Abstract
Background
Epidemiological evidence has suggested a link between beta2‐agonists and increases in asthma mortality. There has been much debate about possible causal links for this association, and whether regular (daily) long‐acting beta2‐agonists are safe.
Objectives
The aim of this review is to assess the risk of fatal and non‐fatal serious adverse events in trials that randomised patients with chronic asthma to regular formoterol versus placebo or regular short‐acting beta2‐agonists.
Search methods
We identified trials using the Cochrane Airways Group Specialised Register of trials. We checked websites of clinical trial registers for unpublished trial data and Food and Drug Administration (FDA) submissions in relation to formoterol. The date of the most recent search was January 2012.
Selection criteria
We included controlled, parallel design clinical trials on patients of any age and severity of asthma if they randomised patients to treatment with regular formoterol and were of at least 12 weeks' duration. Concomitant use of inhaled corticosteroids was allowed, as long as this was not part of the randomised treatment regimen.
Data collection and analysis
Two authors independently selected trials for inclusion in the review. One author extracted outcome data and the second author checked them. We sought unpublished data on mortality and serious adverse events.
Main results
The review includes 22 studies (8032 participants) comparing regular formoterol to placebo and salbutamol. Non‐fatal serious adverse event data could be obtained for all participants from published studies comparing formoterol and placebo but only 80% of those comparing formoterol with salbutamol or terbutaline.
Three deaths occurred on regular formoterol and none on placebo; this difference was not statistically significant. It was not possible to assess disease‐specific mortality in view of the small number of deaths. Non‐fatal serious adverse events were significantly increased when regular formoterol was compared with placebo (Peto odds ratio (OR) 1.57; 95% CI 1.06 to 2.31). One extra serious adverse event occurred over 16 weeks for every 149 people treated with regular formoterol (95% CI 66 to 1407 people). The increase was larger in children than in adults, but the impact of age was not statistically significant. Data submitted to the FDA indicate that the increase in asthma‐related serious adverse events remained significant in patients taking regular formoterol who were also on inhaled corticosteroids.
No significant increase in fatal or non‐fatal serious adverse events was found when regular formoterol was compared with regular salbutamol or terbutaline.
Authors' conclusions
In comparison with placebo, we have found an increased risk of serious adverse events with regular formoterol, and this does not appear to be abolished in patients taking inhaled corticosteroids. The effect on serious adverse events of regular formoterol in children was greater than the effect in adults, but the difference between age groups was not significant.
Data on all‐cause serious adverse events should be more fully reported in journal articles, and not combined with all severities of adverse events or limited to those events that are thought by the investigator to be drug‐related.
Keywords: Adult, Child, Humans, Adrenergic beta‐Agonists, Adrenergic beta‐Agonists/adverse effects, Adrenergic beta‐Agonists/therapeutic use, Age Factors, Albuterol, Albuterol/therapeutic use, Asthma, Asthma/drug therapy, Asthma/mortality, Bronchodilator Agents, Bronchodilator Agents/adverse effects, Bronchodilator Agents/therapeutic use, Chronic Disease, Ethanolamines, Ethanolamines/adverse effects, Ethanolamines/therapeutic use, Formoterol Fumarate, Terbutaline, Terbutaline/therapeutic use
Plain language summary
Does daily treatment with formoterol result in more serious adverse events compared to placebo or daily salbutamol?
Asthma is a common condition that affects the airways – the small tubes that carry air in and out of the lungs. When a person with asthma comes into contact with an irritant (an asthma trigger), the muscles around the walls of the airways tighten, the airways become narrower, and the lining of the airways becomes inflamed and starts to swell. This leads to the symptoms of asthma ‐ wheezing, coughing and difficulty in breathing. They can lead to an asthma attack or exacerbation. People can have underlying inflammation in their lungs and sticky mucus or phlegm may build up, which can further narrow the airways. There is no cure for asthma; however there are medications that allow most people to control their asthma so they can get on with daily life.
Long‐acting beta2‐agonists, such as formoterol, work by reversing the narrowing of the airways that occurs during an asthma attack. These drugs ‐ taken by inhaler ‐ are known to improve lung function, symptoms, quality of life and reduce the number of asthma attacks. However, there are concerns about the safety of long‐acting beta2‐agonists, particularly in people who are not taking inhaled corticosteroids to control the underlying inflammation. We did this review to take a closer look at the safety of people taking formoterol daily compared to people on placebo or the short acting beta2‐agonist salbutamol.
There was no statistically significant difference in the number of people who died during treatment with formoterol compared with placebo or salbutamol. Because so few people die of asthma, huge trials or observational studies are normally required to detect a difference in death rates from asthma. There were more non‐fatal serious adverse events in people taking formoterol compared to those on placebo; for every 149 people treated with formoterol for 16 weeks, one extra non‐fatal event occurred in comparison with placebo. There was no significant difference in serious adverse events in people on formoterol compared to regular salbutamol.
We conclude that regular formoterol should not be taken by people who are not taking regular inhaled steroids due to the increased risk of serious adverse events. Formoterol should not be used as a substitute for inhaled corticosteroids, and adherence with inhaled steroids should be kept under review if separate inhalers are used when formoterol is added to inhaled corticosteroids.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ SAE all ages.
| Regular formoterol versus placebo or salbutamol for chronic asthma: SAEs in adults and children | ||||||
|
Patient or population: patients with chronic asthma Settings: community Intervention: regular formoterol versus placebo or salbutamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular formoterol versus placebo or salbutamol | |||||
| SAEs ‐ formoterol versus placebo (follow‐up: mean 16 weeks) | Medium‐risk population | OR 1.57 (1.05 to 2.37) | 6646 (19) | ⊕⊕⊕⊕ high | ||
| 10 per 1000 | 16 per 1000 (10 to 23) | |||||
| SAEs ‐ formoterol versus salbutamol (follow‐up: mean 13 weeks) | Medium‐risk population | OR 0.72 (0.37 to 1.43) | 2119 (9) | ⊕⊕⊕⊝ moderate1 | ||
| 23 per 1000 | 17 per 1000 (9 to 33) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SAE: serious adverse event | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confidence interval includes the possibility of harm or benefit
Summary of findings 2. Summary of findings ‐ mortality (all‐cause).
| Regular formoterol versus placebo or salbutamol for chronic asthma | ||||||
|
Patient or population: patients with chronic asthma Settings: community Intervention: regular formoterol Comparison: placebo or salbutamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or salbutamol | Regular formoterol | |||||
| Mortality (all‐cause) ‐ formoterol versus placebo (follow‐up: mean 16 weeks) | Medium‐risk population | OR 1.52 (0.24 to 9.71) | 5463 (14) | ⊕⊕⊕⊝ moderate1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Mortality (all‐cause) ‐ formoterol versus salbutamol (follow‐up: mean 13 weeks) | Medium‐risk population | OR 0.5 (0.03 to 8.05) | 1418 (6) | ⊕⊕⊕⊝ moderate1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not enough data to assess this outcome
Summary of findings 3. Summary of findings ‐ SAE children.
| Regular formoterol versus placebo or salbutamol for children with chronic asthma: serious adverse events | ||||||
|
Patient or population: patients with chronic asthma (children) Settings: community Intervention: regular formoterol versus placebo or salbutamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular formoterol versus placebo or salbutamol | |||||
| SAEs ‐ formoterol versus placebo (follow‐up: mean 23 weeks) | Medium‐risk population | OR 2.82 (1.16 to 6.83) | 1335 (5) | ⊕⊕⊕⊕ high | ||
| 12 per 1000 | 33 per 1000 (14 to 77) | |||||
| SAEs ‐ formoterol versus salbutamol | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SAE: serious adverse event | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
There is currently no universally accepted definition of the term 'asthma'. This is in part due to an overlap of asthmatic symptoms with those of other diseases such as chronic obstructive pulmonary disease (COPD), but is also due to the probable existence of more than one underlying pathophysiological process. There are, for example, wide variations in the age of onset, symptoms, triggers, associations with allergic disease and the type of inflammatory cell infiltrate seen in patients diagnosed with severe asthma (Miranda 2004). Patients with all forms and severity of disease will typically have intermittent symptoms of cough, wheeze and/or breathlessness. Underlying these symptoms there is a process of variable, at least partially reversible airway obstruction, airway hyper responsiveness and, in most cases, chronic inflammation.
Airway obstruction
Patients with a history of asthma demonstrate chronic changes within the airways including goblet cell hyperplasia, airway smooth muscle (ASM) hyperplasia and hypertrophy (Ebina 1993; Ordonez 2001; Woodruff 2004) and excess myofibroblasts with increased subepithelial collagen deposition (Brewster 1990). In the acute setting, in patients who have died of status asthmaticus, airway obstruction is evident from air‐trapping and lung hyperinflation with mucus plugging of the small and large airways (Dunnill 1960; Kuyper 2003). There is also shedding of ciliated bronchial mucosal cells, inflammatory cell infiltrates and submucosal oedema with transudation of fluid into the bronchial lumen (Carroll 1993). It is more difficult to measure the degree of ASM contraction (bronchoconstriction) in post‐mortem studies although evidence for a role of bronchoconstriction in airway narrowing comes from other sources.
Airway hyper responsiveness
Patients with asthma typically display a degree of 'airway hyper responsiveness' to inhaled allergens (Cockcroft 2006), and to a variety of chemical stimuli including histamine, serotonin, bradykinin, prostaglandins, methacholine and acetylcholine as well as other triggers such as exercise, deep inhalation and inhalation of cold air (Boushey 1980). Bronchoconstriction is implicated as the primary effector mechanism of airway narrowing in these responses. This is because of both the short time frame of the response and because many of these stimuli typically either cause bronchoconstriction directly in vitro or promote bronchoconstriction through interference with the autonomic control of ASM. Further evidence comes from findings that this response can be abolished or diminished by bronchodilator medications such as atropine and beta2‐agonists (Phillips 1990; Simonsson 1967); although beta2‐agonists in particular may have additional mechanisms of action. Whether airway hyper responsiveness relates primarily to an abnormality of ASM, to increased ASM bulk (Wiggs 1990), to aberrant autonomic control or reflex pathways, or to physical damage to the airway epithelium remains to be established. Regular use of salbutamol has, however, been shown to increase airway hyper responsiveness to allergen exposure and produce tolerance to the protective effect of salbutamol against bronchoconstriction induced by both methacholine and allergens (Cockcroft 1993).
Inflammation
It has long been thought that the histological changes described above and the phenomenon of airway hyper responsiveness are due to a combined acute and chronic inflammatory response (Bousquet 2000). Patients with status asthmaticus have increased numbers of inflammatory cells including eosinophils and neutrophils, as well as a variety of pro‐inflammatory cytokines and chemokines found in bronchial alveolar lavage (Tonnel 2001). In patients with chronic asthma there is also evidence of increased eosinophil numbers (Bousquet 1990), inflammatory cell adhesion molecules (Vignola 1993) and some evidence of an association between the extent of inflammation, disease severity and hyperreactivity. This association has however been questioned on the background of a number of negative results (Brusasco 1998), although it is made difficult to prove by the lack of a consistent marker of a sequential and variable inflammatory response (Haley 1998).
Description of the intervention
Beta2‐agonists and mortality: an historical perspective
Time trend data and case‐control studies
Adrenaline was successfully used in the symptomatic treatment of asthma as far back as 1903 (Tattersfield 2006). Initially given subcutaneously, the inhaled route was tried in 1929 to reduce adverse effects but these remained a problem and in 1940 details of a new agent, isoprenaline (isoproterenol), were published in Germany (Konzett 1940). Although isoprenaline was more selective for beta‐ as opposed to alpha‐adrenoreceptors, adverse effects including palpitations were still a major problem, particularly with oral administration (Gay 1949) and it first became available as atomiser spray for use in the UK in 1948 (Pearce 2001).
Prior to the 1940s, mortality rates from asthma in a number of countries were stable and low at less than 1 asthma death per 100,000 people per year (Pearce 2001; Figure 1). During the 1940s and 50s there was a slight rise in mortality rates and concerns about a possible link to inhaled adrenaline were raised at an early stage (Benson 1948). However, the rise was small and the cause unclear and sales continued to increase with the introduction of aerosol or metered‐dose inhalers in the early 1960s. During this decade there was an epidemic of asthma deaths in at least six countries including England, Wales and New Zealand (Figure 1). In all six countries the epidemics coincided with the licensing of an aerosol called 'Isoprenaline Forte', which contained five times the dose of isoprenaline per administration than the standard preparation (Stolley 1972). In other countries including the Netherlands, where isoprenaline forte was introduced late and sales volumes low, and in the US, where isoprenaline forte was not licensed, no increase in asthma mortality occurred. This was despite an approximate trebling in per capita alternative bronchodilator sales between 1962 and 1968 in the US (Stolley 1972). A detailed review of the epidemic in England and Wales concluded it was not due to changes in death certification, disease classification or an increase in asthma prevalence, but instead was most likely due to new methods of treatment (Speizer 1968). In England and Wales mortality rates fell following health warnings about the overuse of inhalers and banning of over the counter sales in 1968. It was around this time that more selective beta2‐agonists such as terbutaline (Bergman 1969) and salbutamol (albuterol) (Cullum 1969) were being developed.
1.

Changes in asthma mortality (5 to 34 age group) in three countries in relation to the introduction of isoprenaline forte in the UK and New Zealand and of fenoterol in New Zealand. (From Blauw 1995. With permission from the Lancet).
In the late 1970s a second epidemic of asthma deaths occurred in New Zealand (Figure 1). It was later shown that this epidemic coincided with the introduction and rising sales of fenoterol, a new short‐acting beta2‐agonist (Crane 1989; Figure 2). A significant association between mortality and fenoterol use was demonstrated in three consecutive case‐control studies, the latter studies addressing criticisms of the first (Crane 1989; Grainger 1991; Pearce 1990). Furthermore the relative risk of asthma death in patients prescribed fenoterol increased markedly when analysis was restricted to subgroups defined by markers of severity, including previous hospital admission and use of oral corticosteroids. Following the publication of the first case‐control study, the fenoterol market share in New Zealand fell from 30% in 1988 to 3% in 1991 and by the early 1990s the mortality epidemic appeared to be over (Figure 2). During the gradual decline in mortality in New Zealand from its peak in 1979, total sales of alternative beta2‐agonists, including salbutamol, gradually rose and the use of inhaled corticosteroids also increased during the latter half of the 1980s (Pearce 2007).
2.

