Abstract
Background
Traditionally, Maddrey discriminant function (DF) score has been used for stratifying the prognosis of alcoholic hepatitis. Recently, the Model for end-stage liver disease (MELD) score has been applied to alcoholic hepatitis and some investigators consider MELD score as a better prognostic indicator. Another new prognostic approach, Lille model has been also suggested to accurately identify patients at high risk of death. Therefore, this prospective study was aimed to compare MELD, DF, Child–Turcotte–Pugh (CTP) scores and Lille model for predicting the short-term mortality in Indian patients with alcoholic hepatitis.
Methods
We calculated the DF, CTP, MELD and Lille scores in patients hospitalized with alcoholic hepatitis & evaluated if the scores predicted in-hospital mortality.
Results
A total of 104 patients were enrolled and thirty-two (30.7%) patients died during the hospitalization (2–30 days). Admission DF score (OR 1.1, P < 0.04), CTP (OR 2, P < 0.05) MELD score (OR 2.2, P < 0.005) and first week MELD score (OR 1.1, P < 0.05) were independently associated with in-hospital mortality. The area under the receiver-operating curve (AUROC) for the admission and day 7 MELD score was significantly higher than CTP score and was comparable to DF score and Lille model (AUC & 95% CI: 0.97 [0.95–1.0], 0.99 [0.99–1.0], 0.91 [0.83–0.91] and 0.92 [0.86–0.98] for MELD at admission & day 7, admission DF and Lille model, respectively). The MELD score >14 at admission and >12 at day 7 had high sensitivity and specificity in predicting short-term mortality (96%, 89% and 95%, 98% respectively). The cutoff of 0.45 for the Lille model was able to identify 79% of the observed deaths, whereas DF score ≥32 for DF were able to identify 85%.
Conclusion
MELD score, as a predictive model for assessment of short-term mortality in alcoholic hepatitis is better than CTP and comparable to DF and Lille model.
Keywords: DF, CTP, MELD, alcoholic hepatitis
Abbreviations: AH, alcoholic hepatitis; HCC, hepatocellular carcinoma; MELD, Model for end-stage liver disease; PVT, portal vein thrombosis; CTP, Child–Turcotte–Pugh score; SBP, spontaneous bacterial peritonitis; HRS, hepatorenal syndrome; AUC, area under the curve; AUROC, area under the receiver-operating curve
Alcohol is a true hepatotoxin, which contributes to majority of liver disease related deaths across the world.1 Chronic alcohol abuse can result in a spectrum of liver injury that ranges from mild fatty infiltration to alcoholic hepatitis (AH), cirrhosis and hepatocellular carcinoma (HCC). The prognosis among patients with alcoholic hepatitis can vary dramatically and mortality exceeding 50% in some case.2,3 Although AH is considered as acute form of liver injury but nearly 50% of patients with AH have established cirrhosis at the time of clinical presentation.4 Pharmacological therapies including corticosteroids and pentoxifylline need to be considered for patients with more severe disease to improve prognosis.3,5 Recently a provocative study clearly demonstrates the short-term survival benefit of liver transplantation for severe AH not responding to medical therapy.6 Consequently, the earlier identification of subset of patients with significant disease who will not improve with symptomatic therapy is necessary for potentially beneficial treatments to be provided. Various models have been shown to predict short-term prognosis in alcoholic hepatitis patients. The discriminant function (DF) introduced by Maddrey and Boitnott, has proved useful for identifying patients with poor short-term survival rates.3 Patients with a DF ≥32 have a poor prognosis, with one-month mortality rates of 35%–45% in absence of specific pharmacotherapy.7–9 By contrast, patients with a DF < 32 have short-term survival rates of 90%–100%.3,10 DF uses the Prothombin Time (PT), a variable that is poorly standardized across different laboratories and initial validation of DF relationship to mortality is based on patient cohorts from several decades past. The Model for end-stage liver disease (MELD) score has been also shown to predict survival in several western cohorts of patients with severe alcoholic hepatitis.11–14 Because the MELD score include measures of renal function, it appears to be more accurate in patients with concomitant renal injury. Another approach, Lille model, a combination of six reproducible variables, has high sensitivity and specificity for early identification of patients at high risk of death at 6 months.15
We aimed to compare MELD, CTP DF and Lille scores in predicting in-hospital mortality in Indian patients admitted with diagnosis of alcoholic hepatitis.
