Abstract
During hemodialysis and related therapies, removal of waste products from the blood is made possible across a semi-permeable membrane. The microbiological quality of treated water (TW) and dialysate influences a number of dialysis-related complications. This article is a review of the microbiological features of TW and dialysate fluid over a six-year period (February 2007 to December 2012) in the Dialysis Unit, 1st Medico-Surgical Hospital, Agadir, Morocco. Installation of a water treatment unit has been followed by a protocol to check its quality periodically. Results of microbiological monitoring (microorganisms and endotoxins) were collected over a six-year period. Fifty-four samples of TW and 12 samples of dialysate fluid were analyzed for colony forming units (CFU) and endotoxin during this period. All dialysate samples were negative, while in the TW, 9.2% of the samples yielded >100/mL CFU and 16.7% yielded >0.06 EU/mL of endotoxins. These abnormal results happened especially during the first two first years. More frequent disinfection of the distribution loop was the corrective measure. To obtain high-quality water for hemodialysis, the appropriate system must be continuously monitored in order to get high microbiological quality of TW and dialysate fluid.
Introduction
Hemodialysis (HD) patients are exposed to a large volume of water (400 L) used for the production of dialysis fluids (DF). Treated water (TW) and DF used in HD come into direct contact with the bloodstream, with the only interposition being a semi-permeable artificial membrane. Water may also move from the dialysate to blood through back-filtration, and it could be used as an infusion liquid in convective methods.1
Chronic complications associated with microbial contamination of HD water include beta-2 microglobulin-related amyloidosis, resistance to erythropoietin and accelerated atherosclerosis. 1,2
It is important to know and monitor the chemical and microbiological purity of dialysis water. If adequately treated, HD water could contribute to improving the patient’s quality of life. Unfortunately, this feature of HD therapy is often neglected, especially in the developing countries.3
The aim of this retrospective study is to assess the microbiological quality of TW and dialysate with an ultrafilter in the Dialysis Unit, 1st Medico-Surgical Hospital, Agadir, Morocco, during the six-year period from February 2007 to December 2012.
Materials and Methods
The dialysis unit is supplied from the water distribution network of the hospital. The use of appropriate technology has allowed the production of good-quality water during the six years of study (February 2007 to December 2012). The original design included the following:
-
-
Water reserve with a capacity of 250 m3.
-
-
Pre-treatment.
-
-
Reverse osmosis (RO) equipment.
-
-
Distribution system: The material used is cross-linked polyethylene (PEX).
-
-
Dialysis machine with dialysis fluid (DF) filter.
Disinfection and management
Chemical disinfections of the RO membranes and the loop were performed every six weeks. If any changes were made to the RO system, such as membrane replacement or removal, the system was disinfected after this manipulation and samples were sent for microbiological analysis.
Bacteriological and endotoxin quality of treated water
Two samples of TW, one at the beginning and the other at the end of the loop, along with a sample of DF were collected quarterly for microbiological and endotoxin level assay. The microbiological cultures were performed by the SOLUDIA LABORATORY, Salé, Morocco.
Determinations: The plates containing poor nutrient culture medium (Tryptone glucose extract agar, TGEA) were incubated at 17– 23°C for seven days, followed by the counting of colonies after 48–72 h. The number of colonies obtained is expressed as colony forming units per milliliter (CFU/mL). The identification of the isolated microorganisms is based on the usual laboratory methods. The same methodology was applied to each sample.
The method of determination of endotoxins was the limulus amebocyte lysate. The available guidelines were the European Pharmacopoeia (7th edition 2011) and the International Organization for Standardization (ISO)/DIS 23500.4,5
Statistical analysis was performed using SPSS 10.0 statistical software. Quantitative variables were expressed as mean ± standard deviation and categorical variables by percentages.
Results
The reported results correspond to a period of six years: February 2007 to December 2012.
Microbiological parameters and endotoxins in treated water
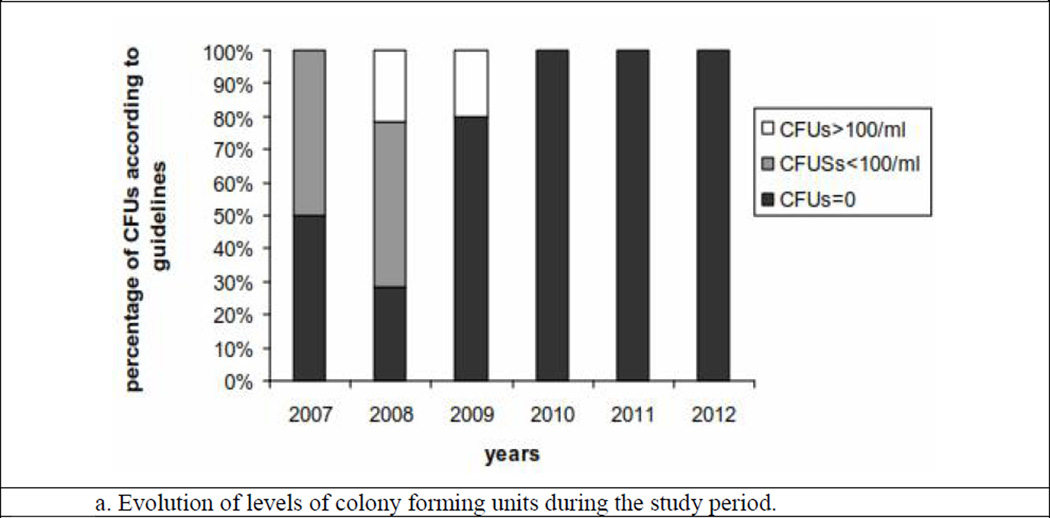
Figures 1a and 1b show the CFU and endotoxin levels in the TW during the six-year study period. A total of 54 samples of TW were analyzed for CFU and endotoxin levels during this period. Negative growth was observed in 4% of the total samples. However, growth of CFU was positive, and was <100 CFU/mL in 20.4% of the samples. In 9.2% of the samples, the CFU growth was >100/mL.
