Introduction
Absent pulmonary valve syndrome (APVS) also known as congenital absence of pulmonary valve or pulmonary valve agenesis is a rare outflow tract anomaly of heart. It is defined as absent or rudimentary pulmonary valve. Variable degree of annular stenosis with dilatation of main pulmonary artery (MPA) is always present. However dilatation of pulmonary artery branches, ventricular septum defect (VSD) and tricuspid atresia may or may not be present. Most of the authors divide APVS in two categories: (a) Absent pulmonary valve with VSD – also known as Fallot type APVS (b) Absent pulmonary valve with intact ventricular septum and possible tricuspid atresia – also known as Non-Fallot type APVS. Most of cases belong to Fallot type APVS category. Overall Fallot type APVS occurs in 0.2–0.4% of live born infants with congenital heart disease.1 Non-Fallot type APVS is rare and its frequency is unknown. There are only a few case reports of its antenatal diagnosis. We report a case of Non-Fallot type of APVS which was diagnosed antenatally on the basis of characteristic ultrasound findings. APVS with these set of findings is extremely rare.
Case report
A 24-year-old primigravida in a non-consanguineous marriage was referred for second trimester ultrasound at 18th wk of gestation. Anomaly scan revealed isolated fetal cardiac anomaly with following description: visceral-atrial situs and 4-chamber view were normal, outflow tract evaluation revealed normal aortic origin, main pulmonary artery (MPA) was dilated (Fig. 1) and had stenotic communication with right ventricle (Fig. 2), small pulmonary valve leaflets were identified, left pulmonary artery showed mild asymmetric dilatation as compared to right pulmonary artery (Fig. 3). Colour Doppler revealed aliasing in region of pulmonary valve and main pulmonary artery (Fig. 4). Pulsed wave triplex examination revealed markedly increased velocity in region of pulmonary valve consistent with pulmonary stenosis. In addition to pulmonary stenosis, pulmonary regurgitation was also seen (Fig. 5). Ductus arteriosus was present. Long axis view for arches revealed no additional abnormality.
Fig. 1.

Modified oblique axial scan just below the level of arch showing dilated MPA (arrow), ascending aorta (A).
Fig. 2.
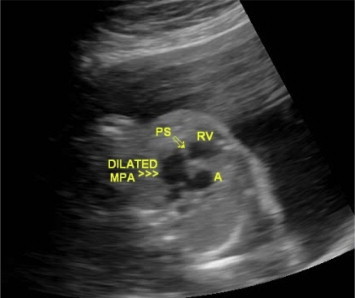
Modified outflow tract view showing dilated MPA (>>>), stenotic pulmonary valve (arrow), right ventricle (RV) and ascending aorta (A).
Fig. 3.

Outflow tract view for pulmonary artery showing dilated left branch of MPA (LPA), asymmetry in size of left and right branch of pulmonary artery (RPA), descending aorta (DAo).
Fig. 4.

Colour Doppler image almost at a level corresponding to Fig. 3 showing aliasing (arrow) in region of pulmonary valve and main pulmonary artery.
Fig. 5.

