Abstract
Background
Intestinal worm infestation is a global health problem. Soil-transmitted helminth (STH) infections form the most important group of intestinal worms affecting two billion people worldwide, causing considerable morbidity and suffering, though entirely preventable. The present study was undertaken to measure the parasite load in the target population and evaluate the efficacy of anthelminthic drugs.
Methods
Current study was undertaken from 01 July 2012 to 30 June 2013. All outdoor as well as indoor patients advised stool examination formed the study population and it included 2656 males and 76 females (including 6 children). Investigations included stool examination and blood counts. A single-oral dose of anthelminthic drug was given to all positive cases. Stool tests were repeated after 14–21 days to evaluate cure rate.
Results
Overall prevalence of intestinal worm infection was found to be 49.38%. Ascaris was the most common parasite (46.88%), followed by Taenia (2.1%) and Hymenolepis nana (0.21%). Cure rate was found to be 66% for Ascaris and 100% in other cases.
Conclusion
The study reveals high prevalence of intestinal helminths in our subject population and calls for immediate control measures, including preventive chemotherapy and treatment of entire ‘at risk’ population and improvement of their living conditions including provision of potable water.
Keywords: Helminth, Prevalence, Infestation, Sanitation, Parasite
Introduction
Intestinal worm infestations are widely prevalent in tropical and subtropical countries and occur where there is poverty and poor sanitation. Soil-transmitted helminth (STH) infections form the most important group of intestinal worms affecting two billion people worldwide and the main species which infect are Ascaris lumbricoides, (roundworms), Trichuris trichiura, (whip worms) and Necator americanus/Ancylostoma duodenale (hookworms)1 According to World Health Organisation (WHO), globally there are 1221–1472 million cases of Ascariasis, 750–1050 million cases of Trichuriasis and 740–1300 million cases of hookworm infestation.2 These STHs are also considered Neglected Tropical Diseases (NTDs) as they inflict considerable morbidity and mortality, though entirely preventable.
The burden of disease due to these intestinal parasites is an estimated 22.1 million disability-adjusted life-years (DALYs) lost for hookworm, 10.5 million for Ascaris; and 6.4 million for Trichuris.3 Approximately 10,500 deaths each year are due to complications of Ascariasis and 65,000 deaths per year are due to anaemia caused by hookworm infection.4 WHO recommends periodic administration of albendazole (ALB) 400 mg or mebendazole (MBZ) 500 mg for control of STH. The global target is to eliminate morbidity due to STH in children by 2020.5
The present station where this study has been carried out is located in a mountainous region in northern part of the country and is known to be highly endemic for Intestinal worm infestations, mainly STH. With this in the backdrop, the present study has been undertaken to assess the parasite load in the target population with primary focus on STH; and evaluate the efficacy of anthelminthic drugs using a protocol which was standardized in terms of the treatment and follow up i.e. repeat stool test 14–21 days after the administration of standard doses of drugs to evaluate the cure rate (CR).6
Material and methods
The current study was carried out from 01 July 2012 to 30 June 2013. All outdoor as well as indoor patients advised stool examination formed the study population. However, owing to the remoteness and the peculiar location of the station, the majority of study population comprised of only adult males, while females and children constituted a very small number i.e. 2656 males and 76 females (including 6 children). Patients suffering from diarrhea/dysentery were excluded from the study. Investigations included macroscopic as well as microscopic stool examination and blood counts.
The patients were provided with wide mouthed clean, dry, labelled plastic containers for collection of samples and were asked to provide 5 g of solid or 10 ml of liquid stool. The fresh stool samples were examined within 1–2 h of collection. Macroscopic examination was carried out to identify structures like proglottids, scolices, adult tapeworm, Enterobius, Ascaris, Trichuris and hookworms. Unstained wet saline mount preparations were done to detect eggs or larvae and Iodine wet mount was done to detect cysts. However cases which were found negative by saline preparation method, Formal-Ether concentration technique was adopted.
A single-oral dose of ALB (400 mg) was given to the patients found positive for A. lumbricoides and T. trichiura. A single-oral dose of 10 mg/kg body weight of praziquantel was given to those found positive for Taenia solium/Taenia saginata. However, Hymenolepis nana was treated with an oral dose of 25 mg/kg body weight of praziquantel, and the dose was repeated after one week. Stool tests were repeated after 14–21 days to evaluate CR. The patients were also educated about personal hygiene and the importance of washing hands, wearing shoes, and cleanliness of surrounding area.
