Abstract
The refractive index (RI) is the fundamental characteristic that affects the optical properties of aerosols, which could be some of the most important factors influencing direct radiative forcing. The secondary organic aerosols (SOAs) generated by the photooxidation of benzene, toluene, ethylbenzene and m-xylene (BTEX) under low-NOx and high-NOx conditions are explored in this study. The particles generated in our experiments are considered to be spherical, based on atomic force microscopy (AFM) images, and nonabsorbent at a wavelength of 532 nm, as determined by ultraviolet-visible light (UV-Vis) spectroscopy. The retrieved RIs at 532 nm for the SOAs range from 1.38–1.59, depending on several factors, such as different precursors and NOx levels. The RIs of the SOAs are altered differently as the NOx concentration increases as follows: the RIs of the SOAs derived from benzene and toluene increase, whereas those of the SOAs derived from ethylbenzene and m-xylene decrease. Finally, by comparing the experimental data with the model values, we demonstrate that the models likely overestimate the RI values of the SOA particles to a certain extent, which in turn overestimates the global direct radiative forcing of the organic particles.
Aerosol particles could affect the global radiative balance of the atmosphere1,2,3,4, but the effects remain poorly quantified5. In contrast to other methods of quantifying scattering and absorption, the refractive index (RI) depends primarily on composition rather than particle size or concentration6; therefore, it is an important and useful characteristic. Using the RI, secondary quantities, such as the absorption and scattering cross sections, can be calculated. Globally, organic compounds compose 50% of the aerosol mass, and in certain polluted areas, this amount can be as high as 90%7. Secondary organic aerosols (SOAs) compose up to 80% of all organic aerosols and contribute to the attenuation of regional visibility, climate change and potential health hazards8,9,10. Considering the ubiquity and predominance of SOAs, understanding the optical properties of SOAs is of great concern. Aromatic hydrocarbons compose 20–50% of the non-methane hydrocarbons in urban air and are considered to be some of the primary precursors to urban SOAs11. Anthropogenic aromatic compounds yield 76% of the calculated secondary organic particles in Beijing City and are thought to be the major source of SOAs, while among the aromatic hydrocarbons, benzene, toluene and the C8 aromatics (e.g., xylene and ethylbenzene) are the most abundant species12. The NOx level in the atmosphere is variable. In different regions (e.g., urban, suburban and rural areas or polluted areas and relatively clean areas) and during different periods (e.g., morning, midday and afternoon), the NOx concentration may change acutely, ranging from ≤2 ppb to several hundred ppb13,14,15, and the level of NOx has been found to be highly influential in SOA formation from a variety of compounds16,17,18,19. Therefore, the investigation of different NOx levels is imperative and has great atmospheric significance.
Many previous studies have been performed on the gas-phase photooxidation of aromatic hydrocarbons. These studies have primarily concentrated on the reaction mechanisms, SOA yields and particle composition, but the optical properties of the SOAs are not well understood20. Recently, certain studies have been performed to investigate the optical properties of SOA particles21,22,23,24,25,26,27, primarily focusing on α-pinene and toluene, which represent biological and anthropogenic volatile organic compounds (VOCs), respectively. Nakayama et al. measured the RIs at 355 and 532 nm of toluene and α-pinene SOAs in the presence of NOx or O3 and found values ranging from 1.46 to 1.6321. These authors have further investigated the optical properties of the SOAs derived from toluene and 1,3,5-trimethylbenzene at wavelengths of 405, 532 and 781 nm and have found that nitroaromatic compounds are the major contributors to the light absorption of the toluene SOA at UV wavelengths22. Redmond and Thompson estimated the RIs of α-pinene and toluene SOAs and found values of 1.49–1.51 and 1.49–1.50, respectively23. Kim et al. have reported the RIs for the SOAs generated by α-pinene, β-pinene and toluene under high or low NOx conditions. The RIs varied from 1.45 to 1.6, depending on the precursor and aerosol mass concentration24. Recently, Kim and Paulson investigated the relative volatility of SOAs by evaporating organics from the particles using a thermo denuder at temperatures between 60 and 100°C and measured the RIs before and after evaporation; they observed no change in the RI value of the toluene SOA during this process26.
Although the optical properties of the SOAs generated from different aromatic hydrocarbons have significant atmospheric implications, these characteristics are unclear22. This study focuses on the optical properties of the SOAs formed by benzene, toluene, ethylbenzene and m-xylene (BTEX), all of which are typical anthropogenic VOCs. We investigate the RIs of these SOA particles under low-NOx and high-NOx conditions (classical and HONO) to determine the influence of the NOx level (see Methods). The NOx level in the high-NOx condition is close to that in the existing atmosphere13,14, and the HC/NOx ratio in the HONO experiments is very similar to ambient values28,29,30, whereas the low-NOx experiments represent certain extreme conditions wherein the NOx level is ≤2 ppb15,30,31,32. The morphology of the particles was also determined in this study. To our knowledge, this is the first investigation of the RIs of the SOAs derived from benzene, ethylbenzene and m-xylene and the first determination of the influence of the NOx level on these SOAs. Finally, a comparison between the model values and the experimental data is presented, and a suggestion for the revision of the model RI values is given.