Pulsed wave triplex sampling at level of pulmonary valve showing markedly increased peak systolic velocity in region of pulmonary stenosis (spectrum below base line) and pulmonary regurgitation (spectrum above base line).
Rest of the cardiac examination was normal. Based on above findings of pulmonary stenosis, pulmonary regurgitation and dilatation of main pulmonary artery, differential diagnosis of absent pulmonary valve syndrome was offered.
Parents was explained prognosis and possible chromosomal anomaly associations however they decided to continue with pregnancy. Follow up examinations at 4 weekly interval revealed no additional abnormalities except that the findings gradually became more conspicuous.
Delivery was conducted at tertiary care hospital. Post natal echocardiography done at 2nd day and 4 weeks of life by pediatric cardiologist confirmed the diagnosis and revealed: rudimentary pulmonary valve, pulmonary artery stenosis, free pulmonary regurgitation, dilated main pulmonary artery and right pulmonary artery, patent foramen ovale, pressure gradient of 35 mm of Hg across pulmonary valve. Child has been put on medical management and has no chromosomal abnormality.
Discussion
First case of APVS was reported by Cheevers in 1847.2 Most of reported cases belong to Fallot type of APVS category. Irrespective of the fact that whether it is Fallot type or Non-Fallot type of absent pulmonary valve syndrome (APVS) both share common features. These common features are: rudimentary or absent pulmonary valve, dilated main pulmonary artery with or without dilatation of its branches, to-and-fro flow at site of absent pulmonary valve and systolic pressure gradient across narrowed pulmonary valve. Additionally Fallot type APVS has features of VSD, overriding of aorta and absent Ductus arteriosus.
Various theories have been postulated for aneurysmal dilatation of pulmonary arteries. Accepted theories for Fallot type APVS are: (A) Agenesis of Ductus arteriosus is responsible for dilatation of pulmonary artery (B) Increased stroke volume due to pulmonary incompetence and pulmonary stenosis lead to post stenotic dilatation.3 For Non-Fallot type APVS accepted theory is: as the Ductus is present, aorta and MPA accommodate bulk of increased flow, hence branch pulmonary arteries are largely spared as are seen in our case. It has also been brought out by other authors that whenever ventricular septum is intact (Non-Fallot type APVS), Ductus arch is always present.4
APVS can be antenatally suspected if we see Bow Tie or balloon like configuration of pulmonary arteries (2). Our case showed balloon like dilated MPA, however it must be kept in mind that in early pregnancy this dilatation may not be seen. These characteristic findings at times become evident during progression of pregnancy. Tricuspid atresia may not be always present with Non-Fallot type of APVS, as is seen in our case. Similar findings have been reported by Volpe et al.5
Absent pulmonary valve has been reported to be associated with chromosomal anomalies in about 25% of cases which include Trisomy 13, Trisomy 21, chromosome 6 and 7 deletions. In about 25% of cases it is seen associated with 22q11 microdeletion.5 Interestingly Non-Fallot type of APVS is not found associated with chromosomal anomalies, very similar to our case. Similar findings have been reported by Razavi et al.6 Extracardiac anomalies like diaphragmatic hernia, cleft lip and palate, polydactyly, neural tube defects, duodenal atresia and hydronephrosis etc. are also seen associated with APVS in about 42.8% of cases.5
Role of umbilical artery Doppler examination in early pregnancy has also been investigated in order to detect cardiac anomalies. Reversal of end diastolic flow (REDF) in patients with APVS at 10–14 wk of gestation has been well documented. Though REDF is a rare finding at 10–14 wk of gestation but when present is mostly associated with major fetal cardiac anomalies. This in particular applies to Fallot type APVS.7
Prognosis of APVS remains poor. Apart from cardiac complications most of the infants develop complications secondary to respiratory distress. Respiratory distress is usually secondary to compression of bronchi by dilated pulmonary arteries and this may lead to massive lobar emphysema. Orientation of infundibulum of pulmonary artery also controls the preferential dilatation of pulmonary arteries. Normally infundibulum is short and vertical. In APVS if orientation of infundibulum is toward right and horizontal it leads to aneurysmal dilatation of right pulmonary artery, which further compresses the middle lobe bronchus. If infundibulum is left orientated it leads to aneurysmal dilatation of left pulmonary artery which compresses left main and upper lobe bronchus. Our case showed dilatation of main pulmonary artery and left branch of pulmonary artery.
Conclusion
APVS is rare congenital conotruncal anomaly with poor prognosis. By demonstrating pulmonary stenosis, pulmonary regurgitation, dilated pulmonary artery with or without its branches and associated cardiac defects if present, this rare anomaly can be diagnosed antenatally with high degree of confidence. Possibility of associated chromosomal anomalies should always be thought of. Though Non-Fallot type APVS is frequently not associated with chromosomal anomalies but still prognosis remains poor in these patients.
Conflicts of interest
All authors have none to declare.
References
- 1.Ferencz C. A case control study of cardiovascular malformations in live born infants: morphogenetic relevance of epidemiological findings. In: Clark E.B., Takao A., editors. Developmental Cardiology: Morphogenesis and Functions. Futura Publication; 1990. pp. 523–539. [Google Scholar]
- 2.Philip S., Varghese M., Manohar K., Cherian K.M. Absent pulmonary valve syndrome: prenatal cardiac ultrasound diagnosis with autopsy correlation. Eur J Echocardiogr. 2011;12:E44. doi: 10.1093/ejechocard/jer155. [DOI] [PubMed] [Google Scholar]
- 3.Joshi Alpana N., Rane Hema S., Kamble Rajesh, Mestry Pravin J., Hemal Maniar, Shah Yatin. Prenatal diagnosis of absent pulmonary valve syndrome. J Ultrsound Med. 2010;29:823–829. doi: 10.7863/jum.2010.29.5.823. [DOI] [PubMed] [Google Scholar]
- 4.Yeager Scott B., Van Der Velde Mary E., Waters Brenda L., Sanders Stephen P. Prenatal role of Ductus arteriosus in absent pulmonary valve syndrome. Echocardiography. 2002;19:489–493. doi: 10.1046/j.1540-8175.2002.00489.x. [DOI] [PubMed] [Google Scholar]
- 5.Volpe P., Paladini D., Marasini M. Characteristics, associations and outcome of absent pulmonary valve syndrome in fetus. Ultrasound Obstet Gynecol. 2004;24:623–628. doi: 10.1002/uog.1729. [DOI] [PubMed] [Google Scholar]
- 6.Razavi R.S., Sharland G.H., Simpson J.M. Prenatal diagnosis by echocardiogram and outcome of absent pulmonary valve syndrome. AM J Cardiol. 2003;91:429–432. doi: 10.1016/s0002-9149(02)03238-1. [DOI] [PubMed] [Google Scholar]
- 7.Berg C., Thomsen Y., Geipel A., Germer U., Gembruch U. Reversed end diastolic flow in umbilical artery at 10–14 wk of gestation is associated with absent pulmonary valve syndrome. Ultrasound Obstet Gynecol. 2007;30:254–258. doi: 10.1002/uog.4098. [DOI] [PubMed] [Google Scholar]


