Abstract
Background
Prescription errors are commonly encountered in health care settings. They can lead to inefficient delivery of health care thus jeopardizing patient care. Knowing the quantum and the possible causes of such errors is the first step in trying to prevent them. We conducted a random audit of prescriptions received in service dispensary of a tertiary care hospital and analyzed them for prescription errors.
Methods
A total of 1000 prescriptions were randomly selected. These prescriptions were analyzed with the help of three qualified pharmacists and were stratified as per the errors encountered.
Results
Out of the total of 1000 prescriptions, 650 prescriptions (65%) were found to have a total of 1012 errors. Type B errors were found in 22.4% prescriptions, type C errors in 9.7% prescriptions and type D in 69.1% prescriptions.
Conclusion
Prescription errors require proactive, continuous and meticulous monitoring so as to minimize them. It requires identification of preventable causes, increasing awareness and sensitizing the prescriber towards this important aspect of health care delivery.
Keywords: Prescription, Medication, Errors, Brand names, Noncompliance, Handwriting
Introduction
Prescribing medicines to patients is an integral part of medical care. It involves decision-making about the choice of medicines, its communication to pharmacist in the form of prescriptions for dispensing and finally, administration of medicines. The whole process requires seamless communication at various stages. However, a decremental knowledge gap exists at each step, with patient being least informed and almost totally unaware of the benefits and risks of medicines. Like any other process involving multiple individuals, this process too is prone to errors with the potential of jeopardizing patient care. Ensuring flawless delivery of correct medication to the patients is drawing long deserved attention from health care professionals. It has become an important part of overall efforts for judiciously using medicines and minimizing their adverse effects.
An error can occur at any stage of the prescription process viz:
- Choosing a medicine:
-
•There may be selection of irrational, inappropriate or ineffective (a medicine that is not effective for the indication in general or for a specific patient) medication.
-
•Under-prescribing (failure to prescribe a medicine that is indicated and appropriate, or use of too low a dose of an appropriate medicine).
-
•Over-prescribing (prescribing a medicine too much, too often or for too long).
-
•
Prescription writing: Omission/mistake in – superscription, dosage form, strength of preparation, improper route and/or illegible handwriting lead to such errors.
Formulation used: Such errors occur due to wrong strength, contaminants, wrong or misleading packaging of formulations involved.
Dispensing of medication: Dispensing wrong medicine or wrong formulation to the patient or dispensing medicines with wrong labeling can result in such errors.
Administering/taking the medicine: Despite correct selection of medicines, meticulous prescription writing and careful dispensing, the patient may still take or be administered medicines in wrong amount, by wrong route, in wrong frequency or for wrong duration.
Monitoring therapy: Medicines need to be prescribed for a defined time period. Even long-term treatments require monitoring and modifications from time to time depending on various factors such as disease progression and changes in patient's physiological parameters. Failing to alter therapy when required or erroneous alteration also account for errors.1
An error occurring at any of these stages is defined as medication error. It may result in failure of the therapy or may cause harm to the patient. Medication errors are recognized to be an important impediment in providing optimum medical care to the patients. A number of studies have been done to assess their magnitude in diverse settings. In one study, inpatient medication errors occurred at the rate of 1.5–5.3 per 100 orders.2 In another study 16% of the patients reported a medication error with two third of them in outpatient department (OPD) patients.3 These errors have a negative impact on patients' health and therefore should be minimized.4–6 Medication errors can give rise to adverse events too. In one study, 11% of adverse events were due to medication errors.7
The frequency of medication errors differs from one set up to another depending on a number of factors such as type of patients, training, patient load, medical audit procedures and sensitization of the health care workers. Therefore, prescriptions (for both admitted and OPD patients) need to be proactively screened for such errors and steps be taken to minimize them.
Prescription errors are an important form of medication errors. Studies have shown that 15–21% prescriptions contain at least one prescribing error.8,9 In India, there are a few published studies pertaining to medication errors and prescription errors. Most of the published studies have addressed the issue of medication errors in indoor admitted patients.10–12 All these studies have been conducted on 300–500 subjects. Patel et al conducted a survey of 999 OPD prescriptions in which they focused mainly on the issue of irrational drug use.13 This study was planned to initiate the process of identification and subsequent minimization of medication errors. As an initial step, this observational study was planned to look only for errors in prescription writing for OPD patients.