Results
SOA formation process and features
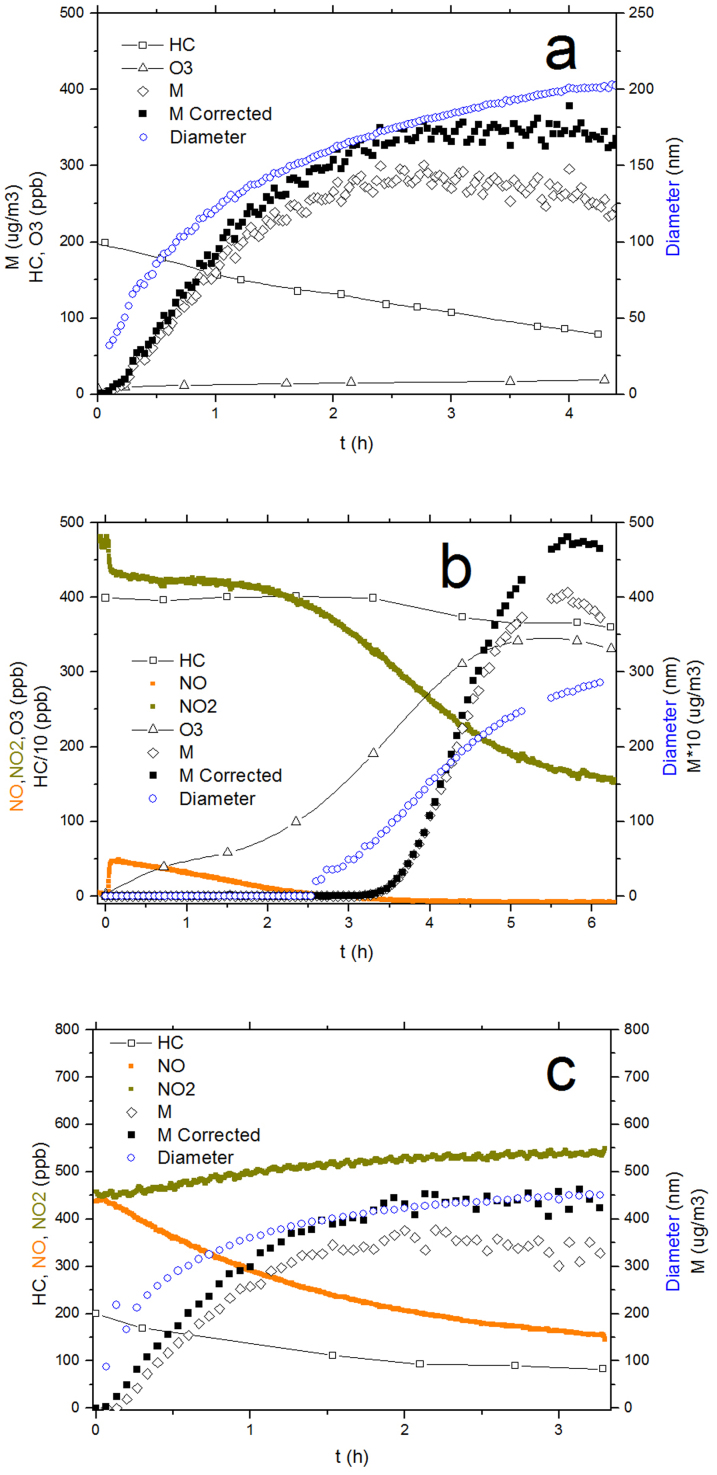
As illustrated in Fig. 1, three different NOx conditions were investigated, the details of which are given in the Methods section. Fig. 1a displays a typical reaction profile under the low-NOx condition. In this experiment, the initial m-xylene and H2O2 concentrations were 200 ppb and 5 ppm, respectively. During the entire process, 121.4 ppb m-xylene was consumed. The particles were generated just within a few minutes after the lights were turned on. A small amount of O3 (<20 ppb) was produced during this reaction. After more than 4 hours of illumination, the particle size tended to be stable at approximately 200 nm, and the mass concentration (M) was approximately 350 μg/m3 after a wall-loss correction using the method introduced by McMurry and Grosjean33. The SMPS data (M) in Fig. 1 shows that the mass concentration plateaued at the end of the experiment, indicating that the status in the chamber tend to stabilize. Fig. 1b shows a representative reaction profile under the classical high-NOx condition. In this experiment, the initial ethylbenzene, NO and NO2 concentrations were 4 ppm, 3 ppb and 480 ppb, respectively. During the photooxidation, 402 ppb ethylbenzene was reacted. No particles were generated during the first 2.5 hours. Particles appeared just as the NO concentration approached zero, which is consistent with other classical high-NOx studies16,19,34. At the end of the experiment, the particle surface mean diameter was approximately 300 nm, and the mass concentration was greater than 40 μg/m3. A considerable amount of O3 was generated during the reaction process, especially after particles were generated, leading to a high concentration of greater than 300 ppb when the reaction was complete. Fig. 1c is the reaction profile of a HONO experiment. The initial m-xylene, NO and NO2 concentrations were 200 ppb, 442 ppb and 465 ppb, respectively. During the entire process, 117.2 ppb m-xylene was consumed, yielding approximately 400 μg of secondary particles. Aerosol generation occurred almost immediately, even when the NO concentration was high. The final size mode was approximately 450 nm, which was significantly larger than with the other two conditions. Because the NO concentration was high, the formation of O3 was restrained19.
Figure 1. Reaction profiles.
(a) The low-NOx experiment (200 ppb m-xylene, 5 ppm H2O2); (b) the classical high-NOx experiment (4 ppm ethylbenzene, 3 ppb NO and 480 ppb NO2); (c) the HONO experiment (200 ppb m-xylene, 442 ppb NO and 465 ppb NO2).
Morphology of the secondary particles
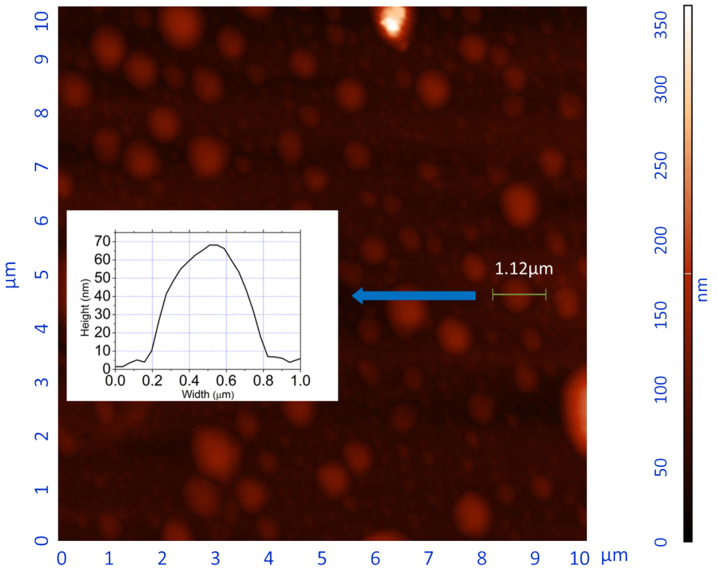
In the Mie theory, it is assumed that the aerosol particles are spheres. Under this premise, the RIs derived according to the Mie theory are correct and accurate; otherwise, a deviation will occur. Therefore, we investigated the morphology of the SOA particles. An atomic force microscopy (AFM) image of a typical aromatic SOA is shown in Fig. 2. The SOA was generated by the photooxidation of ethylbenzene under a low-NOx condition. Most of the particles are less than 1 μm and are approximately 600–800 nm in diameter. The cross-section data of a typical particle are also depicted in Fig. 2. The height of this particle is approximately 70 nm, whereas the width (diameter) is greater than 600 nm; therefore, its shape is similar to a pie. We speculate that the particles were liquid-like droplets when they were suspended in the air and became flattened upon impact with the wafers. This speculation is consistent with certain other studies. Freedman et al. have found that organic particles deform upon impact with a surface35, which is similar to our observation. Marcolli et al. have noted that the SOA particles should be liquid or waxy36. Bahreini et al. have suggested that the SOA formed via cycloalkene ozonolysis is likely in the liquid phase37. In this study, our investigation found that the SOA particles formed from BTEX photooxidation are likely in a liquid state at ambient temperature and pressure.