In accordance with the WHO guidelines, the following formula was used to calculate the prevalence of infection.7
Results
A total of 2732 subjects were included in the study comprising of 2656 males and 76 females, including 6 children. The age/sex breakdown revealed that the majority of males i.e. 44.05% (1170/2656), as well as females 53.94% (41/76) belonged to age group 20–29 years. The highest parasitosis was found in the age group 0–9 years (83.33%). Detailed breakdown of parasite prevalence by age and sex has been given in Table 1.
Table 1.
Breakdown of parasite prevalence by age and sex.
| Age in years | Male |
Female |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
Positive | % | Total |
Positive | % | Total |
Positive | % | |
| Samples | Samples | Samples | |||||||
| 0–09 | 4 | 3 | 75.00 | 2 | 2 | 100.00 | 6 | 5 | 83.33 |
| 10–19 | 9 | 4 | 44.44 | 8 | 3 | 37.50 | 17 | 7 | 41.18 |
| 20–29 | 1170 | 706 | 60.34 | 41 | 29 | 70.73 | 1211 | 735 | 60.69 |
| 30–39 | 1006 | 427 | 42.45 | 14 | 6 | 42.86 | 1020 | 433 | 42.45 |
| 40–49 | 457 | 162 | 35.45 | 6 | 2 | 33.33 | 463 | 164 | 35.42 |
| >50 | 10 | 3 | 30.00 | 5 | 2 | 40.00 | 15 | 5 | 33.33 |
| Total | 2656 | 1305 | 49.13 | 76 | 44 | 57.89 | 2732 | 1349 | 49.38 |
The overall prevalence of intestinal parasitosis was found to be 49.38% (1349/2732). The prevalence of Ascaris lumbricoides was found to be the highest (46.88%), followed by Taenia (2.1%) and H. nana (0.21%). Relative prevalence of parasites detected in the study is given in Fig. 1. Most of the positive cases were asymptomatic. However, absolute eosinophil counts (AEC) were found to be raised in 47.93% of positive cases. The AEC range was recorded to be between 250 and 1750 cells/cu.mm.
Fig. 1.
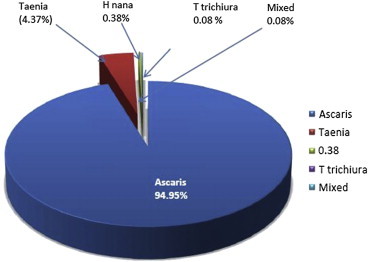
Relative prevalence of intestinal parasites in the study population.
The highest prevalence of parasite was recorded in October (82.7%) while the lowest prevalence was detected in January (30.6%). Month-wise breakdown of the prevalence of various intestinal parasites is given in Table 2.
Table 2.
Month-wise intestinal parasite prevalence.
| S. no | Month | Total sample | Total parasite detected (%) | Ascaris (%) | Taenia (%) | Others (%) |
|---|---|---|---|---|---|---|
| 1 | Jul-2012 | 260 | 101 (38.8) | 89 (34.2) | 11 (10.9) | 1 (0.4) |
| 2 | Aug-2012 | 299 | 110 (36.8) | 106 (35.5) | 4 (3.6) | 0 (0.0) |
| 3 | Sep-2012 | 266 | 131 (49.2) | 127 (47.7) | 3 (2.3) | 1 (0.4) |
| 4 | Oct-2012 | 162 | 134 (82.7) | 122 (75.3) | 12 (9.0) | 0 (0.0) |
| 5 | Nov-2012 | 187 | 127 (67.9) | 123 (65.8) | 3 (2.4) | 1 (0.5) |
| 6 | Dec-2012 | 193 | 140 (72.5) | 134 (69.4) | 6 (4.3) | 0 (0.0) |
| 7 | Jan-2013 | 235 | 72 (30.6) | 72 (30.6) | – | 0 (0.0) |
| 8 | Feb-2013 | 210 | 75 (35.7) | 75 (35.7) | – | 0 (0.0) |
| 9 | Mar-2013 | 262 | 132 (50.4) | 132 (50.4) | – | 0 (0.0) |
| 10 | Apr-2013 | 273 | 159 (58.2) | 146 (53.5) | 10 (6.3) | 3 (1.1) |
| 11 | May-2013 | 199 | 82 (41.2) | 78 (39.2) | 3 (3.7) | 1 (0.5) |
| 12 | Jun-2013 | 186 | 86 (46.2) | 77 (41.4) | 7 (8.1) | 2 (1.1) |
| Total | 2732 | 1349 (49.38) | 1281 (46.88) | 59 (2.16) | 9 (0.33) |
The study also revealed seasonal variations with the highest prevalence in autumn (80.5%), while it was lowest in the months of spring (43.9%). The breakdown of seasonal variation is given in Table 3.