Inhaled fenoterol market share and annual asthma mortality in New Zealand in persons aged 5 to 34
The introduction of long‐acting beta2‐agonists
Given the relatively short action of beta2‐agonists such as salbutamol, in the late 1980s efforts were made to develop longer‐acting compounds. Subsequently the long‐acting beta2‐agonists (LABAs), salmeterol and formoterol were released by GlaxoSmithKline (GSK) and Novartis, respectively. Both drugs cause bronchodilation that lasts for more than 12 hours although formoterol has a faster onset of action (Kemp 1993; Ringdal 1998). Given previous concerns about the safety profile of some of the short acting beta2‐agonists, salmeterol and formoterol were subject to randomised controlled trials on larger numbers of patients. Using these trials several Cochrane reviews have addressed the efficacy of LABAs in addition to inhaled corticosteroids (Ni Chroinin 2004; Ni Chroinin 2005), in comparison with placebo (Walters 2007), short‐acting beta2‐agonists (Walters 2002), leukotriene‐receptor antagonists (Ducharme 2006), and increased doses of inhaled corticosteroids (Greenstone 2005). The beneficial effects of LABAs on lung function, symptoms, quality of life and exacerbations requiring oral steroids have been demonstrated. However, with some studies demonstrating an associated increase in mortality concerns about the safety profile of LABAs have heightened and there has been much debate about the potential protective role of inhaled corticosteroids.
How the intervention might work
We have outlined the pharmacology of beta2‐agonists in detail in Appendix 1. Since the early epidemics in asthma mortality, a number of potential mechanisms have been proposed to explain a relationship to the use of beta2‐agonists. We discuss these mechanisms in detail in Appendix 2; they include direct toxicity, tolerance, delay in seeking help and reduction in use of inhaled corticosteroids.
Why it is important to do this review
We have taken a different approach from Salpeter 2006, in that we have not assumed a class effect of long‐acting beta2‐agonists, but we have considered trials comparing regular formoterol to placebo or regular salbutamol/terbutaline. We have chosen not to include results from trials on salmeterol in this review, as there are known differences in the pharmacological properties of salmeterol and formoterol (Ringdal 1998; Van Noord 1996). This review forms part of a set of reviews on the safety of regular salmeterol and formoterol that has now been published (Cates 2008; Cates 2009; Cates 2009a; Cates 2011). We have also excluded studies which randomised participants to formoterol and inhaled corticosteroids for this review, as these are considered in another review (Cates 2009a).
In view of the difficulty in ascertaining the causation of deaths and serious adverse events (SAEs), we have considered all‐cause fatal and non‐fatal SAEs as the main outcomes of this review, with asthma‐related and cardiovascular events as secondary outcomes.
Objectives
To assess the risk of mortality and non‐fatal serious adverse events in trials which randomise patients with chronic asthma to formoterol alone
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials (RCTs) of parallel design, with or without blinding, in which formoterol alone was randomly assigned to patients with chronic asthma. We excluded studies on acute asthma and exercise‐induced bronchospasm.
Types of participants
We included patients with a clinical diagnosis of asthma of any age group, unrestricted by disease severity, previous or current treatment.
Types of interventions
We included trials that randomised patients to receive inhaled formoterol twice daily for a period of at least 12 weeks, at any dose and delivered by any device (metered‐dose inhalers (MDIs) with chlorofluorocarbons (CFCs) or hydrofluoroalkane (HFAs), or dry powder inhalers (DPIs)). We included studies that used comparison groups with placebo or short‐acting beta2‐agonists, and co‐intervention with leukotriene receptor antagonists, inhaled or oral corticosteroids or theophylline was allowed as long as they are not part of the randomised intervention. We excluded studies that randomised patients to formoterol for intermittent use as a reliever, and studies that compared different doses of formoterol, or different delivery devices or propellants (with no placebo arm). We also excluded studies in which formoterol was randomised together with an inhaled steroid (in separate inhalers or a combined inhaler) from this review; however these are considered in a separate review (Cates 2009a).
Types of outcome measures
Primary outcomes
All‐cause mortality
All‐cause non‐fatal serious adverse events
Secondary outcomes
Asthma‐related mortality
Asthma‐related non‐fatal serious adverse events
Respiratory‐related mortality
Respiratory‐related non‐fatal serious adverse events
Cardiovascular‐related mortality
Cardiovascular‐related non‐fatal serious adverse events
Asthma‐related non‐fatal life‐threatening events (intubation or admission to intensive care)
Respiratory‐related non‐fatal life‐threatening events (intubation or admission to intensive care)
We did not restrict outcomes to those that the trial investigators considered to be related to trial medication.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 3 for details). All records in the Specialised Register coded as 'asthma' were last searched in January 2012 using the following terms:
(((beta* and agonist*) and (long‐acting or "long acting")) or ((beta* and adrenergic*) and (long‐acting or "long acting")) or (bronchodilat* and (long‐acting or "long acting")) or (salmeterol or formoterol or eformoterol or Advair or Symbicort or serevent or Seretide or Oxis)) AND (serious or safety or surveillance or mortality or death or intubat* or adverse or toxicity or complications or tolerability)
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We also checked websites of clinical trial registers for unpublished trial data and FDA submissions in relation to formoterol.
Data collection and analysis
Selection of studies
Both authors (CJC, MJC) independently assessed studies identified in the literature searches by examining titles, abstract and keywords fields. We obtained studies that potentially fulfilled the inclusion criteria in full text. We independently assessed these full‐text trial reports for inclusion. We resolved disagreements by consensus. We kept a record of decisions.
Data extraction and management
We extracted data using a prepared checklist before entering into Rev Man 5.0. We entered data on characteristics of included studies (methods, participants, interventions, outcomes) and results of the included studies. We contacted authors or manufacturers if serious adverse event data were not included in the trial report, and searched manufacturers' and FDA websites for further details of adverse events.
Assessment of risk of bias in included studies
Both authors assessed the included studies for bias protection (including sequence generation for randomisation, allocation concealment, blinding of participants and assessors, loss to follow‐up, completeness of outcome assessment and other possible bias prevention). We resolved disagreements by consensus.
Measures of treatment effect
We recorded the number of participants with a serious adverse event of any cause (fatal and non‐fatal), and in view of the difficulty in deciding whether events are asthma‐related, we noted details of the cause of death and serious adverse events where they were available. We noted the definition of serious adverse events, and sought further information if this was not clear (particularly in relation to hospital admissions and serious adverse events).
Unit of analysis issues
We extracted data using the number of participants who suffered one or more serious adverse events, in order to avoid double‐counting events from the same participant.
Assessment of heterogeneity
We assessed heterogeneity in the pooled odds ratio using the I2 statistic in RevMan 5.0.
Assessment of reporting biases
We assessed reporting bias by comparing the published and unpublished serious adverse events, and by comparing all serious adverse events with those that were thought to be drug‐related. We also compared serious events with all adverse events (whether serious or not).
Data synthesis
The outcomes of this review were dichotomous and we recorded the number of participants with each outcome event, by allocated treated group. We planned to analyse mortality using risk difference, as many studies did not have any deaths in either arm. However, the recently revised Cochrane Handbook for Systematic Reviews of Interventions advises against this approach, so we only used the risk differences to estimate the absolute impact of treatment (Higgins 2008). Although meta‐analysis with Peto odds ratio has advantages when events are rare (Bradburn 2007), it performs less well with unbalanced treatment arms and large effect sizes, and therefore we calculated the results for serious adverse events and mortality in RevMan 5.0 as pooled odds ratios using the Mantel‐Haenszel (MH) fixed‐effect model. We compared the results of this model to the Peto method and MH random‐effects models (as sensitivity analysis), although Bradburn 2007 cautions against the random‐effects model as the variance calculations are based on large sample assumptions. We explored heterogeneity on the basis of the subgroup and sensitivity analyses outlined below. We inspected funnel plots to assess publication bias.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses on the primary outcomes on the basis of dose of formoterol (usual dose versus high dose), age (adults versus children) and comparator used. We planned to carry out subgroup analysis based on reported corticosteroid use, but none of the studies reported whether the patients who actually suffered serious adverse events were taking inhaled corticosteroids or not. We compared subgroups using tests for interaction (Altman 2003).
The definition of serious adverse events was rarely reported in the trials, but there is a standard definition used by industry in clinical study reports (ICHE2a 1995) and this is listed in Appendix 4.
Sensitivity analysis
We checked the overall results for the primary outcomes to assess the impact of removing the results from unblinded studies, and also those participants that were randomised to high‐dose formoterol (24 µg twice daily). We also checked the impact of using fixed or random‐effects models for meta‐analysis.
Results
Description of studies
Results of the search
See Figure 3 for the study flow diagram. We found 512 abstracts from the initial search in October 2007 (reduced to 504 after removing duplicates). We identified 128 relevant abstracts that related to formoterol alone. We included 22 studies (32 references) in the review and excluded 95 others (see Excluded studies). Of those excluded 53 were less than 12 weeks in duration, (including 29 single‐dose studies), three were not RCTs, eight were dose or device comparison studies, nine were on exercise‐induced bronchospasm or acute asthma, nine used formoterol as reliever medication, eight were cross‐over in design, three randomised to formoterol and budesonide and five were excluded for other reasons. One study that was included in Salpeter 2006 was excluded from this review because formoterol was randomised with budesonide (Price 2002) and therefore did not fit the inclusion criteria for this review. Both authors reached consensus on all the included studies after inspection of the full text from papers and websites.
3.

Study flow diagram.
We repeated the search in July 2008, when we found 48 further abstracts. Six were relevant to this review but we excluded five as they were short‐term studies, and one was on exercise‐induced bronchospasm; we found no new studies meeting the inclusion criteria. Similarly a further search in January 2012 (cumulative total of 748 citations) identified three potentially relevant studies (Happonen 2009; Kamenov 2007; Price 2008). On checking the full text, none of these yielded new trials that were suitable for inclusion (see Excluded studies).
We identified one additional included study from other reviews; this was a trial on the AstraZeneca controlled trials register (SD‐037‐0344) which is otherwise unpublished. Additionally two documents on the FDA website provided additional serious adverse event information for four of the included studies (Bensch 2001; Bensch 2002; Pleskow 2003; Wolfe 2006). Additionally 22 studies of at least four weeks duration are listed in the appendix to a Novartis FDA submission (NovartisNDA20‐831 2005), but it is not clear how these relate to the 10 included studies that we found which were supported by Novartis.
Included studies
We included 22 studies on 8032 participants (6693 adults and 1339 children); the characteristics of these studies are fully described in Characteristics of included studies. Sponsorship of the studies is also listed in Table 4.
1. Study sponsors.
| Study ID | Sponsor |
| Bensch 2001 | Novartis |
| Bensch 2002 | Novartis |
| Busse 2004 | Novartis |
| Corren 2007 | AstraZeneca |
| Ekstrom 1998 | AstraZeneca |
| Ekstrom 1998a | AstraZeneca |
| FitzGerald 1999 | Novartis |
| Hekking 1990 | Not reported |
| Kesten 1991 | Novartis |
| Kozlik‐Feldmann 1996 | Not reported |
| LaForce 2005 | Novartis |
| Levy 2005 | Novartis |
| Molimard 2001 | Novartis |
| Noonan 2006 | AstraZeneca |
| Pleskow 2003 | Novartis |
| SD‐037‐0344 | AstraZeneca |
| Steffensen 1995 | Novartis |
| van der Molen 1997 | AstraZeneca |
| van Schayck 2002 | Not reported |
| Von Berg 2003 | AstraZeneca |
| Wolfe 2006 | Novartis |
| Zimmerman 2004 | AstraZeneca |
Eight studies enrolled adults over 18 years of age (Ekstrom 1998; Ekstrom 1998a; FitzGerald 1999; Hekking 1990; Kesten 1991; Molimard 2001; Steffensen 1995; van der Molen 1997), one study adults over 16 years of age (van Schayck 2002), eight studies adults and adolescents over 12 years of age (Bensch 2001; Busse 2004; Corren 2007; LaForce 2005; Noonan 2006; Pleskow 2003; SD‐037‐0344; Wolfe 2006) and five enrolled children from five up to 12 or 16 years of age (Bensch 2002; Kozlik‐Feldmann 1996; Levy 2005; Von Berg 2003; Zimmerman 2004).
All of the studies were of 12 weeks duration with the exception of Bensch 2002 (52 weeks), FitzGerald 1999 (24 weeks), van der Molen 1997 (24 weeks) and Wolfe 2006 (16 weeks). This gives a weighted mean duration of 16 weeks for the 19 studies with a placebo arm.
Since many of the studies randomised patients to more than two treatment arms, we have reported the overall number of patients randomised to treatment categories under investigation. Adults and adolescents are combined, as separate data for adolescents were not provided in any of the studies. In total 8032 participants were randomised (6693 adults and 1339 children); of these 2146 adults and 483 children received placebo, 2483 adults and 568 children received formoterol up to 12 µg twice daily, 921 adults and 277 children received formoterol 24 µg twice daily, and 1143 adults and 11 children received regular salbutamol or terbutaline. Although Molimard 2001 was an open study that did not use a placebo group, we included it in the placebo comparisons as on‐demand salbutamol was used by patients in both the formoterol and the comparison arm.
Doses of formoterol ranged from 4.5 µg to 24 µg twice daily in children and 6 µg to 24 µg twice daily in adults (but different delivery devices mean that the delivered dose may not be identical between studies, even if the nominal dose is the same). We have considered high‐dose formoterol to be 24 µg twice daily.
Concurrent use of inhaled corticosteroids varied in the included studies from zero to 100%; Table 5 lists the inhaled steroid use by study. In almost all the studies at least half of the patients were taking inhaled corticosteroids at baseline.
2. Proportion of participants using inhaled corticosteroids (ICS).
| Study ID | Proportion of participants on ICS |
| Bensch 2001 | 51% |
| Bensch 2002 | 69% |
| Busse 2004 | 64% |
| Corren 2007 | 0% (withdrawn) |
| Ekstrom 1998 | 86% |
| Ekstrom 1998a | 89% |
| FitzGerald 1999 | 100% |
| Hekking 1990 | Not reported |
| Kesten 1991 | 62% |
| Kozlik‐Feldmann 1996 | 0% |
| LaForce 2005 | 67% |
| Levy 2005 | 72% |
| Molimard 2001 | 100% |
| Noonan 2006 | 100% |
| Pleskow 2003 | 44% |
| SD‐037‐0344 | 100% |
| Steffensen 1995 | 87% |
| van der Molen 1997 | 100% |
| van Schayck 2002 | 95% |
| Von Berg 2003 | 82% |
| Wolfe 2006 | 62% |
| Zimmerman 2004 | 100% |
Risk of bias in included studies
Figure 4 show a graphical representation of the domains of risk of bias across each study.
4.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Allocation concealment and sequence generation were often poorly reported in the included studies. However, the majority of the studies are supported by manufacturers of formoterol (see Table 4) and are therefore likely to have had appropriate protection against selection bias.
Blinding
All studies were double‐blind except for Kozlik‐Feldmann 1996, Molimard 2001 and van Schayck 2002, and of these only Molimard 2001 has contributed data to the primary outcomes.
Incomplete outcome data
The included studies generally had withdrawal rates of less than 20% with the exception of Corren 2007 (51% dropout in the placebo group), and Noonan 2006 (60% withdrawals in placebo arm and 51.2% in formoterol arm).
Selective reporting
Although paper reports of studies often did not include usable information on all‐cause serious adverse events, it has proved possible to obtain serious adverse events information from 19 of the 22 included studies. This represents 100% of participants randomised to formoterol or placebo, but only 80% of participants compared to regular salbutamol or terbutaline. There are clear guidelines for the reporting of serious adverse events for industry (ICHE3 2007). These all have to be listed in detail for the regulatory authorities, but there is clearly no similar expectation from medical journals.
Twenty‐two studies of at least four weeks duration are listed in the appendix to a Novartis FDA submission (NovartisNDA20‐831 2005), but it is not clear how many of these would fulfil the criteria for inclusion in this review, or how they related to the 10 Novartis studies included in this review (Table 4). It has not been possible to obtain further information from the sponsors.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcomes
All‐cause mortality
Events were sparse in the trials and the presence or absence of mortality was not always reported in the paper publications.
Formoterol versus placebo
Data were available from 14 studies comparing formoterol (N = 3413) with placebo (N = 2050); this represents 80% of the randomised patients for this comparison. Three deaths occurred in these trials (two adults and one child), and overall there were three deaths on formoterol and none on placebo. The cause of death for one adult was not reported in SD‐037‐0344, there was one adult death from asthma in Pleskow 2003, and one child died of a subarachnoid haemorrhage in Von Berg 2003. The pooled odds ratio (OR) was not statistically significant (Peto OR 4.50; 95% confidence interval (CI) 0.41 to 49.49), see Figure 5. The confidence interval and point estimate are somewhat different for a Mantel‐Haenszel OR 1.52 (95% CI 0.24 to 9.71) using fixed or random‐effects models. This discrepancy is due to the continuity correction required for zero cells in the Mantel‐Haenszel method. The point estimate of the pooled risk difference (RD) was an increase of 4 deaths per 10,000 treated with regular formoterol over 16 weeks, with a confidence interval from 20 fewer deaths to 28 more deaths per 10,000. There was no statistical heterogeneity in this outcome.
5.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 Overall results.
Formoterol versus salbutamol
Data were available from six studies comparing formoterol (N = 847) to salbutamol/albuterol (N = 571), representing 49% of the randomised patients for this comparison. Only two deaths occurred, both in Pleskow 2003; one in the formoterol arm (from asthma as reported above) and one in the salbutamol arm (from pancreatitis), and again the difference was not statistically significant. As only one study contributed to this outcome, we calculated no pooled OR.
Subgroup analyses
No subgroup analyses were possible for all‐cause mortality as the data were too sparse.
Serious adverse events (SAEs) (non‐fatal all‐cause)
An example of the definition of a serious adverse event (SAE) is given in Pleskow 2003: “A serious adverse event was defined as any experience that was fatal or life‐threatening, permanently disabling, requiring in‐patient or prolonged hospitalisation, or was a congenital abnormality, cancer or drug overdose.” This is in line with the definition in Appendix 4, and we have assumed that this definition was used in the other trials (even though this was often not made explicit). In most cases the events were defined as serious because they were associated with hospital admission.
Formoterol versus placebo
Combined data from adults and children
Data were available on non‐fatal SAEs for 19 studies comparing regular formoterol (N = 4017) to placebo (N = 2629); this represents all of the randomised patients from published studies for this comparison. The studies were largely on adults (N = 5311), but five trials were in children (N = 1335).
The overall result indicated an increased risk of SAEs with formoterol (Peto OR 1.57; 95% CI 1.06 to 2.31) with low heterogeneity (I2 = 0%), Figure 6. SAEs were rare in the studies (occurring in 1.2% of patients on placebo over a weighted mean of 16 weeks), so the pooled risk difference is small at 0.007 (95% CI 0.0012 to 0.013) and using a weighted mean of the placebo arms, over a 16‐week period there would need to be 149 patients treated (95% CI 66 to 1407) for one extra serious adverse event to occur; this number needed to treat was calculated by Visual Rx using the pooled odds ratio and baseline risk of 1%. This is illustrated in Figure 7 which shows that for every thousand patients treated over 16 weeks with formoterol there are an extra six patients who will suffer a serious adverse event, so that in comparison to 10 per thousand in the placebo group this rises to 16 per thousand on regular formoterol.
6.

Forest plot of comparison: 2 Adults and children non‐fatal serious adverse events, outcome: 2.1 Formoterol versus placebo or salbutamol.
7.

Serious adverse events with regular formoterol compared to placebo. In the control group 12 people out of 1000 had serious adverse events over 16 weeks, compared to 19 (95% CI 13 to 27) out of 1000 for the active treatment group.
Sensitivity analysis by applying the Mantel‐Haenszel method gave a very similar result, OR 1.57 (95% CI 1.05 to 2.37). Using a random‐effects model (which is not recommended for rare events due to an increased risk of bias (Bradburn 2007)), the point estimate was similar but the confidence interval widened: OR 1.52 (95% CI 0.96 to 2.40). When we excluded the participants receiving high‐dose formoterol (24 µg) arms from the analysis the confidence interval again widened (Peto OR 1.55; 95% CI 0.97 to 2.48)(Analysis 3.1).
3.1. Analysis.

Comparison 3 Adults and children non‐fatal serious adverse events (without formoterol 24 µg arms), Outcome 1 Formoterol versus placebo or salbutamol.
Adults with non‐fatal serious adverse events
When the adult data comparing regular formoterol (N = 3170) with placebo (N = 2137) were considered on their own, the results showed a smaller increase in risk which did not reach statistical significance (Peto OR 1.23; 95% CI 0.76 to 1.99)(Analysis 4.1). The Mantel‐Haenszel method gave very similar results, OR 1.22 (95% CI 0.76 to 1.96).
4.1. Analysis.

Comparison 4 Adults with non‐fatal serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Children with non‐fatal serious adverse events
Although fewer children were studied, regular formoterol (N = 843) compared with placebo (N = 492), the separate results for children showed a larger increase in serious adverse events with regular formoterol (Peto OR 2.48; 95% CI 1.27 to 4.83)(Analysis 5.1). Again the Mantel‐Haenszel method gave a very similar result, OR 2.92 (95% CI 1.26 to 6.74). There was low heterogeneity in this outcome (I2 = 0%), and the result in children remained significant when the data on high‐dose formoterol were excluded (Peto OR 2.52; 95% CI 1.15 to 5.51) and Mantel‐Haenszel OR 2.59 (95% CI 1.08 to 6.19)(Analysis 6.1).
5.1. Analysis.

Comparison 5 Children with non‐fatal serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
6.1. Analysis.
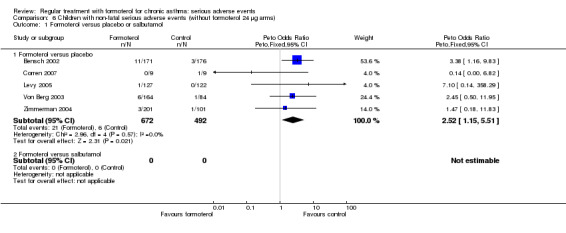
Comparison 6 Children with non‐fatal serious adverse events (without formoterol 24 µg arms), Outcome 1 Formoterol versus placebo or salbutamol.
When the results in children are compared with adults using a test for interaction (Altman 2003), the increased risk in children relative to adults is a relative OR of 2.39 (95% CI 0.91 to 6.27) using the Mantel‐Haenszel odds ratio, which is not statistically significant. The results are very similar when the Peto OR is compared between adults and children (relative OR 2.02; 95% CI 0.89 to 4.59).
High‐dose formoterol versus lower doses
When the study arms using formoterol 24 µg twice daily were compared to those using lower doses (formoterol 12 µg twice daily), no significant difference was found in adults (Peto OR 1.35; 95% CI 0.64 to 2.85)(Analysis 7.1). The confidence interval for this result is too wide to rule out a difference in relation to dose, and although the three adult studies all used the same dry powder delivery device (Aerolizer), there is a high level of heterogeneity between studies (I2 = 74%), which is unexplained.
7.1. Analysis.

