trait impulsivity. Appetite. 2012;58:907–913. doi: 10.1016/j.appet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Levitsky D. The non-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiol Behav. 2005;86:623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 4. Oxford University Press; Oxford; New York: 2004. [Google Scholar]
- Lineweaver TT, Bond MW, Thomas RG, Salmon DP. A normative study of Nelson’s (1976) modified version of the Wisconsin Card Sorting Test in healthy older adults. Clin Neuropsychol. 1999;13:328–347. doi: 10.1076/clin.13.3.328.1745. [DOI] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, Wiers RW, Mann K, Kiefer F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes. 2011 doi: 10.1038/ijo.2011.184. in press. [DOI] [PubMed] [Google Scholar]
- Lokken KL, Boeka AG, Yellumahanthi K, Wesley M, Clements RH. Cognitive performance of morbidly obese patients seeking bariatric surgery. Am Surg. 2010;76:55–59. [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, et al. The power of food scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Thomas G. Measures of restrained eating: Conceptual evolution and psychometric update. In: Allison DB, Baskin ML, editors. Handbook of assessment methods for eating behaviors and weight related problems: Measures, theory and research. SAGE; London: 2009. [Google Scholar]
- Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Macht M, Simons G. Emotional eating. In: Nyklíĉek I, Vingerhoets A, Zeelenberg M, editors. Emotion Regulation and Well-being. Springer New York; New York, NY: 2011. pp. 281–295. [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: Evidence for general rather than specific differences. Psychol Rec. 2011;61:4. doi: 10.1007/bf03395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: an integrative hierarchical approach. J Pers Soc Psychol. 2005;88:139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obes. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr Validation of the five-factor model of personality across instruments and observers. J Pers Soc Psychol. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Kurtz JE, Yamagata S, Terracciano A. Internal consistency, retest reliability, and their implications for personality scale validity. Pers Soc Psychol Rev. 2010;15:28–50. doi: 10.1177/1088868310366253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Löckenhoff CE. Handbook of Personality and Self-regulation. Wiley-Blackwell; 2010. Self-regulation and the Five-Factor Model of personality traits; pp. 145–168. [Google Scholar]
- Mesas AE, Muñoz-Pareja M, López-García E, Rodríguez-Artalejo F. Selected eating behaviours and excess body weight: A systematic review. Obes Rev. 2012;13:106–135. doi: 10.1111/j.1467-789X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Miller J, Flory K, Lynam D, Leukefeld C. A test of the four-factor model of impulsivity-related traits. Pers Individ Dif. 2003;34:1403–1418. [Google Scholar]
- Mitchell JT, Kimbrel NA, Hundt NE, Cobb AR, Nelson-Gray RO, Lootens CM. An analysis of reinforcement sensitivity theory and the five-factor model. Eur J Pers. 2007;21:869–887. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S, Sears MR, Thomson WM, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PR, Gates GR. Assessment of a microcomputer-based version of the Austin maze. J Gen Psychol. 1988;115:307–314. doi: 10.1080/00221309.1988.9710567. [DOI] [PubMed] [Google Scholar]
- Mõttus R, McNeill G, Jia X, Craig LCA, Starr JM, Deary IJ. The associations between personality, diet and body mass index in older people. Health Psychol. 2011 doi: 10.1037/a0025537. in press. [DOI] [PubMed] [Google Scholar]
- Mõttus R, Realo A, Allik J, Deary IJ, Esko T, Metspalu A. Personality traits and eating habits in a large sample of Estonians. Health Psychol. 2012 doi: 10.1037/a0027041. in press. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FTY, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006;47:253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA. Attentional processing of food cues in overweight and obese individuals. Curr Obes Rep. 2012;1:106–113. doi: 10.1007/s13679-012-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA, Muris P. Food-related Stroop interference in obese and normal-weight individuals: Behavioral and electrophysiological indices. Eat Behav. 2010;11:258–265. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Muris P, Euser AS, Franken IHA. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Nilsson E. Overweight and cognition. Scand J Psychol. 2009;50:660–667. doi: 10.1111/j.1467-9450.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hietanen JK, Calvo MG, Hyönä J. Food catches the eye but not for everyone: A BMI–contingent attentional bias in rapid detection of nutriments. PLoS ONE. 2011;6:e19215. doi: 10.1371/journal.pone.0019215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Süss H, Schulze R, Wilhelm O, Wittmann WW. Working memory capacity-facets of a cognitive ability construct. Pers Individ Dif. 2000;29:1017–1046. [Google Scholar]
- Pagoto SL, Spring B, Cook JW, McChargue D, Schneider K. High BMI and reduced engagement and enjoyment of pleasant events. Pers Individ Dif. 2006;40:1421–1431. [Google Scholar]
- Paolo AM, Axelrod BN, Tröster AI. Test-Retest Stability of the Wisconsin Card Sorting Test. Assessment. 1996;3:137–143. [Google Scholar]