Patients and methods
Patient Information
The study involved 104 patients consecutively admitted to the Gastroenterology unit, at Banaras Hindu University hospital, in Varanasi, India from January 2011 to September 2012. The inclusion criterion was admission diagnosis of AH, based on clinical and biochemical data. The diagnosis of alcoholic hepatitis was made when (a) history of heavy alcohol abuse (>40 g/d for male & >20 g/d for female) was present until 1 month of onset of symptoms (b) AST/ALT ratio >2 with an AST level >45 (1.5 times upper limit of normal) but <500 U/L (3) a total bilirubin >2 mg/dl. Patients were excluded from the study if they had positive viral markers or have alternate diagnosis for liver dysfunction, HCC, portal vein thrombosis (PVT), significant comorbidities, and extra hepatic biliary obstruction. Institutional ethical committee approved the study protocol and informed consent was taken from patients or relatives.
Methods and Data Collection
Data collected prospectively of consecutive patients with diagnosis of AH. Detailed clinical history was taken particularly with reference to history of alcohol intake, duration, pattern, and type of liquor. Patients were assessed at admission for severity of liver disease and presence of complications like ascites, jaundice, encephalopathy, variceal-hemorrhage, spontaneous bacterial peritonitis (SBP), and/or hepatorenal syndrome (HRS) and daily progress notes were recorded. MELD, CTP, and DF scores were calculated on admission, and on day 7 respectively. Patients were treated with steroid or pentoxifylline if DF ≥32. Besides counseling for complete abstinence, they were assessed for nutritional, as well as vitamin and mineral deficiencies. Patients were provided multiple feedings including breakfast and a night-time snack, with a diet containing 1.5 g/kg protein and 35–40 kcal/kg energy. Albumin was used as volume replacement therapy, in patients with SBP. Patients with variceal bleed were treated with endoscopic band ligation and adjuvant terlipressin. Terlipressin and albumin were also used in patients with HRS. The data was collected for each patient until the end-point of either hospital discharge or in-hospital mortality.
Statistical Methods
Descriptive statistics for continuous variables included mean with SD or median and for categorical variables frequency distribution with percentage were calculated. Mean values of the individual variables and scores for both survival and death group were compared using Student t test or Mann–Whitney test. The independent association with in-hospital mortality for the individual variables and scores was calculated using bivariate logistic regression analysis. To compare the prognostic value of the different scores, receiver-operating characteristic (ROC) curves were graphed. The area under the curve (AUC) was compared using DeLong's test. The Kaplan–Meier method, constructed cumulative survival curves for the difference in in-hospital mortality. Differences between the curves were tested for significance using the log–rank statistic. Data were analyzed using SPSS (version 16) for windows.
Results
A total 104 patients were included with an admission diagnosis of AH & satisfied inclusion criteria of study. Table 1 summarizes the demographics and clinical characteristics of this cohort of patients. All the patients were male. The mean age was 44.81 ± 9.43 years (range 25–64). Maximum patients (38.46%) were in range of 36–46 years. The most frequent symptoms reported by the patients with alcoholic hepatitis with or without cirrhosis in our study were jaundice (100%), pedal edema (80.8%) and anorexia (80.8%). Other symptoms were diarrhea (19.2%), fever (38.46%), GI bleeding (32.7%). Out of total 104 patients 50 (48.07%) patients had one or more features of liver decompensation at the time of admission. There were total of 32 deaths during the hospitalization, accounting for 30.77% 30-day mortality. At the end of first week 10 patients expired and 22 patients were discharged. Out of remaining 72 patients twenty-two patients expired during same hospitalization and 50 were discharged. The mean duration of hospital stay was 14.1 ± 10.9 (2–30) days. Twenty-two patients (69.0%) died with liver failure with HRS. This was followed by multi-organ failure (17.2%), variceal-hemorrhage (10.3%), and SBP leading to sepsis (3.4%). Table 2 summarizes the means and ranges of the admission and first week laboratory variables and scores and comparison between those who survived and those who died during hospitalization. The mean admission scores were significantly higher in the death group. Similarly, the mean day 7 scores were significantly higher in those who died compared to those who survived. At the end of first week out of remaining 72 hospitalized patients, 50 (69.4%) patients showed decline in MELD score as compare to baseline and only 10 (20%) of them succumbed and 40 (80%) were discharged.