Material and methods
A total of 1000 prescriptions were randomly selected, out of all the prescriptions received in one month at central hospital dispensary from various OPDs. Prescription errors were stratified according to nuisance they may cause by hampering the dispensing work, a method suggested by Neville et al.14 According to this method, prescription errors can be classified as follows:
Type A: Errors which are potentially serious to patient. Such prescription would be dangerous to the patient if dispensed. For e.g. (i) if the dose of a cardiac drug viz. Digoxin is increased by a factor of 10 OR (ii) if the pharmacist is not able to differentiate between ‘Daonil’ (Brand name for glibenclamide, a hypoglycemic medicine) and ‘Digene’ (Brand name of a mixture of antacids and methyl polysiloxone).
Type B: Errors causing major nuisance by making a pharmacist to contact the prescriber in order to dispense the medicine. For e.g. If type of formulation prescribed (e.g. whether conventional tablet or slow release tablet of indapamide is to be dispensed) or strength of formulation (e.g. whether aspirin tablets of 75 mg or 150 mg or 325 mg or Atorvastatin tablets of 10 or 20 mg are to be dispensed) is not mentioned or use of brand name about which the dispensing pharmacist is not aware.
Type C: Errors causing minor nuisance which can be managed by involving other pharmacist to take a professional decision at dispensary level before dispensing. Though such prescription can be correctly dispensed without contacting the prescriber, however such an error causes hindrance in the functioning of the dispensary and delays dispensing of medication to the patient. For e.g. (i) omission of dosing schedules of commonly prescribed medicines like paracetamol, diclofenac (ii) using brand names of commonly used medicines such as ‘Natrilix’ for indapamide, ‘Tixylix’ for promethazine (iii) using abbreviations such as ‘NTP’ for nortriptyline, ‘UDCA’ for ursodeoxycholic acid.
Type D: Trivial errors consisting of spelling errors or omissions such as date, age and/or gender of the patient etc. Such errors do not hamper the execution of prescriptions.
Three pharmacists, who have been working in the hospital dispensary, were asked to screen these randomly selected prescriptions under the supervision of the authors. The prescription errors were identified and were listed as per the type described by Nivelle et al. If the prescription had more than one error, both the type of errors were identified and included in the analysis.
Analysis protocol
The selected prescriptions were screened for the following prescription writing errors by authors at the first instance:
-
1.
Strength of preparation not mentioned.
-
2.
Use of brand names.
-
3.
Incomplete description of dosing schedule and dosing instructions.
-
4.
Illegible handwriting.
-
5.
Diagnosis not mentioned.
-
6.
Age of the patient not mentioned.
-
7.
Gender not mentioned.
Subsequently, these prescriptions were also given to three pharmacists, who have been working in the hospital dispensary for at least two months, to assess the level of difficulty they would face in dispensing these. The screening by pharmacists was done under the supervision of authors. The decisions of pharmacist were recorded by authors and were considered for classifying the errors into various categories.
If the prescription had more than one error, both the type of errors were identified and included in the analysis.
Results
Out of a total of 1000 prescriptions, 650 prescriptions (65%) were found to have one or more errors. The total number of errors was 1012 as many prescriptions had more than one error. All types of errors except type A were observed in this study (Table 1). In 1000 prescriptions analyzed, type B errors were found in 22.4% prescriptions. The main reasons for type B errors were use of brand name (8.9% of prescriptions), no mention of strength of preparation (8.7% of prescriptions), incomplete description of dosing schedule and dosing instructions (2.6% prescriptions) and illegible handwriting (2.2% prescriptions). Type C errors were found in 9.7% prescriptions. They resulted due to use of brand names (0.9% prescriptions) and illegible handwriting (8.8% prescriptions). The most common type of errors was type D and was found in 69.1% prescriptions. Type D errors were due to no mention of diagnosis (60.1% prescriptions) no mention of age (6.5% prescriptions), illegible handwriting (2.3% prescriptions) and gender not mentioned (0.2% prescriptions) (Figs.1 and 2).
Table 1.