Figure 2. AFM image of the SOA particles formed by photooxidation of aromatic hydrocarbons (typically, 200 ppb ethylbenzene, 5 ppm H2O2).
The volume of the pie-like particle was calculated, which allowed calculation of the equivalent diameter when it was a sphere. We assumed that the particles were spheres because the surface tension of the droplets would minimise their surface. The equivalent diameter of the particle we chose in Fig. 2 was 200 nm. We found that most of the particles were similar in size to the selected particle, which is consistent with the diameter data of the scanning mobility particle sizer (SMPS), approximately 200 nm. The AFM images thus suggest that the particles are likely to be spherical droplets; therefore, there is likely to be little deviation when using the Mie theory.
Light absorption of the SOA particles
The light absorption of SOAs at visible wavelengths has been investigated and reported by many researchers in laboratory and field studies38,39,40. Glyoxal, methylglyoxal and related compounds can form light-absorbing substances during aqueous reactions, which could contribute to atmospheric brown carbon41,42. Photochemical experiments with phenol and related compounds in the aqueous phase have revealed a shift in the absorption to longer wavelengths (from 350 to 650 nm)43,44. The evolution of the absorption to longer wavelengths has been explained by the formation of larger conjugated oligomers that exhibit soot-like structures, whereas these types of structures may not be formed in the gas phase in an oxygen-containing atmosphere45. Certain biogenic VOCs, such as limonene and isoprene, can also form light-absorbing SOAs in the presence of acid seeds or aqueous ammonium ions46,47. At the same time, some researchers have found that certain types of SOAs formed by gas-phase oxidation in the absence of seeds have no significant absorption in the visible range21,22,48,49. Liu et al. have investigated the light absorption of SOAs formed by the gas-phase oxidation of α-pinene, limonene and catechol from 220 to 1200 nm and have found negligible absorption in the visible range48. Song et al. have studied the gas-phase oxidation of α-pinene + O3 and α-pinene + NOx + O3 systems and have concluded that these systems do not form visible light-absorbing SOAs under typical atmospheric conditions49. Our results are consistent with these studies. From the light-absorption spectrum over the range of 300–700 nm (see Supplementary Fig. S2 online), we can observe that gas-phase-formed BTEX SOAs absorb light at wavelengths <450 nm but have no obvious absorption at 532 nm with the detection sensitivity of our instrument. In other words, only the scattering properties of the secondary particles generated by the gas-phase reaction in our experiments should be considered, and the value of k in the RI equation (m = n + ki) can be ignored.
RI under different NOx levels
The extinction efficiency at each particle size can be calculated for a given value of n and k using a Mie code written in MATLAB for spherical particles, based on the FORTRAN code from Bohren and Huffman50. The calculated total extinction is then compared to the measured extinction. The best-fit RI is determined by minimising the following reduced merit function (χr):
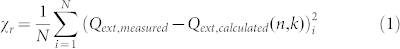 |
where N is the number of diameters to calculate, Qext is the extinction efficiency of the particles (see Methods) and n and k denote the real and imaginary parts of the RI, respectively.
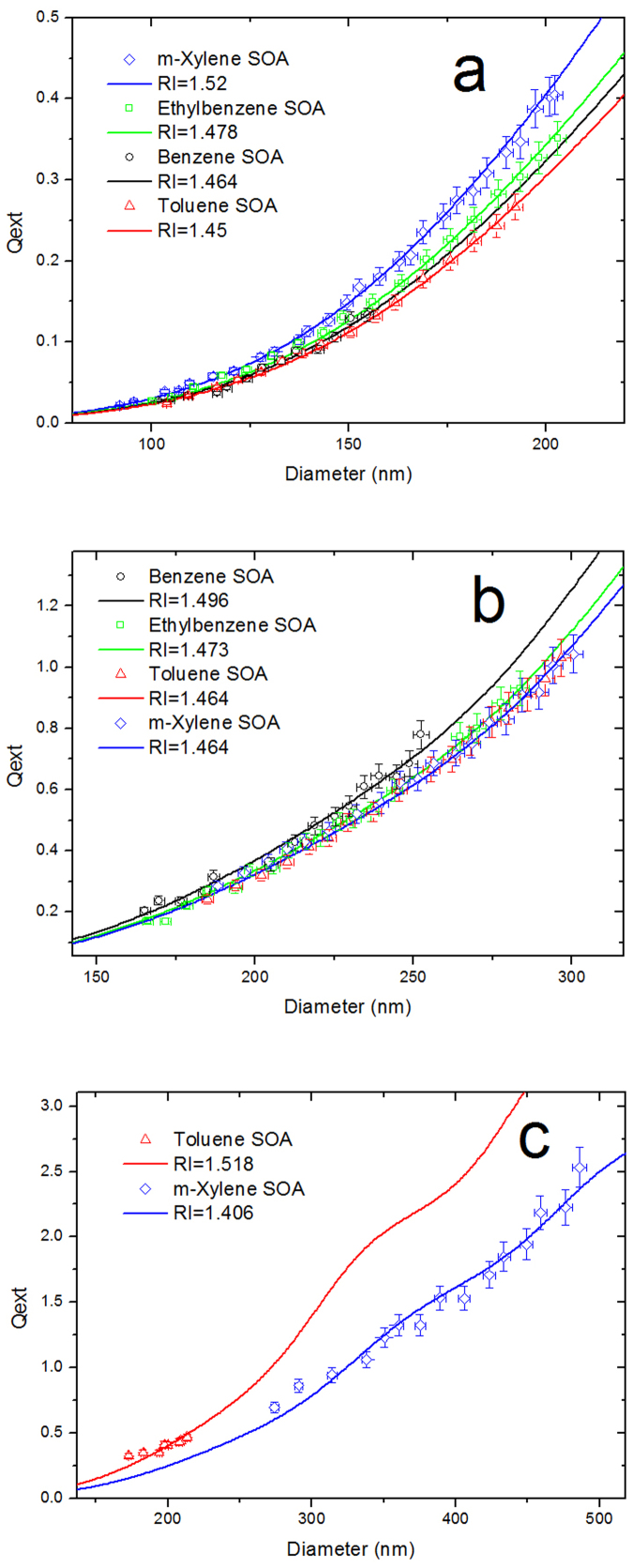
Fig. 3a depicts the dependence of Qext on the surface mean diameter and the retrieved RIs of the BTEX SOAs under the low-NOx condition. The concentrations of hydrocarbon and oxidant are identical. The temperature and relative humidity (RH) conditions are similar (with a fluctuation of <2°C and <4% RH, respectively). As illustrated in the figure, the measured extinction efficiencies generally conform to the Mie scattering curves, and the RI values are in the range of 1.45–1.52. Studies that explore the RIs of these four aromatic hydrocarbons under low-NOx conditions are lacking; therefore, only limited data can be contrasted. Note that the m-xylene SOA has the largest RI value at 1.52, followed by the ethylbenzene SOA at 1.478, the benzene SOA at 1.464 and the toluene SOA, which has the smallest RI value at 1.45. It is well known that hydrocarbons react with OH to produce RO2, and the RO2 can then react with HO2, NO and RO251. Under this condition, there is no detectable NO; therefore, we can attribute these RI values to the RIs of the BTEX SOAs generated through the RO2 + RO2 and RO2 + HO2 pathways. The different RI values are attributed to the different components of the products. Although undergoing similar reaction pathways under the low-NOx condition, m-xylene tends to produce products with high RI values. The data also reveal that the benzene SOA particles are smaller in size than the other particles, leading to markedly smaller final extinction efficiencies. This result is likely due to the different structure of the precursors. With no alkyl group attached to the benzene ring, benzene is less reactive than the other alkyl-substituted aromatics34.
