Abstract
Background
Vector monitoring in military stations would help in protecting the armed forces from vector borne diseases such as malaria, Japanese encephalitis and filariasis.
Methods
Adult mosquitoes were collected from four villages around a military station in India using light traps and the species composition was estimated. Insecticide susceptibility of disease vectors against DDT, deltamethrin and permethrin was established using WHO kits.
Results
The known malaria vectors constituted 4.9% of the total mosquito collections and Anopheles philippinensis/nivipes (2.05%) was the most abundant. Japanese encephalitis and dengue vectors constituted 25.3 and 0.05% whereas the known vectors of both Japanese encephalitis and filariasis formed 50.9%. The mean (±SEmean) of annual parasitic index, slide positivity and Plasmodium falciparum percentage among the civilian population during the study period were 1.46 ± 0.37, 1.65 ± 0.77 and 50.2 ± 10.7. The filariasis vector Culex quinquefasciatus was resistant to DDT with 65.4% mortality whereas the DDT resistance in the Japanese encephalitis vector Culex vishnui gr. with 91.9% mortality needs to be confirmed. All other species tested were susceptible to DDT, deltamethrin and permethrin.
Conclusion
Targeted interventions are needed to reduce the disease burden and vector activity in the villages adjoining the military station. The use of insect repellents, bed nets and repellent impregnated uniforms by the troops should be ensured for protection from vector borne diseases.
Keywords: Military station, Disease vector, Insecticide resistance
Introduction
Mosquitoes are insects of medical importance since they transmit many diseases such as malaria, Japanese encephalitis, filariasis and dengue. The reduction of mosquito populations is an integral part of our attempts to manage these vector borne diseases.1,2 The knowledge about the species composition, seasonal prevalence and insecticide susceptibility of mosquitoes is vital for the planning and implementation of vector control activities in an area. The control of mosquito vectors assumes much significance in the areas where there is a high incidence and transmission of malaria and other mosquito borne diseases.3,4
The military and paramilitary personnel are highly vulnerable to the incidence of malaria. The loss of man-days resulting from morbidity and mortality may adversely affect the security operations. The armed forces personnel are more prone to disease incidence due to their patrolling activities and increased exposure to the environment.5,6 The mapping of disease vectors in each geographical area is needed to protect the troops and their families from vector borne diseases.7 The villages situated around the cantonment areas often serve as the reservoirs for malaria infections apart from offering sufficient breeding grounds for mosquito proliferation.8 Chemical insecticides remain the most commonly used method of mosquito control in India. However, the development of insecticide resistance in mosquito vectors due to the indiscriminate use of insecticides is a matter of public health concern in the country.9
The present study was undertaken to monitor the activity of the vectors of malaria and other mosquito borne diseases in a military station in India so as to estimate the risk of disease transmission to the military personnel. The insecticide susceptibility of disease vectors against the commonly used insecticides was also established.
Material and methods
The studies were conducted during March 2009–February 2011 in four villages namely, Balitika, Paruwa, Rupkuria, and Udmari situated around a military station in India. The study period was categorised into two – March–August and September–February.
Adult mosquitoes were collected from human dwellings during 1800–0600 h using CDC light traps on monthly basis. Indoor resting collections were made using aspirators and flashlights during 0500–0700 h. The mosquitoes were brought to the laboratory and identified based on standard taxonomic keys.10,11 The species composition was estimated as the percent contribution of each mosquito species to the total number of mosquitoes collected. WHO kits were used as per the guidelines12 for establishing the insecticide susceptibility status. The percent mortality 24 h after exposure to DDT (4%), deltamethrin (0.05%) and permethrin (0.75%) impregnated papers was recorded. The data on annual parasitic index (API), slide positivity rate (SPR) and Plasmodium falciparum percentage (Pf%) from the public health centres (PHC) around the military station during 2009–2011 was collected from the office of the District Malaria Officer.
Results
Twenty five mosquito species were collected and identified from the study areas. This included 13 species of Anopheles, 5 species of Culex, 4 species of Mansonia and one species each of Coquilletidia, Armigeres and Aedes. Culex quinquefasciatus was the predominant species constituting 33.7% of the total collections. Culex vishnui gr. (14.7%) and Mansonia uniformis (12.2%) were also recorded in high numbers. The percent compositions of Culex bitaeniorhynchus, Culex gelidus and Culex malayi were 1.55, 3.3 and 3.9 respectively. Mansonia annulifera, Mansonia indiana and Mansonia longipalpis constituted 5.05, 3.45 and 3.2% of the collections. Aedes albopictus, was the only Aedes species recorded in the study although in very low numbers (0.05%). Armigeres subalbatus and Coquilletidia crassipes formed 7.1 and 0.35% of the adult collections (Table 1).
