Abstract
Hemorrhage from downhill varices is a rare manifestation. The etiology of downhill varices is due to superior vena cava obstruction while uphill varices are secondary to portal hypertension. We report a rare case of 55-year-old female with bleeding downhill varices not associated with obstruction or compression of superior vena cava, but was due to severe pulmonary artery hypertension secondary to chronic rheumatic heart disease.
Keywords: downhill varices, pulmonary artery hypertension, rheumatic heart disease, uphill varices
Abbreviations: SVC, superior vena cava; PAH, pulmonary artery hypertension; CRHD, chronic rheumatic heart disease; AST, aspartate aminotransferase; ALT, alanine transferase; RA, right atrium; LA, left atrium; RVH, right ventricular hypertrophy; LV, left ventricular; TR, severe tricuspid regurgitation; RCS, red colour signs; MDCT, multidetector computed tomography
Esophageal varices are classified as downhill or uphill varices. Uphill varices are common, found at the lower end of the esophagus, extend upwards and develop as a consequence of portal hypertension. Downhill varices are rare, found at upper esophagus, extend downwards and usually develop due to superior vena cava obstruction. Very rarely they can develop due to pulmonary artery hypertension. We report a case presenting with upper gastrointestinal bleed from the downhill varices, which was also found to have uphill varices due to portal hypertension. The downhill varices in this patient were due to moderate pulmonary artery hypertension as a consequence of chronic rheumatic heart disease with severe mitral regurgitation, mild mitral stenosis and severe tricuspid regurgitation.
Case report
A 55-year-old female was admitted with repeated episodes of hematemesis and melena of 15 days duration. Patient was a diagnosed case of liver cirrhosis. General physical examination revealed pallor, raised jugular venous pressure, pedal edema and icterus. Her pulse was 82/min, regular and a blood pressure of 110/70 mmHg. Her abdomen was distended and shifting dullness was present. Cardiovascular examination revealed apical impulse in 6th intercostal space 2 cm outside mid-clavicular line, grade II parasternal heave, palpable pulmonary artery pulsations and palpable 2nd heart sound at parasternal 2nd left intercostals space, loud P2, opening snap and grade III/VI pansystolic murmur in mitral and tricuspid area. Respiratory and central nervous system examination were normal. On investigation her hemoglobin was 8 g/dL, platelet counts 0.76 lakh/mm3, TLC 7200/mm3 with normal differentials, aspartate aminotransferase (AST) 40 U/L, alanine transferase (ALT) 41 U/L, alkaline phosphatase 150 mg/dL, total bilirubin 2.4 mg/dL with unconjugated fraction of 1.6 mg/dL, total serum proteins 6.2 g/dL, albumin 2.1 g/dL, prothrombin time 20 s, urea 19 mg/dL, creatinine 1 mg/dL, Na 143 meq/l and K was 3.7 meq/L. Her X-ray chest showed cardiomegaly, straightening of left heart border, findings suggestive of right atrium (RA) and left atrium (LA) enlargement, dilated pulmonary artery and superior vena cava (SVC) [Figure 1]. Her ECG showed normal sinus rhythm with evidence of RA and LA enlargement with evidence of right ventricular hypertrophy (RVH) and left ventricular (LV) volume overload. Her serology was negative for ANA, HBsAg, anti HCV antibodies and HIV. On upper gastrointestinal endoscopy she had large varices 2 columns extending from post cricoid region downwards decreasing in caliber to small varices in mid esophagus with an adherent clot suggestive of recent bleeding site [Figure 2]. She also had large varices 3 column in lower esophagus extending upwards becoming small varices in mid esophagus without any stigmata of recent bleed [Figure 3].
Figure 1.
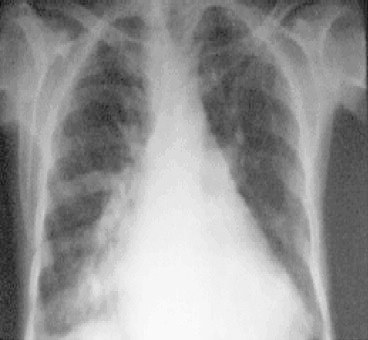
Chest X-ray showing cardiomegaly.
Figure 2.