Figure 3. Dependence of the extinction efficiencies of the polydispersed SOA particles on the surface mean diameter at a wavelength of 532 nm and the retrieved RIs for the following.
(a) the L1, L2, L5 and L7 low-NOx experiments; (b) the classical high-NOx experiments; (c) the HONO experiments.
The retrieved RI values and the dependence of the extinction efficiencies on the surface mean diameter of the BTEX SOAs under the classical high-NOx condition are shown in Fig. 3b. Fig. 3c represents the HONO experiments for toluene and m-xylene; benzene and ethylbenzene do not produce detectable particles under this condition. The RI values are in the range of 1.406–1.518. In a classical high-NOx experiment with toluene, Kim et al. measured a range of 1.4–1.6 for the high-NOx toluene SOA24; Nakayama et al. measured this RI value at 1.483 ± 0.03621. We measure 1.464 for this RI value, which agrees well with previous studies. We notice that the RI value of the classical high-NOx SOA ranges from 1.464 to 1.496, which is a narrower range than in the low-NOx condition. The RI values of the SOAs generated from different aromatic hydrocarbons respond differently when the NOx level increases from low to high as follows: the RIs of SOAs generated from benzene and toluene oxidation increase, whereas the RIs of the ethylbenzene and m-xylene SOAs decrease. Unexpectedly, the toluene and m-xylene SOA particles have the same RI value under the classical high-NOx condition, whereas the RI values are clearly different in the HONO experiment. We infer that the RO2 + NO pathway is likely to play an important role in leading to this result because the NO concentration in the HONO experiment is significantly higher (Fig. 1b, c). The details of this phenomenon will be discussed in the following section.
Changes in the RIs as the particles grow
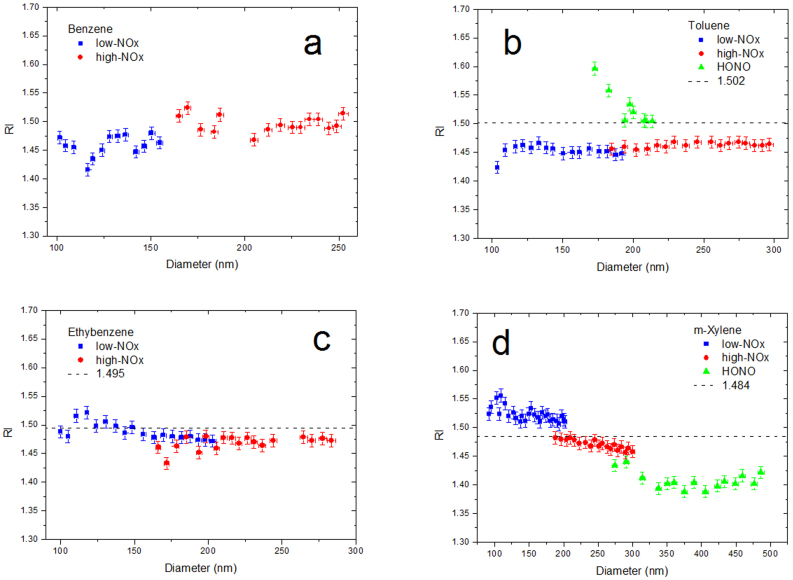
Certain previous studies have assumed that the RI values of the SOA particles remain constant as the particles grow21,22; however, in our experiment (as shown in Fig. 3), certain data points do not fit well with the extinction curves using the uniform RI value. Therefore, we retrieve the RI for every point. The results in Fig. 4 indicate that the products of different aromatic hydrocarbons display different trends as the particle size increases, and the details are described below. For the benzene SOA, the RI values slightly increase as the diameter increases under both the low and high NOx levels, with the high-NOx condition higher than the low-NOx condition. For the toluene SOA, the RI values change slightly under the low and high NOx levels, with the high NOx condition being slightly higher, but the SOA under the HONO condition gives a significantly higher RI value with a decreasing trend. For the ethylbenzene SOA, the RI values have a decreasing trend as the particles grow under the low-NOx condition and do not show any clear change under the high-NOx condition. The most obvious phenomenon of fluctuation occurs for the m-xylene SOA with decreasing RI values as the particles grow and the NOx level increases. Changing RI values have also been observed in other studies on certain biogenic SOAs24,25,26, indicating that the RI values are not constant.
Figure 4. Dependence of the RIs on the surface mean diameter of the SOA particles generated under different NOx levels.
(a) Benzene; (b) toluene; (c) ethylbenzene; (d) m-xylene. The RI values calculated by the QSPR (black dashed lines) are also given in (b), (c) and (d).
We infer that the products of the RO2 + NO pathway with BTEX precursors have different optical properties, leading to the results in Fig. 4. As we mentioned in the previous section, the reaction pathways of the low-NOx experiments are RO2 + RO2 and RO2 + HO2, whereas for the high-NOx experiments, the RO2 + NO pathway is added to compete with the other two pathways. Fig. 3a shows the optical properties of the products of the RO2 + RO2 and RO2 + HO2 pathways, whereas the difference between Fig. 3b and Fig. 3a is possibly caused by the different optical properties of the RO2 + NO products compared with the former two pathways. For the data shown in Fig. 3c, the proportion of the RO2 + NO pathway is larger than in Fig. 3b because the NO concentration is significantly higher during the photooxidation process; in Fig. 4, this trend is much clearer. The RI values of the RO2 + NO pathway products can be placed in order, taking the low-NOx RI values as the baseline. The order is as follows: benzene (higher than the baseline) > toluene (slightly higher than the baseline) > ethylbenzene (slightly lower than the baseline) > m-xylene (significantly lower than the baseline). The HONO experiments agree well with this order, as shown in Fig. 4b and 4d. For the toluene SOA, the HONO experiments (higher NO) yield a higher RI value, whereas for the m-xylene SOA, the HONO experiments yield a lower RI value. The different performance of BTEX under the high-NOx condition is likely due to the different molecular structures of the hydrocarbons, and the details of these differences should be studied in future research.