Types and causes of observed prescription errors.
| Cause of error | Number of errors in 1000 prescriptions |
|||
|---|---|---|---|---|
| Type B errors | Type C errors | Type D errors | Total | |
| Strength of preparation not mentioned | 87 | 87 | ||
| Use of brand names | 89 | 9 | 98 | |
| Incomplete description of dosing schedule and dosing instructions | 26 | 26 | ||
| Illegible handwriting | 22 | 88 | 23 | 133 |
| Diagnosis not mentioned | 601 | 601 | ||
| Age of the patient not mentioned | 65 | 65 | ||
| Gender not mentioned | 02 | 02 | ||
| Total | 224 | 97 | 691 | 1012 |
Fig. 1.
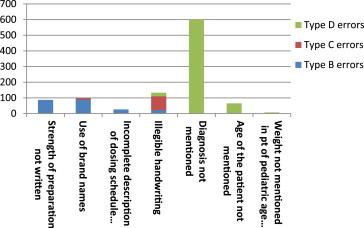
Types and causes of observed prescription errors.
Fig. 2.
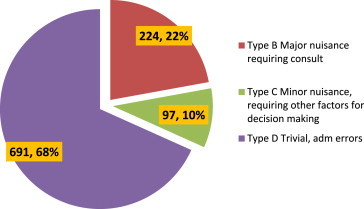
Pie diagram showing percentage of various types of errors.
Discussion
Medication errors form an important cause of patient morbidity and mortality and a number of studies confirm their existence worldwide. Ingrim et al15 audited 7858 prescriptions and found errors in 1070 prescriptions (13.6%). A total of 1130 errors were recorded in 7858 prescriptions. In another study, conducted in Indonesia at a Government Hospital, prescription errors were found in 99.1% prescriptions (n = 229).16
In our study, an error rate of 65% was observed with most of the errors as trivial i.e. type D. Such errors are not likely to hamper correct execution of the prescriptions. Most of the type D errors resulted due to absence of diagnosis in the prescriptions. Mentioning diagnosis in the superscription is a part of correct prescription writing and is mandated by WHO.17 Mention of a diagnosis may help the pharmacist to correlate and interpret the correct medicine or formulation if the handwriting is not completely understood. Omission of mentioning ‘age’ of the patient was the next contributor for type D errors. Mentioning patient's age apparently appears superfluous but is vital from patho-physiological view especially for patients in extremes of age. It is also important if two patients of the same name, gender and diagnosis are attending OPD or dispensary at one time.
Type B errors resulted mainly due to –
-
–
Use of brand names like Tablet ‘Pangraf’, ‘Lospot’, ‘Natrilam’ etc.
-
–
Strength of preparations not mentioned viz. Tablet ‘Ecospirin’ 75 mg or 150 mg, Tablet Methylcobalamin 500 mcg or 1500 mcg.
-
–
Failure to clearly outline dosing schedule and dosing instructions like tapering schedule for Tablet Prednisolone or specific time to take Tablet Omeprazole which is most effective if taken on empty stomach.
Medicines must be prescribed only by generic names to maintain uniformity, clarity, ease of understanding and reduce the cost of medical care. However, the brand names are usually catchy, suggestive, and easy to remember making their use common. Since a medicine may have more than one brand name, problem occurs when an uncommon medicine is prescribed by the brand name or a common medicine is prescribed by a not so popular or a newer brand name. In such cases, there may be delay in dispensing if the pharmacist is not well familiar with that brand as he will have to contact the prescriber or consult some other source like medicine indices (e.g. CIMS, MIMS, Drug Today etc) to know the ingredients or he may simply not dispense the medicine considering it as non-available. The problem becomes all the more important in tertiary care hospitals where a single dispensary handles prescriptions from a wide range of OPDs (from neurology to nephrology and from reconstructive surgery to psychiatry). For example Tablet ‘Cellcept’ (Brand name for mycophenolate mofetil) may be a common medicine for nephrologists but the dispensing pharmacist may not be conversant with the brand name. In this analysis, unfamiliar brand names in 8.9% prescriptions would have required pharmacist to contact prescriber for clarification about the name of the medicine before dispensing it. In government health care set ups, usually the procurement is done as per the generic name of the drug. As a result, medicine from the same manufacturer may not be procured every time and thus the brands of medicines may change with fresh procurement. Hence, it becomes even more important to prescribe using generic names only.