Comparison 7 Dose comparison: formoterol 24 µg versus 12 µg twice daily, Outcome 1 Serious adverse events.
Moreover the data from the Novartis database of published and unpublished placebo‐controlled studies of at least four weeks duration has been included for comparison (Novartis 2005); the Novartis data show a significantly higher risk of asthma‐related serious adverse events with formoterol 24 µg twice daily in comparison to 12 µg twice daily (Peto OR 2.16; 95% CI 1.13 to 4.11) and Mantel‐Haenszel OR 2.08 (95% CI 1.11 to 3.89).
Formoterol versus salbutamol
Adults with non‐fatal serious adverse events
In contrast, the results from nine studies in adults comparing regular formoterol (N = 1119) to salbutamol (N = 920), representing 80% of the randomised patients for this comparison, showed a non‐significant reduction in the risk of SAEs (Peto OR 0.73; 95% CI 0.37 to 1.43)(Analysis 4.1). However, when the results from patients using high‐dose formoterol were excluded, there was a significant reduction in risk for formoterol in comparison with salbutamol or terbutaline (Peto OR 0.41; 95% CI 0.19 to 0.90) and Mantel‐Haenszel OR 0.40 (95% CI 0.17 to 0.94) (Analysis 3.1). A test for interaction between the results comparing formoterol with placebo and salbutamol was not significant; the risk in trials against regular salbutamol compared to those against placebo using Mantel‐Haenszel OR gives a relative OR of 0.46 (95% CI 0.21 to 1.01), whilst for the Peto method the relative OR is 0.59 (95% CI 0.26 to 1.36).
No studies were found comparing regular formoterol to regular salbutamol or terbutaline in children.
SAEs all‐cause (fatal and non‐fatal combined)
When fatal and non‐fatal SAEs are considered together the findings are very similar to those for the non‐fatal events, with a significant increase in risk with regular formoterol in comparison with placebo (Peto OR 1.61; 95% CI 1.09 to 2.37) and Mantel‐Haenszel OR 1.63 (95% CI 1.08 to 2.44), but not in comparison with regular salbutamol (see Analysis 8.1).
8.1. Analysis.

Comparison 8 Adults and children fatal and non‐fatal serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Secondary outcomes
Mortality by cause of death
Asthma mortality and cardiovascular mortality
Only one death related to asthma was reported using formoterol 24 µg in Pleskow 2003, and one death in Von Berg 2003 from sub‐arachnoid haemorrhage on formoterol 9 µg, so there are insufficient data to assess the impact of formoterol on disease‐specific mortality. The third death in SD‐037‐0344 has no reported cause.
SAEs related to asthma and the cardiovascular system
When formoterol is compared to placebo, there is a significant increase in asthma‐related serious adverse events on regular formoterol (Peto OR 1.99; 95% CI 1.12 to 3.53) and Mantel‐Haenszel OR 1.90 (95% CI 1.03 to 3.48)(Analysis 11.1). This finding is also apparent from the Novartis integrated database (Novartis 2005), which is concordant with the results of the 10 Novartis studies included in the review as shown in Analysis 12.1.
11.1. Analysis.
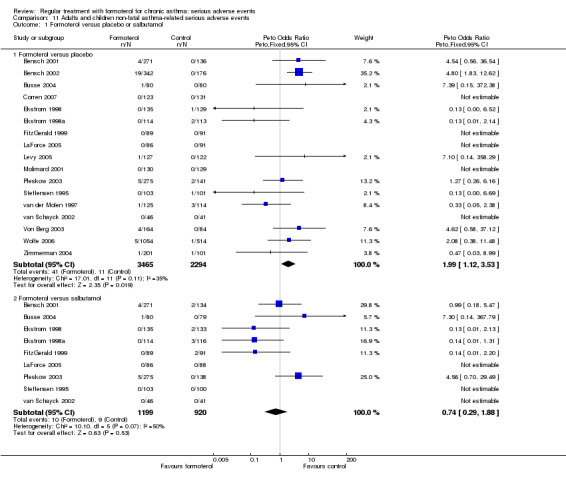
Comparison 11 Adults and children non‐fatal asthma‐related serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
12.1. Analysis.

Comparison 12 Adults and children non‐fatal asthma‐related serious adverse events (Novartis data), Outcome 1 Formoterol versus placebo or salbutamol.
In comparison with regular salbutamol there is a decrease in asthma‐related serious adverse events on regular formoterol but this is not significant (Peto OR 0.74; 95% CI 0.29 to 1.88) and Mantel‐Haenszel OR 0.72 (95% CI 0.32 to 1.76)(Analysis 11.1).
There is a significant increase in hospital admissions for asthma when regular formoterol is compared to placebo: Peto OR 3.28; 95% CI 1.65 to 6.52 and Mantel‐Haenszel OR 4.28; 95% CI 1.60 to 11.46 (Analysis 13.1). For this outcome we assumed that all the patients with asthma‐related SAE documented in the FDA submission were admitted to hospital; in Pleskow 2003 this is clearly stated to be the case for two patients on placebo and five on formoterol 24 µg, but is not clearly reported for the single patient on formoterol 12 µg (Mann 2003). No significant difference was seen when regular formoterol is compared to regular salbutamol.
13.1. Analysis.
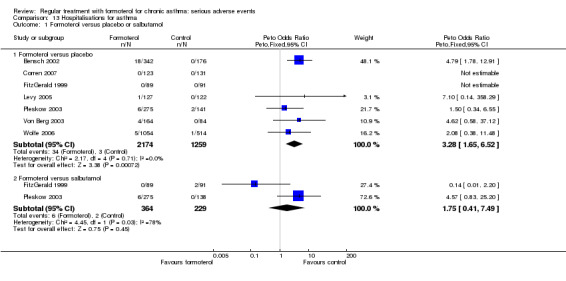
Comparison 13 Hospitalisations for asthma, Outcome 1 Formoterol versus placebo or salbutamol.
Very few events relating to the cardiovascular system were reported, so although the direction of effect is in favour of formoterol the confidence intervals in comparison to placebo and salbutamol are very wide (Analysis 14.1).
14.1. Analysis.

Comparison 14 Adults and children non‐fatal cardiovascular serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Impact of inhaled corticosteroids
It has not been possible to assess whether inhaled corticosteroids have an impact on SAEs with regular formoterol from the included studies, as this would require individual patient data relating inhaled corticosteroid usage to those patients who suffered the events, and these data are not available. However, data are presented in Novartis 2005 in which patients on inhaled corticosteroids are compared with those who are not, for asthma‐related serious adverse events. Although the increased risk is larger without inhaled corticosteroids (Peto OR 3.22; 95% CI 1.17 to 8.89) and Mantel Haenszel OR 7.31 (95% CI 0.97 to 55.06), than with inhaled corticosteroids (Peto OR 2.58; 95% CI 1.21 to 5.49) and Mantel‐Haenszel OR 3.47 (95% CI 1.21 to 9.97), the confidence intervals are wide and there is no statistically significant interaction between the increased risk and use of inhaled corticosteroids. Moreover there remains a significant three‐fold increase in odds for patients who were taking inhaled corticosteroids, and this is maintained when the data on formoterol 24 µg twice daily are excluded (Peto OR 2.76; 95% CI 1.06 to 7.15) and Mantel‐Haenszel OR 3.11 (95% CI 1.01 to 9.55)(Analysis 15.1).
15.1. Analysis.

Comparison 15 Impact of inhaled corticosteroids on asthma‐related SAEs, Outcome 1 Patients with at least one asthma‐related SAE.
Discussion
Summary of main results
Three deaths occurred on regular formoterol and none on placebo; this difference was not statistically significant. It was not possible to assess disease‐specific mortality in view of the small number of deaths.
Non‐fatal serious adverse events (SAEs) were significantly increased in comparison with placebo. These events were rare, occurring in 1.0% of patients in the placebo arms over an average of 16 weeks; in comparison 1.6% of those given regular formoterol suffered a SAE (Figure 7). One additional participant with a SAE was found to occur for every 149 people treated with regular formoterol over 16 weeks, and the play of chance is compatible with one extra event for every 66 to 1407 given regular formoterol. The majority of these extra events appear to be asthma‐related, and data from unpublished studies on the Novartis integrated database of placebo‐controlled trials suggest that there may be a significant increase in the risk of asthma‐related SAEs with regular formoterol even in those patients who were taking inhaled corticosteroids (Analysis 15.1).
The increased risk is larger in children than in adults, but the difference between the results in children and adults is not statistically significant. No significant overall differences were found for all‐cause mortality or non‐fatal SAEs in trials comparing regular formoterol with salbutamol or terbutaline.
Overall completeness and applicability of evidence
Although large numbers of participants have been treated with regular formoterol, the rarity of mortality and SAEs means that there is still considerable uncertainty in relation to the size of the effects being investigated. There is insufficient evidence to be sure whether the larger increase in SAEs found in children is significantly different from the smaller increase in adults. We have received additional information relating to eight studies from authors and manufacturers, but data have not been forthcoming for three of the included studies. The missing data represent a moderate proportion of the participants who are in trials comparing formoterol with salbutamol, but they could alter the point estimates and confidence intervals for this comparison. Whilst it has not been possible to obtain details of unpublished Novartis studies submitted to the FDA, the pooled data from the Novartis trials has been used in this review (but not combined with the included studies as there is an unknown degree of overlap).
Information from papers published in medical journals
All‐cause non‐fatal SAEs were published in the paper reports of studies for 31 patients on formoterol and seven patients on placebo; this less than half of the total number of events found from all sources. Considering only the data from paper reports there is still a significant increase with regular formoterol (Peto odds ratio (OR) 2.31; 95% confidence interval (CI) 1.17 to 4.53), see Analysis 16.1.
16.1. Analysis.

Comparison 16 Adults and children published non‐fatal serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Serious and non‐serious adverse events
All adverse events are shown in Analysis 17.1, and published adverse events in Analysis 18.1. Both show small increases with regular formoterol that do not reach statistical significance, which may be because the larger number of minor adverse events are not altered by formoterol. Published drug‐related adverse events showed a significant increase as shown in Analysis 19.1.
17.1. Analysis.
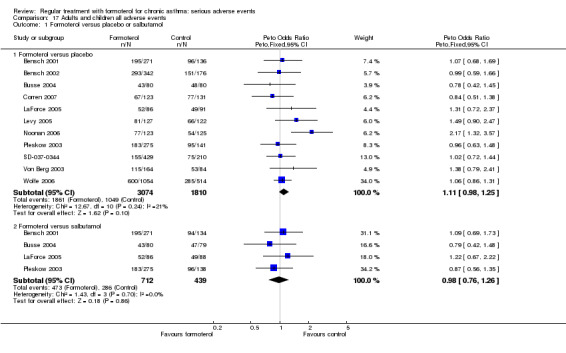
Comparison 17 Adults and children all adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
18.1. Analysis.

Comparison 18 Adults and children published adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
19.1. Analysis.

Comparison 19 Adults and children all published drug‐related adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Drug‐related serious adverse events
If the analysis had been confined to SAEs that were thought to be drug‐related, only five of the 136 SAEs would have been included, and because there is a wide confidence interval around this small number of events, no significant increase in drug‐related SAEs was found (Peto OR 1.45; 95% CI 0.18 to 11.61) and Mantel‐Haenszel OR 0.90 (95% CI 0.19 to 4.24) (Analysis 20.1).
20.1. Analysis.