To quantitatively compare the measured RIs with the composition measured by Forstner et al.52, we calculate the RIs for the SOA components with a quantitative structure-property relationship (QSPR) approach, as developed by Redmond and Thompson23. The results are depicted in Fig. 4. The QSPR was built with the 589 nm data, whereas our measurements were made at 532 nm; however, this difference has little influence because the RIs of organic materials have only a very slight dependence on the wavelength in the mid-visible range, changing by less than 0.002–0.005 between 589 and 532 nm53. We observe that there are certain deviations between the experimental data and the calculated values. Because Forstner et al. identified only 15–30% of the collected aerosol mass, and several identified compounds were thought to be vapour adsorption to the quartz filter rather than aerosol-phase constituents52, the deviations are reasonable. In addition, the deviations may be caused by the different experimental conditions, such as the addition of propene, the light type and intensity, the temperature and the RH. Under different initial conditions, the components of the products may change.
We notice that the RI value of the m-xylene SOA has a significant fluctuation. This phenomenon may be attributed to the following reasons. Initially, the density variation may change the RI values. Commonly, there is a positive correlation between the RI and the density. A denser material generally tends to have a larger RI54. From certain earlier studies55,56, we know that the m-xylene SOA obtains a higher density at the onset of aerosol formation, and the density decreases as the particles become larger; the decreasing density leads to the decrease in the RIs, and another name for the RI is optical density54. The second explanation for the fluctuation is heterogeneous reaction and the ageing process. Aromatics react with OH radicals in the gas phase and produce multifunctional carbonyls that have a high reactivity in particle-phase reactions57. Reactions (e.g., hydration, polymerisation, formation of hemiacetal/acetal and cationic rearrangement) of gas-phase species and an aerosol surface can change the composition of the particle phase58. This effect may change the proportion of the particle components, thereby altering the RI. In addition, the ageing process might play a role in the fluctuation. For example, m-tolualdehyde and m-toluic acid are products of m-xylene, and m-tolualdehyde could be oxidised to m-toluic acid during the ageing process of aerosols52. According to the CRC handbook, the RIs of m-tolualdehyde and m-toluic acid are 1.541 and 1.509, respectively59. Therefore, during this type of conversion, the RI decreases.
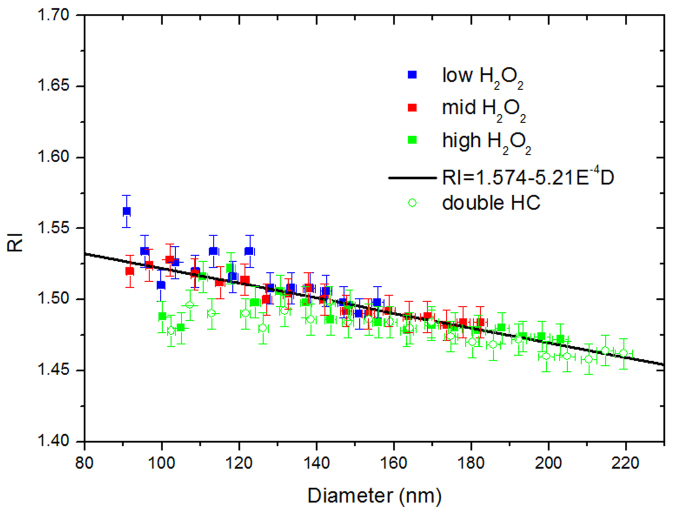
The dependence of the RI value on the initial HC and oxidiser concentrations under the low-NOx condition was also investigated. As shown in Fig. 5, the RI values decrease as the particles grow for the ethylbenzene SOA. The solid points represent the L3–L5 experiments with different concentrations of hydrogen peroxide, whereas the green (solid and open) points represent the L5 and L6 experiments with different hydrocarbon concentrations. Clearly, the oxidiser concentration does not influence the RI value under the same diameter but influences the final size mode; the hydrocarbon concentration slightly influences the RI value with a negative correlation. Generally, the different initial concentrations exert only a slight influence on the RIs.
Figure 5. Dependence of the RI values on the surface mean diameter of the ethylbenzene SOA generated in the L3–L6 experiments.
The solid data points represent the L3–L5 experiments with different concentrations of hydrogen peroxide, whereas the green (solid and open) data points represent the L5 and L6 experiments with different hydrocarbon concentrations.
Comparison with the model values
As illustrated in Fig. 6, we compared our experimental data to the model values. The RI values of the SOA generated by BVOCs measured by Kim et al.24,25,26 and the data of the toluene SOA measured by Nakayama et al.21 are also shown in this figure. The three lines are the values of real RIs of seven common models60. ECHAM4, GOCART, GISS, Sprintars and Grantour perform better than ULAQ and MIRAGE compared with the laboratory studies. Nearly all of the experimental data are lower than the model values, regardless of whether the SOAs are generated by biological or anthropogenic VOCs. Kim et al. have noted that changing the RI value from 1.4 to 1.5 can produce an increase in the radiative forcing by at least 12% for non-absorbing particles25. Because aromatic hydrocarbons, limonene, α-pinene and β-pinene contribute most of the SOA content, current models may overestimate the RI values of organic aerosols to a certain extent.
Figure 6. RIs of the different SOA particles, and the comparison between the experimental data and the model values.
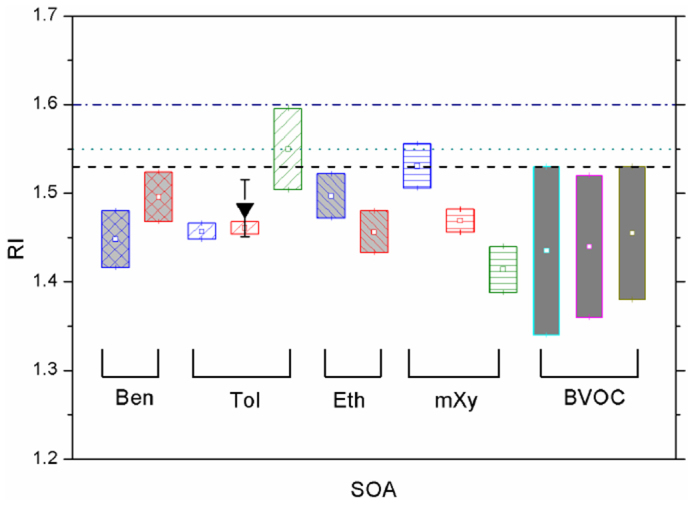
The BTEX data are the current results; the toluene result (inverted triangle) is from Nakayama et al.20, and the data of the BVOCs are from Kim et al.25,26,27. The blue boxes are the results of the low-NOx experiments; the red boxes are the results of the classical high-NOx experiments, and the green boxes are the results of the HONO experiments. The three BVOCs are limonene (cyan), α-pinene (pink) and β-pinene (dark yellow). The three lines are the RI values of organic aerosols in different models: the blue dash/dot line is for the ULAQ; the green dotted line is for MIRAGE; and the black dashed line is for ECHAM4, GOCART, GISS, Sprintars and Grantour51.