Strength of preparation was missing in a total of 87 prescriptions. Omitting the strength of the preparation will not have much effect on dispensing medicines which are available in single strength (e.g. Capsule doxycycline, Tablet mebendazole). However, a large number of the medicines are available in multiple strengths such as glimepiride which is available as 1, 2 and 4 mg tablets; warfarin as 1, 2 and 5 mg tablets; atorvastatin as 10, 20 and 40 mg tablets; omeprazole as 20 and 40 mg tablets. Mentioning strength of a medicine becomes inescapable especially for medicines with narrow margin of safety (e.g. glimepiride, warfarin) which if taken in overdose, may cause more harm than good. In our analysis, Aspirin, atorvastatin and methylcobalamin were three most commonly prescribed medicines without mentioning the strength of the tablet. While indicating the strength of the formulation, only the internationally accepted abbreviations should be used: ‘g’ for gram, ‘ml’ for milliliter. Use of decimal should also be avoided as it may get inconspicuously written and, wherever necessary, full words should be written to avoid misunderstanding i.e. write 50 μg instead of 0.05 mg or 50 μg.
Incomplete dosing schedule/dosing instruction were found in 2.6% prescriptions leading to type B errors. The problem is more important in cases where medicines needs to be prescribed as loading and maintenance dose (e.g. Chloroquine), in sliding scale (e.g. corticosteroids), or when a medicine needs to be taken on as and when required basis (e.g. analgesics like paracetamol). While indicating dosage schedules, it is better to avoid Latin words such as BD, TDS. Clear instructions such as two times a day for BD, three times a day for TDS should be used preferably along with the time of administration.
Poorly legible or illegible handwriting was another cause of prescription errors encountered in a total of 133 prescriptions (13.3% prescriptions), leading to all types of errors (22 type B, 88 type C and 23 type D) depending on the ability of the pharmacist to understand the prescription correctly. If medicines are prescribed with generic name with age, sex and diagnosis of the patient mentioned, the pharmacist may dispense with less difficulty. However, in absence of such details, he will have to seek clarification from the prescriber more frequently. Writing a legible prescription is the legal responsibility of the prescriber and he is responsible for wrong interpretation of the prescription by the pharmacist.18
The use of computers for prescription writing also may reduce prescription errors.19,20 The computerized system can be integrated with patient details including his/her physiological parameters, known allergies, real time information regarding availability of medicines, and the system can be tailor-made to offer best possible therapy. Before the prescription is finalized, such an integrated system can also provide medicine specific information, alerts regarding possible over dosage, interactions among medicines etc. Besides technical inputs, these electronic prescriptions can expedite repeated monthly prescriptions for patients who are on long term treatment. Such prescription, once generated, can be sent electronically to the dispensary where dispensing packages can be prepared before the patient reaches the dispensary, thus reducing waiting time. However, evolving and applying such technology across a large number of hospitals and training of manpower remain major impediments in such an endeavor. Once implemented, updating the database in light of emerging evidence, data protection, maintaining confidentiality and routine trouble shooting will be major challenges in ensuring routine use of such a technology.
Since this study was a retrospective observational study, all the pertinent information required for identifying prescribing errors such as suboptimal dose, suboptimal duration, overmedication was not available. Hence analysis of such prescribing errors was deliberately left out. However, some of the glaring errors were noted. It was observed that there is a general tendency to overprescribe nutritional supplements. Multivitamins, calcium preparations were found to be prescribed in most of the prescriptions irrespective of the diagnosis. In fact, in two prescriptions, Capsule ‘Autrin’ (a combination of iron, vitamin B12 and folic acid), Tablet multivitamin and Tablet ‘B’ complex were prescribed together. Some prescriptions had both syrup and tablet preparations of iron prescribed concurrently. Since other relevant details regarding patho-physiological state of the patient (such as renal functions, liver functions) etc were not available, a detailed analysis of the choice of medicine and its dose etc could not be undertaken. Such an analysis can be subsequently taken up in specific specialties with the inclusion of treating physicians.
Conclusion
Prescription errors are recognized as a cause of concern that requires proactive, continuous and meticulous monitoring in a non-punitive manner with emphasis on what mistake was made rather than who made the mistake. The problem can be further minimized by sensitizing the prescribers to follow prescription writing practices as per ‘WHO Guidelines on Good Prescribing’.17 As this study demonstrates, there is a requirement of undertaking regular structured prescription audits to minimize prescription errors. Subsequently, the scope of such audits can be widened to include in – hospital prescriptions and medication errors resulting from choice of medicines, drug–drug interactions, food drug interactions, etc.