Table 1.
Baseline Demographics and Clinical Characteristics of the Patients.
| Variables | N (total) | Mean ± SD (range) or % |
|---|---|---|
| Age (years) | 104 | 44.81 ± 9.3 (25–64) |
| Cirrhosis | 55 (104) | 52.3% |
| Steroid/pentoxifylline treatment | 10/35 (104) | 9.6%/33.6% |
| Length of hospital stay (days) | 14.1 ± 10.9 (2–30) | |
| In-hospital death | 32 (104) | 30.77% |
| Hepatomegaly | 46 (104) | 45% |
| Presence of ascites | 45 (104) | 43% |
| Hepatic encephalopathy | 40 (104) | 38% |
Table 2.
Comparison of Admission and First Week Survival and Death Variables and Scores.
| Variables (units) | Total Mean ± SD (range) |
Survival (N = 72) Mean ± SD (range) |
Death (N = 32) Mean ± SD (range) |
P-value |
|---|---|---|---|---|
| Admission [104] | ||||
| Age (years) | 44.81 ± 9.391 (25–64) | 42.3 ± 9.4 (25–64) | 47.0 ± 9.4 (25–63) | 0.014 |
| S Br (mg/dL) | 9.6 ± 6.2 (2.2–32.9) | 6.6 ± 4.2 (2.2–22.) | 15.6 ± 6.2 (2.2–32.9) | <0.001 |
| Albumin (g/dL) | 2.55 ± 0.46 (1.5–3.7) | 2.95 ± 0.46 (1.5–3.7) | 2.25 ± 0.46 (1.5–3.7) | <0.001 |
| Na (meq/l) | 128.4 ± 8.9 (108–145) | 132.4 ± 7.9 (110–145) | 127.4 ± 7.4 (108–145) | <0.05 |
| International Normalized Ratio (INR) | 1.4 ± 0.4 (1.02–3.7) | 1.4 ± 0.4 (1.02–3.1) | 2.4 ± 0.8 (1.02–3.7) | <0.001 |
| S Cr (mg/dL) | 1.40 ± 0.72 (0.50–4.7) | 1.20 ± 0.72 (0.50–2.7) | 2.80 ± 0.98 (0.80–4.7) | <0.001 |
| CTP | 10.3 ± 1.8 (7–15) | 9.6 ± 1.3 (7–15) | 12.3 ± 1.8 (7–15) | <0.001 |
| DF | 30.1 ± 15.1 (5.3–83.5) | 23.7 ± 17.0 (5.3–33) | 40.1 ± 18.1 (11.3–83.5) | <0.001 |
| MELD | 13.6 ± 7.5 (0.21–31.6) | 10.9 ± 8.2 (0.21–22.5) | 23.6 ± 7.5 (8.2–31.6) | <0.001 |
| First week [72] | Survival (N = 50) | Death (N = 22) | ||
| S Br (mg/dL) | 10.07 ± 8.04 (2.1–33.7) | 6.06 ± 3.15 (2.1–13) | 19.12 ± 8.64 (8–33.7) | <0.001 |
| Albumin (g/dL) | 2.7 ± 0.56 (1.6–3.8) | 2.90 ± 0.53 (1.9–3.8) | 2.22 ± 0.34 (1.6–2.8) | <0.001 |
| Na (meq/l) | 130.4 ± 6.2 (113–139.9) | 131.7 ± 5.7 (113–139.9) | 115.69 ± 7.65 (113–139.9) | <0.01 |
| INR | 1.34 ± 0.38 (1.02–3.1) | 1.16 ± 0.07 (1.02–1.30) | 1.76 ± 0.47 (1.1–3.1) | <0.001 |
| S Cr (mg/dL) | 1.21 ± 0.57 (0.55–3.1) | 0.94 ± 0.25 (0.55–1.56) | 1.84 ± 0.62 (1.08–3.1) | <0.001 |
| Day 7 CTP | 9.91 ± 2.02 (7–15) | 8.8 ± 1.1 (7–12) | 12.3 ± 1.3 (10–15) | <0.001 |
| Day 7 DF | 27.7 ± 18.2 (4.1–73.7) | 17.6 ± 6.2 (4.1–33) | 51.9 ± 14.4 (27.4–73.7) | <0.001 |
| Day 7 MELD | 11.73 ± 7.9 (0.24–33.1) | 7.12 ± 3.2 (0.24–15.6) | 22.19 ± 4.9 (14.7–33.1) | <0.001 |
Risk Factors for In-hospital Death
