Abstract
Background and aims
Bilirubin, a breakdown product of heme metabolism, has been shown to be protective against cardiovascular mortality; however, it is also a marker of liver function. There are limited data on the longitudinal changes in bilirubin with aging in a population-based cohort of older adults. This study was designed to determine whether serum bilirubin changes with age in older adults, and to evaluate whether age attenuates the association between bilirubin and mortality.
Methods
This is a prospective cohort study of 2364 participants with a mean age of 70 years, who completed a research clinic visit from 1984 to 1987, and 1703 participants who returned for a second research visit approximately 8 years later. Cross-sectional and longitudinal multivariable-adjusted analyses were performed to examine the association between serum bilirubin, aging, and mortality.
Results
In cross-sectional analyses, when the cohort was divided into quartiles of age, higher baseline serum bilirubin levels were associated with older age in analyses adjusted for sex, body mass index (BMI), alanine aminotransferase (ALT), albumin, and metabolic traits (P-value <0.001). In longitudinal analyses, among the subset of participants who had two research visits, aging remained significantly associated with an increase in bilirubin in multivariable-adjusted models (P-value <0.0001). When the longitudinal cohort was divided into bilirubin quartiles, Kaplan–Meier analysis showed an incremental reduction in survival with higher bilirubin levels (P-value = 0.002); however, this association between bilirubin quartile and mortality was no longer significant after adjusting for age (P-value 0.30), suggesting higher bilirubin in older age does not confer survival advantage.
Conclusions
Serum bilirubin levels gradually increase with age in older adults. Elevated bilirubin in older individuals is not associated with improved survival as previously reported in middle-aged populations.
Keywords: bilirubin, aging, liver, mortality
Abbreviations: UGT, uridine diphosphate-glucuronosyltransferase; LDL, low-density lipoprotein; MRP2, multi drug resistance protein 2; MELD, model for end stage liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transpeptidase
Bilirubin is a breakdown product of heme metabolism and is subsequently degraded by uridine diphosphate-glucuronosyltransferase (UGT). In vitro studies show that bilirubin acts as a potent antioxidant under physiologic conditions and may have anti-inflammatory effects.1–8 Bilirubin inhibits low-density lipoprotein (LDL) and lipid oxidation, preventing oxidized LDL and lipids that would otherwise contribute to atherosclerotic plaque, opposing the development of cardiovascular disease.4,9–13 Results from observational cohort studies as well as studies of Gilbert's disease have demonstrated that slightly elevated bilirubin levels are associated with a reduced risk of cardiovascular disease, supporting the concept of bilirubin as a protective antioxidant.7,14–22 In contrast to the protective role in cardiovascular disease, bilirubin levels are directly related to liver-related mortality. The process of bilirubin catabolism is dependent upon liver function. Bilirubin must be taken up into hepatocytes where uridine diphosphate-glucuronosyltransferase (UGT) converts water-insoluble bilirubin into a conjugated, water-soluble form that can be excreted into bile. Bilirubin secretion is mediated by an ATP-dependent multiple drug resistance protein 2(MRP2).23,24 Elevated levels of bilirubin indicate hepatocellular dysfunction. Bilirubin is a sensitive index of liver disease, serving as one of three variables used to calculate the MELD score that determines all-cause mortality and organ allocation for liver transplantation.25–27
While bilirubin levels have been characterized in middle-aged populations and individuals with liver disease, limited studies have reported the relationship between bilirubin and mortality in an older population.28 Fleming et al was one of the first to describe the prevalence of abnormal liver tests in an older population. In a cohort of 13,276 individuals who were aged 75 years and older, there was a 5.4% prevalence of abnormal bilirubin levels. Elevated bilirubin levels were associated with a modest increase in mortality; however, the relationship between absolute bilirubin levels and mortality was not certain because laboratories with different standards performed the liver tests.29
This study aims, first, to determine whether bilirubin changes with age in a well-characterized population based cohort of older individuals and, second, to evaluate whether age alters the relationship between bilirubin and mortality.