Table 1.
Seasonal prevalence of mosquito species around a military station in India during 2009–2011.
| Mosquito species | Percent composition |
||
|---|---|---|---|
| March–August | September–February | Yearly mean | |
| Anopheles annularis | 0.40 | 1.3 | 0.85 |
| An. barbirostris | 1 | 3.3 | 2.25 |
| An. crawfordi | 0.8 | 2.4 | 1.6 |
| An. culicifacies | 0.60 | 3.4 | 2 |
| An. philippinensis/nivipes | 0.20 | 4.1 | 2.05 |
| An. vagus | 3.2 | 1.9 | 2.55 |
| Other anophelinesa | 0.2 | 0.1 | 0.15 |
| Culex bitaeniorhynchus | 2.7 | 0.4 | 1.55 |
| Cx. gelidus | 2.4 | 4.2 | 3.3 |
| Cx. malayi | 5.7 | 2.1 | 3.9 |
| Cx. quinquefasciatus | 40.3 | 27.1 | 33.7 |
| Cx. vishnui gr. | 17.2 | 12.3 | 14.7 |
| Mansonia annulifera | 4 | 6.1 | 5.05 |
| Ma. indiana | 2.7 | 4.2 | 3.45 |
| Ma. longipalpis | 2 | 4.4 | 3.2 |
| Ma. uniformis | 9.9 | 14.4 | 12.2 |
| Armigeres subalbatus | 6 | 8.2 | 7.1 |
| Coquilletidia crassipes | 0.6 | 0.1 | 0.35 |
| Aedes albopictus | 0.1 | 0 | 0.05 |
An. aconitus, An. jamesi, An. karwari, An. subpictus, An. minimus, An. fluviatilis and An. kochi.
Culex mosquitoes were predominant in all the four villages surveyed. The percent composition of Cx. quinquefasciatus was the highest in Rupkuria (43.8) whereas the lowest in Udmari (24.4). Cx. vishnui gr. constituted 21.3% of the total collections from Rupkuria whereas only 10.2% of collections from Udmari. Udmari recorded the highest percent composition of Ma. annulifera (9.85), Ma. indiana (5.3) and Ma. longipalpis (5.1). The percentage of Ma. uniformis was the highest in Paruwa (21.7) while Balitika recorded the highest percent composition of Armigeres (11.7). Cq. crassipes was recorded from Balitika (0.5%) and Udmari (0.9%) whereas Ae. albopictus was recorded only from Balitika (0.2%). The percent composition of Anopheles annularis and Anopheles philippinensis/nivipes were the highest in Balitika (1.48 and 4.18) while the percent composition of Anopheles culicifacies was the highest in Udmari (4.45) (Table 2).
Table 2.
Species composition of mosquitoes in villages around a military station in India during 2009–2011.
| Mosquito species | Percent composition |
|||
|---|---|---|---|---|
| Balitika | Paruwa | Rupkuria | Udmari | |
| Anopheles annularis | 1.48 | 0.33 | 0.19 | 1.41 |
| An. barbirostris | 2.71 | 1.31 | 0.67 | 4.3 |
| An. crawfordi | 2.61 | 0.3 | 0.87 | 2.62 |
| An. culicifacies | 1.84 | 1.06 | 0.65 | 4.45 |
| An philippinensis/nivipes | 4.18 | 0.63 | 1.98 | 1.41 |
| An. vagus | 2.84 | 0.47 | 1.28 | 5.59 |
| Other anophelinesa | 0.28 | 0.04 | 0.1 | 0.19 |
| Culex bitaeniorhynchus | 1.28 | 1.38 | 2.66 | 0.87 |
| Cx. gelidus | 4.33 | 1.51 | 5.2 | 2.15 |
| Cx. malayi | 4.6 | 2.9 | 5.8 | 2.3 |
| Cx. quinquefasciatus | 32.9 | 33.8 | 43.8 | 24.4 |
| Cx. vishnui gr. | 12.7 | 14.6 | 21.3 | 10.2 |
| Mansonia annulifera | 3.05 | 4.83 | 2.45 | 9.85 |
| Ma. indiana | 3.4 | 4.48 | 0.61 | 5.3 |
| Ma. longipalpis | 1.6 | 4.4 | 1.7 | 5.1 |
| Ma. uniformis | 7.8 | 21.7 | 7.1 | 12.2 |
| Armigeres subalbatus | 11.7 | 6.26 | 3.64 | 6.8 |
| Coquilletidia crassipes | 0.5 | 0 | 0 | 0.9 |
| Aedes albopictus | 0.2 | 0 | 0 | 0 |
An. aconitus, An. jamesi, An. karwari, An. subpictus, An. minimus, An. fluviatilis and An. kochi.