- Paquet C, Daniel M, Knäuper B, Gauvin L, Kestens Y, Dubé L. Interactive effects of reward sensitivity and residential fast-food restaurant exposure on fast-food consumption. Am J Clin Nutr. 2010;91:771–776. doi: 10.3945/ajcn.2009.28648. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Performance tests in human psychopharmacology (1): Test reliability and standardization. Human Psychopharmacology: Clinical and Experimental. 1991;6:1–9. [Google Scholar]
- Phelan S, Hassenstab J, McCaffery JM, Sweet L, Raynor HA, Cohen RA, Wing RR. Cognitive interference from food cues in weight loss maintainers, normal weight, and obese individuals. Obes. 2010;19:69–73. doi: 10.1038/oby.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti R, Bertella L, Albani G, Mauro A, Molinari E, Semenza C. Decision-making in obesity: A study using the Gambling Task. Eat Weight Disord. 2006;11:126–132. doi: 10.1007/BF03327557. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EM, Tapper K, Calitri R. Cognitive and behavioral correlates of BMI among male and female undergraduate students. Appetite. 2009;52:797–800. doi: 10.1016/j.appet.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Provencher V, Bégin C, Gagnon-Girouard MP, Tremblay A, Boivin S, Lemieux S. Personality traits in overweight and obese women: associations with BMI and eating behaviors. Eat Behav. 2008;9:294–302. doi: 10.1016/j.eatbeh.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Lawyer SR, Reilly W. Percent body fat is related to delay and probability discounting for food in humans. Behav Processes. 2010;83:23–30. doi: 10.1016/j.beproc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ratcliff KL. PhD thesis. 2010. Using Adiposity Change in College Freshman to Examine the Comorbidity of ADHD and Obesity. [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Pers Individ Dif. 2009;47:973–978. [Google Scholar]
- Roberts ME, Demetriou L, Treasure JL, Tchanturia K. Neuropsychological profile in the overweight population: An exploratory study of set-shifting and central coherence. Therapy. 2007;4:821–824. [Google Scholar]
- Roefs A, Huijding J, Smulders FTY, MacLeod CM, de Jong PJ, Wiers RW, Jansen ATM. Implicit measures of association in psychopathology research. Psychol Bull. 2011;137:149–193. doi: 10.1037/a0021729. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell SL, David AS. Improving performance on the WCST: variations on the original procedure. Schizophr Res. 1997;28:63–76. doi: 10.1016/s0920-9964(97)00093-5. [DOI] [PubMed] [Google Scholar]
- Rydén A, Sullivan M, Torgerson JS, Karlsson J, Lindroos AK, Taft C. Severe obesity and personality: A comparative controlled study of personality traits. Int J Obes Relat Metab Disord. 2003;27:1534–1540. doi: 10.1038/sj.ijo.0802460. [DOI] [PubMed] [Google Scholar]
- Rydén A, Sullivan M, Torgerson JS, Karlsson J, Lindroos AK, Taft C. A comparative controlled study of personality in severe obesity: A 2-y follow-up after intervention. Int J Obes. 2004;28:1485–1493. doi: 10.1038/sj.ijo.0802768. [DOI] [PubMed] [Google Scholar]
- Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: The Whitehall II Cohort Study. Am J Clin Nutr. 2009;89:601. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Schachter S, Goldman R, Gordon A. Effects of fear, food deprivation, and obesity on eating. J Pers Soc Psychol. 1968;10:91. doi: 10.1037/h0026284. [DOI] [PubMed] [Google Scholar]
- Schweiger A, Abramovitch A, Doniger GM, Simon ES. A clinical construct validity study of a novel computerized battery for the diagnosis of ADHD in young adults. J Clin Exp Neuropsychol. 2007;29:100–111. doi: 10.1080/13803390500519738. [DOI] [PubMed] [Google Scholar]
- Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protocols. 2006;1:892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- Siegrist M. Reliability of the Stroop test with single-stimulus presentation. Percept Mot Skills. 1995;81:1295–1298. doi: 10.2466/pms.1995.81.3f.1295. [DOI] [PubMed] [Google Scholar]
- Siervo M, Arnold R, Wells JCK, Tagliabue A, Colantuoni A, Albanese E, Brayne C, Stephan BCM. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12:968–983. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- Silva JR, Pizzagalli DA, Larson CL, Jackson DC, Davidson RJ. Frontal brain asymmetry in restrained eaters. J Abnorm Psychol. 2002;111:676–681. doi: 10.1037//0021-843x.111.4.676. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Jackson CJ. The appetitive motivation scale and other BAS measures in the prediction of approach and active avoidance. Pers Individ Dif. 2005;38:981–994. [Google Scholar]
- Smith E, Hay P, Campbell L, Trollor J. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes Rev. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Soreni N, Crosbie J, Ickowicz A, Schachar R. Stop Signal and Conners’ Continuous Performance Tasks: Test-retest reliability of two inhibition measures in ADHD Children. J Atten Disord. 2009;13:137–143. doi: 10.1177/1087054708326110. [DOI] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, Potter J. The developmental psychometrics of big five self-reports: acquiescence, factor structure, coherence, and differentiation from ages 10 to 20. J Pers Soc Psychol. 2008;94:718–737. doi: 10.1037/0022-3514.94.4.718. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek KM. PhD thesis. 2011. Body mass index, age, and neurocognitive functioning. [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Zald D, Dagher A. Dopamine-based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci. 2011;6:81–93. doi: 10.1007/7854_2010_89. [DOI] [PubMed] [Google Scholar]
