Abstract
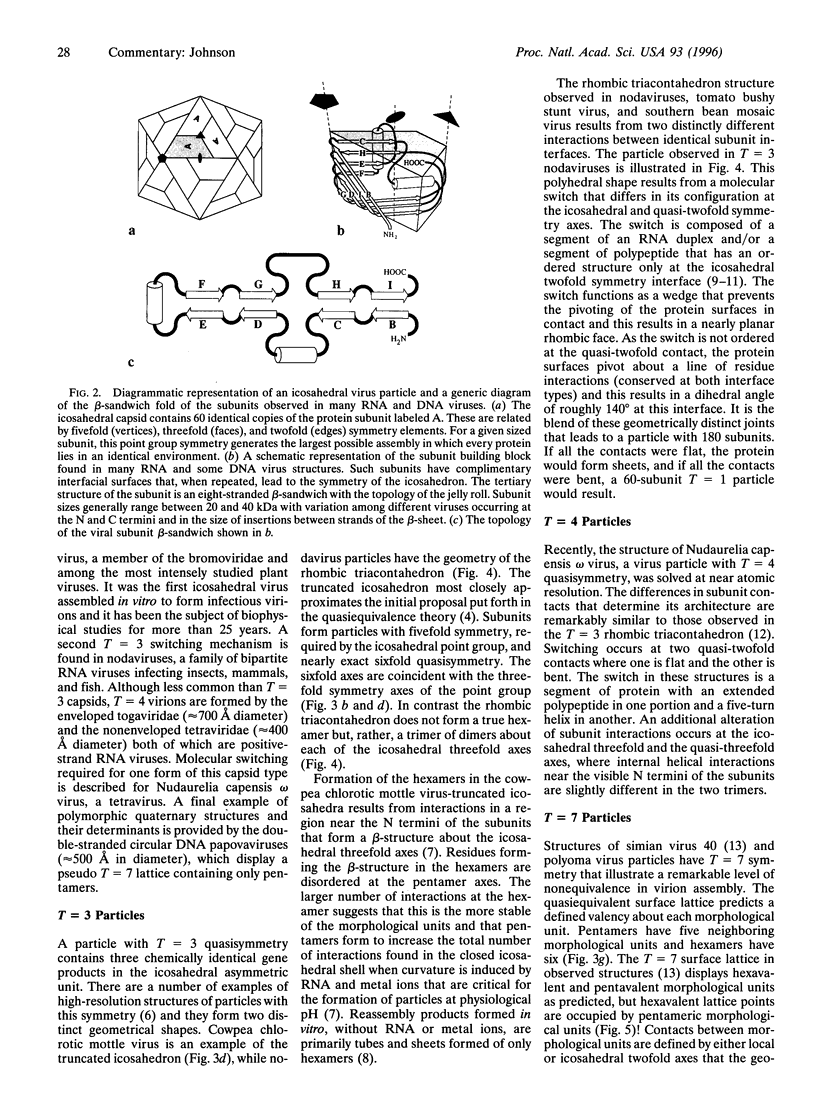
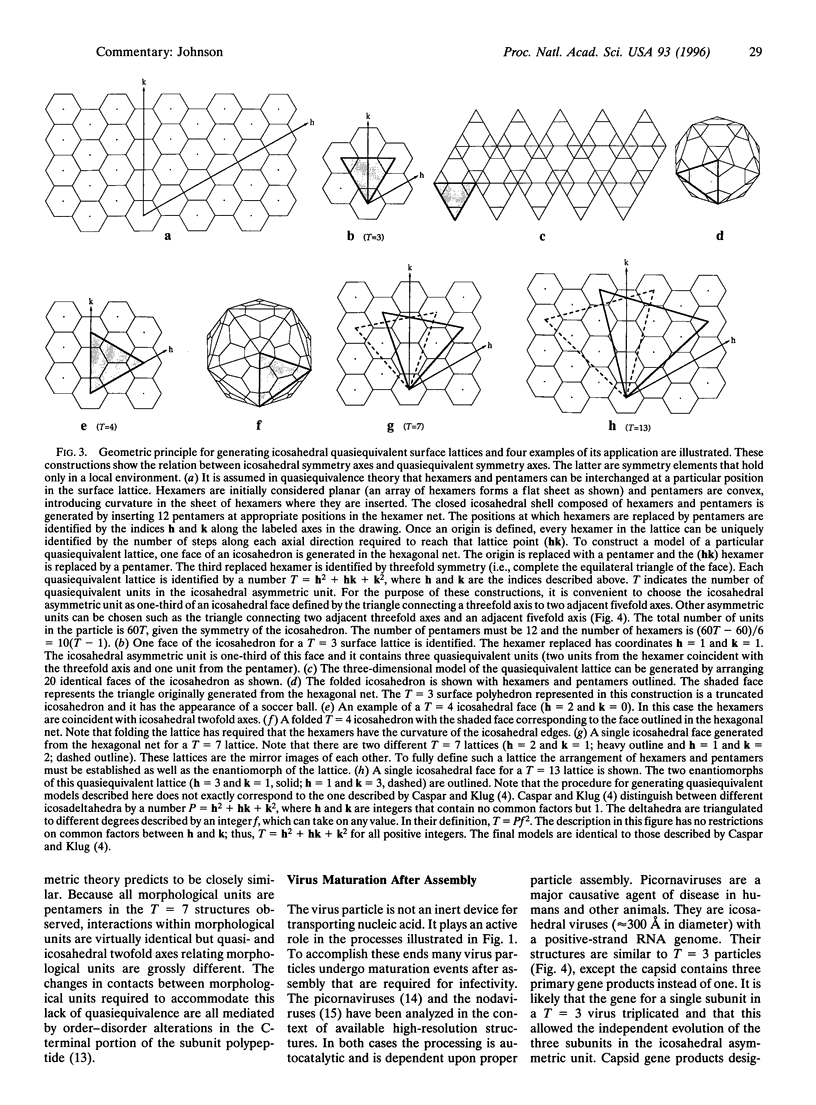
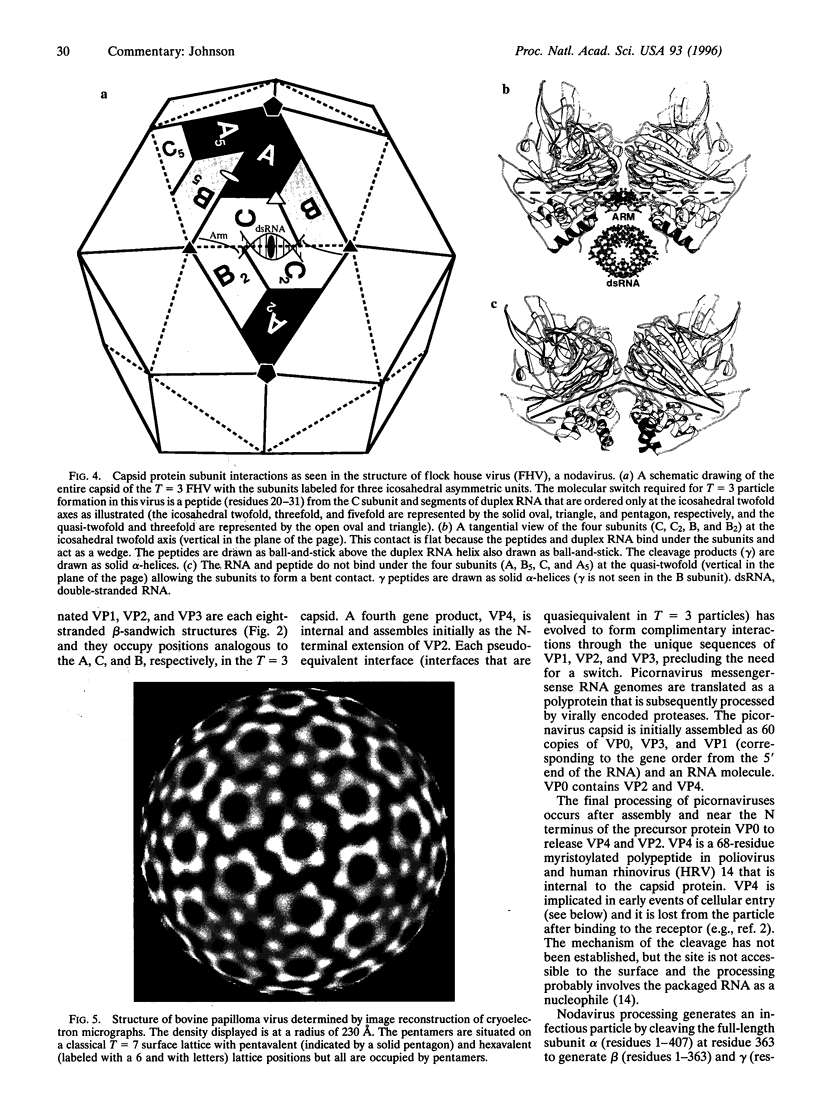
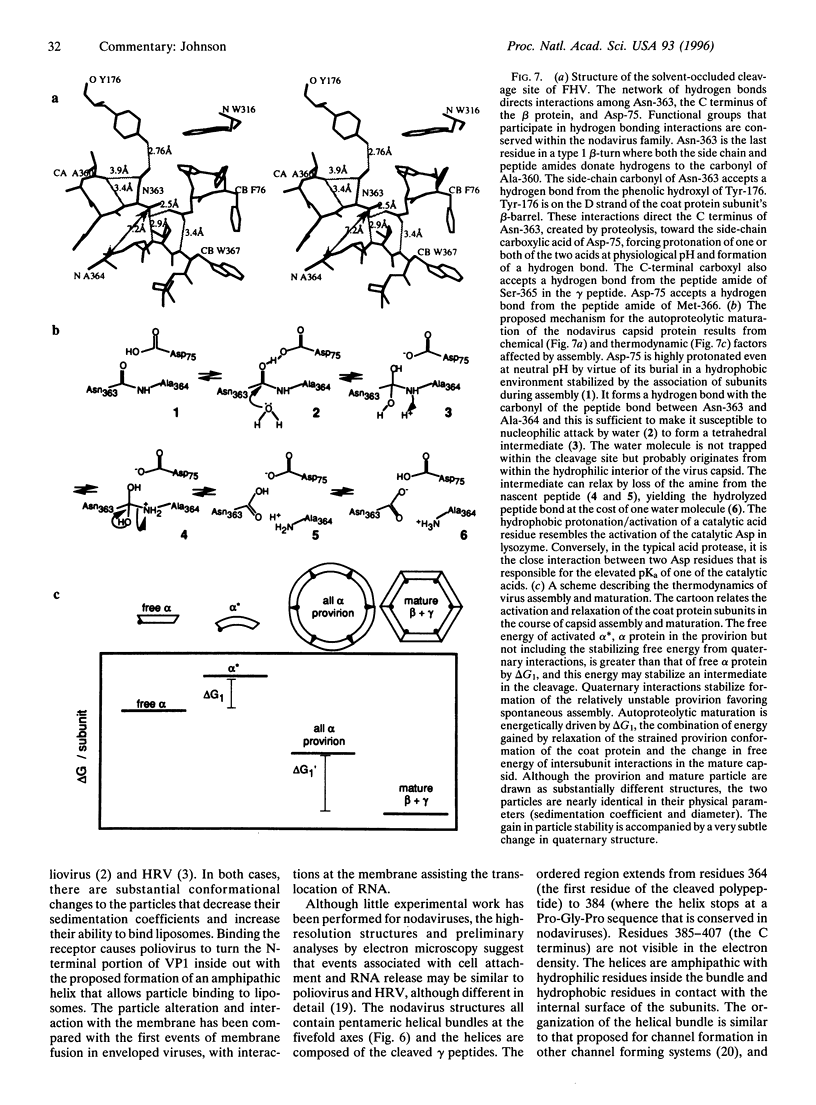
Biological processes often require that a single gene product participate in multiple types of molecular interactions. Viruses with quasiequivalent capsids provide an excellent paradigm for studying such phenomena because identical protein subunits are found in different structural environments. Differences in subunit joints may be controlled by protein segments, duplex or single-stranded RNA, metal ions, or some combination of these. Each of the virus groups examined display a distinctive mechanism for switching interface interactions, illustrating the magnitude of options that are likely to be found in other biological systems. In addition to determining capsid morphology, assembly controls the timing of autocatalytic maturation cleavage of the viral subunits that is required for infectivity in picorna-, noda-, and tetraviruses. The mechanism of assembly-dependent cleavage is conserved in noda- and tetraviruses, although the quaternary structures of the capsids are different as are the molecular switches that control subunit interfaces. The function of the cleavage in picorna-, noda-, and tetraviruses is probably to release polypeptides that participate in membrane translocation of RNA.