Comparison 20 Adults and children serious drug‐related adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Quality of the evidence
All the studies were double‐blind with the exception of LaForce 2005, Molimard 2001 and van Schayck 2002. Of these, only Molimard 2001 contributed data to SAEs with regular formoterol in comparison with placebo, and if the meta‐analysis is confined to the double‐blind trials the increase remains significant (Peto OR 1.52; 95% CI 1.02 to 2.25). There are methodological concerns in relation to data from the Novartis integrated database, as these are not presented for the individual studies, but where it has been possible to compare results from the database with the Novartis studies included in the review the results are very similar (Analysis 12.1).
Agreements and disagreements with other studies or reviews
The findings of this review are similar to those of our review comparing regular salmeterol to placebo and regular salbutamol (Cates 2008). Both reviews show that, in comparison with placebo, there are significant increases in all‐cause SAEs with the use of regular long‐acting beta2‐agonists. The size of this increase is comparable for regular salmeterol (Peto OR 1.15; 95% CI 1.02 to 1.29) and regular formoterol (Peto OR 1.57; 95% CI 1.06 to 2.31). The placebo group event rates were different in the two reviews, so it would be misleading to try to compare the absolute increases in risk between salmeterol and formoterol. Similarly, although the increase in events reached statistical significance in adults (but not children) for salmeterol, and in children (but not adults) for formoterol, these age group differences may be due to the play of chance, as the test for interaction between age group and treatment effect was not significant in either case (Altman 2003).
The number of participants is too small to assess the impact of regular formoterol on all‐cause or asthma‐related mortality, so it is not possible to compare these results with the increased asthma mortality found in SMART 2006. However, data submitted by Novartis to the FDA (Novartis 2005) do indicate a significant increase in asthma‐related serious adverse events, even in patients taking regular inhaled corticosteroids (Analysis 15.1).
A recent meta‐analysis of individual patient data from trials of all FDA‐approved LABAs found a higher risk of serious asthma‐related events in children as compared to adults (McMahon 2011), which is in keeping with the results of this review. This increased risk in children persisted in those who were given concomitant ICS, but not in the trials in which participants were assigned to ICS as part of their randomised treatment.
Authors' conclusions
Implications for practice.
In comparison with placebo, we have found an increased risk of serious adverse events with regular formoterol, and this does not appear to be abolished in patients taking inhaled corticosteroids. The effect on serious adverse events of regular formoterol in children was greater than the effect in adults, but the difference between age groups was not significant.
Implications for research.
Data on all‐cause serious adverse events should be more fully reported in medical journals, and not combined with all adverse events or limited to those events that are thought by the investigator to be drug‐related.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement added |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 5 January 2012 | New search has been performed | No new studies found. Minor edits made and plain language summary revised. |
| 5 January 2012 | New citation required but conclusions have not changed | New search carried out in January 2012 but no new studies included. |
| 10 November 2008 | Amended | The 'Summary of findings' tables have been reordered. Contents are unchanged. An additional reference has also been added for Corren 2007. The Primary Analysis has been changed to Peto Odds Ratio. |
Acknowledgements
We thank Susan Hansen and Elizabeth Stovold of the Cochrane Airways Group for their assistance in searching for trials and obtaining the abstracts and full reports, and extraction of data on trial characteristics, Toby Lasserson for assistance in checking outcome data and Anne Tattersfield for helpful comments. We are grateful to Martin Shaw (Novartis) for providing data in relation to FitzGerald 1999, Onno van Schayck for providing data for van Schayck 2002, Vincent Le Gros (Novartis) for providing data in relation to Molimard 2001, Andrea von Berg for providing data in relation to Von Berg 2003 and Robyn Von Maltzahn (AstraZeneca) for providing data in relation to Ekstrom 1998; Ekstrom 1998a; van der Molen 1997; Zimmerman 2004.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Pharmacology of beta2‐agonists
Beta2‐agonists are thought to cause bronchodilation primarily through binding beta2‐adrenoceptors on airways smooth muscle (ASM), with subsequent activation of both membrane‐bound potassium channels and a signalling cascade involving enzyme activation and changes in intracellular calcium levels following a rise in cyclic adenosine monophosphate (cAMP) (Barnes 1993). However, beta2‐adrenoceptors are also expressed on a wide range of cell types where beta2‐agonists may have a clinically significant effect including airway epithelium (Morrison 1993), mast cells, post capillary venules, sensory and cholinergic nerves and dendritic cells (Anderson 2006). Beta2‐agonists will also cross‐react to some extent with other beta‐adrenoceptors including beta1‐adrenoceptors on the heart.
The in vivo effect of any beta2‐agonist will depend on a number of factors relating to both the drug and the patient. The degree to which a drug binds to one receptor over another is known as selectivity, which can be defined as absolute binding ratios to different receptors in vitro, whilst functional selectivity is measured from downstream effects of drugs in different tissue types in vitro or in vivo. All of the beta2‐agonists described thus far are more beta2 selective than their predecessor isoprenaline in vitro. However, because attempts to differentiate selectivity between the newer agents are confounded by so many factors, it is difficult to draw conclusions about in vitro selectivity studies and probably best to concentrate on specific adverse side effects in human subjects at doses which cause the same degree of bronchodilatation. The potency of a drug refers to the concentration that achieves half the maximal receptor activation of which that drug is capable but it is not very important clinically as for each drug, manufacturers will alter the dose to try to achieve a therapeutic ratio of desired to undesired effects. In contrast efficacy refers to the ability of a drug to activate its receptor independent of drug concentration. Drugs that fully activate a receptor are known as full agonists and those that partially activate a receptor are known as partial agonists. Efficacy also is very much dependent on the system in which it is being tested and is affected by factors including the number of receptors available and the presence of other agonists and antagonists. Thus whilst salmeterol acts as a partial agonist in vitro it causes a similar degree of bronchodilation to the strong agonist formoterol in stable asthmatic patients (Van Noord 1996), presumably because there are an abundance of well‐coupled beta2‐adrenoceptors available with few downstream antagonising signals. In contrast, with repetitive dosing formoterol is significantly better than salmeterol at preventing methacholine‐induced bronchoconstriction (Palmqvist 1999). These differences have led to attempts to define the 'intrinsic efficacy' of a drug independent of tissue conditions (Hanania 2002), as shown in Table 26. The clinical significance of intrinsic efficacy remains unclear.
3. Intrinsic efficacy of beta‐agonists.
| Drug | Intrinsic efficacy (%) |
| Isoprenaline, adrenaline | 100 |
| Fenoterol | 42 |
| Formoterol | 20 |
| Salbutamol | 4.9 |
| Salmeterol | < 2 |
Adapted from Hanania 2002. The authors acknowledge that it is difficult to determine the intrinsic efficacy of salmeterol given its high lipophilicity.
Appendix 2. Possible mechanisms of increased asthma mortality with beta‐agonists
Direct toxicity
This hypothesis states that direct adverse effects of beta2‐agonists are responsible for an associated increase in mortality and most research in the area has concentrated on effects detrimental to the heart. Whilst it is often assumed that cardiac side effects of beta2‐agonists are due to cross‐reactivity with beta1‐adrenoceptors (i.e. poor selectivity), it is worth noting that human myocardium also contains an abundance of beta2‐adrenoceptors capable of triggering positive chronotropic and inotropic responses (Lipworth 1992). Indeed, there is good evidence that cardiovascular side effects of isoprenaline (Arnold 1985) and other beta2‐agonists including salbutamol (Hall 1989) are mediated predominantly via cardiac beta2‐adrenoceptors thus making the concept of in vitro selectivity less relevant. Generalised beta2‐adrenoceptor activation can also cause hypokalaemia (Brown 1983) and it has been proposed that, through these and other actions, beta2‐agonists may predispose to life‐threatening dysrhythmias or cause other adverse cardiac effects.
During the 1960s epidemic most deaths occurred in patients with severe asthma and it was originally assumed that asthma and its sequelae, including hypoxia, were the primary cause of death. However, mucus plugging and hypoxia does not preclude a cardiac event as the final cause of death, and one might expect those with severe asthma to take more doses of a prescribed inhaler. As noted by Speizer and Doll most deaths in the 1960s were in the 10 to 19 age group and “at these ages children have begun to act independently and may be particularly prone to misuse a self‐administered form of treatment” (Speizer 1968). If toxicity were related to increasing doses of beta2‐agonists one might expect most deaths to occur in hospital where high doses are typically used and this was not the case. One possible explanation for this anomaly was provided by animal experiments in which large doses of isoprenaline caused little ill effect in anaesthetised dogs with normal arterial oxygenation whereas much smaller doses caused fatal cardiac depression and asystole (although no obvious dysrhythmia) when hypoxic (Collins 1969; McDevitt 1974). It has been hypothesised therefore that such events would be less likely in hospital where supplemental oxygen is routinely given. The clinical relevance of these studies remains unclear although there is some evidence of a synergistic effect between hypoxia and salbutamol use in asthmatic patients in reducing total peripheral vascular resistance (Burggraaf 2001) – another beta2‐mediated effect which could be detrimental to the heart during an acute asthma attack through a reduction in diastolic blood pressure. Other potential mechanisms of isoprenaline toxicity include a potential increase in mucous plugging and worsening of ventilation perfusion mismatch despite bronchodilation (Pearce 1990).
Further concerns about a possible toxic effect of beta2‐agonists were raised during the New Zealand epidemic in the 1970s. In 1981 Wilson et al, who first reported the epidemic, reviewed 22 fatal cases of asthma and noted “In 16 patients death was seen to be sudden and unexpected. Although all were experiencing respiratory distress, most were not cyanosed and the precipitate nature of their death suggested a cardiac event, such as an arrest, inappropriate to the severity of their respiratory problem” (Wilson 1981). In humans, fenoterol causes significantly greater chronotropic, inotropic and electrocardiographic side effects than salbutamol in asthmatic patients (Wong 1990). Interestingly, across the same parameters fenoterol also causes more side effects than isoprenaline (Burgess 1991).
In patients with mild asthma and without a bronchoconstrictor challenge, salmeterol and salbutamol cause a similar degree of near maximal bronchodilation at low doses (Bennett 1994). However, whilst as a one‐off dose salbutamol is typically used at two to four times the concentration of salmeterol, the dose equivalences for salmeterol versus salbutamol in increasing heart rate and decreasing potassium concentration and diastolic blood pressure were 17.7, 7.8 and 7.6 respectively (i.e. salmeterol had a greater effect across all parameters). Given the lower intrinsic efficacy of salmeterol (Table 4), these results highlight the importance of in vivo factors; one possible explanation for the difference is the increased lipophilicity of salmeterol compared to salbutamol contributing to higher systemic absorption (Bennett 1994).
When comparing increasing actuations of standard doses of formoterol and salmeterol inhalers in stable asthmatic patients, relatively similar cardiovascular effects are seen at lower doses (Guhan 2000). However, at the highest doses (above those recommended by the manufacturers) there were trends towards an increase in systolic blood pressure with formoterol; in comparison there was a trend towards a decrease in diastolic blood pressure and an increase in QTc interval with salmeterol although no statistical analysis of the difference was performed. In contrast in asthmatic patients with methacholine‐induced bronchoconstriction there was no significant difference between salmeterol and formoterol in causing increased heart rate and QTc interval although formoterol caused significantly greater bronchodilation and hypokalaemia (Palmqvist 1999). Whilst there is good evidence of cardiovascular and metabolic side effects with increasing doses of beta2‐agonists, it is a little difficult to envisage serious adverse effects of this nature when using LABAs at manufacturer‐recommended preventative doses. However, it is possible that some patients choose to use repeated doses of LABAs during exacerbations.
Tolerance
In this setting, the term tolerance refers to an impaired response to beta2‐agonists in patients who have been using regular beta2‐agonist treatment previously (Haney 2006). Tolerance is likely to result from a combination of reduced receptor numbers secondary to receptor internalisation and reduced production and also uncoupling of receptors to downstream signalling pathways following repeated activation (Barnes 1995). This phenomenon is likely to explain the beneficial reduction in systemic side effects seen with regular use of beta2‐agonists including salbutamol after one to two weeks (Lipworth 1989). However, the same effect on beta2‐adrenoceptors in the lung might be expected to produce a diminished response to the bronchodilating activity of beta2‐agonists following regular use. In patients with stable asthma, whilst there is some evidence of tolerance to both salbutamol (Nelson 1977) and terbutaline (Weber 1982) other studies have been less conclusive (Harvey 1982; Lipworth 1989). However, evidence of tolerance to short and long‐acting beta2‐agonists in both protecting against and reducing bronchoconstriction is much stronger in the setting of an acute bronchoconstrictor challenge with chemical, allergen and 'natural' stimuli (Haney 2006; Lipworth 1997).
Studies comparing salmeterol and formoterol have shown that both cause tolerance compared to placebo but there was no significant difference between the drugs (van der Woude 2001). There also appears to be little difference in the tolerance induced by regular formoterol and regular salbutamol treatment (Hancox 1999; Jones 2001). To the authors' knowledge no studies have looked specifically at the degree of tolerance caused by isoprenaline and fenoterol in the setting of acute bronchoconstriction. Tolerance to bronchodilation has been shown to clearly occur with addition of inhaled corticosteroids to salmeterol and formoterol (Lee 2003) and terbutaline (Yates 1996). There is conflicting evidence as to whether high‐dose steroids can reverse tolerance in the acute setting (Jones 2001; Lipworth 2000).
At first glance the toxicity and tolerance hypotheses might appear incompatible as systemic and cardiovascular tolerance ought to protect against toxicity in the acute setting and there is good evidence that such tolerance occurs in stable asthmatic patients (Lipworth 1989). However, whilst this study showed that changes in heart rate and potassium levels were blunted by previous beta2‐agonist use, they were not abolished; furthermore, at the doses studied these side effects appear to follow an exponential pattern (Lipworth 1989). In contrast, in the presence of bronchoconstrictor stimuli the bronchodilator response to beta2‐agonists follows a flatter curve (Hancox 1999; Wong 1990) and as previously discussed this curve is shifted downwards by previous beta2‐agonist exposure (Hancox 1999). Thus, it is theoretically possible that in the setting of an acute asthmatic attack and strong bronchoconstricting stimuli, bronchodilator tolerance could lead to repetitive beta2‐agonist use and ultimately more systemic side effects than would otherwise have occurred. Of course, other sequelae of inadequate bronchodilation including airway obstruction will be detrimental in this setting.
Whilst the tolerance hypothesis is often cited as contributing towards the asthma mortality epidemics it is difficult to argue that reduced efficacy of a drug can cause increased mortality relative to a time when that drug was not used at all. However, tolerance to the bronchodilating effect of endogenous circulating adrenaline is theoretically possible and there is also evidence of rebound bronchoconstriction when stopping fenoterol (Sears 1990), which may be detrimental. Furthermore, it appears that regular salbutamol treatment can actually increase airway responsiveness to allergen (Cockcroft 1993) a potentially important effect that could form a variant of the toxicity hypothesis. Differences between beta2‐agonists in this regard are unclear, but the combination of rebound hyper responsiveness and tolerance of the bronchodilator effect with regular beta2‐agonist exposure has been recently advocated as a possible mechanism to explain the association between beta2‐agonists and asthma mortality (Hancox 2006).
Other explanations
Confounding by severity
Historically, this hypothesis has been used extensively to try to explain the association between mortality and the use of fenoterol during the 1970s New Zealand epidemic (see Pearce 2007) and is still quoted today. The hypothesis essentially relies on the supposition that patients with more severe asthma are more likely to take either higher doses of beta2‐agonists or a particular beta2‐agonist (such as fenoterol) thereby explaining the association. This hypothesis was carefully ruled out in the three case‐control studies by comparing the association between fenoterol and mortality in patients with varying severity of disease (Crane 1989; Grainger 1991; Pearce 1990). Furthermore, the hypothesis cannot explain the overall increase in mortality in the 1960s and 1970s nor can it explain any significant increase in mortality (whether taking inhaled steroids or not) from randomised controlled trial data.
The delay hypothesis
This hypothesis accepts that beta2‐agonists or a particular beta2‐agonist cause an increased risk of mortality but indirectly by causing patients to delay before getting medical help and further treatments including high dose steroids and oxygen. There is evidence that both salmeterol and formoterol can reduce awareness of worsening underlying inflammation (Bijl‐Hofland 2001; McIvor 1998). It is difficult to rule out the delay hypothesis in either explaining or contributing towards both the asthma mortality epidemics and an association with regular use of LABAs. There is evidence that beta2‐agonists with higher intrinsic efficacy are more effective at relieving bronchoconstriction in the acute setting (Hanania 2007) and could paradoxically cause patients to delay seeking medical help for longer. For the delay hypothesis to explain the increase in mortality during the 1960s and 1970s one has to imply that hospital treatment of asthma when mortality rates were low during the earlier years of the 20th century was effective. It is difficult to say exactly how effective such treatment is likely to have been.
Reduced corticosteroid treatment
A slight but significant variation of the delay hypothesis suggests that patients who have separate beta2‐agonists and corticosteroid inhalers may choose to take less corticosteroid because of better symptom control from the inhaled beta2‐agonists and it is reduced corticosteroid treatment that contributes to a rise in mortality. It is rather difficult to see how this hypothesis explains the epidemics of asthma deaths in the 1960s and 1970s relative to the 1920s and 30s (Figure 1), given that corticosteroids were not used for the treatment of asthma in the earlier decades. If this hypothesis were to explain increased mortality from more recent randomised controlled trial data one would not expect to see an increase in mortality in those taking LABAs alone.
Appendix 3. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (T he Cochrane Library) | Quarterly |
| PSYCINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 4. Definition of serious adverse events
The Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) define serious adverse events as follows (ICHE2a 1995):
"A serious adverse event (experience) or reaction is any untoward medical occurrence that at any dose:
Results in death,
Is life‐threatening,
Requires inpatient hospitalisation or prolongation of existing hospitalisation,
Results in persistent or significant disability/incapacity, or
Is a congenital anomaly/birth defect.
NOTE: The term "life‐threatening" in the definition of "serious" refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe."
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall results | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 14 | 5463 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.50 [0.41, 49.49] |
| 1.2 Formoterol versus salbutamol | 6 | 1418 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.02, 8.98] |
1.1. Analysis.

Comparison 1 All‐cause mortality, Outcome 1 Overall results.
Comparison 2. Adults and children non‐fatal serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 19 | 6646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [1.06, 2.31] |
| 1.2 Formoterol versus salbutamol | 9 | 2119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.37, 1.43] |
2.1. Analysis.