Among the anophelines, Anopheles vagus was the predominant species forming 22.3% of the anophelines collected. It was followed by Anopheles barbirostris (19.6%) and An. philippinensis/nivipes (17.9%) and while An. culicifacies, Anopheles crawfordi and An. annularis formed 17.5, 14 and 7.4% of anophelines. The other anopheline species recorded were Anopheles aconitus, Anopheles jamesi, Anopheles karwari, Anopheles subpictus, Anopheles minimus, Anopheles fluviatilis and Anopheles kochi, which together formed 1.31% of the collections (Fig. 1). The data on malaria incidence among the civilian population around the military station during 2009–2011 showed that the mean API was 1.46 ± 0.37 whereas the mean SPR was 1.65 ± 0.77. The mean Pf% during the period was 50.2 ± 10.7 (Table 3).
Fig. 1.
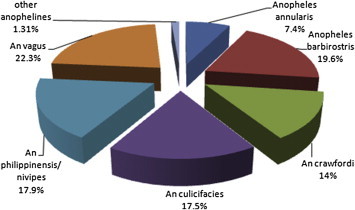
Species composition of Anopheles mosquitoes in villages around a military station in India during 2009–2011.
Table 3.
Malaria incidence among the civilian population around a military station in India during 2009–2011.
| Malaria indices | 2009 | 2010 | 2011 | Mean ± SEmean |
|---|---|---|---|---|
| Annual parasitic index | 2.08 | 1.5 | 0.8 | 1.46 ± 0.37 |
| Slide positivity rate | 3.18 | 1.07 | 0.7 | 1.65 ± 0.77 |
| Plasmodium falciparum (%) | 30.4 | 53.2 | 67 | 50.2 ± 10.7 |
The malaria vectors including An. annularis, An. culicifacies and An. philippinensis/nivipes constituted 4.9% of the total collections. The percentage of mosquitoes which are vectors of both filariasis and Japanese encephalitis was 50.9. This included Cx. quinquefasciatus, Ma. annulifera and Ma. uniformis. JE vectors, which include An. barbirostris, Culex bitaeniorhynchus, Cx. gelidus, Cx. vishnui gr., and Ma. indiana formed 25.3%. The dengue vector, Ae. albopictus was in very low-percentage (0.05) while 18.8% of the mosquitoes were not disease vectors (Fig. 2).
Fig. 2.
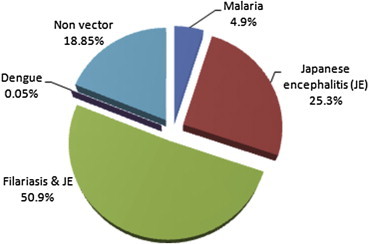
Prevalence of mosquito vectors in villages around a military station in India during 2009–2011.
The tests carried out to evaluate the insecticide susceptibility revealed that most of the species were susceptible to the commonly used insecticides. Cx. quinquefasciatus was resistant to DDT with 65.4% mortality. DDT resistance was the highest in Rupkuria (48.4% mortality) followed by Udmari (59.2% mortality) and Balitika (72.6% mortality). The DDT susceptibility status of Cx. quinquefasciatus in Paruwa with 81.2% mortality suggested the possibility of resistance, which needs to be confirmed. Similarly, the susceptibility status of Cx. vishnui gr. against DDT (91.9% mortality) needs confirmation. This species showed 100% mortality against DDT in Balitika and Paruwa whereas 82.7% and 84.9% mortality in Rupkuria and Udmari. The percent mortality of An. barbirostris against DDT was 98.9 with confirmation of the susceptibility status needed from Udmari (95.7%) while it was susceptible to deltamethrin and permethrin in all the four villages. The percent mortality of Cx. quinquefasciatus against deltamethrin and permethrin were 99.7 and 98.8 respectively indicating its susceptibility. An. philippinensis/nivipes, An. culicifacies and Ma. uniformis were susceptible with 100% mortality against all the three insecticides tested (Table 4).