Full text
PDF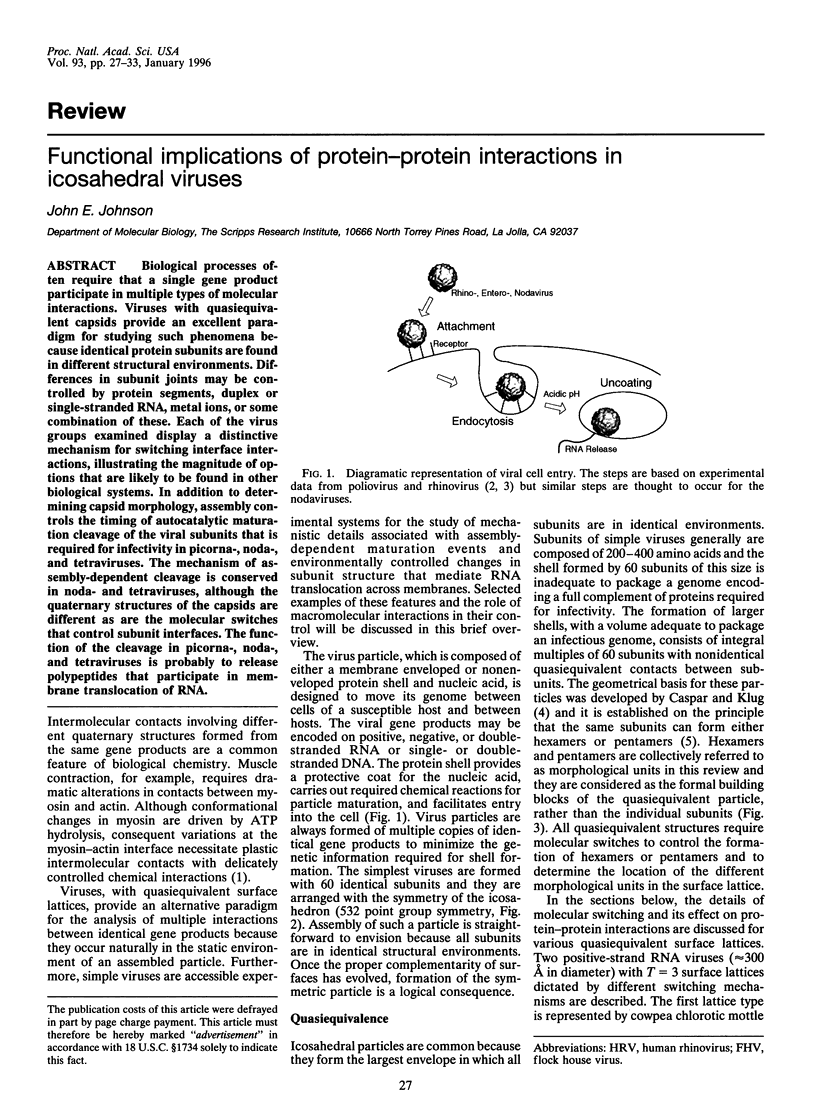


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft J. B. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Reddy V. S., Olson N. H., Fisher A. J., Baker T. S., Johnson J. E. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure. 1994 Apr 15;2(4):271–282. doi: 10.1016/s0969-2126(00)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. J., Johnson J. E. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature. 1993 Jan 14;361(6408):176–179. doi: 10.1038/361176a0. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Rueckert R. R. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J Virol. 1988 Sep;62(9):3399–3406. doi: 10.1128/jvi.62.9.3399-3406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Munshi S., Liljas L., Agrawal D., Olson N. H., Reddy V., Fisher A., McKinney B., Schmidt T., Baker T. S. Comparative studies of T = 3 and T = 4 icosahedral RNA insect viruses. Arch Virol Suppl. 1994;9:497–512. doi: 10.1007/978-3-7091-9326-6_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Milligan R. A. Protein-protein interactions in the rigor actomyosin complex. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):21–26. doi: 10.1073/pnas.93.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. J., Bricogne G., Harrison S. C. Structure of tomato busy stunt virus IV. The virus particle at 2.9 A resolution. J Mol Biol. 1983 Nov 25;171(1):61–93. doi: 10.1016/s0022-2836(83)80314-3. [DOI] [PubMed] [Google Scholar]
- Olson N. H., Kolatkar P. R., Oliveira M. A., Cheng R. H., Greve J. M., McClelland A., Baker T. S., Rossmann M. G. Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):507–511. doi: 10.1073/pnas.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Pattus F. Rendering a membrane protein soluble in water: a common packing motif in bacterial protein toxins. Trends Biochem Sci. 1993 Oct;18(10):391–395. doi: 10.1016/0968-0004(93)90096-6. [DOI] [PubMed] [Google Scholar]
- Roenhorst J. W., Verduin B. J., Goldbach R. W. Virus-ribosome complexes from cell-free translation systems supplemented with cowpea chlorotic mottle virus particles. Virology. 1989 Jan;168(1):138–146. doi: 10.1016/0042-6822(89)90412-1. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. Viral cell recognition and entry. Protein Sci. 1994 Oct;3(10):1712–1725. doi: 10.1002/pro.5560031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir J. A., Munshi S., Wang G., Baker T. S., Johnson J. E. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure. 1995 Jan 15;3(1):63–78. doi: 10.1016/s0969-2126(01)00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick A., Reddy V. S., Dasgupta R., Schneemann A., Ray W. J., Jr, Rueckert R. R., Johnson J. E. Capsid assembly in a family of animal viruses primes an autoproteolytic maturation that depends on a single aspartic acid residue. J Biol Chem. 1994 May 6;269(18):13680–13684. [PubMed] [Google Scholar]