Table 3.
Seasonal variations in intestinal parasite prevalence.
| SN | Season | Total sample | Total parasite | Prevalence ratio |
|---|---|---|---|---|
| 1 | Monsoon | 785 | 370 | 47.1 |
| 2 | Autumn | 389 | 313 | 80.5 |
| 3 | Winter | 521 | 275 | 52.7 |
| 4 | Spring | 472 | 207 | 43.9 |
| 5 | Summer | 565 | 284 | 50.3 |
Summer – (April to mid June).
Monsoon – (Mid June to Mid Sep).
Autumn – (Mid Sep to Mid Nov).
Winter – (Mid Nov to Jan).
Spring – (Feb to Mar).
The CR after one time administration of recommended doses of anthelminthic drugs was found to be 66% for Ascaris lumbricoides and 100% for other parasites.
Discussion
Intestinal worm infestation is a global health problem and is a matter of serious concern for the third world countries. Overcrowding, contamination of water, poor sanitation and migration of people to cities greatly favour transmission of parasitic infection resulting in high endemicity. STH infections form the most important group of intestinal worms and account for 27% of entire school-age and preschool-age children population in the World, who are in need of anthelminthic treatment.5
WHO recommends preventive chemotherapy (PC) to all at-risk people living in endemic areas once a year where the prevalence of STH in the community is over 20%, and twice a year where it is over 50%. However, administration of ALB and MBZ is not recommended in pregnancy during its first trimester for safety reasons; but is permissible in the 2nd and 3rd trimester.8
Present study revealed 49.38% parasitosis in the study population with Ascaris lumbricoides as the commonest parasite (46.88%), followed by Taenia (2.1%) and H. nana (0.21%). Wani et al (2009),9 in a study in an adjoining district in Kashmir valley, also reported high prevalence of intestinal parasitic infection i.e. 73.36% with the highest prevalence of A. lumbricoides (69.84%), followed by T. trichiura (31.65%), Enterobius vermicularis (16.80) and T. saginata (3.01%).
Prevalence of intestinal parasites as reported by some of the authors in India and neighbouring countries is given in Table 4.
Table 4.
Relative prevalence of Ascariasis as reported by some authors in their studies.
| Name of author (Year) | Total parasite prevalence | Prevalence of Ascariasis |
|---|---|---|
| Present study (2013)a | 49.38 | 46.88 |
| Wani et al (2009)a,9 | 73.36 | 69.84 |
| Vinod Kumar et al (2003)19 | 71.73 | 23.73 |
| Bisht et al (2011)a,20 | 38.20 | 6.25 |
| Shrestha (2001)b,21 | 81.94 | 72.62 |
| Khanal L K et al (2004)b,22 | 17.60 | 03.52 |
| Singh et al (2013)b,23 | 15.17 | 05.72 |
| Gunawardena et al (2011)c,24 | 29.04 | 24.44 |
| Raghunathan et al (2010)c,25 | 34.56 | 14.93 |
Studies conducted in India.
Studies conducted in Nepal.
Studies conducted in Sri Lanka.
The present study also reveals seasonal variations in the prevalence of intestinal parasites, with the highest prevalence (80.5%) in autumn (Mid Sep to mid Nov); and lowest (43.9%) in the months of spring (Feb to Mar). However, Khanum et al (2010),10 in their study among the outdoor patients including teacher, students and staff of the Dhaka University, reported the highest prevalence (29.3%) during the rainy season and the lowest (19.38%) in the winter months. In another study by Avhad et al (2012)11 on “Effect of Climatic Factors on the Prevalence of Intestinal Helminths from Aurangabad District (M.S), India” highest prevalence was recorded in rainy season (8.59%–25.69%) while lowest in summer months (1.96%–8.59%).