Methods
Study Cohort
The Rancho Bernardo Study (RBS) is a prospective cohort that was established in 1972 in order to characterize cardiovascular disease risk factors in an older population. The study enrolled 82% of the residents living in a geographically defined suburban neighborhood in Southern California. Participants completed a more extensive research study clinic visit between 1984 and 1987 at which time blood samples were obtained. A subset of the cohort returned for a second research study clinic visit between 1992 and 1996 at which time a second blood sample was obtained. The current analysis includes 2364 participants who completed the 1984–87 study visit, and the subset of 1073 participants who completed both study visits and had available longitudinal data. This cohort and its subsets have been well characterized and followed in a longitudinal manner for long term mortality. The initial objectives, inclusion criteria, and cohort characteristics have been described at length in other publications.12,30 The institutional review board at the University of California, San Diego approved this study and all participants gave written consent.
Clinical and Laboratory Assessment
Qualified interviewers obtained complete medical histories at each research visit. Current medical problems, medical history, including history of chronic liver disease, and medication use were reviewed. Self-reported medications were validated by review of pills and prescriptions brought to the clinic for that purpose. Participants self-reported their alcohol use, including quantity and frequency. Comparison of self-reported use and quantitative responses to a separate nutrition interviewer yielded similar results, providing internal validation for alcohol responses. Morning fasting venous blood samples were obtained during each research study visit, and metabolic parameters were measured in a routine hospital laboratory. Total serum bilirubin level (milligram/deciliter) was measured by photometry after the addition of 3,5-dichlorophenyl diazonium. Height (meters) and weight (kilogram) were measured, and body mass index (BMI) was calculated as weight in kg divided by height in meter squared (kg/m2).12,30–33
Follow Up
Rancho Bernardo Study participants were followed on an annual basis with a mailed questionnaire and a clinical research visit approximately every 4 years where health assessment and vitals were performed. All participants were followed for an average (±SD) of 13.7 (±6.2) years for the longitudinal analyses.
Statistical Analysis
The cohort was divided into quartiles of age: 30–62 (quartile 1), 63–71 (quartile 2), 72–77 (quartile 3), and 78–93 (quartile 4) and subsequently into quartiles of bilirubin (mg/dL): 0.1–0.3 (quartile 1), 0.4–0.5 (quartile 2), 0.6–0.6 (quartile 3), 0.7–2.3 (quartile 4). Descriptive statistics, including BMI, alcohol use, aminotransferases, and lipid levels, were defined for each quartile. Average bilirubin levels were calculated as geometric means, and least square means were used to evaluate trends in bilirubin. Mean ± standard deviation was reported for variables with a normal distribution, and geometric means with 95% confidence intervals were reported for variables with a skewed distribution. In the subset of the cohort who was seen twice, descriptive statistics for each individual were compared from the 1984–1987 and 1992–1997 visits using paired t-test, using logarithmic transformation of bilirubin. The hazard ratio for all-cause mortality based on bilirubin quartile was evaluated before and after adjustment for age. Kaplan–Meier survival curves for each bilirubin quartile were calculated using the log-rank test of equality. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Statistical significance was defined as a two-tailed P-value of less than 0.05.
Results
Population Characteristics
This study included 2364 adults (55% women) with an average (±SD) age of 69.7 (±10.5) years. The average BMI for men and women was 25.8 (±3.3) kg/m2 and 24.3 (±3.8) kg/m2, respectively. The baseline total serum bilirubin level was 0.5 mg/dl (95% confidence interval, 0.49–0.51) with a range from 0.1 to 2.3. Table 1 shows the comprehensive baseline characteristics of the cohort. Analyses of the variables by age quartile revealed significant positive trends in systolic blood pressure, diabetes, and aspartate aminotransferase (AST) and negative trends in BMI, ALT, albumin, triglycerides, and cholesterol. Table 2 shows the baseline characteristics stratified by bilirubin quartile, revealing positive associations with systolic blood pressure, diabetes, AST, ALT, and gamma glutamyl transpeptidase (GGT) and negative associations with percent women, total cholesterol, HDL, and triglycerides.