Table 4.
Abundance of mosquito vectors and their susceptibility to DDT in villages around a military station in India.
| Balitika |
Paruwa |
Rupkuria |
Udmari |
Mean |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC (%) | Mortality (%) | SC (%) | Mortality (%) | SC (%) | Mortality (%) | SC (%) | Mortality (%) | SC (%) | Mortality (%) | |
| Anopheles philippinensis/nivipes | 4.18 | 100 | 0.63 | 100 | 1.98 | 100 | 1.41 | 100 | 2.05 | 100 |
| An. culicifacies | 1.84 | 100 | 1.06 | 100 | 0.65 | 100 | 4.45 | 100 | 2 | 100 |
| An. barbirostris | 2.71 | 100 | 1.31 | 100 | 0.67 | 100 | 4.3 | 95.7# | 2.25 | 98.9 |
| Culex quinquefasciatus | 32.9 | 72.6* | 33.8 | 81.2# | 43.8 | 48.4* | 24.4 | 59.2* | 33.7 | 65.4* |
| Cx. vishnui gr. | 12.7 | 100 | 14.6 | 100 | 21.3 | 82.7# | 10.2 | 84.9# | 14.7 | 91.9# |
| Mansonia uniformis | 7.8 | 100 | 21.7 | 100 | 7.1 | 100 | 12.2 | 100 | 12.2 | 100 |
SC: Species composition; *resistant, #confirmation needed.
Discussion
The major vectors of malaria in India are An. culicifacies, Anopheles dirus, An. fluviatilis, An. minimus, Anopheles sundaicus and Anopheles stephensi. Other anophelines such as An. philippinensis/nivipes, Anopheles varuna, An. annularis and Anopheles jeyporiensis are malaria vectors of local importance.13 The studies on the vectors of defence importance would help in the adoption of preventive measures thereby reducing the morbidity and mortality due to vector borne diseases in the armed forces.7 The light trap collections from the villages around the military station revealed the mosquito density and diversity in the area. It was observed that 4.9% of the total mosquito collections were constituted by the known malaria vectors. Although malaria control measures such as indoor residual sprays with DDT are being undertaken by the district health authorities, cases are reported every year from these villages. The malaria data in these areas during the study period indicated the occurrence of both P. falciparum and Plasmodium vivax infections. The trend of malaria incidence clearly shows that API and SPR have decreased over the years. However, there was a two fold increase in Pf% during this period. The malaria incidence rates during the study period did not vary considerably among the study villages. The epidemiological and entomological studies in the villages along an interstate border in the region have revealed high-risk of malaria.14 The villages around military stations play an important role in the epidemiology of malaria among the service personnel.8 Hence, proper implementation of vector control measures such as indoor residual spays with DDT and the use of bed nets impregnated with synthetic pyrethroids should be ensured in the villages.
Perennial transmission of malaria in the region is aided by the mosquito vectors An. minimus, An. dirus and An. fluviatilis.15 In the present study, An. minimus and An. fluviatilis were recorded in very low numbers. However earlier studies indicated that these efficient vector anophelines were present in high densities in the forest fringe villages.13 An. philippinensis/nivipes, which probably plays a role in malaria transmission in the region16 was the predominant species among the known malaria vectors. An. culicifacies widely prevalent in the study areas was incriminated earlier during a malaria outbreak.17 However, the other known malaria vector An. annularis was recorded in relatively low numbers.
Japanese encephalitis has recently emerged as a public health concern in the region where the climate, agricultural practices and sociocultural behaviour of people are conducive for the disease transmission.4 The major vectors of JE in India belong to Cx. vishnui group comprising of Culex tritaeniorhynchus, Cx. vishnui and Culex pseudovishnui.18 These species breed in wetlands which are abundant in the region. Cx. vishnui gr. mosquitoes were relatively more abundant in the study villages during March–August. This high prevalence indicated high-risk of disease transmission as JE incidence in the region peaks during June–August.4 High densities of these mosquitoes also indicate the availability of breeding habitats in the villages. The other vectors of JE namely, Ma. indiana, Cx. gelidus and An. barbirostris were also recorded in the light trap catches. All JE vectors together formed more than one-fourth of the adult mosquito collections from the study areas.