The present study also reveals raised AEC in 47.93% subjects found positive for STH. Range of AEC was found to be between 250 and 1750 cells/cu.mm. Since eosinophilia is a marker of Th2 cell response, it can be used to predict Helminthic infections.12 Therefore, one may suspect intestinal parasitic infection even in asymptomatic individuals with raised AEC. There are several studies, which support this fact. In United Kingdom, raised AEC is investigated for helminthic infection among migrants and travellers.13
In the present study the CR obtained for Ascaris lumbricoides was 66% and 100% for other parasites. Similar results were also obtained by Jozef Vercruysse et al (2011)14 who while evaluating CR of albendazole for STHs, carried out seven trials among school children in Brazil, Cameroon, Cambodia, Ethiopia, India, Tanzania and Vietnam and achieved a CR of 98.2% for A. lumbricoides, followed by hookworm (87.8%) and T. trichiura (46.6%). However, Soukhathammavong et al (2012)15 in a trial to assess the efficacy of single-dose of albendazole and mebendazole against hookworm in school children in Lao PDR obtained a disappointingly low CR of 36.0% and 17.6% respectively.
In a similar study to assess efficacy of ALB using triple dose therapy, Yap et al (2013)16 in a study of school-age children in Yunnan, observed a CR of 96.7%, 91.5%, and 19.6% for hookworm, A. lumbricoides, and T. trichiura, respectively; while Steinmann et al (2011)17 in single-dose versus triple dose treatment trial against hookworm and other STHs in a community based randomized controlled trial in the People's Republic of China, observed albendazole cured significantly more hookworm infections than mebendazole in both treatment regimens (single-dose: respective CRs – 69% (55–81%) and 29% (20–45%); triple dose: respective CRs 92% (81–98%) and 54% (46–71%)).
The above studies suggest that the CRs obtained with single-oral dose of ALB & MBZ against STH and other nematodes seldom achieve complete cure. Therefore, there is a need to closely monitor anthelminthic drug efficacy and to develop standards and establish guidelines for monitoring a network of laboratories as highlighted in a World Health Organization–World Bank meeting on “Monitoring of Drug Efficacy in Large Scale Treatment Programmes for Human Helminthiasis” in Washington DC at the end of 2007.18
High prevalence of helminthic infection in our study population may be attributed to their prevailing low standards of living conditions, poor sanitation, lack of personal hygiene, paucity of potable water, open defecation due to lack of proper (water seal) latrines and lack of proper disposal of sewage leading to soil contamination and high endemicity of intestinal helminthiasis.
Conclusion
Morbidity due to intestinal worm infestation, particularly STH, is a global health problem affecting nearly two billion people in more than 100 countries. High prevalence of intestinal worm infestation is an indicator of poor living conditions and low standards of sanitation in a society. The present study reveals high prevalence of intestinal worms in the study population and calls for long term control measures to improve their sanitary and living conditions, including treatment of infected individuals and provision of potable water. The impact of these measures would be further enhanced through an organized health education programme, which will encourage healthy behaviour and lead to reduction in soil contamination and morbidity. Needless to say, that with existing understanding of helminth ecology and the availability of low cost drugs; goal to eliminate intestinal helminthiasis as a public health problem is achievable.
Conflicts of interest
All authors have none to declare.
References
- 1.WHO. Soil-transmitted Helminth Infections. Fact sheetN°366 at: www. Who.int/media centre/factsheets/fs366/en/.Accessed 21.07.13.
- 2.WHO . World Health Organisation; 2012. Technical Report Series-972. Research Priorities for Helminth Infections. Technical Report of the TDR Disease Reference Group on Helminth Infections.http://apps. who.int/iris/bit stream/10665/75922/1/WHO_ TRS_972_eng.pdf Accessed 21.07.13. [PubMed] [Google Scholar]
- 3.Stephenson L.S., Latham M.C., Ottesen E.A. Malnutrition and parasitic helminth Infections. Parasitology. 2000;(121 suppl l):S 23–S 38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 4.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers – a review. Int J Parasitol. 2010 Aug 15;40(10):1137–1144. doi: 10.1016/j.ijpara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . World Health Organisation; Geneva: 2012. Soil-transmitted Helminthiases. Eliminating Soil-transmitted Helminthiasis as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020; pp. 3–4. [Google Scholar]
- 6.WHO . World Health Organization; Geneva: 2013. Assessing the Efficacy of Anthelminthic Drugs Against Schistosomiasis and Soil-transmitted Helminthiasis; pp. 3–4. [Google Scholar]
- 7.Montresor A, Crompton DWT, Hall A, Bundy DAP and Savioli L. Guidelines for the Evaluation of Soil-transmitted Helminthiasis and Schistosomiasis at Community Level. WHO/CTD/SIP/98.1:27–28.