Comparison 2 Adults and children non‐fatal serious adverse events, Outcome 1 Formoterol versus placebo or salbutamol.
Comparison 3. Adults and children non‐fatal serious adverse events (without formoterol 24 µg arms).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 18 | 5438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.97, 2.48] |
| 1.2 Formoterol versus salbutamol | 9 | 1848 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.19, 0.90] |
Comparison 4. Adults with non‐fatal serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 15 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 15 | 5311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.76, 1.99] |
| 1.2 Formoterol versus salbutamol | 9 | 2119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.37, 1.43] |
Comparison 5. Children with non‐fatal serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 5 | 1335 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.48 [1.27, 4.83] |
| 1.2 Formoterol versus salbutamol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Children with non‐fatal serious adverse events (without formoterol 24 µg arms).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 5 | 1164 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.52 [1.15, 5.51] |
| 1.2 Formoterol versus salbutamol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Dose comparison: formoterol 24 µg versus 12 µg twice daily.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults (all‐cause) | 3 | 1598 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.64, 2.85] |
| 1.2 Children (all‐cause) | 1 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.52, 2.74] |
| 1.3 Asthma SAE | 1 | 3104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.16 [1.13, 4.11] |
Comparison 8. Adults and children fatal and non‐fatal serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 19 | 6646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.61 [1.09, 2.37] |
| 1.2 Formoterol versus salbutamol | 9 | 2119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.37, 1.38] |
Comparison 9. Asthma mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 12 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 12 | 4522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.54 [0.07, 285.25] |
| 1.2 Formoterol versus salbutamol | 6 | 1418 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.49 [0.07, 286.29] |
9.1. Analysis.
Comparison 9 Asthma mortality, Outcome 1 Formoterol versus placebo or salbutamol.
Comparison 10. Cardiovascular mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 12 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 12 | 4522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.54 [0.07, 285.29] |
| 1.2 Formoterol versus salbutamol | 6 | 1418 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
Comparison 10 Cardiovascular mortality, Outcome 1 Formoterol versus placebo or salbutamol.
Comparison 11. Adults and children non‐fatal asthma‐related serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 17 | 5759 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [1.12, 3.53] |
| 1.2 Formoterol versus salbutamol | 9 | 2119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.29, 1.88] |
Comparison 12. Adults and children non‐fatal asthma‐related serious adverse events (Novartis data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo (Novartis published data) | 10 | 4138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.06 [1.57, 5.96] |
| 1.2 Formoterol versus placebo (Novartis integrated database) | 1 | 5631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.81 [1.54, 5.14] |
Comparison 13. Hospitalisations for asthma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 7 | 3433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.28 [1.65, 6.52] |
| 1.2 Formoterol versus salbutamol | 2 | 593 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.41, 7.49] |
Comparison 14. Adults and children non‐fatal cardiovascular serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 8 | 3049 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.09, 5.21] |
| 1.2 Formoterol versus salbutamol | 5 | 803 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.33] |
Comparison 15. Impact of inhaled corticosteroids on asthma‐related SAEs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with at least one asthma‐related SAE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Patients taking inhaled corticosteroids (all doses of formoterol)) | 1 | 3807 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.58 [1.21, 5.49] |
| 1.2 Patients not taking inhaled corticosteroids (all doses of formoterol) | 1 | 1824 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.22 [1.17, 8.89] |
| 1.3 Patients taking inhaled corticosteroids (formoterol 12 mcg twice daily) | 1 | 2708 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.76 [1.06, 7.15] |
| 1.4 Patients not taking inhaled corticosteroids (formoterol 12 mcg twice daily) | 1 | 1103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.71 [0.75, 18.48] |
Comparison 16. Adults and children published non‐fatal serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 7 | 1792 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.17, 4.53] |
| 1.2 Formoterol versus salbutamol | 3 | 536 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.15, 2.44] |
Comparison 17. Adults and children all adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 11 | 4884 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.98, 1.25] |
| 1.2 Formoterol versus salbutamol | 4 | 1151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.76, 1.26] |
Comparison 18. Adults and children published adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 8 | 3743 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| 1.2 Formoterol versus salbutamol | 4 | 1151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.26] |
Comparison 19. Adults and children all published drug‐related adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 4 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [1.07, 1.88] |
| 1.2 Formoterol versus salbutamol | 3 | 542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.48, 1.18] |
Comparison 20. Adults and children serious drug‐related adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Formoterol versus placebo or salbutamol | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol versus placebo | 9 | 3618 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.18, 11.61] |
| 1.2 Formoterol versus salbutamol | 3 | 746 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.04, 12.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bensch 2001.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, multicentre parallel‐group study over 12 weeks at 26 trial sites in the USA. 2‐week, single‐blind, placebo lead‐in period. | |
| Participants |
Population: 541 adolescents and adults (12 to 75) years with mild to moderate persistent asthma Baseline characteristics: mean age 35.5 years. FEV1 66% predicted. Concomitant inhaled corticosteroids used by 51% of participants. Inclusion criteria: mild to moderate persistent asthma requiring daily use of an inhaled B2‐selective adrenergic agent (short‐ or long‐acting). FEV1 between 40% and 80% predicted after an 8‐hour period of abstinence from short‐acting B2‐ adrenergic agonist use. Bronchodilator reversibility at least a 15% increase in FEV1 within 30 minutes after inhalation of 180 µg of albuterol delivered via MDI. Co‐interventions permitted: ICS 51%, slow release theophylline 17%. Exclusion criteria: URTI, hospitalisation/asthma exacerbation < 4 weeks, serious illness |
|
| Interventions |
Formoterol delivered by Aerolizer (dry powder) and albuterol by MDI |
|
| Outcomes | Primary outcome: FEV1 . Safety was evaluated by adverse event reports. Paper report: "Adverse events, whether or not trial drug related, were reported by 68% of patients on formoterol 12 mcg, 76% on formoterol 24 mcg, 70% on albuterol and 71% on placebo. No deaths occurred during the study." No SAE data reported but the numbers match FDA submission for study 040 (http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf). This lists all (asthma‐related in brackets) SAEs as: 1 (0) on formoterol 12 µg, 5 (4) on formoterol 24 µg, 2 (2) on albuterol and 1 (0) on placebo. |
|
| Notes | Supported by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy. Matching capsules and devices |
| Incomplete outcome data (attrition bias) Adverse Events | Unclear risk | All patients included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | No SAE data in paper publication, but FDA submission provided SAE information |
Bensch 2002.
| Methods | A randomised, double‐blind, placebo‐controlled, multinational, multicentre, paediatric study over 52 weeks at 42 centres. Initial run‐in period of 2 weeks. | |
| Participants |
Population: 518 children (5 to 12) years with persistent asthma on preventer therapy Baseline characteristics: mean age 9 years. FEV1 71% predicted. At least 69% were taking ICS, with 26% on sodium cromoglycate and 5% nedocromil sodium. Inclusion criteria: persistent asthma diagnosed by ATS criteria. Sodium cromoglycate, nedocromil sodium and/or ICS for at least 4 weeks before entry in the study, but still required daily use of inhaled salbutamol (albutamol) to control symptoms. Baseline FEV1 ranged from 50% to 85% of predicted and increased by 15% or more within 30 minutes after inhaling 200 µg salbutamol (180 µg albuterol in the United States) delivered by a metered‐dose inhaler. Co‐interventions: 70% on ICS, 30% on cromones (all stable dosage) Exclusion criteria: unstable asthma, URTI, OS course or exacerbation asthma within 4 weeks, QT interval on ECG > 0.46 sec |
|
| Interventions |
|
|
| Outcomes | Primary outcome: FEV1 (area under curve) Paper reports: "There were no deaths in this study." Table 4 in the paper reports SAE data but not in a way that allows all‐cause SAE data to be extracted. Full SAE data found on FDA website. |
|
| Notes | Supported by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 407/518 (78%) completed the study |
| Selective reporting (reporting bias) | Low risk | All‐cause SAE data not extractable from published paper, but present in FDA web report |
Busse 2004.
| Methods | Randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study over 12 weeks in outpatient clinics at 18 US centres. Run‐in 2 weeks single‐blind placebo. | |
| Participants | 239 adolescents and adults (13 to 85) years with persistent asthma. (9 patients were aged less than 18 years old) Baseline characteristics: mean age 38 years. FEV1 65% predicted. Concomitant inhaled corticosteroids used by 64% of participants. Inclusion criteria: persistent asthma, requiring regular or on‐demand bronchodilators, FEV1 % predicted at least 40%, bronchodilator reversibility by either an increase of at least 15% in FEV1 over baseline or increases of at least 12% and 200 mL in FEV1 over baseline within 30 minutes of inhaling albuterol 180 to 360 µg (2 to 4 puffs) via pMDI. Co‐interventions permitted: ICS, cromolyn, montelukast, theophyllines, intra nasal ICS. Exclusion criteria: RT infection, use of OCS, parenteral CS or hospitalisation for asthma due to exacerbation < 1 month, use of unstable anti‐inflammatory regime, corrected QT interval > 460 ms, current smoker, ex smoker > 10 Pack years, serious medical condition, pregnancy, lactation, use of oral beta blocker, non‐potassium sparing diuretic, anti‐arrhythmic, tricyclic antidepressant, MAOI, NSAIDS |
|
| Interventions | 1. Formoterol 10µg BD
2. Placebo BD
3. Albuterol 180 µg QDS Delivery was DPI for formoterol and MDI for albuterol (salbutamol) |
|
| Outcomes | The primary efficacy variable was the 12‐hour AUC of FEV1 after 12 weeks' treatment No deaths in the study. Non‐fatal SAEs: formoterol 2 (1 asthma exacerbation and 1 basal cell carcinoma). Albuterol 3 (1 eating disorder, 1 renal calculi and 1 pneumonia). Placebo 0. No events were considered to be related to study medication. Any AE: formoterol 43, albuterol 47 and placebo 48 |
|
| Notes | Funding Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 ratio using computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐dummy matched devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 209/239 (87%) completed the study; SAE reported for both those who withdrew and those who continued in the study |
| Selective reporting (reporting bias) | Low risk | Serious adverse events (fatal and non‐fatal) documented in the paper publication |
Corren 2007.
| Methods | Randomised, double‐blind, double‐dummy, multicentre, placebo‐controlled study over 12 weeks at 56 US centres from July 2002 to September 2003. Run‐in 7 to 21 days in which usual asthma therapy was withdrawn. | |
| Participants |
Population: 480 adolescents and adults (12 to 78) years with mild to moderate persistent asthma. The web report also includes results from a further 31 children aged 6 to 11 years. Baseline characteristics: mean age 36 years. FEV1 75% predicted. Concomitant inhaled corticosteroids used by 100% of participants at baseline but withdrawn for the formoterol and placebo arms of this study. Inclusion criteria: mild to moderate persistent asthma for at least 6 months, treated with inhaled corticosteroids for at least 4 weeks before screening, FEV1 between 60% and 90% predicted on ICS at screening and between 50% and 85% predicted after discontinuation of ICS during run‐in period. Bronchodilator reversibility of at least 12% and 0.20 L in FEV1 over baseline within 15 to 30 minutes after administration of albuterol pMDI (2 to 4 inhalations (90 µg per inhalation)). Exclusion criteria: reasons for exclusion from the study included severe asthma (as judged by the investigator), asthma requiring hospitalisation once or emergency treatment more than once within the 6 months before the study or requiring treatment with systemic corticosteroids within the 4 weeks before screening, and/or a > 10 pack‐year smoking history at screening. Pregnant or breastfeeding. |
|
| Interventions | 1. Formoterol 9 µg (DPI)
2. Placebo BID (pMDI) The Symbicort and budesonide arms of this study are not included in this review |
|
| Outcomes | The co‐primary efficacy variables were changes from baseline in morning predose FEV1 and 12‐hour mean FEV1 (from serial spirometry) after administration of the morning dose of study medication 2 serious adverse events in the placebo group (intestinal obstruction, abdominal pain) reported on the website. One of these presumably occurred in a child under 12 years, as the paper reports 1 serious adverse event in those aged over 12 years. No cardiac‐related serious adverse events were reported in any group. No deaths occurred in any group (website data). |
|
| Notes | Study sponsored by AstraZeneca http://www.astrazenecaclinicaltrials.com/Article/525476.aspx accessed 16 June 2008 for web data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By study site, computer‐generated allocation schedule using balanced blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐dummy. Patients received both a pMDI and DPI containing either active treatment or placebo of the alternative active treatment as appropriate. |
| Incomplete outcome data (attrition bias) Adverse Events | Unclear risk | ITT analysis used for adverse events. High dropout rate in placebo group (51%) |
| Selective reporting (reporting bias) | Low risk | Serious adverse events reported in paper publication |
Ekstrom 1998.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, multicentre, multinational study (Sweden, Norway, Spain, Italy) over 12 weeks at 25 centres. Run‐in: 2 weeks single‐blind placebo. | |
| Participants |
Population: 397 adults (18 to 79) with mild to moderate asthma Baseline characteristics: mean age 47 years. FEV1 62% predicted. Concomitant inhaled corticosteroids used by 86% of participants. Inclusion criteria: diagnosis asthma by ATS criteria. FEV1 of 40% to 80% predicted, bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline and at least 200 ml, measured after inhalation of 0.5 mg terbutaline via Turbuhaler Exclusion criteria: not reported |
|
| Interventions | 1. Formoterol 6 µg BD 2. Terbutaline 500 µg QDS 3. Placebo: placebo QDS Delivery was dry powder device |
|
| Outcomes | Primary outcome PEF. Adverse events were assessed by asking patients if they had experienced any health problems or symptoms not usually associated with their asthma. "Asthma aggravation accounted for two serious adverse events in the terbutaline group and one serious adverse event in the placebo group." Manufacturers asked for all‐cause serious adverse event data, and these have been provided as 1 patient on formoterol, 3 patients on terbutaline and 2 patients on placebo. |
|
| Notes | Author affiliation includes Astra Draco | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy, matching devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 359/397 (90%) completed the study |
| Selective reporting (reporting bias) | Low risk | All‐cause SAE data have been provided by sponsors |
Ekstrom 1998a.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, multicentre study over 12 weeks at 28 centres in Scandinavia. Run‐in 1 week single blind. | |
| Participants |
Population: 343 adults (18 to 82) with moderate stable asthma Baseline characteristics: mean age 48 years. FEV1 61% predicted. Concomitant inhaled corticosteroids used by 89% of participants. Inclusion criteria: diagnosis asthma by ATS criteria. FEV1 of 40% to 80% predicted, bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline 15 minutes after inhalation of 0.5 mg terbutaline sulphate via Turbuhaler. Co‐interventions: ICS 89%, cromones 2% ‐ stable doses OS 2 participants Exclusion criteria: not reported |
|
| Interventions |
Delivery was dry powder device ‐ Turbuhaler |
|
| Outcomes | FEV1, PEF, rescue use, asthma symptom score, adverse events, asthma deterioration Primary outcome PEF. Adverse events were assessed by asking patients if they had experienced any health problems or symptoms not usually associated with their asthma. No SAE reporting at all in the paper. Manufacturers asked for all‐cause serious adverse event data: 4 patients with SAE in terbutaline group and 2 on placebo. Of these 3 and 2 were asthma‐related. |
|
| Notes | Author affiliation includes Astra Draco | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random order, balanced blocks |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy, matching devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 311/343 (90%) completed the study |
| Selective reporting (reporting bias) | Low risk | SAE results provided by sponsors |
FitzGerald 1999.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, multicentre study over 24 weeks at 15 centres in Canada. Run‐in 2 weeks single‐blind. | |
| Participants |
Population: 271 adults with moderate to severe asthma
Baseline characteristics: mean age 36 years. FEV1 79% predicted. Concomitant inhaled corticosteroids used by all the participants. Inclusion criteria: diagnosis asthma by ATS criteria. Using ICS at a constant dose of 400 to 1200 mg/day and inhaled SABA for at least 1 month. Bronchodilator reversibility as a 15% or greater increase in FEV1 15 to 30 minutes after inhalation of a B2‐agonist, and increased sensitivity to inhaled methacholine, defined as a PC20 less than or equal to 8 mg/mL. Rescue albuterol required on 5 out of 7 days of run‐in period. Exclusion criteria: URTI/change in asthma medication/exacerbation of asthma within 2 months. Smoking. |
|
| Interventions | 1. Formoterol 12 µg BD
2. Placebo QDS
3. Albuterol 200 µg QDS Delivery was dry powder device |
|
| Outcomes | The primary end point of this trial was the methacholine PC20 at the end of the double‐blind phase (visit 6) No SAE data published for this study, but unpublished serious adverse event data provided by the authors "Drug‐related adverse events were reported by 15%, 12% and 10% of the formoterol, regular albuterol and on‐demand albuterol patients, respectively." |
|
| Notes | Supported by a grant from Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy, matching devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | |
| Selective reporting (reporting bias) | Low risk | No SAE reporting in paper and no web data found, but data provided by the authors |
Hekking 1990.
| Methods | Randomised, double‐blind, double‐dummy, multicentre, parallel‐group study of outpatients over 12 weeks in The Netherlands. Washout period of 12 hours for inhaled and 24 hours for oral medication. | |
| Participants |
Population: 301 adults (18 to 70) years with stable‐phase asthma Baseline characteristics: mean age 40 years. FEV1 not reported. Concomitant inhaled corticosteroids use not reported (but allowed in protocol). Inclusion criteria: stable‐phase asthma, FEV1 % predicted less than 80%, bronchodilator reversibility greater than 15% in FEV1 over baseline Exclusion criteria: use of theophyllines, oral beta2 agonists or anticholinergics |
|
| Interventions | 1. Formoterol 12 µg BD
2. Salbutamol 200 µg QDS Delivery was MDI |
|
| Outcomes | PEF, rescue use, asthma attacks, efficacy rating No report in paper in relation to serious adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) Adverse Events | Unclear risk | 15 of 150 formoterol patients and 30 of 151 salbutamol patients were withdrawn |
| Selective reporting (reporting bias) | High risk | No SAE reporting in the paper |
Kesten 1991.
| Methods | A randomised, double‐blind, double‐dummy, parallel‐group, multicentre, multinational study (Canada, UK), over 12 weeks at 7 centres. Run‐in 4 weeks double‐blind cross‐over. | |
| Participants |