Lymphatic filariasis is a major socioeconomic and public health problem due to its considerable morbidity and social stigma.19 Cx. quinquefasciatus, the major vector of bancroftian filariasis in urban areas of India,20 was abundant throughout the year with the highest percent contribution to the total collections. Apart from filariasis, this species is known to transmit JE in India.21 Ma. uniformis was the most abundant among the Mansonia species and is a vector of both filariasis and JE.21,22 More than half of the mosquito collections in the present study were potential vectors of both JE and filariasis.
Aedes albopictus, which formed 0.05% of the mosquito samples is an important vector of many arboviral diseases such as dengue and chikungunya.23 These mosquitoes are active during the day time and hence were rarely recorded in the light trap collections. Ae. albopictus breeds in natural and manmade containers and is widely prevalent in the region.24 Surveys of water holding containers in the study villages had indicated the breeding of dengue vectors in them. Surveillance of Aedes mosquitoes in these areas is important in the context of the recent incidence of dengue in many parts of the region.
The development of insecticide resistance in mosquito vectors adversely affects the efficacy of vector control activities. DDT remains the insecticide of choice for public health use in India. Synthetic pyrethroids especially deltamethrin is being used for the treatment of bed nets in India.25 Most of the species tested except Cx. quinquefasciatus were susceptible to DDT and synthetic pyrethroids. The highest DDT resistance was observed in Rupkuria, which had the highest species composition of Cx. quinquefasciatus. The resistance shown by Cx. quinquefasciatus indicated that DDT spraying is no longer effective against this widely prevalent disease vector in this region. The use of chemical larvicides such as temephos or indoor residual spraying with malathion or synthetic pyrethroids could be recommended for Culex control in these areas. DDT resistance in Cx. quinquefasciatus was reported earlier from army field units and adjacent villages in the region.26 The probability of resistance development in Cx. vishnui gr. mosquitoes against DDT is a matter of concern. However, more studies are needed to confirm DDT resistance in these important vectors of JE.
The armed forces personnel need to be protected from the incidence of mosquito borne diseases such as malaria, JE, filariasis and dengue. The information on the diversity and abundance of disease vectors and their insecticide susceptibility as revealed by the present study could help in adopting vector control measures for the military station in future. Any efforts for managing vector borne diseases among the troops should encompass targeted interventions to reduce the disease burden among the surrounding civilian population and to prevent vector breeding in the adjoining areas. The adoption of personal protective measures such as the use of insect repellents, bed nets and repellent impregnated uniforms by the service personnel should be ensured to combat mosquitoes and the diseases transmitted by them.
Conflicts of interest
All authors have none to declare.
Acknowledgements
The authors are thankful to the District Malaria Officer for providing the data on malaria incidence.
References
- 1.Boratne A.V., Jayanthi V., Datta S.S., Singh Z., Senthilvel V., Joice Y.S. Predictors of knowledge of selected mosquito-borne diseases among adults of selected peri-urban areas of Puducherry. J Vector Borne Dis. 2010;47:249–256. [PubMed] [Google Scholar]
- 2.Hay S.I., Myers M.F., Burke D.S. Etiology of interepidemic periods of mosquito-borne disease. Proc Nat Acad Sci. 2000;97:9335–9339. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dev V., Sangma B.M., Dash A.P. Persistent transmission of malaria in Garo hills of Meghalaya bordering Bangladesh, north-east India. Malar J. 2010;9:263. doi: 10.1186/1475-2875-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phukan A.C., Borah P.K., Mahanta J. Japanese encephalitis in Assam, northeast India. Southeast Asian J Trop Med Public Health. 2004;35:618–622. [PubMed] [Google Scholar]
- 5.Patra S.S., Dev V. Malaria related morbidity in central reserve police force personnel located in the north-eastern states of India. J Hum Ecol. 2004;15:255–259. [Google Scholar]