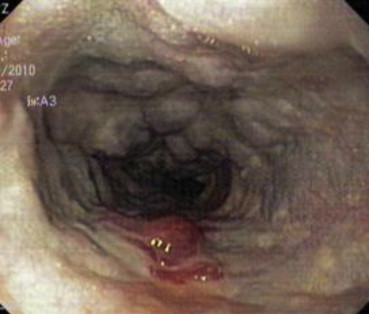
Downhill varices with red colour signs (RCS) in upper esophagus.
Figure 3.
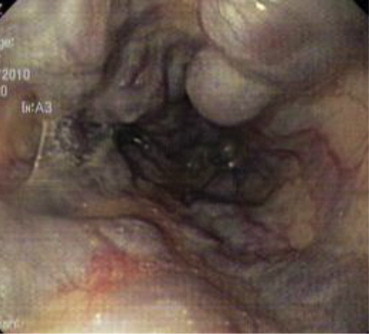
Uphill varices in lower esophagus.
In view of downhill varices contrast enhanced computed tomography of chest was done which ruled out intramural or extramural SVC obstruction/compression but it revealed cardiomegaly with enlargement of all the heart chambers, dilated SVC and thoracic esophageal collateral veins [Figure 4]. CECT upper abdomen depicted chronic hepatic parenchymal disease with portal vein thrombosis, extensive collaterals, splenomegaly, splenic infract and ascites. Her echocardiography revealed chronic rheumatic heart disease, severe mitral regurgitation, mild mitral restenosis, and severe tricuspid regurgitation (TR) with moderate pulmonary arterial hypertension (PAH) (RVSP 60 mmHg) with all 4 chambers of heart dilated. USG abdomen and splenoportal Doppler revealed normal liver size with diffusely altered echotexture, thrombosis of portal vein and superior mesenteric vein with collaterals at porta, there was splenomegaly with few collaterals at splenic hilum, gastroesophageal junction, splenorenal area and retroperitoneum. Patient was admitted in ICU and somatostatin infusion, blood transfusions and other supportive treatment was given. Cardiologist's opinion was sought and accordingly diuretics and other supportive medicines were started. In view of the repeated UGI bleed and an adherent clot on downhill varix in upper 1/3 of esophagus, variceal ligation was performed. Her condition improved and there were no further episodes of bleeding and she was discharged after 6 days. She was put on 3 weekly schedule of variceal ligation only for lower esophageal varices and regular follow-up in cardiology clinic.
Figure 4.
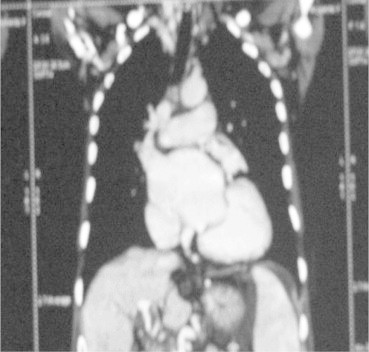
Multidetector computed tomography (MDCT) showing cardiomegaly, no superior vena cava (SVC) obstruction and dilated pulmonary artery.