Univariate analysis of the different scores is shown in Table 3. All scores were associated with mortality. Multivariate analysis of individual variables showed no independent association with mortality on admission and at the end of first week. Multivariate analyses of the different scores were done and shown in Table 4. At admission CTP, DF and MELD scores were independently associated with in-hospital mortality. However, at day 7 only MELD score was independently associated with in-hospital mortality on multivariate analysis.
Table 3.
Univariate Analysis of the Different Scores in Predicting Short-term Mortality.
| Scores | N | Admission OR (95% CI) |
P-value | N | Day 7 OR (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| CTP | 104 | 2.6 (1.8–3.8) | <0.001 | 72 | 2.4 (1.8–3.5) | 0.001 |
| DF | 104 | 1.2 (1.1–1.3) | <0.001 | 72 | 1.5 (1.1–2.2) | 0.007 |
| MELD | 104 | 2.4 (1.4–4.1) | 0.001 | 72 | 1.2 (1.1–1.3) | 0.001 |
Table 4.
Multivariate Analysis of Scores in Predicting Short-term Mortality.
| Scores | N | Admission OR (95% CI) |
P-value | N | Day 7 OR (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| CTP | 104 | 2.0 (1.3–3.1) | <0.05 | 72 | 1.3 (0.4–4.4) | 0.21 |
| DF | 104 | 1.1 (0.9–1.2) | 0.04 | 72 | 1.3 (1.1–1.4) | 0.76 |
| MELD | 104 | 2.2 (1.3–3.7) | 0.005 | 72 | 1.1 (1.0–1.2) | <0.05 |
Receiver-Operating Characteristic Curves of Different Scores
The ROC curves for the scores are shown in Figures 1–3. The AUC for admission MELD was highest and statistically different from admission CTP and comparable to DF. The AUC of day 7 MELD score was also highest but not statistically different compared to both CTP and DF score. At admission DF score of ≥32 yielded the sensitivity of 83% and specificity of 85% in predicting in-hospital mortality. Ideal cutoff for admission MELD score of 14 with highest prediction value yielded 96% sensitivity and 89% specificity (Table 5). The MELD score of 12 at day 7 had 95% sensitivity and 98% specificity in predicting mortality (Table 5). The AUROC value of the Lille model was 0.92 (95% CI 0.86–0.98) (Figure 3). AUROC was highest for MELD both at admission and at day 7 (Figure 3). In terms of number of predicted deaths, the cutoff of 0.45 for the Lille model was able to identify 79% of the observed deaths, whereas DF score ≥32 for DF were able to identify 85%.
Figure 1.
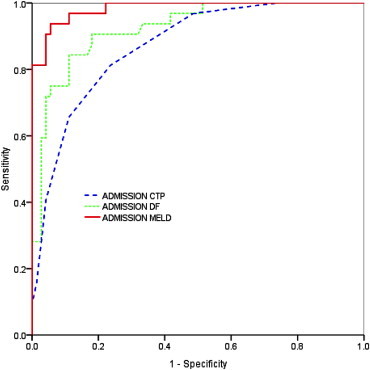
ROC curves for admission CTP, DF and MELD in predicting short-term mortality.