Table 1.
Cohort Baseline Characteristics by Age Quartile.
| Total | Age quartiles (Years) |
P-value | ||||
|---|---|---|---|---|---|---|
| Q1 (30–62) | Q2 (63–71) | Q3 (72–77) | Q4 (78–93) | |||
| Number of individuals | 2364 | 597 | 574 | 589 | 604 | |
| Women (%) | 55.8 | 57.0 | 57.8 | 58.2 | 50.5 | 0.0357 |
| BMI (kg/m2) (mean ± SD) | 24.9 ± 3.7 | 25.5 ± 4.0 | 25.2 ± 3.7 | 24.7 ± 3.6 | 24.4 ± 3.4 | <0.0001 |
| Alcohol use (%) | <0.0001 | |||||
| None | 36.8 | 36.5 | 34.8 | 32.1 | 43.4 | |
| <1 drink/day | 22.0 | 25.0 | 18.8 | 22.6 | 21.4 | |
| 1–2 drinks/day | 26.8 | 22.5 | 27.0 | 31.4 | 26.5 | |
| >2 drinks/day | 14.5 | 16.1 | 19.3 | 13.9 | 8.8 | |
| Bilirubin (mg/dl) (95% CI)a | 0.50 (0.49–0.51) | 0.43 (0.41–0.44) | 0.49 (0.47–0.51) | 0.53 (0.51–0.55) | 0.56 (0.54–0.58) | <0.0001 |
| Systolic blood pressure (mmHg) (mean ± SD) | 138.9 ± 21.9 | 124.0 ± 18.4 | 136.3 ± 18.7 | 144.5 ± 19.2 | 150.7 ± 21.5 | <0.0001 |
| Total cholesterol (mg/dl) (mean ± SD) | 219.9 ± 40.0 | 218.7 ± 36.6 | 228.1 ± 41.5 | 219.6 ± 40.5 | 213.5 ± 39.9 | 0.0009 |
| HDL cholesterol (mg/dL) (mean ± SD) | 61.7 ± 18.7 | 60.9 ± 18.2 | 62.3 ± 19.9 | 62.9 ± 20.1 | 60.9 ± 16.7 | 0.8538 |
| Total/HDL cholesterol ratio (mean ± SD) | 3.9 ± 1.3 | 3.9 ± 1.3 | 4.0 ± 1.3 | 3.8 ± 1.3 | 3.9 ± 1.2 | 0.0120 |
| Triglycerides (mg/dl) (95% CI)a | 102.0 (99.8–104.3) | 100.2 (95.9–104.7) | 111.3 (106.4–116.4) | 101.7 (97.3–106.3) | 95.8 (91.7–100.1) | 0.0245 |
| Smoking (%)b | 58.2 | 55.4 | 65.0 | 61.3 | 51.3 | 0.0755 |
| Diabetes (%) | 14.3 | 7.4 | 12.5 | 17.7 | 19.7 | <0.0001 |
| AST (IU/L) (95% CI)a | 24.6 (24.3–25.0) | 23.4 (22.7–24.2) | 24.7 (24.0–25.5) | 25.0 (24.3–25.8) | 25.4 (24.6–26.1) | 0.0003 |
| ALT (IU/L) (95% CI)a | 17.4 (17.1–17.8) | 19.6 (18.9–24.2) | 18.1 (17.4–18.9) | 16.9 (16.2–17.6) | 15.4 (14.8–16.1) | <0.0001 |
| GGT (IU/L) (95% CI)a | 9.8 (9.5–10.1) | 9.7 (9.1–10.2) | 10.5 (9.9–11.1) | 9.9 (9.3–10.4) | 9.2 (8.8–9.9) | 0.2000 |
| Albumin (g/dL) (mean ± SD) | 4.3 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.3 | 4.2 ± 0.3 | 4.1 ± 0.2 | <0.0001 |
Geometric means (95% CI).