Population: 145 adults (18 to 65) years with stable, symptomatic asthma Baseline characteristics: mean age not reported. Mean baseline FEV1 for the formoterol group was 2.14 L and 1.98 L for the albuterol group. Concomitant inhaled corticosteroids used by 62% of participants. Inclusion criteria: diagnosis of asthma requiring daily treatment with an inhaled B2‐agonist. FEV1 % predicted at least 40%, bronchodilator reversibility by an increase of at least 15% in FEV1 30 minutes after inhalation of 200 µg of albuterol by metered‐dose inhaler. Use of rescue albuterol greater during the placebo treatment period than during the albuterol treatment period by an average of 2 puffs per day or more. Co‐interventions: none 32%, ICS 62 %, cromones 7%, theophylline 32% Exclusion criteria: use of OS, exacerbation asthma within 1 month, significant non‐respiratory illnesses and women of childbearing potential |
|
| Interventions | 1. Formoterol 10 µg BD
2. Albuterol 180 µg QDS Delivery was MDI |
|
| Outcomes | FEV1, FVC, FEV 25% to 75%, PEF, rescue use, rate asthma attacks day and night, adverse events, exacerbations, efficacy rating. No serious adverse event data reported in the paper. No web data found. |
|
| Notes | Supported by Ciba‐Geigy (now Novartis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy, matching inhalers |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 133/145 (91%) completed the study |
| Selective reporting (reporting bias) | High risk | No published SAE data |
Kozlik‐Feldmann 1996.
| Methods | A randomised, parallel‐group, single‐centre study over 12 weeks in Germany | |
| Participants |
Population: 22 children with clinically stable asthma Baseline characteristics: mean age 10 years. Before treatment, all values for FEV1 were found beyond 100% of that predicted. Inclusion criteria: mild asthma, requiring no preventive therapy. The patients needed no further therapy, i.e. with corticosteroids or theophyllines. Exclusion criteria: requiring additional therapy for asthma |
|
| Interventions | 1. Formoterol 24 µg BD 2. Salbutamol 200 µg QDS Delivery was not reported | |
| Outcomes | FEV1, FVC, histamine provocation, beta‐receptor binding sites on mononuclear leucocytes, adverse events "No side effects due to beta2‐agonist therapy were seen". |
|
| Notes | No indication of sponsorship for this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | High risk | Open |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | No serious adverse event data documented |
LaForce 2005.
| Methods | Randomised, double‐blind, multicentre, parallel‐group study over 12 weeks at 22 sites in the US. Run‐in 2 weeks. | |
| Participants |
Population: 265 adolescents and adults (13 to 81) years with persistent asthma Baseline characteristics: mean age 37 years. FEV1 68% predicted. Concomitant inhaled corticosteroids used by 67% of participants. Inclusion criteria: persistent asthma. FEV1 % predicted at least 40%, bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline within 30 minutes of inhaling albuterol (180 to 320 µg) (still eligible if the increase in FEV1 was 12% or greater and at least 0.2 L over baseline) Exclusion criteria: pregnant/lactating women; smoking history > 10 pack‐years; history of malignancy; RTI or hospitalisation with exacerbation in previous month; treatment with systemic steroids |
|
| Interventions | 1. Formoterol 10 µg BID
2. Placebo
3. Salbutamol 180 µg QID Delivery was DPI (pMDI for salbutamol) |
|
| Outcomes | The primary efficacy variable was 12‐hour AUC of FEV1 after 12 weeks' treatment. "There were no deaths in the study. A total of three patients experienced serious adverse events and were withdrawn from the study." Formoterol 1 small cell lung cancer, albuterol 1 increase in heart rate and 1 coronary artery stenosis. |
|
| Notes | Sponsored by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 using a computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 235/265 (89%) completed the study |
| Selective reporting (reporting bias) | Low risk | Deaths and serious adverse events reported in the paper |
Levy 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre, parallel‐group study over 12 weeks in 22 US centres. Run‐in 2 weeks single‐blind placebo. | |
| Participants |
Population: 249 children (5 to 13) years with mild to moderate persistent asthma Baseline characteristics: mean age 9 years. FEV1 75% predicted. Concomitant inhaled corticosteroids used by 72% of participants. Inclusion criteria: mild to moderate persistent asthma. FEV1 % predicted at least 50%, bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline within 30 min of inhaling albuterol 180 µg via MDI, regular use of an on‐demand bronchodilator Exclusion criteria: history of respiratory tract infection, hospitalisation due to an asthma exacerbation in the month prior to visit 1, clinically significant medical condition, history of allergy to any inhaled medications, QTc interval > 0.46 seconds, or used parenteral or oral corticosteroids in the month prior to visit 1 |
|
| Interventions | 1. Formoterol 10 µg BD
2. Placebo BD Delivery was DPI |
|
| Outcomes | The primary efficacy variable was the 12‐hour AUC of FEV1 at 12 weeks "One formoterol‐treated patient experienced a serious adverse event (asthma exacerbation), which led to hospitalisation and discontinuation from the study. The event was not suspected as study‐drug related by the investigator." No web report found |
|
| Notes | Sponsored by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, matched placebo |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 227/249 (91%) completed the study |
| Selective reporting (reporting bias) | Low risk | Serious adverse events reported in the paper |
Molimard 2001.
| Methods | Randomised, outpatients, open, parallel‐group study over 3 months from February 1998 to March 1999 in France. Run‐in 2 to 3 weeks. | |
| Participants |
Population: 259 adults (18+) years with moderate persistent asthma Baseline characteristics: mean age 39 years. FEV1 73% predicted. Concomitant inhaled corticosteroids used by 100% of participants. Inclusion criteria: moderate persistent asthma, daily treatment with an inhaled corticosteroid (the same product at a stable dose for at least 1 month prior to the first visit) and daily treatment with inhaled bronchodilators (taken regularly or on demand). FEV1 % predicted at least 60%, bronchodilator reversibility by an increase of at least 10% in FEV1 over baseline to be documented at the first visit or 3 months prior. Exclusion criteria: known hypersensitivity to sympathic amines or to lactose, pregnancy or breast‐feeding, women of childbearing potential who did not use a reliable contraceptive method, significant change in the regular asthma medication, asthma exacerbation or respiratory tract infection in the month prior to the first visit, incapacity to use a metered‐dose inhaler correctly or to complete the patient diary |
|
| Interventions | 1. Formoterol dry‐powder capsule 12 µg BD 2. Salbutamol on‐demand via MDI | |
| Outcomes | The primary efficacy variable was the mean change in morning predose PEF for the entire treatment period "No drug‐related serious AE was reported". No report in paper of mortality or all‐cause SAE, but authors have provided additional information: “If we consider all‐cause SAE, no SAE was reported in the on‐demand salbutamol group and two SAEs were reported in the formoterol group (not suspected to be drug‐related): one case of hospitalisation due to a malignant tumour of breast and one case of hospitalisation due to an uterine haemorrhage. No death was reported in this study.” |
|
| Notes | Supported by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Centralised phone randomisation was used to avoid inclusion bias |
| Blinding (performance bias and detection bias) Adverse Events | High risk | Open |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 229/259 (88%) completed the study |
| Selective reporting (reporting bias) | Low risk | All‐cause SAE information provided by authors |
Noonan 2006.
| Methods | Randomised, double‐blind, double‐dummy, multicentre, placebo‐controlled study over 12 weeks from July 2002 to January 2004 at 84 US centres (respiratory or allergy speciality clinical practice). Run‐in 2 weeks. | |
| Participants |
Population: 596 adolescents and adults (12 to 87) years with moderate to severe persistent asthma Baseline characteristics: mean age 41 years. FEV1 67% predicted. Concomitant inhaled corticosteroids used by 100% of participants. Inclusion criteria: moderate to severe persistent asthma chronically treated with a medium to high dose of ICS, FEV1 % predicted within the entrance range of 45% to 85%, bronchodilator reversibility of FEV1 of at least 12% and 0.20 L from the pre‐albuterol baseline value within 15 to 30 minutes after administration of a standard dose of salbutamol Exclusion criteria: requiring hospitalisation once or emergency treatment more than once in the preceding 6 months, greater than 10‐pack per year smoking history |
|
| Interventions | 1. Symbicort 320 µg/9 µg BD pMDI 2. Budesonide 320 µg BD pMDI 3. Formoterol 9 µg BD DPI 4. Placebo DPI and pMDI Only the formoterol and placebo arms are included in this review |
|
| Outcomes | The co‐primary efficacy variables were baseline adjusted average 12‐hour FEV I and predose FEV I. No all‐cause SAE data reported in the paper but website indicates 2 SAEs on formoterol and none on placebo. There were no deaths. Web data found on AstraZeneca clinical trials website SD‐039‐0717 |
|
| Notes | Sponsored by AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Low risk | Identical packages shipped to centres |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 60% withdrawals in placebo arm and 51.2% in formoterol arm |
| Selective reporting (reporting bias) | Low risk | Full SAE data on website |
Novartis 2005.
| Methods | This represents results from placebo‐controlled trials of at least 4 weeks duration from the Integrated Database of Novartis Clinical Trials | |
| Participants | ||
| Interventions |
|
|
| Outcomes | Asthma‐related SAE data | |
| Notes | These data have been entered as a separate subgroup and not combined with other studies as 5 of the 22 studies are already included in this review, but no separate information has yet been received in relation to the other 17 studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No individual study data available |
| Allocation concealment (selection bias) | Unclear risk | No individual study data available |
| Blinding (performance bias and detection bias) Adverse Events | Unclear risk | No individual study data available |
| Incomplete outcome data (attrition bias) Adverse Events | Unclear risk | No individual study data available |
| Selective reporting (reporting bias) | Unclear risk | No individual study data available |
Pleskow 2003.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, multicentre, parallel‐group study over 12 weeks | |
| Participants |
Population: 554 adults and adolescents (12 to 75) years with mild to moderate persistent asthma Baseline characteristics: mean age 33 years. FEV1 66% predicted. Concomitant inhaled corticosteroids used by 44% of participants Inclusion criteria: mild‐to‐moderate asthma, inhaled B2‐selective adrenoreceptor agonist on a daily basis for 2 or more months, FEV1 % predicted between 40% to 80%, bronchodilator reversibility by an increase of at least 15% in FEV1 within 30 min after inhalation of albuterol 180 µg, chest x‐ray with normal findings or findings consistent with asthma Exclusion criteria: pregnant women/women child‐bearing potential who did not have reliable form of contraception; significant coronary heart disease; prior MI; uncontrolled hypertension; diabetes; convulsive disorder; intolerance of beta‐agonists; URTI within 1 month of study entry; hospitalisation for acute asthma within 1 month of study entry or during run‐in; non‐compliance to medical regimes; parenteral/oral steroids in month prior to visit 1; newly instituted/modified ICS therapy (including discontinuation); disodium cromoglycate; oral/inhaled anticholinergics; desensitisation therapy; recent use of astemizole; use of theophylline in month prior to visit 1; use of antiarrhythmics; use of Prozac; vaccination with live virus in month prior to visit 1; weight 35% above or 25% predicted; significant smoking history; malignancy |
|
| Interventions |
Formoterol delivered by Aerolizer (dry powder) and albuterol by MDI |
|
| Outcomes | Primary outcome: FEV1. Safety was evaluated by adverse event reports. 13 serious adverse events are reported in the paper but not attributed to treatment groups. 2 deaths are fully described as below. No Novartis web report found for this study. FDA submission for study 041 (http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf). This lists all (asthma‐related in brackets) SAE as: 1 (1) on formoterol 12 µg, 7 (5) on formoterol 24 µg, 2 (0) on albuterol and 3 (2) on placebo FDA documents also show 1 death from asthma with formoterol 24 µg and 1 death from pancreatitis with albuterol (which are included in the above SAE totals) |
|
| Notes | Sponsored by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 484/554 (87%) completed the study |
| Selective reporting (reporting bias) | Low risk | Full SAE data from FDA submission |
SD‐037‐0344.
| Methods | Randomised, double‐blind, double‐dummy, parallel‐group, placebo‐controlled, multicentre, 12‐week study with a 2‐week run‐in period. This study was performed in 48 centres across 7 countries (Argentina, Brazil, Greece, Mexico, Philippines, Poland, South Africa). | |
| Participants |
Population: 639 adolescents and adults (12 to 80) years with asthma Baseline characteristics: mean age 35 years. FEV1 not reported. Concomitant inhaled corticosteroids used by 100% of participants. Inclusion criteria: treated with 200 to 1000 µg/day of inhaled steroids for the previous 3 months and on a stable dose for 30 days prior to the start of the run‐in period. For inclusion to the treatment period, total asthma symptom score (night‐time plus day‐time) of greater than 1 on at least 4 of the last 7 days of the run‐in period. |
|
| Interventions | 1. Formoterol (HFA) 9 µg BD (pMDI)
2. Formoterol 9 µg BD (Turbuhaler) 2. Placebo BD |
|
| Outcomes | Primary variable was the change from baseline (mean over last 10 days of run‐in period) to treatment (mean for the 12‐week treatment period) in morning PEF before inhalation of study treatment "One subject, a 66‐year‐old male, died during this study, after 55 days of treatment with formoterol HFA pMDI 9 mg; the investigator considered that there was no relationship between study medication and death. Six subjects experienced a serious adverse event (SAE) during treatment." No paper publication. "The HFA product will not be available on the market." |
|
| Notes | Sponsored by AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 630/639 (97%) completed the study |
| Selective reporting (reporting bias) | Low risk | Full SAE data reported |
Steffensen 1995.
| Methods | A randomised, double‐blind, double‐dummy, placebo‐controlled, multicentre, parallel‐group study over 12 weeks at 20 centres in Scandinavia. Run‐in 2 weeks. | |
| Participants |
Population: 304 adolescents and adults (18 to 79) years with clinically stable asthma Baseline characteristics: mean age 48 years. Concomitant inhaled corticosteroids used by 87% of participants. FEV1 66% predicted. Inclusion criteria: clinically stable and use of inhaled, short‐acting B2‐agonist at least 1 month before the start of the trial, clinical history of reversible obstructive airway disease, FEV1 % predicted at least 40%, bronchodilator reversibility of 15% in FEV1 over baseline 15 to 30 min after inhalation of salbutamol 400 µg Exclusion criteria: unstable asthma, altered dose medication |
|
| Interventions | 1. Formoterol 12 µg BD
2. Salbutamol 400 µg QDS
3. Placebo QDS Delivery was dry powder device |
|
| Outcomes | FEV1, FVC, PEF, rescue use, asthma symptom score. Adverse events, including asthma exacerbations, efficacy rating | |
| Notes | Funding from Ciba Geigy (later Novartis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, double‐dummy, matching devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 260/304 (85%) completed the study |
| Selective reporting (reporting bias) | Low risk | All‐cause SAE described in paper by treatment group |
van der Molen 1997.
| Methods | Randomised, double‐blind, multicentre, placebo‐controlled, parallel‐group study over 24 weeks in 16 centres in Canada and The Netherlands. Patients were recruited from 10 outpatient hospital clinics in Canada and 5 out‐patient clinics and 1 co‐ordinating centre for 44 Dutch general practitioners in The Netherlands. Run‐in 4 weeks. | |
| Participants |
Population: 239 adults with mild to moderate asthma Baseline characteristics: mean age 43 years. FEV1 67% predicted. Concomitant inhaled corticosteroids used by 100% of participants. Inclusion criteria: mild to moderate asthma, regular use of any dose of inhaled corticosteroids, at least 5 inhalations of a short acting B2‐agonist per week before the entry visit, bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline after 2 inhalations of 250 µg terbutaline or the equivalent dose of salbutamol Exclusion criteria: oral corticosteroids at any time in the last month, smoking history of greater than 20 pack‐years, FEV1 less than 40% predicted, or an exacerbation of asthma symptoms during the previous month |
|
| Interventions | 1. Formoterol 24 µg BD
2. Placebo BD Delivery was DPI |
|
| Outcomes | The primary variable in the study was the total daily score of asthma symptoms. No reporting in the paper of serious adverse events. No website found. Manufacturers asked for all‐cause serious adverse event data. These data gave no indication of any deaths. 4 patients with SAE on formoterol and 5 on placebo. 1 and 3 had asthma‐related SAE. |
|
| Notes | Supported by Astra Draco SD‐037‐3008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Blocks of 4 to 1 of the 2 treatment groups of equal size |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 208/239 (87%) completed the study |
| Selective reporting (reporting bias) | Low risk | SAE data provided by sponsors |
van Schayck 2002.
| Methods | Randomised study over 12 weeks at a lung function laboratory in The Netherlands. Run‐in 8 weeks. | |
| Participants |
Population: 162 adolescents and adults (16 to 60) years Baseline characteristics: mean age 35 years, FEV1 86% predicted. Concomitant inhaled corticosteroids used by 95% of participants. Inclusion criteria: history of bronchial symptoms or a clinical diagnosis of asthma, FEV1 % predicted at least 50%, and either PC20 on histamine < 8 mg/mL‐1 or bronchodilator reversibility by an increase of at least 15% in FEV1 over baseline after inhalation of 800 µg salbutamol Exclusion criteria: not reported At the start of the 8‐week washout period, patients to cease all their pulmonary medication (inhaled corticosteroids, cromoglycates, bronchodilators) and to use only rescue medication on demand |
|
| Interventions |
Delivery was MDI |
|
| Outcomes | The effects of bronchodilator use on the perception of airway obstruction There is no indication that data on adverse events were collected. Author has confirmed that no serious adverse events were reported in this study. |
|
| Notes | No support declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | High risk | Open? |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 128/162 (79%) completed the study |
| Selective reporting (reporting bias) | Low risk | No information on SAEs in paper but data provided by author |
Von Berg 2003.
| Methods | A randomised, double‐blind, placebo‐controlled study over 12 weeks at 32 centres in 5 countries in Europe. Run‐in 2 weeks. | |
| Participants |
Population: 248 children (6 to 17) years with mild to moderate asthma Baseline characteristics: mean age 11 years. FEV1 81% predicted. Concomitant inhaled corticosteroids used by 82% of participants. Inclusion criteria: 6 to 17 years, diagnosis of asthma, ATS guidelines, at least 6 months previously, FEV1 % predicted at least 40%, receiving anti‐inflammatory agents (ICS, OCS, nedocromil, cromones) at stable dose for 30 days, able to use Turbuhaler, and peak flow meter, bronchodilator reversibility by either an increase of at least 15% in FEV1 over baseline or an increase of at least 9% in FEV1 predicted or an increase of at least 15% am PEF 4/8 days run‐in Exclusion criteria: use of astemizole within 60 days before inclusion, regular nasal corticosteroids or antihistamines within 30 days, immunotherapy less than 90 days, significant seasonal asthma or allergy, significant medical condition |
|
| Interventions | 1. Formoterol 9 µg BD
2. Formoterol 4.5 µg BD
3. Placebo Delivery was Turbuhaler |
|
| Outcomes | The primary efficacy variable was morning PEF, recorded in patients' diaries "Nine serious AEs (SAEs) were reported in eight patients in the formoterol groups (n=3 formoterol 4.5 mcg and n=5 in formoterol 9 mcg) and one SAE in the placebo group; none of the SAEs was considered causally related to the study drug. One death was reported in the formoterol 9 mcg group, from a subarachnoid haemorrhage, which was judged unlikely to be due to the study drug." Further information has been provided by the author to clarify that 2 patients in the formoterol 9 µg arm suffered 2 serious adverse events (asthma and haematuria in 1 patient, and abdominal pain and appendicitis in 1 patient), so 3 patients in each formoterol arm and 1 in the placebo arm had any SAE. The author also confirmed that the participant who died was not included in the 9 SAE events, but was one of the 8 patients who suffered a SAE. |
|
| Notes | No sponsorship reported in the paper but the author confirms that the study was sponsored by Astra | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive patient number, 1:1:1 ratio using computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind, identical devices |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 225/248 (91%) completed the study |