- 8.World Health Organization . 2006. Preventive Chemotherapy in Human Helminthiasis: Co-ordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; pp. 10–11. [Google Scholar]
- 9.Wani S.A., Ahmad F. Intestinal helminths and associated risk factors in children of district Pulwama, Kashmir, India. Indian J Med Microbiol. 2009;27(01):81–82. [PubMed] [Google Scholar]
- 10.Khanum H., Rahman M.M., Uddin M.H. Intestinal parasitic infestation among the outdoor patients of Dhaka University Medical Centre, Bangladesh. Univ j.zool. Rajshah. 2010;28:45–49. i. Univ. [Google Scholar]
- 11.Avhad S.B., Wahule V.K., Hiware C.J. Effect of climate factors on the prevalence of intestinal helminths from Aurangabad district (MS), India. Int J Basic Appl Res. Dec 2012;02(02):49–55. [Google Scholar]
- 12.Donnelly Sheila, Stack Colin M., O'Neill Sandra M., Sayed Ahmed A., Williams David L., Dalton John P. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22:4022–4032. doi: 10.1096/fj.08-106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checkley A.M., Chiodini P.L., Dockrell D.H. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010 Jan;60(1):1–20. doi: 10.1016/j.jinf.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Vercruysse J., Behnke J.M., Albonico M. Assessment of the anthelminthic efficacy of albendazole in school children in seven countries where soil transmitted helminths are endemic. PLoS Negl Trop Dis. 2011:29. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soukhathammavong P.A., Sayasone S., Phongluxa K. Low efficacy of single-dose albendazole and mebendazole against hookworm and effect on concomitant helminth infection in Lao PDR. PLoS Negl Trop Dis. 2012 Jan;(1):6. doi: 10.1371/journal.pntd.0001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap P., Du Z.W., Wu F.W. Rapid re-infection with soil-transmitted helminths after triple-dose albendazole treatment of school-aged children in Yunnan, People's Republic of China. Am J Trop Med Hyg. 2013 Jul;89(1):23–31. doi: 10.4269/ajtmh.13-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmann P., Utzinger J., Du Z.W. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Deworming for Health and Development. Reports Third Global Meeting the Partners Parasite Control. WHO/CDS/CPE/PVC/2005.14. https://extranet. who.int/iris/restricted/handle/10665/69005. Accessed 21.07.13.
- 19.Vinod Kumar C.S., Anand Kumar H., Sunita V., Kapur Indu. Prevalence of anaemia and worm infestation in school going girls at Gulbarga. Karnataka Indian Pediatr. 2003;40:70–72. [PubMed] [Google Scholar]
- 20.Bisht D., Verma A.K., Bhardwaj H.H.D. Intestinal parasitic infestation among children in a semi-urban Indian population. Trop Parasitol. 2011;1(2):104–107. doi: 10.4103/2229-5070.86946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha B. Intestinal parasitic infestation in healthy school children of Lalitpur district. J Nepal Med Assoc. 2001;41:266–270. [Google Scholar]
- 22.Khanal L.K., Choudhury D.R., Rai S.K. Prevalence of intestinal worm infestations among school children in Kathmandu, Nepal. Nepal Med Coll J. 2011 Dec;13(4):272–274. [PubMed] [Google Scholar]
- 23.Singh G.K., Parajuli K.P., Shrestha M. The prevalence of intestinal parasitic infestation in a Tertiary Care Hospital – a retrospective study. J Nobel Med Coll. 2013;2:13–17. [Google Scholar]
- 24.Gunawardena K., Kumarendran B., Ebenezer R., Gunasingha M.S., Pathmeswaran A., de Silva N. Soil-transmitted helminth infections among plantation sector school children in Sri Lanka: prevalence after ten years of preventive chemotherapy. PLoS Negl Trop Dis. 2011;5(9) doi: 10.1371/journal.pntd.0001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragunathan L., Kalivaradhan S.K., Ramadass S. Helminthic infections in school children in Puducherry, South India. J Microbiol Immunol Infect. 2010;43(3):228–232. doi: 10.1016/S1684-1182(10)60036-9. [DOI] [PubMed] [Google Scholar]