Current or prior smoking Q1: quartile 1, Q2: quartile 2, Q3: quartile 3, Q4: quartile 4, BMI: body mass index, kg: kilogram, m: meter, SD: standard deviation, mg: milligram, dl: deciliter, CI: confidence interval, mmHg: millimeter mercury, HDL: high density lipoprotein, AST: aspartate aminotransferase, ALT: alanine aminotransferase, IU: international units, L: liter, GGT: gamma-glutamyl transferase, g: grams.
Table 2.
Cohort Baseline Characteristics by Bilirubin Quartile.
| Total | Bilirubin quartiles (mg/dl) |
P-value | ||||
|---|---|---|---|---|---|---|
| Q1 (0.1–0.3) | Q2 (0.4–0.5) | Q3 (0.6–0.6) | Q4 (0.7–2.3) | |||
| Number of Individuals | 2363 | 465 | 895 | 334 | 669 | |
| Women (%) | 55.8 | 75.1 | 63.4 | 50.6 | 35.0 | <0.0001 |
| BMI (kg/m2) (Mean ± SD) | 24.9 ± 3.7 | 25.0 ± 4.1 | 24.8 ± 3.5 | 25.0 ± 3.9 | 25.0 ± 3.5 | 0.8271 |
| Alcohol use (%) | 0.0059 | |||||
| None | 36.8 | 43.4 | 38.2 | 32.9 | 32.1 | |
| <1 drink/day | 21.9 | 20.2 | 22.6 | 24.0 | 21.2 | |
| 1-2 drinks/day | 26.8 | 25.2 | 24.9 | 28.1 | 29.9 | |
| >2 drinks/day | 14.5 | 11.2 | 14.3 | 15.0 | 16.7 | |
| Systolic blood pressure (mmHg) (Mean ± SD) | 138.9 ± 21.9 | 135.2 ± 23.7 | 138.5 ± 21.7 | 139.3 ± 20.6 | 142.0 ± 21.1 | <0.0001 |
| Total cholesterol (mg/dl) (mean ± SD) | 219.9 ± 40.0 | 222.6 ± 40.0 | 224.4 ± 39.7 | 219.2 ± 39.9 | 212.1 ± 39.2 | <0.0001 |
| HDL cholesterol (mg/dL) (mean ± SD) | 61.7 ± 18.7 | 63.4 ± 18.6 | 62.4 ± 18.9 | 61.3 ± 17.4 | 59.8 ± 19.0 | 0.0012 |
| Total/HDL cholesterol ratio (mean ± SD) | 3.9 ± 1.3 | 3.8 ± 1.3 | 3.9 ± 1.4 | 3.8 ± 1.2 | 3.8 ± 1.3 | 0.9369 |
| Triglycerides (mg/dl) (95% CI)a | 102.0 (99.8–104.3) | 107.8 (102.5–113.3) | 103.0 (99.3–106.7) | 102.1 (96.3–108.3) | 97.0 (93.0–101.1) | 0.0021 |
| Smoking (%)b | 58.2 | 58.5 | 57.2 | 56.9 | 59.9 | 0.5030 |
| Diabetes (%) | 14.3 | 12.5 | 13.6 | 11.4 | 18.1 | 0.0092 |
| AST (IU/L) (95% CI)a | 24.6 (24.3–25.0) | 22.4 (21.6–23.1) | 24.2 (23.6–24.8) | 25.8 (24.8–26.9) | 26.3 (25.6–27.1) | <0.0001 |
| ALT (IU/L) (95% CI)a | 17.4 (17.1–17.8) | 16.3 (15.6–17.1) | 17.5 (17.0–18.1) | 17.9 (16.9–18.9) | 17.9 (17.2–18.6) | 0.0034 |
| GGT (IU/L) (95% CI)a | 9.8 (9.5–10.1) | 8.9 (8.4–9.5) | 9.6 (9.2–10.1) | 9.8 (9.1–10.6) | 10.7 (10.1–11.3) | <0.0001 |
| Albumin (g/dL) (mean ± SD) | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.0955 |
Geometric means (95% confidence interval).