Figure 2.
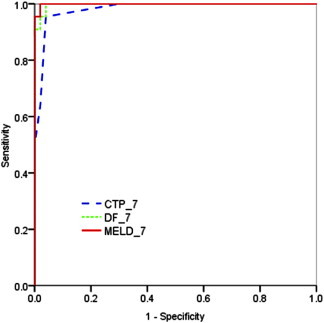
ROC curves for day 7 MELD, DF and CTP scores in predicting short-term mortality.
Figure 3.
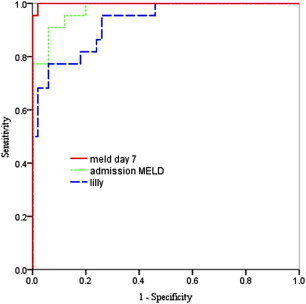
ROC curves for admission MELD, day 7 MELD and lille score in predicting short-term mortality.
Table 5.
Operational Value of MELD Score in Predicting In-hospital Mortality.
| Score | Cutoff | Sensitivity % | Specificity % | PPV % | NPV % | OR |
|---|---|---|---|---|---|---|
| Admission MELD | 14 | 96 | 89 | 54 | 94 | 8.2 |
| First week MELD | 12 | 95 | 98 | 57 | 96 | 11.2 |
Survival curve—Kaplan–Meier survival analysis shown in Figure 4 compares in-hospital mortality based on the admission MELD score >14 & <14 (P < 0.05), the day 7 MELD score of >12 & <12 (P < 0.05). A higher MELD score significantly increases the risk of in-hospital mortality.
Figure 4.
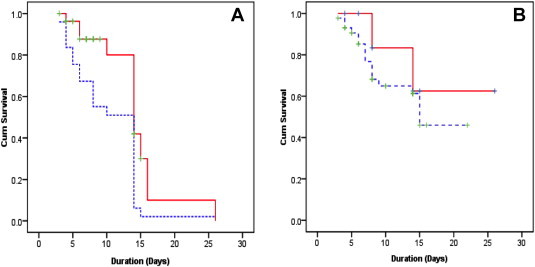
Kaplan–Meier survival curves of [A] Admission MELD score >14 (Blue line) and <14 (Red line), [B] Day 7 MELD score >12 (Blue line) and <12 (Red line) in predicting short-term mortality.
Discussion
The continues effort to develop prognostic models in patients with AH is for identifying subset of patients with high short-term mortality, so that they can be offered specific treatment to improve prognosis. DF score >32 is predictor of >50% one month mortality and currently used as a threshold to start either corticosteroid or pentoxifylline therapy.3,5 However, 10–17% of patients with DF <32 may still die of AH and the cutoff of 32 with 50% mortality was based on risk benefit ratio specific to corticosteroid more than a decade ago. MELD score is now widely used for the prediction of mortality from end-stage liver disease and for organ allocation in transplant candidates. Furthermore, the individual components of the MELD score have been described as individual predictors of mortality from alcoholic hepatitis in various studies.16,17 The most recent approach is based on Lille model to identify persons at high risk of death. Patients above the ideal cutoff of 0.45 showed a marked decrease in 6-month survival and this cutoff was able to identify approximately 75% of the observed deaths.15 Using the 0.45 Lille model cutoff, close to 40% of patients do not benefit from corticosteroids.15 However, proposed cutoff of those scores needs to be tested outside the initial population of their development. We compared different scores as predictive models to assess in-hospital mortality in a cohort of Indian patients with AH.
The association between adverse outcome and bilirubin, INR, ascites, and/or hepatic encephalopathy has been previously reported.16,18,19 We found that patients who died during hospitalization, presented with more deranged laboratory parameters and more advanced liver disease in form of ascites and higher grade of hepatic encephalopathy. However individual laboratory parameter did not show independent association with in-hospital mortality. Two previous studies compared scores based on these laboratory variables showed that admission MELD score was as good as DF score or may be MELD score was better than DF score in predicting in-hospital mortality.11,12 Srikureja et al showed that the first week and the first week increase change in MELD score, but not DF scores were independently associated with mortality.11 In accordance with these results we also noted that admission MELD and DF scores and at the end of first week only MELD score independently predict in-hospital mortality. The change in MELD over 1 week (δ MELD) did not emerge as a predictor of mortality on univariate analysis in our study. From the ROC curves, AUC was highest for MELD both at the admission and at the end of first week.