Conflicts of interest
All authors have none to declare.
References
- 1.Aronson J.K. Medication errors: what they are, how they happen, and how to avoid them. Q J M. 2009;102:513–521. doi: 10.1093/qjmed/hcp052. [DOI] [PubMed] [Google Scholar]
- 2.Dean B., Schachter M., Vincent C., Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual Saf Health Care. 2002;11:340–344. doi: 10.1136/qhc.11.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis K, Schoenbaum SC, Collins KS, Tenney K, Hughes DL, Audet AJ. Room for Improvement: Patients Report on the Quality of their Health Care. Available at: http://www.cmwf.org. Accessed 01.11.03.
- 4.Lapointe N.M., Jollis J.G. Medication errors in hospitalized cardiovascular patients. Arch Intern Med. 2003;163:1461–1466. doi: 10.1001/archinte.163.12.1461. [DOI] [PubMed] [Google Scholar]
- 5.Jenkison M.L. Prescribing errors. Lancet. 2002;360:256–259. doi: 10.1016/S0140-6736(02)09471-0. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy S., Bilkar W.B., Zhou L. Retrospective drug utilization review, medication errors, and clinical outcomes. JAMA. 2003;290:1494–1499. doi: 10.1001/jama.290.11.1494. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi T.K., Weingart S.N., Borus J. Adverse drug events in ambulatory care. New Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T.A. Improving the quality of the order-writing process for inpatient orders and outpatient prescriptions. Am J Health Syst Pharm. 2000;57(suppl 4):4–22. doi: 10.1093/ajhp/57.suppl_4.S18. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy A.F., Nickel R.O. Prescription-writing patterns and errors in a family medicine residency program. J Fam Pract. 1989;29:290–295. [PubMed] [Google Scholar]
- 10.Pote S., Tiwari P., D'Cruz S. Medication prescribing errors in a public teaching hospital in India: a prospective study. Pharm Pract. 2007;5(1):17–20. doi: 10.4321/s1886-36552007000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy L.K.V., Modi A.G., Chaudhary B., Modi V., Patel M. Medication errors – a case study. J Acad Hosp Adm. 2009;21(1,2):28–34. [Google Scholar]
- 12.Karna K., Sharma S., Inamdar S., Bhandari A. Study and evaluation of medication errors in a tertiary care teaching hospital – a baseline study. Int J Pharm Sci. 2012;4(5):587–593. [Google Scholar]
- 13.Patel V., Vaidya R., Naik D., Borker P. Irrational drug use in India: a prescription survey from Goa. J Postgrad Med. 2005 Jan–Mar;51(1):9–12. [PubMed] [Google Scholar]
- 14.Neville R.G., Robertson F., Livingstone S. A classification of prescription errors. J R Coll Gen Prac. 1989;39:110–112. [PMC free article] [PubMed] [Google Scholar]
- 15.Ingrim N.B., Hokanson J.A., Guernsey B.G. Physician noncompliance with prescription-writing requirements. Am J Hosp Pharm. 1983;40:414–417. [PubMed] [Google Scholar]
- 16.Perwitasari D.A., Arbor J., Wahyuningsih I. Medication errors in outpatients of a Government Hospital in Yogyakarta Indonesia. Int J Pharma Sc Rev Res. 2010;1:8–10. [Google Scholar]
- 17.M deVries TPG, Henning RH, Hogerzeil HV, Fresle DA. Guide to Good Prescribing: A Practical Manual. Available at: http/apps.who.int/medicinedocs/en/d/Jwhozip23e. Accessed 18.02.13.
- 18.Mullan K. Importance of legible prescriptions. J R Coll Gen Pract. 1989;39:347–348. [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman R., Singer M., Goldstone J., Bellingan G. Medication errors: a prospective cohort study of hand-written and computerized physician order entry in the intensive care unit. Crit Care. 2005;9:516–521. doi: 10.1186/cc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A Call to Action: Eliminate Handwritten Prescriptions Within 3 Years. Electronic Prescribing Can Reduce Medication Errors (White Papers from the Institute for Safe Medication Practices). Available at www.ismp.org. Accessed 19.02.13.