- 6.Banerjee A., Nayak B. Epidemiological and entomological correlation of malaria transmission in an air force station. Med J Armed Forces India. 2001;57:191–193. doi: 10.1016/S0377-1237(01)80040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilak R., Gupta K.K.D., Verma A.K. Vector databank in the Indian armed forces. Med J Armed Forces India. 2008;64:36–39. doi: 10.1016/S0377-1237(08)80143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhiman S., Gopalakrishnan R., Goswami D., Rabha B., Baruah I., Singh L. Malaria incidence among paramilitary personnel in an endemic area of Tripura. Indian J Med Res. 2011;133:665–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar M., Bhattacharyya I.K., Borkotoki A., Baruah I., Srivastava R.B. Development of physiological resistance and its stage specificity in Culex quinquefasciatus after selection with deltamethrin in Assam, India. Mem Inst Oswaldo Cruz. 2009;104:673–677. doi: 10.1590/s0074-02762009000500001. [DOI] [PubMed] [Google Scholar]
- 10.Wattal B.L., Kalra N.L. Regionwise pictorial keys to the female Indian Anopheles. Bull Nat Soc India Malar Other Mosq Borne Dis. 1961;9:85–138. [Google Scholar]
- 11.Nagpal B.N., Srivastava A., Saxena R., Ansari M.A., Dash A.P., Das S.C. Malaria Research Centre (ICMR); Delhi, India: 2005. Pictorial Identification Key for Indian Anophelines. [Google Scholar]
- 12.World Health Organization . WHO; Geneva: 1998. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-efficacy and Persistence of Insecticides on Treated Surfaces. WHO/CDS/CPC/MAL.98.12. [Google Scholar]
- 13.Das N.G., Gopalakrishnan R., Talukdar P.K., Baruah I. Diversity and seasonal densities of vector anophelines in relation to forest fringe malaria in district Sonitpur, Assam (India) J Parasit Dis. 2011;35:123–128. doi: 10.1007/s12639-011-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das N.G., Talukdar P.K., Kalita J., Baruah I., Srivastava R.B. Malaria situation in forest-fringed villages of Sonitpur district (Assam), India bordering Arunachal Pradesh during an outbreak. J Vector Borne Dis. 2007;44:213–218. [PubMed] [Google Scholar]
- 15.Dev V., Phookan S., Sharma V.P., Anand S.P. Physiographic and entomologic risk factors of malaria in Assam, India. Am J Trop Med Hyg. 2004;71:451–456. [PubMed] [Google Scholar]
- 16.Prakash A., Bhattacharyya D.R., Mohapatra P.K., Mahanta J. Potential of Anopheles philippinensis-nivipes complex mosquitoes as malaria vector in north-east India. J Environ Biol. 2005;26:719–723. [PubMed] [Google Scholar]
- 17.Bhuyan M., Das N.G., Chakraborty B.C. Role of Anopheles culicifacies during an outbreak of malaria in Garubandha PHC, Assam. J Commun Dis. 1997;29:243–246. [PubMed] [Google Scholar]
- 18.Samuel P.P. Japanese encephalitis virus infection in mosquitoes and its epidemiological implications. ICMR Bull. 2000;30 [Google Scholar]
- 19.Mukhopadhyay A.K. Lymphatic filariasis in Andhra Pradesh Paper Mill Colony, Rajahmundry, India after nine rounds of MDA programme. J Vector Borne Dis. 2010;47:55–57. [PubMed] [Google Scholar]
- 20.Samuel P.P., Arunachalam N., Hiriyan J., Thenmozhi V., Gajanana A., Satyanarayana K. Host feeding pattern of Culex quinquefasciatus Say and Mansonia annulifera (Theobald) (Diptera: Culicidae) the major vectors of filariasis in a rural area of South India. J Med Entomol. 2004;41:442–446. doi: 10.1603/0022-2585-41.3.442. [DOI] [PubMed] [Google Scholar]
- 21.Kanojia P.C. Ecological study on mosquito vectors of Japanese encephalitis virus in Bellary district, Karnataka. Indian J Med Res. 2007;126:152–157. [PubMed] [Google Scholar]
- 22.Agrawal V.K., Sashindran V.K. Lymphatic filariasis in India: problems, challenges and new initiatives. Med J Armed Forces India. 2006;62:359–362. doi: 10.1016/S0377-1237(06)80109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gratz N.G. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 24.Dutta P., Mahanta J. Potential vectors of dengue and the profile of dengue in the north-eastern region of India: an epidemiological perspective. Dengue Bull. 2006;30:234–242. [Google Scholar]
- 25.Joshi R.M., Ghose G., Som T.K., Bala S. Study of the impact of deltamethrin impregnated mosquito nets on malaria incidence at a military station. Med J Armed Forces India. 2003;59:12–14. doi: 10.1016/S0377-1237(03)80095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar M., Borkotoki A., Baruah Indra, Bhattacharyya I.K., Srivastava R.B. Molecular analysis of knock down resistance (kdr) mutation and distribution of kdr genotypes in a wild population of Culex quinquefasciatus from India. Trop Med Int Health. 2009;2009(14):1097–1104. doi: 10.1111/j.1365-3156.2009.02323.x. [DOI] [PubMed] [Google Scholar]