Current or prior smoking Q1: quartile 1, Q2: quartile 2, Q3: quartile 3, Q4: quartile 4, BMI: body mass index, kg: kilogram, m: meter, SD: standard deviation, mg: milligram, dl: deciliter, CI: confidence interval, mmHg: millimeter mercury, HDL: high density lipoprotein, AST: aspartate aminotransferase, ALT: alanine aminotransferase, IU: international units, L: liter, GGT: gamma-glutamyl transferase, g: grams.
In the subset of the cohort, comprised of 1073 participants who completed two research study visits, the mean age at study visit 1 (between 1984 and 1987) was 65.7 years (range 30–89), and the mean age at study visit 2 (between 1992 and 1997) was 74.1 years (range 37–96).
All participants were followed for an average (±SD) of 13.7 (±6.2) years for a total of 32,387 person-years of follow up. The cumulative mortality was 56.2% (N = 1329) during the study period. Vital statistics are known for 96% of the study participants, and death certificates have been obtained for 90% of decedents.
Q1. Cross Sectional Analysis: does Bilirubin Change with Age?
In order to evaluate whether bilirubin changes in older adults, we divided the population into quartiles of age (Quartile 1: age 30–62, Quartile 2: age 63–71, Quartile 3: age 72–77, Quartile 4: 78–93). Bilirubin level was calculated as geometric means (in order to normalize the data) and analyzed as a continuous variable in the cross-sectional analysis; higher bilirubin levels were associated with older age (P-value 0.001). Multivariate analysis adjusting by sex, BMI, alcohol use, systolic blood pressure, cholesterol, triglycerides, diabetes, ALT, and albumin did not change the association of increasing bilirubin with age (P < 0.001).
Q2. Longitudinal: does Bilirubin Change with Aging in the Same Population?
In order to examine the change in bilirubin with age for an individual, a sub-group analysis was performed using the paired t-test. 1073 participants completed 2 research study visits approximately 8 years apart. Baseline data for this subset is presented in Table 3. Total bilirubin levels increased from 0.48 to 0.68 from visit 1 to visit 2 (P-value <0.0001). Increasing bilirubin with age was a stepwise effect that remained statistically significant after multivariate adjustment for sex, BMI, alcohol use, systolic blood pressure, cholesterol, triglycerides, diabetes, ALT, and albumin (P < 0.001).
Table 3.
Characteristics of the Sub-cohort at Study Visits.
| 1984–1987 Visit | 1992–1997 Visit | P-value | |
|---|---|---|---|
| Number of Individuals | 1073 | 1073 | |
| Age (yr), mean (range) | 65.7 (30–89) | 74.1 (37–96) | |
| Women (%) | 59.0 | 59.0 | |
| BMI (kg/m) | 24.9 (24.7–25.2) | 25.2 (25.0–25.5) | <0.0001 |
| Alcohol use (%) | <0.0001 | ||
| None | 34.5 | 33.2 | |
| <1 drink/day | 21.3 | 35.9 | |
| 1-2 drinks/day | 28.2 | 20.6 | |
| >2 drinks/day | 16.1 | 10.4 | |
| Bilirubin (mg/dl) (Mean ± SD)a | 0.48 (0.47–0.49) | 0.68 (0.67–0.70) | <0.0001 |
| Systolic blood pressure (mmHg) (Mean ± SD) | 133.4 (132.2–134.6) | 139.0 (137.7–140.3) | <0.0001 |
| Total cholesterol (mg/dl) (Mean ± SD) | 221.6 (219.2–224.0) | 206.9 (204.6–209.1) | <0.0001 |
| HDL cholesterol (mg/dL) (Mean ± SD) | 63.1 (62.0–64.2) | 57.4 (56.4–58.4) | <0.0001 |
| Total/HDL cholesterol ratio (Mean ± SD) | 3.8 (3.7–3.9) | 4.0 (3.9–4.0) | <0.0001 |
| Triglycerides (mg/dl) (95% CI)a | 99.5 (96.3–102.8) | 103.8 (100.6–107.1) | 0.0009 |
| Smoking (%)b | 56.7 | 54.5 | 0.0032 |
| Diabetes (%) | 12.0 | 20.1 | <0.0001 |
| AST (IU/L) (95% CI)a | 24.1 (23.6–24.7) | 19.8 (19.2–20.1) | <0.0001 |
| ALT (IU/L) (95% CI)a | 18.1 (17.6–18.6) | 16.2 (15.7–16.6) | <0.0001 |
| GGT (IU/L) (95% CI)a | 9.4 (9.0–9.8) | 21.7 (20.9–22.5) | <0.0001 |
| Albumin (g/dL) (Mean ± SD) | 4.4 (4.3–4.4) | 4.1 (4.1–4.1) | <0.0001 |
Geometric means (95% confidence interval).