Figure 1.
In the same context, endotoxin levels were <0.03 EU/mL in 75.9% of the samples analyzed and >0.06 EU/mL in 16.7% of the cases.
Microbiological parameters and endotoxins in the dialysate
Twelve dialysate samples were collected during the study and, in all samples, the CFU and endotoxin levels were negative.
Evolution of CFU and endotoxin levels in treated water
Figures 2a and 2b show the evolution of the CFU and endotoxin levels in TW from the beginning of the study. The CFU and endotoxin levels were above the acceptable limits during the first two first years, following which the disinfections became more frequent (twice per quarter) in order to obtain water of better microbiological quality.
Figure 2.
Discussion
From the beginning of the dialysis activities in our unit, the production and use of highly purified TW for dialysis over a long period of time was a serious consideration. We believe that the established protocols have made it possible to reach these objectives.6
The microbiological quality of water in dialysis units is critically dependent upon the presence of bio-film in the distribution network. 5 Achieving a high microbiological quality necessitates attention to the cultivation medium, incubation temperature and incubation time.5–11 Additionally, microbiological analyses require careful sampling to avoid contamination during manipulation.6–10
A number of published studies have shown that improving the microbiological quality of TW and dialysate fluid is associated with a range of clinical benefits.7–12 Quality standards for water and concentrate used to produce dialysate are well established. The European Renal Association Standard stipulates that the microbiological contamination of delivered water should comply with the recommendations of the European Pharmacopeia.8
There is generalized concern over improving the microbiological quality of dialysis water.10 In our study, five of the 54 samples (9.2%) analyzed showed a value slightly above 100/mL for CFU, and nine of the 54 samples (16.7%) analyzed showed a value above 0.06 EU/mL for endotoxins; all the samples during the last 3 years were negative for both tests. This was because after the initial experience, we increased the frequency of disinfection of the water treatment plant.
The use of additional filters is essential for the purity of the dialysate.10 The production of purified water and ultra-pure dialysis fluid could prevent or delay the complications associated with long-term HD therapy.10
Footnotes
Conflict of interest: None
References
- 1.Pontoriero G, Pozzoni P, Andrulli S, Locatelli F. The quality of dialysis water. J Ital Nefrol. 2004;21(Suppl 30):S42–S45. [PubMed] [Google Scholar]
- 2.Ward RA. Dialysis water as a determinant of the adequacy of dialysis. Semin Nephrol. 2005;25:102–111. doi: 10.1016/j.semnephrol.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Penne EL, Visser L, van den Dorpel MA, et al. Microbiological quality and quality control of purified water and ultrapure dialysis fluids for online hemodiafiltration in routine clinical practice. Kidney Int. 2009;76:665–672. doi: 10.1038/ki.2009.245. [DOI] [PubMed] [Google Scholar]
- 4.International Organization for Standarization. Guidance for the preparation and quality management of fluids for hemodialysis and related therapies. ISO 23500. 2011 [Google Scholar]
- 5.Nic Hoenich and col. Guideline on water treatment facilities, dialysis water and dialysis fluid quality for haemodialysis and related therapies. Clinical Practice Guideline by the UK Renal Association and Association of Renal Technologists. the Renal Association. The Association of Renal Technologists [Google Scholar]
- 6.Pontoriero G, Pozzoni P, Andrulli S, Locatelli F. The quality of dialysis water. Nephrol Dial Transplant. 2003;18(Supll 7):vii21–vii25. doi: 10.1093/ndt/gfg1074. [DOI] [PubMed] [Google Scholar]
- 7.The EBPG Expert Group on Haemodialysis. European Best Practice Guidelines for Hemodialysis (Part 1); Section IV, Dialysis fluid purity. Nephrol Dial Transplant. 2002;17(Suppl 7):45–62. [PubMed] [Google Scholar]
- 8.Hoenich NA, Ronco C, Levin R. The importance of water quality and hemodialysis fluid composition. Blood Purif. 2006;24:11–18. doi: 10.1159/000089430. [DOI] [PubMed] [Google Scholar]
- 9.Pontoriero G, Pozzoni P, Tentori F, Scaravilli P, Locatelli F. Maintenance and monitoring of water treatment system. G Ital Nefrol. 2005;22:562–568. [PubMed] [Google Scholar]
- 10.Ragon A. Standards, circulars and recommendations on dialysis fluids: how to practice guidelines 14th Joint Meeting of the Society of Nephrology and the Francophone Society of Dialysis; October 2–5, 2012; Geneva, Switzerland. [Google Scholar]
- 11.Lonnemann G. Assessment of the quality of dialysate. Nephrol Dial Transplant. 1998;13(Suppl 5):17–20. doi: 10.1093/ndt/13.suppl_5.17. [DOI] [PubMed] [Google Scholar]
- 12.Rahmati MA, Homel P, Hoenich NA, Levin R, Kaysen GA, Levin NW. The role of improved water quality on inflammatory markers in patients undergoing regular dialysis. Int J Artif Organs. 2004;27:723–727. doi: 10.1177/039139880402700811. [DOI] [PubMed] [Google Scholar]