| Selective reporting (reporting bias) | Low risk | SAE report in the paper |
Wolfe 2006.
| Methods | Randomised, double‐blind, multicentre, placebo‐controlled, parallel‐group study over 16 weeks in 194 outpatient clinics in the US. Run‐in 2 weeks single‐blind placebo. | |
| Participants |
Population: 2085 adolescents and adults (12 to 82) years with stable, persistent asthma Baseline characteristics: mean age 38 years. FEV1 69% predicted. Concomitant inhaled corticosteroids used by 62% of participants. Inclusion criteria: persistent asthma, use of inhaled beta‐agonist for 2 months prior to study entry, FEV1 at least 40% of predicted normal following washout from inhaled bronchodilator treatment, FEV1 reversibility at least 12% after inhalation of up to 4 puffs of albuterol (360 µg) at screening or documented within the past year Exclusion criteria: hospitalisation within 1 month/treatment of exacerbation of asthma in month prior to study entry; evidence/array of other systemic diseases; pregnancy/failure to use reliable contraception; change to ongoing medication used to treat chronic asthma |
|
| Interventions | 1. Formoterol 12 µg BID
2. Placebo
3. Formoterol 24 µg BID 4. Open‐label formoterol 12 µg BID (with up to 2 uses prn per day). This arm was not included in the review. Delivery was DPI (Aerolizer) |
|
| Outcomes | Serious asthma exacerbations (life‐threatening or requiring hospitalisation) were the primary end point "There were no deaths in the study." SAE data not reported for all causes in the paper but available from FDA documentation. Web data available from documents on FDA website but not yet on Novartis Clinical Trials (16 June 2008) |
|
| Notes | Sponsored by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 ratio using computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) Adverse Events | Low risk | 1791/2085 (86%) completed the study |
| Selective reporting (reporting bias) | Low risk | Paper report is not transparent on serious adverse events from any cause in each arm of the trial, but these data are available from FDA documentation |
Zimmerman 2004.
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre, parallel‐group study over 12 weeks at 27 centres in Canada. Run‐in 2 weeks. | |
| Participants |
Population: 302 children (6 to 11) years with asthma not optimally treated with inhaled corticosteroids alone Baseline characteristics: mean age 9 years. FEV1 78% predicted. Concomitant inhaled corticosteroids used by 100% of participants. Inclusion criteria: aged 6 to 11 years with a clinical diagnosis of asthma according to American Thoracic Society criteria for at least 6 months, FEV1 % predicted of 50% to 90%, bronchodilator reversibility of at least 15%, or at least 9% of predicted normal, treatment with regular inhaled corticosteroids for at least 3 months before trial entry, asthma symptoms sufficient to suggest that additional therapy might be needed Exclusion criteria: known or suspected hypersensitivity to formoterol or inhaled lactose, deteriorating asthma or a respiratory infection, clinically significant concurrent disease, significant seasonal allergy, or if smokers, taken disallowed asthma medications before trial entry, oral corticosteroids or antileukotrienes within 30 days, astemizole within 60 days, sodium cromoglycate or ketotifen within 7 days, salmeterol or formoterol within 72 hours, xanthines or antihistamines within 48 hours |
|
| Interventions | 1. Formoterol 4.5 µg or 9 µg BD
2. Placebo BD Delivery was DPI |
|
| Outcomes | The primary efficacy variable was change from baseline in morning peak expiratory flow (PEF) No information in the paper on SAEs. Manufacturers asked for all‐cause serious adverse event data: no deaths were reported. Placebo ‐ 1 patient with SAE due to asthma. Formoterol 4.5 ‐ 1 patient with SAE due to dog bite. Formoterol 9 ‐ 2 patients with SAE due to URTI and asthma AZ study code DC‐037‐0002 |
|
| Notes | No support declared in the paper, but data on file with AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) Adverse Events | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) Adverse Events | Unclear risk | 267/302 (88%) completed the study |
| Selective reporting (reporting bias) | Low risk | SAE data provided by sponsors |
AE: adverse event; ATS: American Thoracic Society; AUC: area under curve; BD: twice a day; BDP: budesonide dipropionate; CS: corticosteroids; DPI: dry powder inhaler; ECG: electrocardiogram; FDA: Food and Drug Administration (USA); FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICS: inhaled corticosteroids; ITT: intention‐to‐treat; MAOI: monoamine oxidase inhibitors; MDI: metered‐dose inhaler; MI: myocardial infarction; NSAIDs: nonsteroidal anti‐inflammatory drugs; OS: oral steroids; PEF: peak expiratory flow; pMDI: pressurised metered‐dose inhaler; QDS: four times a day; RT: respiratory tract; SABA: short‐acting beta2‐agonist; SAE: serious adverse event; URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 2006 | 2‐week study |
| Akpinarli 1999 | 6‐week treatment periods |
| Ankerst 2003 | Short‐term high‐dose tolerance study |
| Arvidsson 1989 | 2‐week study |
| Aziz 1998 | Volunteers (not asthmatics) |
| Bauer 1995 | Single dose |
| Behling 1999 | 3‐week study |
| Boner 1994 | Single dose |
| Bousquet 2005 | Single dose |
| Bousquet 2005a | Single dose |
| Brambilla 2003 | 4‐week study |
| Bronsky 2002 | Single dose |
| Bronsky 2004 | Single dose |
| Brusasco 2002 | Single dose |
| Burgess 1998 | Single dose |
| Cazzola 2002 | Single dose |
| Ceylan 2004 | Formoterol versus montelukast |
| Chetta 1993 | Single dose |
| Cheung 2006 | 3‐week study |
| Chuchalin 2002 | Formoterol randomised with budesonide |
| Chuchalin 2005 | 1‐week study |
| Chuchalin 2005a | Formoterol randomised with budesonide |
| Chung 1995 | Cross‐over design |
| Dahl 2004 | Device comparison |
| Daugbjerg 1996 | Single dose |
| Dey 2005 | Cross‐over design |
| Dietrich 2006 | Device comparison |
| Dubakiene 2006 | Device comparison |
| Dusser 2005 | HFA versus DPI delivery comparison |
| Ericsson 2006 | Randomised with budesonide |
| Eryonucu 2005 | Single dose |
| Ferrari 2000 | Single dose |
| Fitoussi 2002 | 3‐day study |
| Gessner 2003 | 8‐week study |
| Graff‐Lonnevig 1990 | Cross‐over design |
| Green 2006 | Cross‐over design |
| Happonen 2009 | Cross‐over study comparing formoterol Easyhaler with Foradil Aerolizer |
| Hedenstrom 1992 | Cross‐over design |
| Hermansen 2006 | EIB single doses |
| Houghton 2004 | Single dose |
| Ind 2002 | Formoterol used as reliever |
| Jain 2004 | Formoterol used as reliever |
| Jenkins 2005 | 6‐week treatment periods |
| Kamenov 2007 | Formoterol ‐ HFA metered‐dose inhaler compared to formoterol dry powder inhaler |
| Kesten 1992 | Not RCT |
| Kohler 2003 | Acute asthma |
| Kruse 2005 | Cross‐over design |
| Lebecque 1994 | Single dose |
| Lebecque 1994a | Single dose |
| Lee‐Wong 2008 | Acute asthma |
| Lemaigre 2006 | Single dose |
| Lotvall 1997 | Single dose |
| Lotvall 2005 | Formoterol as reliever |
| Lotvall 2008 | Single dose |
| Lyseng‐Williamson 2003 | Not RCT |
| Maesen 1992 | Short‐term study over 3 days |
| Maesen 1992a | Delivery device and dose |
| Malo 1990 | Single dose |
| Malolepszy 2001 | Formoterol versus theophylline |
| Malolepszy 2002 | Acute asthma |
| Mann 2003 | Overview of 3 included studies: Bensch 2001; Bensch 2002; Pleskow 2003 |
| Marzo 2000 | Healthy volunteers |
| Matthys 2004 | 3‐week study |
| Midgren 1992 | 4‐week study |
| Molimard 2005 | Cumulative dose study |
| Najafizadeh 2007 | Acute asthma |
| Nandeuil 2006 | 8‐day study |
| Newnham 1994 | 4‐week study |
| Newnham 1995 | 4‐week study |
| Novartis 2005b | Both arms received formoterol maintenance |
| Otto‐Knapp 2008 | 4‐week study |
| Patessio 1991 | EIB |
| Pauwels 2003 | Formoterol as reliever |
| Pearlman 2002 | Overview of other studies |
| Pohunek 2004 | Single dose |
| Price 2002 | Randomised together with budesonide |
| Price 2008 | Overview of safety data from AstraZeneca‐sponsored trials of duration of at least 4 weeks |
| Randell 2005 | Device comparison |
| Richter 2007 | Dosage comparison |
| Rico‐Mendez 1999 | Study included patients with chronic bronchitis |
| Ringdal 1998 | Single dose |
| Rosenborg 2000 | Single dose |
| Rosenborg 2002 | Short‐term dose‐response study |
| Rosenborg 2002a | Short‐term dose‐response study |
| Rubinfeld 2006 | Acute asthma |
| Schlimmer 2002 | Acute asthma |
| Sprogoe 1992 | 6‐week study |
| Stahl 2003 | Formoterol as reliever |
| Stelmach 2008 | Study on exercise‐induced bronchospasm |
| Tattersfield 1999 | Description of exacerbations in FACET study |
| Tattersfield 2001 | As‐needed formoterol |
| Totterman 1998 | 3‐day study |
| van den Berg 1995 | 8‐day study |
| van der Woude 2004 | Single dose |
| van Veen 2003 | 2‐week cross‐over |
| Verini 1998 | 5‐day study |
| Villa 2002 | As‐needed formoterol |
| Villa 2002a | As‐needed formoterol |
| Vilsvik 2001 | EIB |
| Wallin 1999 | 8‐week study |
| Wegener 1992 | Single dose |
| Wong 1992 | Single dose |
| Yasuo 1984 | 4‐week study |
| Yasuo 1984a | Single dose |
| Yasuo 1984b | Single dose |
| Yates 1995 | 2‐week cross‐over |
| Yurdakul 2002 | No placebo or SABA control |
DPI: dry powder inhaler; EIB: exercise induced bronchoconstriction; HFA: hydrofluoroalkane; RCT: randomised controlled trial; SABA: short‐acting beta2‐agonist
Differences between protocol and review
We did not use risk difference as the primary metric for analysis of rare events, due to new advice in the latest revision of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). One of the peer reviewers also pointed out that Bradburn 2007 cautions against the use of inverse‐variance and DerSimonian and Laird methods for sparse data, so for the 2009 update we used the Peto OR for the primary analysis, as no continuity correction is required for zero cells. This brings the analysis for this review in line with the other reviews in this series.
Also we investigated reporting bias by comparing published and unpublished serious adverse events, and investigating the impact of using drug‐related adverse events and the combined results from serious and non‐serious adverse events.
Contributions of authors
CJC: conception of the idea, study selection and data collection, statistical analysis and co‐writing of the review. MJC: background information (including Appendices), study selection and co‐writing of the review.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
Programme grant
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bensch 2001 {published data only}
- Bensch G, Lapidus RJ, Levine BE, Lumry W, Yegen U, Kiselev P, et al. A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Annals of Allergy, Asthma, and Immunology 2001; Vol. 86, issue 1:19‐27. [DOI] [PubMed]
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. [DOI] [PubMed]
- Novartis. A twelve‐week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered‐dose inhaler (MDI) versus placebo in patients with mild to moderate asthma. FDA Application 20‐831 (Trial reference 040) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
Bensch 2002 {published data only}
- Bensch G, Berger WE, Blokhin BM, Socolovsky AL, Thomson MH, Till MD, et al. One‐year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy, Asthma and Immunology 2002; Vol. 89, issue 2:180‐90. [DOI] [PubMed]
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. [DOI] [PubMed]
- Novartis. A twelve‐month, double‐blind, between patient placebo controlled trial comparing the safety, tolerability and efficacy of 12 mcg and 24 mcg twice daily dry powder capsules for inhalation delivered by a single‐dose inhaler (AerolizerTM) in children with asthma in need of daily treatment with inhaled bronchodilators and anti‐inflammatory treatment. FDA Application 20‐831 (Trial reference 049) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
Busse 2004 {published data only}
- Busse W, Levine B, Andriano K, Lavecchia C, Yegen U. Efficacy, tolerability, and effect on asthma‐related quality of life of formoterol BID via multidose dry powder inhaler and albuterol QID via metered dose inhaler in patients with persistent asthma: a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2004; Vol. 26, issue 10:1587‐98. [DOI] [PubMed]
Corren 2007 {published data only}
- AstraZeneca. SD‐039‐0716. A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐controlled trial of SYMBICORT® pMDI (80/4.5 μg) versus its monoproducts (budesonide and formoterol) in children (≥ 6 years of age) and adults with asthma – SPRUCE 80/4.5. http://www.astrazenecaclinicaltrials.com/sites/133/imagebank/typeArticleparam528362/SD‐039‐0716.pdf Accessed November 2008.
- Corren J, Korenblat PE, Miller CJ, O'Brien CD, Mezzanotte WS. Twelve‐week, randomized, placebo‐controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered‐dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clinical Therapeutics 2007; Vol. 29, issue 5:823‐43. [DOI] [PubMed]
Ekstrom 1998 {published data only}
Ekstrom 1998a {published data only}
FitzGerald 1999 {published data only}
- FitzGerald JM, Chapman KR, Della Cioppa G, Stubbing D, Fairbarn MS, Till MD, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy and Clinical Immunology 1999; Vol. 103, issue 3 I:427‐35. [DOI] [PubMed]
Hekking 1990 {published data only}
Kesten 1991 {published data only}
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. A three‐month comparison of twice daily inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991; Vol. 144, issue 3 Pt 1:622‐5. [DOI] [PubMed]
- Sculpher MJ, Buxton MJ. The episode‐free day as a composite measure of effectiveness: an illustrative economic evaluation of formoterol versus salbutamol in asthma therapy [see comments]. Pharmacoeconomics 1993; Vol. 4, issue 5:345‐52. [DOI] [PubMed]
Kozlik‐Feldmann 1996 {published data only}
- Kozlik‐Feldmann R, Berg A, Berdel D, Reinhardt D. Long‐term effects of formoterol and salbutamol on bronchial hyperreactivity and beta‐adrenoceptor density on lymphocytes in children with bronchial asthma. European Journal of Medical Research 1996; Vol. 1, issue 10:465‐70. [PubMed]
LaForce 2005 {published data only}
- LaForce C, Prenner BM, Andriano K, Lavecchia C, Yegen U. Efficacy and safety of formoterol delivered via a new multidose dry powder inhaler (Certihaler) in adolescents and adults with persistent asthma. Journal of Asthma 2005; Vol. 42, issue 2:101‐6. [PubMed]
Levy 2005 {published data only}
- Levy R, Pinnas J, Milgrom H, Smith J, Yegen U. Safety and efficacy in children with persistent asthma treated with formoterol 10 mug BID delivered via Certihaler: a novel multidose dry‐powder inhaler. Pediatric Asthma Allergy & Immunology 2005; Vol. 18, issue 1:25‐35.
Molimard 2001 {published data only}
Noonan 2006 {published data only}
Novartis 2005 {published data only}
- Novartis. Pulmonary‐Allergy Drugs Advisory Committee on the safety of long‐acting beta‐agonist bronchodilators NDA 20‐831 Briefing Document. http://www.fda.gov/ohrms/dockets/AC/05/briefing/2005‐4148B1_02_01‐Novartis‐Foradil.pdf Accessed July 2008.
Pleskow 2003 {unpublished data only}
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003; Vol. 124, issue 1:70‐4. [DOI] [PubMed]
- Novartis. A twelve‐week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered‐dose inhaler (MDI) versus placebo in patients with mild to moderate asthma. FDA Application 20‐831 (Trial reference 041) Accessed 2 June 2008. [http://www.fda.gov/cder/foi/nda/2001/20831_Foradil_medr_P1.pdf ]
- Pleskow W, LaForce CF, Yegen U, Matos D, Della Cioppa G. Formoterol delivered via the dry powder Aerolizer inhaler versus albuterol MDI and placebo in mild‐to‐moderate asthma: a randomized, double‐blind, double‐dummy trial. Journal of Asthma 2003; Vol. 40, issue 5:505‐14. [DOI] [PubMed]
SD‐037‐0344 {unpublished data only}
- AstraZeneca. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis Turbuhaler in subjects with asthma. www.astrazenecaclinicaltrials.com/Article/515841.aspx Accessed 19 June 2008.
- AstraZeneca. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised,parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis® Turbuhaler® in subjects with asthma. www.astrazenecaclinicaltrials.com/sites/133/imagebank/typeArticleparam528785/D5125C00344.pdf Accessed 19 June 2008.
Steffensen 1995 {published data only}
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3‐month, placebo‐controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12‐month trial with formoterol. Allergy 1995; Vol. 50, issue 8:657‐63. [DOI] [PubMed]
- Steffensen IE, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996; Vol. 158, issue 49:7092‐6. [PubMed]
van der Molen 1997 {published data only}
- Molen T. Formoterol and asthma exacerbations [5]. Chest 2004; Vol. 125, issue 4:1591. [PubMed]
- Molen T, Postma DS, Turner MO, Jong BM, Malo JL, Chapman K, et al. Effects of the long acting beta agonist formoterol on asthma control in asthmatic patients using inhaled corticosteroids. The Netherlands and Canadian Formoterol Study Investigators. Thorax 1997; Vol. 52, issue 6:535‐9. [DOI] [PMC free article] [PubMed]
- Molen T, Sears MR, Graaff CS, Postma DS, Meyboom‐de Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. Canadian and the Dutch Formoterol Investigators. European Respiratory Journal 1998; Vol. 12, issue 1:30‐4. [DOI] [PubMed]
van Schayck 2002 {published data only}
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta2‐agonists in allergic asthmatic patients. Chest 2001; Vol. 119, issue 5:1306‐15. [DOI] [PubMed]
- Schayck CP, Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C. Potential masking effect on dyspnoea perception by short‐ and long‐acting beta2‐agonists in asthma. European Respiratory Journal 2002; Vol. 19, issue 2:240‐5. [DOI] [PubMed]
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002; Vol. 96, issue 3:155‐62. [DOI] [PubMed]
Von Berg 2003 {published data only}
- Berg A, Papageorgiou Saxoni F, Wille S, Carrillo T, Kattamis C, Helms PJ. Efficacy and tolerability of formoterol turbuhaler in children. International Journal of Clinical Practice 2003; Vol. 57, issue 10:852‐6. [PubMed]
Wolfe 2006 {published data only}
- Friedman B, Sokoi W, Ziehmer B, Orevillo C, Till D. Comparable efficacy and tolerability of formoterol 12 µg or 24 µg twice daily in asthma patients [Abstract]. European Respiratory Journal 2005; Vol. 26, issue Suppl 49:Abstract No. 435.
- Gunkel H. FDA Clinical Review: Foradil Aerolizer. www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005‐4148B1_03_03‐FDA‐Clinical‐Review.pdf Accessed 16 June 2008.
- Wolfe J, LaForce C, Ostrom NK, Korenblat P, Orevillo C, Ziehmer B, et al. Formoterol 24 µg is not associated with an increase in serious asthma exacerbations in patients with persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2005; Vol. 115, issue Suppl 2:S150.
- Wolfe J, LaForce C, Ziehmer B, Orevillo C, Till D. No evidence for increase in serious asthma exacerbations with formoterol 24ug twice daily [Abstract]. European Respiratory Journal 2005; Vol. 26, issue Suppl 49:Abstract No. 434.
- Wolfe J, Laforce C, Friedman B, Sokol W, Till D, Della Cioppa G, et al. Formoterol, 24 microg bid, and serious asthma exacerbations: similar rates compared with formoterol, 12 microg bid, with and without extra doses taken on demand, and placebo. Chest 2006; Vol. 129, issue 1:27‐38. [DOI] [PubMed]
Zimmerman 2004 {published data only}
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol turbuhaler(R) when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004; Vol. 37, issue 2:122‐7. [DOI] [PubMed]
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol Turbuhaler in 6‐11 year old children with asthma, not adequately controlled with inhaled corticosteroids [abstract]. European Respiratory Society Annual Congress 2002 2002:Abstract no: P2734.
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol turbuhaler compared with placebo in children (6‐11 years) with asthma poorly controlled with inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A746.
References to studies excluded from this review
Adler 2006 {published data only}
Akpinarli 1999 {published data only}
- Akpinarli A, Tuncer A, Saraclar Y, Sekerel BE, Kalayci O. Effect of formoterol on clinical parameters and lung functions in patients with bronchial asthma: a randomised controlled trial. Archives of Disease in Childhood 1999; Vol. 81, issue 1:45‐8. [DOI] [PMC free article] [PubMed]
Ankerst 2003 {published data only}
Arvidsson 1989 {published data only}
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Wahlander L, Svedmyr N. Formoterol, a new long‐acting bronchodilator for inhalation. European Respiratory Journal 1989; Vol. 2, issue 4:325‐30. [PubMed]
Aziz 1998 {published data only}
Bauer 1995 {published data only}
Behling 1999 {published data only}
- Behling B, Matthys H. Comparison of efficacy and safety of formoterol with terbutaline in children with mild to moderate asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain 1999:P369.
Boner 1994 {published data only}
Bousquet 2005 {published data only}
- Bousquet J, Guenole E, Duvauchelle T, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, single‐dose, crossover trial evaluating the efficacy and safety profiles of two dose levels (12 and 24 microg) of formoterol‐HFA (pMDI) vs. those of a dose level (24 microg) of formoterol‐DPI (Foradil/Aerolizer) and of placebo (pMDI or Aerolizer) in moderate to severe asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:13‐9. [DOI] [PubMed]
Bousquet 2005a {published data only}
- Bousquet J, Huchon G, Leclerc V, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, single‐dose, efficacy crossover trial comparing formoterol‐HFA (pMDI) versus formoterol‐DPI (Aerolizer) and placebo (pMDI or Aerolizer) in asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:6‐12. [DOI] [PubMed]