Discussion
Our laboratory chamber results demonstrate that the RIs of the BTEX SOAs are closely related to the initial NOx concentration, with different aromatics displaying different trends. For the benzene and toluene SOAs, the RI value increases as the NOx level rises, whereas for the ethylbenzene and m-xylene SOAs, the RI value decreases correspondingly. We infer that this result is caused by the different molecular structures and the RO2 + NO pathway that occurs under the high-NOx condition. The dependence of the RI value on the initial hydrocarbon and oxidant concentrations was also investigated, revealing that the initial concentrations of these reagents have little influence on the optical properties of the SOA. Under our experimental conditions (gas-phase oxidation with no seed particles), the BTEX SOAs have no obvious absorption at 532 nm. However, the SOA particles formed under other conditions may absorb light in the visible range, and further studies examining the underlying mechanism should be performed in the future.
The RI fluctuations in relation to the level of NOx indicate that a single value for the RI of SOA is insufficient to accurately model radiative transfer. In addition, the commonly used model values for the RIs of organic aerosols are generally higher than the experimental data for the SOAs formed from both biological and anthropogenic VOCs, which may cause the overestimation of direct radiative forcing of organic particles to a certain extent.
Methods
SOA formation
The rationale of the experimental design is illustrated online in Supplementary Fig. S1. The SOAs were generated in a 1 m3 Teflon smog chamber in the absence of seed particles. The temperature and RH were continuously monitored. The temperature was maintained at 27–29°C, and cooling fans were in operation to prevent overheating of the chamber. The RH was comparatively dry (10–20%) during the experiments. The mixing ratios of NO, NO2 and O3 were monitored with Thermo 42 i and 49 i gas analyzers, and the concentration of the parent hydrocarbon was determined via gas chromatography with flame ionisation detection (GC-FID, Agilent 6820). The particle size distribution was measured with an SMPS, which was composed of an electrostatic classifier (EC, TSI 3080), a differential mobility analyser (DMA, TSI 3081) and a condensation particle counter (CPC, TSI 3776).
The BTEX reagents were used without further purification. Prior to each experiment, the chamber was flushed with dry, purified air several times to ensure that no detectable VOCs, NOx or O3 were present. A known volume of the parent hydrocarbon was injected into a glass U-tube and introduced into the chamber with an air stream. For the low-NOx experiments, H2O2 (35 wt% water solution) was used as the OH precursor. The background NOx level in the chamber during the experiment was <1 ppb, and according to the injection volume, the initial concentration of H2O2 was estimated to be 1–5 ppm. For the high-NOx experiments, NO2 was introduced from a 50 ppm standard gas cylinder. In some of these experiments, HONO was added to serve as the OH precursor, which is similar to the method of Ng et al.19. To distinguish between these two conditions, we called the experiments without HONO the classical high-NOx experiments, and the others were called the HONO experiments. For the classical high-NOx experiments, the NOx concentration was 100–500 ppb, whereas for the HONO experiments, the NOx concentration was approximately 1 ppm. The HONO was prepared by the dropwise addition of 1 mL of 1 wt% NaNO2 into 2 mL of 10 wt% H2SO4 in a glass bulb. The bulb was then attached to the chamber, and a stream of dry air was passed through the bulb into the chamber. NO and NO2, formed as byproducts in the preparation of the HONO, were also introduced into the chamber. Additional NO2 from a 50 ppm gas cylinder was introduced into the chamber after the addition of the HONO to achieve a total NOx level of approximately 1 ppm. The initial conditions of the low-NOx and high-NOx experiments are listed in Table 1 and Table 2, respectively.
Table 1. Initial conditions of the low-NOx experiments.
| Exp. No. | Parent HC | [HC]0 (ppb) | [H2O2]0 (ppm) | RH (%) |
|---|---|---|---|---|
| L1 | benzene | 200 | 5 | 15.8–14.3 |
| L2 | toluene | 200 | 5 | 14.8–14.2 |
| L3 | ethylbenzene | 200 | 1 | 17.2–17.1 |
| L4 | ethylbenzene | 200 | 3 | 16.4–16.0 |
| L5 | ethylbenzene | 200 | 5 | 18.1–16.8 |
| L6 | ethylbenzene | 400 | 5 | 18.4–16.7 |
| L7 | m-xylene | 200 | 5 | 17.1–16.1 |
Table 2. Initial conditions of the high-NOx experiments.
| Exp. No. | Parent HC | [HC]0 (ppb) | [NO]0 (ppb) | [NO2]0 (ppb) | [NOx]0 (ppb) | RH (%) |
|---|---|---|---|---|---|---|
| H1 | benzenea | 4000 | 4 | 104 | 108 | 20.2–15.1 |
| H2 | toluenea | 4000 | 39 | 398 | 437 | 15.4–12.6 |
| H3 | tolueneb | 230 | 213 | 925 | 1138 | 14.1–12.2 |
| H4 | ethylbenzenea | 4000 | 3 | 480 | 483 | 15.4–13.7 |
| H5 | m-xylenea | 4000 | 28 | 312 | 340 | 18.7–14.0 |
| H6 | m-xyleneb | 200 | 183 | 751 | 934 | 16.1–13.9 |
| H7 | m-xyleneb | 200 | 442 | 465 | 907 | 16.2–14.8 |
a. The classical high-NOx experiments;
b. the HONO experiments.
After the parent hydrocarbon, the NOx and the OH precursors were placed into the chamber, and black lights were turned on to start the photoreaction. For the low-NOx experiments, a set of four lamps with a narrow peak at 254 nm was used, whereas for the high-NOx experiments, 36 lamps with a maximum at 365 nm were used.
Determination of the optical properties
The extinction coefficients of the secondary particles were measured with a custom-built cavity ring-down spectrometer (CRDS). The details of this instrument have been provided elsewhere5; therefore, it is introduced briefly in this study. The wavelength of the laser is 532 nm. The CRDS directly provides the decay time τ, and the extinction coefficient of the particles inside the cavity, αext, can then be derived according to the following equation:
 |
where L is the length of the cavity (the distance between the two mirrors), c is the velocity of light, l is the actual distance in the cavity filled with sample and τ0 is the initial decay time when the cavity is filled with zero air.