- Strauss GP. Test-retest reliability of standard and emotional Stroop tasks: An investigation of color-word and picture-word versions. Assessment. 2005;12:330–337. doi: 10.1177/1073191105276375. [DOI] [PubMed] [Google Scholar]
- Streiner DL. Starting at the beginning: An introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80:99–103. doi: 10.1207/S15327752JPA8001_18. [DOI] [PubMed] [Google Scholar]
- Strimas R, Davis C, Patte K, Curtis C, Reid C, McCool C. Symptoms of attention-deficit/hyperactivity disorder, overeating, and body mass index in men. Eat Behav. 2008;9:516–518. doi: 10.1016/j.eatbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. 2011;101:579–592. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tate RL, Perdices M, Maggiotto S. Stability of the Wisconsin Card Sorting Test and the determination of reliability of change in scores. Clin Neuropsychol. 1998;12:348–357. [Google Scholar]
- Terracciano A, Sutin AR, McCrae RR, Deiana B, Ferrucci L, Schlessinger D, Uda M, Costa PT. Facets of personality linked to underweight and overweight. Psychosom Med. 2009;71:682–689. doi: 10.1097/PSY.0b013e3181a2925b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FE, Midthune D, Subar AF, Kahle LL, Schatzkin A, Kipnis V. Performance of a short tool to assess dietary intakes of fruits and vegetables, percentage energy from fat and fibre. Public Health Nutr. 2004;7:1097–1105. doi: 10.1079/PHN2004642. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Āvila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers Individ Dif. 2001;31:837–862. [Google Scholar]
- Tsuchida A, Fellows LK. Are core component processes of executive function dissociable within the frontal lobes? Evidence from humans with focal prefrontal damage. Cortex. doi: 10.1016/j.cortex.2012.10.014. Submitted. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci. 2009;21:2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Tsukayama E, Duckworth AL, Kim B. Resisting everything except temptation: Evidence and an explanation for domain-specific impulsivity. Eur J Pers. 2012;26:318–334. [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti SV, Treasure J, Campbell IC, McLoughlin DM, Schmidt U. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol Psychiatry. 2005;58:840–842. doi: 10.1016/j.biopsych.2005.05.043. [DOI] [PubMed] [Google Scholar]
- van den Bos R, de Ridder D. Evolved to satisfy our immediate needs: Self-control and the rewarding properties of food. Appetite. 2006;47:24–29. doi: 10.1016/j.appet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- van den Bree M, Przybeck TR, Robert Cloninger C. Diet and personality: Associations in a population-based sample. Appetite. 2006;46:177–188. doi: 10.1016/j.appet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- van Strien T, Herman CP, Anschutz D. The predictive validity of the DEBQ-external eating scale for eating in response to food commercials while watching television. Int J Eat Disord. 2012;45:257–262. doi: 10.1002/eat.20940. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, Pappas N. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Waldorf M. PhD thesis. 2010. Krankheitseinsicht, dynamisch getestete Exekutivfunktionen und defensive Bewältigung bei Schizophrenie [Insight into illness, dynamically assessed executive functions and defensive coping style in people with diagnoses of schizophrenia] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Ward M, Carlsson C, Trivedi M, Sager M, Johnson S. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly JN, Derenne A, Terrell HK. Testing the reliability of delay discounting of ten commodities using the fill-in-the-blank method. Psychol Rec. 2011;61:7. [Google Scholar]
- Weller RE, Cook EW, III, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A. Can(not) take my eyes off it: attention bias for food in overweight participants. Health Psychol. 2011;30:561–569. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. Eur J Pers. 2005;19:559–574. [Google Scholar]
- Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H, Ravussin E. Measurement of dietary restraint: Validity tests of four questionnaires. Appetite. 2007;48:183–192. doi: 10.1016/j.appet.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M. Longitudinal data analysis. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA Handbook of Research Methods in Psychology, Vol 3: Data Analysis and Research Publication. American Psychological Association; Washington: 2012. pp. 245–266. [Google Scholar]
- Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67:132. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: Importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- Wong CL, Mullan BA. Predicting breakfast consumption: An application of the theory of planned behaviour and the investigation of past behaviour and executive function. Br J Health Psychol. 2009;14:489–504. doi: 10.1348/135910708X360719. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–476. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.