Current or prior smoking yr: year, BMI: body mass index, kg: kilogram, m: meter, mmHg: millimeter mercury, mg: milligram, dL: deciliter, HDL: high density lipoprotein, g: gram.
Q3. Is there an Association Between Bilirubin and Mortality?
Total bilirubin quartile was significantly associated with a stepwise increase in all-cause mortality (P-value 0.0017, Table 4). The positive association between bilirubin quartile and mortality was no longer statistically significant after adjustment for age (P-value 0.3). Kaplan–Meier analysis by bilirubin quartile showed incremental reduction in survival with higher bilirubin values (P-value of <0.0001, Figure 1). Wald test for interaction between sex and bilirubin was not significant for all-cause mortality (P-value 0.5).
Table 4.
Hazard Ratios of All-cause Mortality Stratified by Bilirubin Quartiles.
| N | Bilirubin quartiles (mg/dL) |
P-value | ||||
|---|---|---|---|---|---|---|
| Q1 (0.1–0.3) | Q2 (0.4–0.5) | Q3 (0.6–0.6) | Q4 (0.7–2.3) | |||
| Unadjusted Hazard Ratio | 2363 | Referent | 1.41 (1.19–1.66) | 1.62 (1.33–1.97) | 1.96 (1.65–2.32) | 0.0017 |
| Age-Adjusted Hazard Ratio | 2363 | Referent | 0.94 (0.80–1.11) | 1.01 (0.82–1.23) | 1.14 (0.96–1.35) | 0.2494 |
| Number of deaths | 1329 | 195 | 491 | 199 | 444 | |
N: number of participants, Q1: quartile 1, Q2: quartile 2, Q3: quartile 3, Q4: quartile 4.
Figure 1.
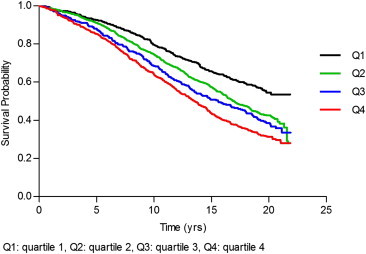
Kaplan–Meier survival by bilirubin quartile.
Discussion
Main Findings
In this older population-based Rancho Bernardo study cohort, small but incremental increases in bilirubin occurred with increasing age. This association between age and bilirubin was observed in the cross-sectional analyses of the baseline cohort and over time in longitudinal analyses. Moreover, even though the majority of the values are within the normal ranges for bilirubin, higher levels are directly associated with higher all-cause mortality. This relationship was eliminated with adjustment for age, supporting the finding that rising bilirubin is a marker of increasing age.