In our cohort, the ideal cutoff obtained for admission MELD was 14 to predict in-hospital mortality with 96% sensitivity and 89% specificity. Moreover, the day 7 MELD score cutoff at 12 had the comparable sensitivity (95%); highest specificity (98%) and better odds ratio. MELD score was superior to CTP and comparable to DF and Lille model in predicting mortality rate in AH patients.
There are some limitations in our study, as we included patients with ascites and few patients also underwent paracentesis during their admission. Serum albumin infusions were given in management of Large Volume Paracentesis (LVP), HRS, and SBP. Thus the significance of these variables might be lost. Almost half of the patients in our cohort have underlying liver cirrhosis, based on clinical and imaging data, so it was not possible to conclude whether the MELD may be more useful in those patients with coexisting cirrhosis and AH. However, diagnosis of cirrhosis was not based on histology. Presence of cirrhosis in 55 patients may have been an over estimation.
To conclude, alcoholic hepatitis remains associated with a high mortality in hospitalized patients. MELD model is better than CTP models and comparable to DF and Lille model in predicting in-hospital mortality. The admission & the day 7 MELD scores are all independently associated with in-hospital mortality. Accurate and validated cutoff yet to be defined.
Conflicts of interest
All authors have none to declare.
References
- 1.Rehm J., Mathers C., Popova S. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 2.Porter H.P., Simon F.R., Pope C.E. Corticosteroid therapy in severe alcoholic hepatitis, a double-blind trial. N Engl J Med. 1971;284:1350–1355. doi: 10.1056/NEJM197106172842404. [DOI] [PubMed] [Google Scholar]
- 3.Maddrey W.C., Boitnott J.K., Bedine M.S. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 4.O'Shea R.S., Dasarathy S., McCullough A.J. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 5.Akriviadis E., Botla R., Briggs W. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 6.Mathurin P., Moreno C., Samuel D. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 7.Helman R.A., Temko M.H., Nye S.W., Fallon H.J. Alcoholic hepatitis: natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311–321. doi: 10.7326/0003-4819-74-3-311. [DOI] [PubMed] [Google Scholar]
- 8.Carithers R.L., Jr., Herlong H.F., Diehl A.M. Methylprednisolone therapy in patients with severe alcoholic hepatitis: a randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 9.Ramond M.J., Poynard T., Rueff B. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 10.Mathurin P., Mendenhall C.L., Carithers R.L. Glucocorticoids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of glucocorticoids in severe AH. J Hepatol. 2002;36:480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 11.Srikureja W., Kyulo N.L., Runyon B.A. MELD score is a better prognostic model that Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Sheth M., Riggs M., Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. doi: 10.1186/1471-230X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soultati A.S., Dourakis S.P., Alexopoulou A. Predicting utility of a model for end stage liver disease in alcoholic liver disease. World J Gastroenterol. 2006;12(25):4020–4025. doi: 10.3748/wjg.v12.i25.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn W., Jamil L.H., Brown L.S. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41(2):353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 15.Louvet A., Naveau S., Abdelnour M. Hepatology. 2007 Jun;45(6):1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 16.Chedid A., Mendenhall C.L., Gartside P. Prognostic factors in alcoholic liver disease. Am J Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 17.Orrego H., Israel Y., Blake J.E., Medline A. Assessment of prognostic factors in alcoholic liver disease: toward a global quantitative expression of severity. Hepatology. 1983;3:896–905. doi: 10.1002/hep.1840030602. [DOI] [PubMed] [Google Scholar]
- 18.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 19.Kumashiro R., Sata M., Ishii K. Prognostic factors for short-term survival in alcoholic hepatitis in Japan: analysis by logistic regression. Alcohol Clin Exp Res. 1996;20:383A–386A. [PubMed] [Google Scholar]