For monodispersed spheres, αext is described by the following equation:
 |
where C is the number concentration, σext is the extinction cross-section and the extinction efficiency Qext is the ratio of the Beer's law extinction cross-section to the geometric area of the particle and is dimensionless.
When the particles are not monodispersed, e.g., a Gaussian distribution of particles, then CπD2 in equation (3) can be replaced by the total surface concentration Stot, and in this situation, the diameter is the surface mean diameter Dsm. Then, the equation is changed to
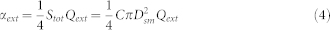 |
Stot, and the Dsm data can be obtained with the SMPS; therefore, the Qext value with different Dsm values can be calculated using equation (4). With the Qext data, the RIs are derived using a MATLAB program.
Ultraviolet-visible light (UV-Vis) spectroscopy
The UV-Vis absorption of the SOAs was also investigated to clarify whether the aromatic SOA particles absorb light at a wavelength of 532 nm. The SOA particles were collected with PTFE filters (0.2 μm pore size) and extracted with 5 mL of a CH2Cl2:CH3CN (1:1) solution. The filtered extracts were placed in a 1 cm quartz cuvette for the absorption measurements. The UV-Vis absorption spectra were obtained with a fibre optic spectrometer (Avantes 2048F) using pure solvent as the reference.
Morphology
The aerosol particles were analysed via AFM to determine their morphology. The dry, polydispersed aerosol particles were collected on SiOx/Si(100) wafers. The aerosol samples were analysed at an ambient RH (~40%) with a tapping mode atomic force microscope (NT-MDT Ntegra solar upright).
Author Contributions
M.G., W.W. and K.L. authored the manuscript. M.G., W.W. and K.L. designed the study. J.L. and D.W. contributed the morphology data. All five authors performed the experiments. M.G., W.W. and K.L. analysed the data.
Supplementary Material
Supplementary Information
Acknowledgments
This project was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB05010400), the National Basic Research Program of China (973 Program, No. 2011CB403401), the Ministry of Science and Technology of China and the National Natural Science Foundation of China (Major Program: 21190052, Contract No. 40925016, 41173112, 41227805).
References
- Penner J. E., Dong X. Q. & Chen Y. Observational evidence of a change in radiative forcing due to the indirect aerosol effect. Nature 427, 231–234 (2004). [DOI] [PubMed] [Google Scholar]
- Cowan T., Cai W. J., Purich A., Rotstayn L. & England M. H. Forcing of anthropogenic aerosols on temperature trends of the sub-thermocline southern Indian Ocean. Sci. Rep. 3, 2245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. C. et al. One hundred years of Arctic surface temperature variation due to anthropogenic influence. Sci. Rep. 3, 2645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand D., Wood R., Anderson T. L., Satheesh S. K. & Charlson R. J. Satellite-derived direct radiative effect of aerosols dependent on cloud cover. Nat. Geosci. 2, 181–184 (2009). [Google Scholar]
- Wang L., Wang W. & Ge M. Extinction efficiencies of mixed aerosols measured by aerosol cavity ring down spectrometry. Chinese Sci. Bull. 57, 2567–2573 (2012). [Google Scholar]
- Zarzana K. J., De Haan D. O., Freedman M. A., Hasenkopf C. A. & Tolbert M. A. Optical properties of the products of alpha-dicarbonyl and amine reactions in simulated cloud droplets. Environ. Sci. Technol. 46, 4845–4851 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Q. et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 34, L13801 (2007). [Google Scholar]
- Davidson C. I., Phalen R. F. & Solomon P. A. Airborne particulate matter and human health: A review. Aerosol Sci. Tech. 39, 737–749 (2005). [Google Scholar]
- Eldering A. & Cass G. R. Source-oriented model for air pollutant effects on visibility. J. Geophys. Res. 101, 19343–19369 (1996). [Google Scholar]
- Turpin B. J. & Huntzicker J. J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos. Environ. 29, 3527–3544 (1995). [Google Scholar]
- Singh H. B., Salas L. J., Cantrell B. K. & Redmond R. M. Distribution of aromatic hydrocarbons in the ambient air. Atmos. Environ. 19, 1911–1919 (1985). [Google Scholar]
- Zi-feng L., Ji-ming H. A. O., Jing-chun D. & Jun-hua L. I. Estimate of the formation potential of secondary organic aerosol in Beijing summertime. Huan Jing Ke Xue 30, 969–975 (2009). [PubMed] [Google Scholar]
- Pandey S. K. et al. Long-term study of NOx behavior at urban roadside and background locations in Seoul, Korea. Atmos. Environ. 42, 607–622 (2008). [Google Scholar]
- Gardner M. W. & Dorling S. R. Neural network modelling and prediction of hourly NOx and NO2 concentrations in urban air in London. Atmos. Environ. 33, 709–719 (1999). [Google Scholar]
- Clapp L. J. & Jenkin M. E. Analysis of the relationship between ambient levels Of O-3, NO2 and NO as a function of NO chi in the UK. Atmos. Environ. 35, 6391–6405 (2001). [Google Scholar]
- Johnson D., Jenkin M. E., Wirtz K. & Martin-Reviejo M. Simulating the Formation of Secondary Organic Aerosol from the Photooxidation of Toluene. Environ. Chem. 1, 150–165 (2004). [Google Scholar]
- Johnson D., Jenkin M. E., Wirtz K. & Martin-Reviejo M. Simulating the Formation of Secondary Organic Aerosol from the Photooxidation of Aromatic Hydrocarbons. Environ. Chem. 2, 35–48 (2005). [Google Scholar]
- Ng N. L. et al. Effect of NO(x) level on secondary organic aerosol (SOA) formation from the photooxidation of terpenes. Atmos. Chem. Phys. 7, 5159–5174 (2007). [Google Scholar]
- Ng N. L. et al. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 7, 3909–3922 (2007). [Google Scholar]
- Hallquist M. et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 9, 5155–5236 (2009). [Google Scholar]
- Nakayama T. et al. Laboratory studies on optical properties of secondary organic aerosols generated during the photooxidation of toluene and the ozonolysis of α-pinene. J. Geophys. Res. 115, D24204 (2010). [Google Scholar]