Relevance
Diverging from the majority of the studies and literature describing the cardio-protective role of bilirubin levels in middle aged adults, this study describes the changes in bilirubin with age and the interaction with mortality in older individuals. This study supports the findings of Fleming et al who described the prevalence of abnormal liver enzymes in a population over the age of 75 where 5.4% had abnormal bilirubin values.29 Our study expands our knowledge by examining absolute bilirubin values, and re-evaluating the use of normal ranges that may not be as applicable in older populations. This is particularly relevant as the US population is aging. The number of Americans over the age of 65 is projected to rise from 40 million in 2010 to more than 70 million by 2030.34–36
This cohort of older adults is a large, well-characterized group that enables preliminary but sound observations to be made. While this is an observational study and cannot determine causality, our results suggest that small incremental increases in total serum bilirubin levels occur with age. While bilirubin may be cardio-protective in a middle-aged population, the putative anti-oxidant effect may be too weak to significantly influence mortality in an older population where more dominant factors drive survival.
Limitations and Strengths of the Study
Several limitations are inherent to this study. The RBS cohort is an observational study without intervention. This population is a homogenous group of white individuals of Western European background. Given the lack of diversity, results may be difficult to generalize to a non-white, geographically distinct population. This study is also limited by fundamental design of the RBS cohort. Since this was a longitudinal prospective study of cardiovascular risk factors, there are certain items or variables that were not initially of interest but subsequently became interesting but could not be measured. Chronic liver disease was self-reported, and testing for viral hepatitis was not available at the time of the study. However, this cohort is unique with the median age of 72 years old and provides novel and significant contributions that certainly could be generalized to older, white individuals.
Conclusion
This study reveals that serum bilirubin levels modestly increase with age. Though the absolute bilirubin levels in older adults may be within normal ranges, small but clinically meaningful increases in bilirubin may be associated with increased mortality. Aging attenuates the association between bilirubin that is protective in middle-aged population but not so in the elderly. This study provides novel observations that could begin to change the way that bilirubin levels are interpreted and used in older populations.
Role of funding agencies
Funding agencies did not have any role in the design and conduct of the study, collection, management, or interpretation of the data; preparation, review, or approval of the manuscript.
Conflicts of interest
All authors have none to declare.
Acknowledgments
R.L. is supported in part by the American Gastroenterological Association (AGA) Foundation–Sucampo–ASP. Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303, and P30CA23100-27.
The Rancho Bernardo Study was funded by the National Institutes of Health/National Institute on Aging Grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK31801.
References
- 1.Stocker R., Glazer A.N., Ames B.N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocker R., Yamamoto Y., McDonagh A.F. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil J., Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 4.Wu T.W., Fung K.P., Wu J. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51:859–862. doi: 10.1016/0006-2952(95)02395-x. [DOI] [PubMed] [Google Scholar]
- 5.Giampapa V.C. Clinical treatment of the aging process presently and immediate future trends. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:97–104. doi: 10.1081/bio-120020164. [DOI] [PubMed] [Google Scholar]
- 6.Lester R., Schmid R. Bilirubin metabolism. N Engl J Med. 1964;270:779–786. doi: 10.1056/NEJM196404092701507. [DOI] [PubMed] [Google Scholar]
- 7.Bulmer A.C., Blanchfield J.T., Toth I. Improved resistance to serum oxidation in Gilbert's syndrome: a mechanism for cardiovascular protection. Atherosclerosis. 2008;199:390–396. doi: 10.1016/j.atherosclerosis.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Maeda Y., Inoguchi T. [Oxidative stress] Nihon Rinsho. 2010;68:814–818. [PubMed] [Google Scholar]