Discussion
This case had moderate pulmonary artery hypertension along with severe TR. If tricuspid valve is competent, increased pulmonary pressures are usually not transmitted to RA and other backward venous channels. But in presence of incompetent tricuspid valve, as in our case, increased pulmonary pressures of PAH are transmitted back to RA, SVC, IVC, liver and hence forth. In our case, increased RA pressure, secondary to PAH and TR, led to transmission of increased pressure and congestion in SVC resulting in congestion of upper esophageal venous channels. This long-term congestion and increased pressure probably resulted in formation of upper esophageal varices. Similarly hepatic congestion secondary to PAH and TR resulted in cardiac cirrhosis and portal hypertension causing uphill lower esophageal varices. However even at best this is a hypothesis, as we know that not all patients of cardiac cirrhosis who have PAH and TR, develop downhill varices. In our experience this is the 1st case of uphill and downhill varices together presenting with hematemesis from downhill varices as evident by adherent clot on upper esophageal varix on endoscopy. Further, bleeding from downhill varices is quite rare. Hundred cases of downhill varices are reported in literature,1 most of them are due to SVC obstruction. To best of our knowledge ours is the 2nd case in literature due to pulmonary artery hypertension, the 1st case was reported by M. Areia et al. in 2006.2
There is another case report from Taiwan where in a young child with infra-cardiac total anomalous pulmonary venous congestion draining in to portal vein developed uphill lower esophageal varices. This case also underlines the role of increased pressure and congestion in a venous channel leading to esophageal varices.3
The normal venous drainage of the esophagus occurs predominantly by the azygous and hemiazygous systems. The downward extension of the downhill varices depends on the level and duration of obstruction in SVC. If the obstruction is proximal to the azygous veins, blood flows to the heart through mediastinal collaterals and downhill varices will be confined only to upper esophagus. When the obstruction is below or involving the azygous vein, blood returns by the hemiazygous, esophageal and portal veins, with varices extending to the entire organ.4–6 One possible etiology for downhill varices apart from SVC obstruction is PAH and TR leading to increase in SVC pressure which may cause downhill varices. Uphill varices are due to liver cirrhosis secondary to congestive hepatopathy due to PAH and TR related to chronic rheumatic heart disease. Other causes of downhill varices are lung and thyroid carcinoma, chronic mediastinal fibrosis, metastatic carcinoma, venulitis, central hemodialysis catheter and lymphoma7 which were ruled out in this case. Generally downhill varices are non hemorrhagic. The uphill varices can often bleed as they are associated with coagulopathy due to chronic liver disease, they are subepithelial (in contrast to submucosal downhill varices) and are likely to affect by gastroesophageal reflux disease.
We report this case as combined downhill and uphill varices are rare, bleeding from downhill varices is very uncommon, severe PAH and TR (due to chronic rheumatic heart disease) producing downhill varices is still rare (SVC obstruction is the usual cause) and successful variceal ligation of downhill varix to prevent further bleed make it a unique presentation and hence, worth reporting.
Conclusion
Combined downhill and uphill varices in a single patient are a rare phenomenon. Pulmonary arterial hypertension should be thought as one possible source of downhill varices in absence of SVC obstruction or compression which is the commonest cause of downhill varices. Rare bleed from downhill varix responds well to variceal ligation.
Conflicts of interest
All authors have none to declare.
References
- 1.Serin E., Ozer B., Gumurdulu Y., Yildirim T., Barutcu O., Boyacioglu S. A case of Castleman's disease with “downhill” varices in the absence of superior vena cava obstruction. Endoscopy. 2002;34(2):160–162. doi: 10.1055/s-2002-19840. [DOI] [PubMed] [Google Scholar]
- 2.Areia M., Romãozinho J.M., Ferreira M., Amaro P., Freitas D. “Downhill” varices. A rare cause of esophageal hemorrhage. Rev Esp Enferm Dig. 2006;98:359–361. doi: 10.4321/s1130-01082006000500006. [DOI] [PubMed] [Google Scholar]
- 3.Chen H.Y., Chen S.J., Li Y.W. Esophageal varices in congenital heart disease with total anomalous pulmonary venous connection. Int J Card Imaging. 2000 Oct;16(5):405–409. doi: 10.1023/a:1026578412327. Source Department of Diagnostic Radiology, Shin Kong Wu Ho-Su Memorial Hospital, Taiwan, Republic of China. [DOI] [PubMed] [Google Scholar]
- 4.Otto D.L., Kurtzman R.S. Esophageal varices in superior vena caval obstruction. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1000–1012. [PubMed] [Google Scholar]
- 5.Martorell F. Esophageal varices due to superior caval hypertension. Angiologia. 1955;7(2):49–53. [PubMed] [Google Scholar]
- 6.Johnson L.S., Kinnear D.G., Brown R.A., Mulder D.S. Downhill esophageal varices. A rare cause of upper gastrointestinal bleeding. Arch Surg. 1978;113(12):1463–1464. doi: 10.1001/archsurg.1978.01370240085016. [DOI] [PubMed] [Google Scholar]
- 7.Gerstenberg E., Taenzer V., Bachmann D., Woellgens P. Downhill-Varizen: oesophagusvarizen ohne portale Hypertension. Med Welt. 1971;22:1433–1436. [PubMed] [Google Scholar]