Brambilla 2003 {published data only}
- Brambilla C, Gros V, Bourdeix I, Efficacy of Foradil in Asthma French Study G. Formoterol 12 microg BID administered via single‐dose dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on‐demand salbutamol: a multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2003; Vol. 25, issue 7:2022‐36. [DOI] [PubMed]
Bronsky 2002 {published data only}
Bronsky 2004 {published data only}
Brusasco 2002 {published data only}
- Brusasco V, Crimi E, Gherson G, Nardelli R, Oldani V, Francucci B, et al. Actions other than smooth muscle relaxation may play a role in the protective effects of formoterol on the allergen‐induced late asthmatic reaction. Pulmonary Pharmacology & Therapeutics 2002; Vol. 15, issue 4:399‐406. [DOI] [PubMed]
Burgess 1998 {published data only}
Cazzola 2002 {published data only}
Ceylan 2004 {published data only}
Chetta 1993 {published data only}
- Chetta A, Donno M, Maiocchi G, Pisi G, Moretti D, Olivieri D. Prolonged bronchodilating effect of formoterol versus procaterol in bronchial asthma. Annals of Allergy 1993; Vol. 70, issue 2:171‐4. [PubMed]
Cheung 2006 {published data only}
Chuchalin 2002 {published data only}
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN, Epoch Study G. The safety and efficacy of formoterol OxisR TurbuhalerR plus budesonide PulmicortR Turbuhaler in mild to moderate asthma: A comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002; Vol. 56, issue 1:15‐20. [PubMed]
Chuchalin 2005 {published data only}
Chuchalin 2005a {published data only}
- Chuchalin AG, Manjra AI, Rozinova NN, Skopkova O, Cioppa GD, Till D, et al. Formoterol delivered via a new multi‐dose dry powder inhaler (Certihaler) is as effective and well tolerated as the formoterol dry powder inhaler (Aerolizer) in children with persistent asthma. Journal of Aerosol Medicine 2005; Vol. 18, issue 1:63‐73. [DOI] [PubMed]
Chung 1995 {published data only}
- Chung KF. Eformoterol and bronchial hyperresponsiveness in mild asthma. British Journal of Clinical Practice. Supplement 1995, issue 81:14‐5. [PubMed]
Dahl 2004 {published data only}
- Dahl R, Creemers JP, Noord J, Sips A, Della Cioppae G, Thomson M, et al. Comparable efficacy and tolerability of formoterol (Foradil) administered via a novel multi‐dose dry powder inhaler (Certihaler) or the Aerolizer dry powder inhaler in patients with persistent asthma. Respiration 2004; Vol. 71, issue 2:126‐33. [DOI] [PubMed]
Daugbjerg 1996 {published data only}
Dey 2005 {published data only}
- Dey. Study in patients with asthma. Clinicaltrials.gov 2005.
Dietrich 2006 {published data only}
- Dietrich H, Nguyen TD, Buermann U, Petzold U, Munzel U, Maus J. Pharmacodynamic (PD) pharmacokinetic (PK) and safety profile of formoterol delivered via two different dry different dry powder inhalers (DPIs) [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:614s [E3606].
Dubakiene 2006 {published data only}
Dusser 2005 {published data only}
- Dusser D, Vicaut E, Lefrancois G. Double‐blind, double‐dummy, multinational, multicenter, parallel‐group design clinical trial of clinical non‐inferiority of formoterol 12 microg/unit dose in a b.i.d. regimen administered via an HFA‐propellant‐pMDI or a dry powder inhaler in a 12‐week treatment period of moderate to severe stable persistent asthma in adult patients. Respiration 2005; Vol. 72, issue Suppl 1:20‐7. [DOI] [PubMed]
Ericsson 2006 {published data only}
Eryonucu 2005 {published data only}
Ferrari 2000 {published data only}
Fitoussi 2002 {published data only}
- Fitoussi S, Dobson C, Ayre G, Langholff W. Safety and tolerability profile of high dose formoterol (Aerolizer®) and salbutamol (MDI) given for three days in patients with mild persistent asthma. Chest 2002:P117.
Gessner 2003 {published data only}
Graff‐Lonnevig 1990 {published data only}
Green 2006 {published data only}
Happonen 2009 {published data only}
- Happonen P, Harkonen M, Vaheri R, Rytila P. Long‐term safety of inhaled formoterol in adult asthma patients [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16 2009:[P1964].
Hedenstrom 1992 {published data only}
Hermansen 2006 {published data only}
Houghton 2004 {published data only}
- Houghton CM, Langley SJ, Singh SD, Holden J, Monici Preti AP, Acerbi D, et al. Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo‐controlled, crossover study. British Journal of Clinical Pharmacology 2004; Vol. 58, issue 4:359‐66. [DOI] [PMC free article] [PubMed]
Ind 2002 {published data only}
Jain 2004 {published data only}
- Jain A, Raghuram J. Randomized controlled study of the safety and efficacy of PRN formoterol compared to PRN albuterol for the management of asthma [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando 2004:B36 Poster G17.
Jenkins 2005 {published data only}
Kamenov 2007 {published data only}
- Kamenov A, Kamenov S, Kamenov B. The efficacy and safety formoterol‐HFA pMDI in school children with persistent asthma [Abstract]. European Respiratory Journal 2007; Vol. 30, issue Suppl 51:460s [E2712].
Kesten 1992 {published data only}
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. Sustained improvement in asthma with long‐term use of formoterol fumarate. Annals of Allergy 1992; Vol. 69, issue 5:415‐20. [PubMed]
Kohler 2003 {published data only}
- Kohler D, Schmidt F, Wiese C, Dellweg D, Haidl P. Safety concerns regarding the use of the formoterol Turbohaler® for acute asthma attacks [abstract]. American Thoracic Society 99th International Conference 2003:B036 Poster H74.
Kruse 2005 {published data only}
Lebecque 1994 {published data only}
- Lebecque P, Vliers S, Saint‐Moulin T, Godding V. The effect of a single dose of formoterol using an aerosol inhaler in asthmatic children. A randomized, controlled, double‐blind study. Revue des Maladies Respiratoires 1994; Vol. 11, issue 1:47‐50. [PubMed]
Lebecque 1994a {published data only}
- Lebecque P, Vliers S, De SMT, Godding V. Response to a single dose of inhaled formoterol in asthmatic children: a double‐blind randomized controlled study. Revue des Maladies Respiratoires 1994; Vol. 11, issue 1:47‐50. [PubMed]
Lee‐Wong 2008 {published data only}
Lemaigre 2006 {published data only}
Lotvall 1997 {published data only}
- Lotvall J, Persson G, Larsson P, Mardell C, Rosenborg J. Tolerability of inhaled high doses of formoterol and salbutamol in asthmatic patients maintained on twice daily formoterol 12 (9µg) delivered [abstract]. European Respiratory Journal. Supplement 1997; Vol. 10 Suppl 25:103S.
Lotvall 2005 {published data only}
Lotvall 2008 {published data only}
Lyseng‐Williamson 2003 {published data only}
Maesen 1992 {published data only}
Maesen 1992a {published data only}
Malo 1990 {published data only}
Malolepszy 2001 {published data only}
Malolepszy 2002 {published data only}
- Malolepszy J. Efficacy and tolerability of oral theophylline slow‐release versus inhalative formoterol in moderate asthma poorly controlled on low‐dose steroids. Atemwegs‐ und Lungenkrankheiten 2002; Vol. 28, issue 2:78‐87.
Mann 2003 {published data only}
Marzo 2000 {published data only}
Matthys 2004 {published data only}
- Matthys H, Behling B, Behling E. Comparison of formoterol (12 and 6 mug b.i.d) vs terbutaline (0.5 mg b.i.d) with equal doses of inhaled budesonide (0.2 mg bid) in 246 children with mild to moderate asthma. Atemwegs‐ und Lungenkrankheiten 2004; Vol. 30, issue 5:251‐6.
Midgren 1992 {published data only}
Molimard 2005 {published data only}
- Molimard M, Guenole E, Duvauchelle T, Vicaut E, Lefrancois G. A randomized, double‐blind, double‐dummy, safety crossover trial comparing cumulative doses up to 96 microg of formoterol delivered via an HFA‐134a‐propelled pMDI vs. same cumulative doses of formoterol DPI and placebo in asthmatic patients. Respiration 2005; Vol. 72, issue Suppl 1:28‐34. [DOI] [PubMed]
Najafizadeh 2007 {published data only}
- Najafizadeh K, Pour HS, Ghadyanee M, Shiehmorteza M, Jamali M, Majdzadeh S. A randomized, double‐blind, placebo‐controlled study to evaluate the role of formoterol in the management of acute asthma. Emergency Medicine Journal 2007; Vol. 24, issue 5:317‐21. [DOI] [PMC free article] [PubMed]
Nandeuil 2006 {published data only}
- Nandeuil A, Kottakis I, Raptis H, Roslan H, Ivanov Y, Woodcock A. Safety and tolerability of the novel very long acting B2 agonist carmoterol given as a 2µg qd dose 8 days comparison with formoterol and placebo in patients with persistent asthma [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:665s [P3859].
Newnham 1994 {published data only}
Newnham 1995 {published data only}
- Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax 1995; Vol. 50, issue 5:497‐504. [DOI] [PMC free article] [PubMed]
Novartis 2005b {published data only}
- Novartis. A 12‐month multicenter, randomized, double‐blind, double‐dummy trial to examine the long‐term tolerability of fomoterol 10µg via the multiple dose dry powder inhaler (MDDPI), both as twice daily maintenance therapy and as on‐demand use in addition to maintenance in patients with persistent asthma. http://pharma.us.novartis.com/ 2005.
Otto‐Knapp 2008 {published data only}
- Otto‐Knapp R, Conrad F, Hosch S, Metzenauer P, Maus J, Noga O, et al. Efficacy and safety of formoterol delivered through the Novolizer, a novel dry powder inhaler (DPI) compared with a standard DPI in patients with moderate to severe asthma. Pulmonary Pharmacology and Therapeutics 2008; Vol. 21, issue 1:47‐53. [DOI] [PubMed]
Patessio 1991 {published data only}
- Patessio A, Podda A, Carone M, Trombetta N, Donner CF. Protective effect and duration of action of formoterol aerosol on exercise‐induced asthma. European Respiratory Journal 1991; Vol. 4, issue 3:296‐300. [PubMed]
Pauwels 2003 {published data only}
Pearlman 2002 {published data only}
Pohunek 2004 {published data only}
Price 2002 {published data only}
- Price D, Dutchman D, Mawson A, Bodalia B, Duggan S, Todd P. Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose. Thorax. 2002/08/30 2002; Vol. 57, issue 9:791‐8. [0040‐6376: (Print)] [DOI] [PMC free article] [PubMed]
Price 2008 {published data only}
- Price J, Radner F, Hultquist C, Lindberg B. Safety of formoterol in children and adolescents: experience from asthma clinical trials [Abstract]. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8 2008:[E3053].
Randell 2005 {published data only}
Richter 2007 {published data only}
Rico‐Mendez 1999 {published data only}
- Rico‐Mendez FG, Ochoa G, Rocio Chapela M, Diaz M, Cohen V, Ibarra J, et al. Dry powdered formoterol, twice a day versus aerosolized salbutamol, four times a day, in patients with stable asthma. Revista Alergia Mexico 1999; Vol. 46, issue 5:130‐5. [PubMed]
Ringdal 1998 {published data only}
Rosenborg 2000 {published data only}
Rosenborg 2002 {published data only}
Rosenborg 2002a {published data only}
- Rosenborg J, Larsson P, Rott Z, Bocskei C, Poczi M, Juhasz G. Assessment of a relative therapeutic index between inhaled formoterol and salbutamol in asthma patients. European Journal of Clinical Pharmacology 2002; Vol. 58, issue 4:S61‐7. [PubMed]
Rubinfeld 2006 {published data only}
Schlimmer 2002 {published data only}
Sprogoe 1992 {published data only}
- Sprogoe Jakobsen U, Viktrup L, Davidsen O, Viskum K. Bronchial asthma treated with long‐acting beta 2 agonist. Comparison between formoterol 12 mu/g inhaled twice daily and salbutamol 200. Ugeskrift for Laeger 1992; Vol. 154, issue 47:3325‐8. [PubMed]
Stahl 2003 {published data only}
Stelmach 2008 {published data only}
Tattersfield 1999 {published data only}
Tattersfield 2001 {published data only}
Totterman 1998 {published data only}
van den Berg 1995 {published data only}
van der Woude 2004 {published data only}
van Veen 2003 {published data only}
Verini 1998 {published data only}
Villa 2002 {published data only}
- Villa J, Kuna P, Egner J, Brander R. A 6‐month comparison of the safety profiles of formoterol (Oxis®) Turbuhaler® as needed and terbutaline (Bricanyl®) Turbuhaler® as needed in asthmatic children on anti‐inflammatory medication [abstract]. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A746.
Villa 2002a {published data only}
- Villa J, Kuna P, Egner J, Brander R. Safety of formoterol reliever therapy compared with terbutaline in asthmatic children taking anti‐inflammatory therapy [abstract]. European Respiratory Society Annual Congress 2002 2002:Abstract no: P2739.
Vilsvik 2001 {published data only}
Wallin 1999 {published data only}
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory and Critical Care Medicine 1999; Vol. 159, issue 1:79‐86. [DOI] [PubMed]
Wegener 1992 {published data only}
Wong 1992 {published data only}
Yasuo 1984 {published data only}
- Yasuo K, TakaoI S, Mitsuru W, Terumi T, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a new beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind repeated‐dose study at 37 institutions. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:697‐727.
Yasuo 1984a {published data only}
- Yasuo K, Terumi T, Mitsuru I, Takao S, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind single‐dose study at 37 institutions Part 2. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:671‐95.
Yasuo 1984b {published data only}
- Yasuo K, TerumiI T, Kazuhiko TO, Takao S, Shigenori N, Eisei N, et al. Evaluation of therapeutic effects of formoterol, a beta‐receptor stimulating bronchodilator, on bronchial asthma ‐ a joint double‐blind single‐dose study at 30 institutions Part 1. Rinsho Hyoka (Clinical Evaluation) 1984; Vol. 12, issue 3:647‐70.
Yates 1995 {published data only}
Yurdakul 2002 {published data only}
Additional references
Altman 2003
- Altman DG, Bland JM. Statistics notes: interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2006
Arnold 1985
- Arnold JMO, O'Connor PC, Riddell JG, Harron DWG, Shanks RG, McDevitt DG. Effects of the beta‐2‐adrenoceptor antagonist ici‐118,551 on exercise tachycardia and isoprenaline‐induced beta‐adrenoceptor responses in man. British Journal of Clinical Pharmacology 1985; Vol. 19, issue 5:619‐30. [DOI] [PMC free article] [PubMed]
Barnes 1993
Barnes 1995
Bennett 1994
- Bennett JA, Smyth ET, Pavord ID, Wilding PJ, Tattersfield AE. Systemic effects of salbutamol and salmeterol in patients with asthma. Thorax 1994; Vol. 49, issue 8:771‐4. [DOI] [PMC free article] [PubMed]
Benson 1948
Bergman 1969
Bijl‐Hofland 2001
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 164, issue 5:764‐9. [DOI] [PubMed]
Blauw 1995
- Blauw GJ, Westendorp RGJ. Asthma deaths in New Zealand ‐ whodunnit. The Lancet 1995;345:2‐3. [DOI] [PubMed] [Google Scholar]
Boushey 1980
Bousquet 1990
Bousquet 2000
Bradburn 2007
Brewster 1990
Brown 1983
Brusasco 1998
- Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax 1998; Vol. 53, issue 11:992‐8. [DOI] [PMC free article] [PubMed]
Burgess 1991
- Burgess CD, Windom HH, Pearce N, Marshall S, Beasley R, Siebers RWL, et al. Lack of evidence for beta‐2 receptor selectivity ‐ a study of metaproterenol, fenoterol, isoproterenol, and epinephrine in patients with asthma. American Review of Respiratory Disease 1991; Vol. 143, issue 2:444‐6. [DOI] [PubMed]
Burggraaf 2001
- Burggraaf J, Westendorp RGJ, in't Veen J, Schoemaker RC, Sterk PJ, Cohen AF, et al. Cardiovascular side effects of inhaled salbutamol in hypoxic asthmatic patients. Thorax 2001; Vol. 56, issue 7:567‐9. [DOI] [PMC free article] [PubMed]
Carroll 1993
Cates 2008
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2009
- Cates CJ, Lasserson TJ. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007695.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2009a
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006924.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2011
- Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007694.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockcroft 1993
Cockcroft 2006
Collins 1969
- Collins JM, McDevitt DG, Shanks RG, Swanton JG. Cardio‐toxicity of isoprenaline during hypoxia. British Journal of Pharmacology 1969; Vol. 36, issue 1:35‐45. [DOI] [PMC free article] [PubMed]
Crane 1989
Cullum 1969
- Cullum VA, Farmer JB, Jack D, Levy GP. Salbutamol ‐ a new selective beta‐adrenoceptive receptor stimulant. British Journal of Pharmacology 1969; Vol. 35, issue 1:141‐51. [DOI] [PMC free article] [PubMed]
Ducharme 2006
- Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long‐acting beta2‐agonists versus anti‐leukotrienes for chronic asthma. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD003137] [DOI] [PubMed] [Google Scholar]
Dunnill 1960
- Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. Journal of Clinical Pathology 1960; Vol. 13, issue 1:27‐33. [DOI] [PMC free article] [PubMed]
Ebina 1993
Gay 1949
Grainger 1991
- Grainger J, Woodman K, Pearce N, Crane J, Burgess C, Keane A, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981‐7 ‐ a further case‐control study. Thorax 1991; Vol. 46, issue 2:105‐11. [DOI] [PMC free article] [PubMed]
Greenstone 2005
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] [DOI] [PubMed] [Google Scholar]
Guhan 2000
- Guhan AR, Cooper S, Oborne J, Lewis S, Bennett J, Tattersfield AE. Systemic effects of formoterol and salmeterol: a dose‐response comparison in healthy subjects. Thorax 2000; Vol. 55, issue 8:650‐6. [DOI] [PMC free article] [PubMed]
Haley 1998
Hall 1989
Hanania 2002
Hanania 2007
Hancox 1999
Hancox 2006
Haney 2006
Harvey 1982
- Harvey JE, Tattersfield AE. Airway response to salbutamol ‐ effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax 1982; Vol. 37, issue 4:280‐7. [DOI] [PMC free article] [PubMed]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
ICHE2a 1995
- Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Clinical safety data management: definitions and standards for expedited reporting. http://www.fda.gov/cder/guidance/iche2a.pdf 1995.
ICHE3 2007
- Expert working group (efficacy) of the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use. FDA Center for Drug Evaluation and Research. Guideline for Industry Structure and Content of Clinical Study Reports. http://www.fda.gov/cder/guidance/iche3.pdf 25 August 2007.
Jones 2001
Kemp 1993
- Kemp JP, Bierman CW, Cocchetto DM. Dose‐response study of inhaled salmeterol in asthmatic‐patients with 24‐hour spirometry and Holter monitoring. Annals of Allergy 1993; Vol. 70, issue 4:316‐22. [PubMed]
Konzett 1940
- Konzett H. Neue broncholytisch hochwirksame Körper der Adrenalinreihe. Naunyn‐Schmiedeberg's Archives of Pharmacology 1940; Vol. 197:27‐40.
Kuyper 2003
Lee 2003
- Lee DKC, Jackson CM, Currie GP, Cockburn WJ, Lipworth BJ. Comparison of combination inhalers vs inhaled corticosteroids alone in moderate persistent asthma. British Journal of Clinical Pharmacology 2003; Vol. 56, issue 5:494‐500. [DOI] [PMC free article] [PubMed]
Lipworth 1989
Lipworth 1992
- Lipworth BJ, McDevitt DG. Inhaled beta‐2‐adrenoceptor agonists in asthma ‐ help or hindrance. British Journal of Clinical Pharmacology 1992; Vol. 33, issue 2:129‐38. [DOI] [PMC free article] [PubMed]
Lipworth 1997
- Lipworth BJ. Airway subsensitivity with long‐acting beta 2‐agonists: is there cause for concern?. Drug Safety 1997;16(5):295‐308. [DOI] [PubMed] [Google Scholar]
Lipworth 2000
McDevitt 1974
- McDevitt DG, Shanks RG, Swanton JG. Further observations on cardiotoxicity of isoprenaline during hypoxia. British Journal of Pharmacology 1974; Vol. 50, issue 3:335‐44. [DOI] [PMC free article] [PubMed]
McIvor 1998
McMahon 2011
Miranda 2004
Morrison 1993
Nelson 1977
Ni Chroinin 2004
- Ni Chroinin M, Greenstone IR, Ducharme FM. Addition of long‐acting beta2‐agonists to inhaled steroids as first line therapy for persistent asthma in steroid‐naive adults and children. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD005307] [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
NovartisNDA20‐831 2005
- Novartis. Foradil® Aerolizer® (formoterol fumarate inhalation powder) 12 mcg. NDA 20‐831. Appendix 1 – List of pre‐defined terms. http://www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005‐4148B1_02_02‐Novartis‐Appendix‐1.pdf Accessed 4 June 2008.
Ordonez 2001
Palmqvist 1999
Pearce 1990
- Pearce N, Grainger J, Atkinson M, Crane J, Burgess C, Culling C, et al. Case‐control study of prescribed fenoterol and death from asthma in New Zealand, 1977‐81. Thorax 1990; Vol. 45, issue 3:170‐5. [DOI] [PMC free article] [PubMed]
Pearce 2001
- Pearce N BR, Crane J, Burgess C. Epidemiology of asthma mortality. Asthma and Rhinitis. Oxford: Blackwell Scientific, 2001:56‐69. [Google Scholar]
Pearce 2007
- Pearce N. Adverse Reactions: the Fenoterol Story. 1st Edition. Auckland: Auckland University Press, 2007:215. [9781869403744] [Google Scholar]
Phillips 1990
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990; Vol. 85, issue 4:755‐62. [DOI] [PubMed]
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. [DOI] [PubMed] [Google Scholar]
Sears 1990
Simonsson 1967
- Simonsson BG, Jacobs FM, Nadel JA. Role of autonomic nervous system and cough reflex in increased responsiveness of airways in patients with obstructive airway disease. Journal of Clinical Investigation 1967; Vol. 46, issue 11:1812‐8. [DOI] [PMC free article] [PubMed]
SMART 2006
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15‐26. [DOI] [PubMed] [Google Scholar]
Speizer 1968
- Speizer FE, Doll R, Heaf P. Observations on recent increase in mortality from asthma. British Medical Journal 1968; Vol. 1, issue 5588:335‐9. [DOI] [PMC free article] [PubMed]
Stolley 1972
Tattersfield 2006
Tonnel 2001
van der Woude 2001
- Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta(2) agonists. Thorax 2001; Vol. 56, issue 7:529‐35. [DOI] [PMC free article] [PubMed]
Van Noord 1996
Vignola 1993
Walters 2002
- Walters EH, Walters JAE, Gibson PW. Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