- 9.Baranano D.E., Rao M., Ferris C.D. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong M.H., Bettencourt R., Barrett-Connor E. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PLoS One. 2010;5:e14254. doi: 10.1371/journal.pone.0014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei R., Curtin L.R., Arias E. U.S. decennial life tables for 1999–2001: methodology of the United States Life Tables. Natl Vital Stat Rep. 2008;57:1–9. [PubMed] [Google Scholar]
- 12.Loomba R., Bettencourt R., Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muchova L., Kraslova I., Lenicek M. [Gilbert's syndrome–myths and reality] Cas Lek Cesk. 2004;143:375–380. [PubMed] [Google Scholar]
- 14.Djousse L., Levy D., Cupples L.A. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol. 2001;87:1196–1200. doi: 10.1016/s0002-9149(01)01494-1. A4, 7. [DOI] [PubMed] [Google Scholar]
- 15.Novotny L., Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 16.Black M., Billing B.H. Hepatic bilirubin udp-glucuronyl transferase activity in liver disease and Gilbert's syndrome. N Engl J Med. 1969;280:1266–1271. doi: 10.1056/NEJM196906052802303. [DOI] [PubMed] [Google Scholar]
- 17.Loomba R.S., Aggarwal S., Shah P.H. Influenza vaccination and cardiovascular morbidity and mortality: analysis of 292,383 patients. J Cardiovasc Pharmacol Ther. 2012;17:277–283. doi: 10.1177/1074248411429965. [DOI] [PubMed] [Google Scholar]
- 18.de Vries H.S., Te Morsche R.H., Jenniskens K. A functional polymorphism in UGT1A1 related to hyperbilirubinemia is associated with a decreased risk for Crohn's disease. J Crohns Colitis. 2012;6:597–602. doi: 10.1016/j.crohns.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Breimer L.H., Wannamethee G., Ebrahim S. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504–1508. [PubMed] [Google Scholar]
- 20.Hopkins P.N., Wu L.L., Hunt S.C. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol. 1996;16:250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- 21.Arias E. United States life tables by Hispanic origin. Vital Health Stat. 2010;2:1–33. [PubMed] [Google Scholar]
- 22.Schwertner H.A., Jackson W.G., Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 23.Kullak-Ublick G.A., Stieger B., Hagenbuch B. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- 24.Bloomer J.R., Berk P.D., Howe R.B. Interpretation of plasma bilirubin levels based on studies with radioactive bilirubin. JAMA. 1971;218:216–220. [PubMed] [Google Scholar]
- 25.Kamath P.S., Kim W.R. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 26.Malinchoc M., Kamath P.S., Gordon F.D. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.M., Wai C.T., Da Costa M. Bilirubin appears to be the only independent variable affecting mortality on liver transplant waiting list if waiting time exceeds 1 year. Transplant Proc. 2005;37:4365–4366. doi: 10.1016/j.transproceed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Temme E.H., Zhang J., Schouten E.G. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887–894. doi: 10.1023/a:1013794407325. [DOI] [PubMed] [Google Scholar]
- 29.Fleming K.M., West J., Aithal G.P. Abnormal liver tests in people aged 75 and above: prevalence and association with mortality. Aliment Pharmacol Ther. 2011;34:324–334. doi: 10.1111/j.1365-2036.2011.04718.x. [DOI] [PubMed] [Google Scholar]
- 30.Langenberg C., Bergstrom J., Laughlin G.A. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol. 2005;162:1189–1197. doi: 10.1093/aje/kwi338. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E., Laughlin G.A., Connor C. Coronary artery calcium versus intima-media thickness as a measure of cardiovascular disease among asymptomatic adults (from the Rancho Bernardo Study) Am J Cardiol. 2007;99:227–231. doi: 10.1016/j.amjcard.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.K., Bettencourt R., Brenner D. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7:e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong M.H., Bettencourt R., Brenner D.A. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol. 2012;10:285–290. doi: 10.1016/j.cgh.2011.10.014. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias E., Curtin L.R., Wei R. U.S. decennial life tables for 1999-2001, United States life tables. Natl Vital Stat Rep. 2008;57:1–36. [PubMed] [Google Scholar]
- 35.Arias E., Rostron B.L., Tejada-Vera B. United States life tables, 2005. Natl Vital Stat Rep. 2010;58:1–132. [PubMed] [Google Scholar]
- 36.Trends in aging–United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–104. 106. [PubMed] [Google Scholar]