- Nakayama T. et al. Wavelength and NOx dependent complex refractive index of SOAs generated from the photooxidation of toluene. Atmos. Chem. Phys. 13, 531–545 (2013). [Google Scholar]
- Redmond H. & Thompson J. E. Evaluation of a quantitative structure-property relationship (QSPR) for predicting mid-visible refractive index of secondary organic aerosol (SOA). Phys. Chem. Chem. Phys. 13, 6872–6882 (2011). [DOI] [PubMed] [Google Scholar]
- Kim H., Barkey B. & Paulson S. E. Real refractive indices of α- and β-pinene and toluene secondary organic aerosols generated from ozonolysis and photo-oxidation. J. Geophys. Res. 115, D24212 (2010). [Google Scholar]
- Kim H., Barkey B. & Paulson S. E. Real refractive indices and formation yields of secondary organic aerosol generated from photooxidation of limonene and alpha-pinene: the effect of the HC/NO(x) ratio. J. Phys. Chem. A 116, 6059–6067 (2012). [DOI] [PubMed] [Google Scholar]
- Kim H. & Paulson S. E. Real refractive indices and volatility of secondary organic aerosol generated from photooxidation and ozonolysis of limonene, α-pinene and toluene. Atmos. Chem. Phys. 13, 7711–7723 (2013). [Google Scholar]
- Yu Y. et al. Photooxidation of α-pinene at high relative humidity in the presence of increasing concentrations of NOx. Atmos. Environ. 42, 5044–5060 (2008). [Google Scholar]
- Geng F. et al. Characterizations of ozone, NOx, and VOCs measured in Shanghai, China. Atmos. Environ. 42, 6873–6883 (2008). [Google Scholar]
- Zhang Y., Mu Y., Liu J. & Mellouki A. Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J. Environ. Sci. 24, 124–130 (2012). [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang X.-l., Xu X.-f. & Xu H.-h. Comparison analysis of variation characteristics of SO2, NOx, O3 and PM2.5 between rural and urban areas, Beijing. Huan Jing Ke Xue 29, 1059–1065 (2008). [PubMed] [Google Scholar]
- Grant D. D. et al. Volatile organic compounds at a rural site in western Senegal. J. Atmos. Chem. 60, 19–35 (2008). [Google Scholar]
- Rohrer F. et al. Mixing ratios and photostationary state of NO and NO2 observed during the POPCORN field campaign at a rural site in Germany. J. Atmos. Chem. 31, 119–137 (1998). [Google Scholar]
- McMurry P. H. & Grosjean D. Gas and aerosol wall losses in teflon film smog chambers. Environ. Sci. Technol. 19, 1176–1182 (1985). [DOI] [PubMed] [Google Scholar]
- Martin-Reviejo M. & Wirtz K. Is benzene a precursor for secondary organic aerosol? Environ. Sci. Technol. 39, 1045–1054 (2005). [DOI] [PubMed] [Google Scholar]
- Freedman M. A., Baustian K. J., Wise M. E. & Tolbert M. A. Characterizing the morphology of organic aerosols at ambient temperature and pressure. Anal. Chem. 82, 7965–7972 (2010). [DOI] [PubMed] [Google Scholar]
- Marcolli C., Luo B. P. & Peter T. Mixing of the organic aerosol fractions: Liquids as the thermodynamically stable phases. J. Phys. Chem. A 108, 2216–2224 (2004). [Google Scholar]
- Bahreini R. et al. Measurements of secondary organic aerosol from oxidation of cycloalkenes, terpenes, and m-xylene using an Aerodyne aerosol mass spectrometer. Environ. Sci. Technol. 39, 5674–5688 (2005). [DOI] [PubMed] [Google Scholar]
- Kirchstetter T. W., Novakov T. & Hobbs P. V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. 109, D21208 (2004). [Google Scholar]
- Barnard J. C., Volkamer R. & Kassianov E. I. Estimation of the mass absorption cross section of the organic carbon component of aerosols in the Mexico City Metropolitan Area. Atmos. Chem. Phys. 8, 6665–6679 (2008). [Google Scholar]
- Volkamer R., Ziemann P. J. & Molina M. J. Secondary Organic Aerosol Formation from Acetylene (C2H2): seed effect on SOA yields due to organic photochemistry in the aerosol aqueous phase. Atmos. Chem. Phys. 9, 1907–1928 (2009). [Google Scholar]
- Shapiro E. L. et al. Light-absorbing secondary organic material formed by glyoxal in aqueous aerosol mimics. Atmos. Chem. Phys. 9, 2289–2300 (2009). [Google Scholar]
- Sareen N., Schwier A. N., Shapiro E. L., Mitroo D. & McNeill V. F. Secondary organic material formed by methylglyoxal in aqueous aerosol mimics. Atmos. Chem. Phys. 10, 997–1016 (2010). [Google Scholar]
- Gelencser A. et al. In-situ formation of light-absorbing organic matter in cloud water. J. Atmos. Chem. 45, 25–33 (2003). [Google Scholar]
- Chang J. L. & Thompson J. E. Characterization of colored products formed during irradiation of aqueous solutions containing H2O2 and phenolic compounds. Atmos. Environ. 44, 541–551 (2010). [Google Scholar]
- Ervens B., Turpin B. J. & Weber R. J. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies. Atmos. Chem. Phys. 11, 11069–11102 (2011). [Google Scholar]
- Limbeck A., Kulmala M. & Puxbaum H. Secondary organic aerosol formation in the atmosphere via heterogeneous reaction of gaseous isoprene on acidic particles. Geophys. Res. Lett. 30, 19 (2003). [Google Scholar]
- Bones D. L. et al. Appearance of strong absorbers and fluorophores in limonene-O-3 secondary organic aerosol due to NH4+-mediated chemical aging over long time scales. J. Geophys. Res. 115, D05203 (2010). [Google Scholar]
- Liu P. F., Zhang Y. & Martin S. T. Complex Refractive Indices of Thin Films of Secondary Organic Materials by Spectroscopic Ellipsometry from 220 to 1200 nm. Environ. Sci. Technol. 47, 13594–13601 (2013). [DOI] [PubMed] [Google Scholar]
- Song C., Gyawali M., Zaveri R. A., Shilling J. E. & Arnott W. P. Light absorption by secondary organic aerosol from alpha-pinene: Effects of oxidants, seed aerosol acidity, and relative humidity. J. Geophys. Res. 118, 11741–11749 (2013). [Google Scholar]
- Bohren C. F. & Huffman D. R. Absorption and Scattering of Light by Small Particles. (John Wiley, 1983). [Google Scholar]
- Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 34, 2063–2101 (2000). [Google Scholar]
- Forstner H. J. L., Flagan R. C. & Seinfeld J. H. Secondary organic aerosol from the photooxidation of aromatic hydrocarbons: Molecular composition. Environ. Sci. Technol. 31, 1345–1358 (1997). [Google Scholar]
- Moreels E., Degreef C. & Finsy R. Laser-light refractometer. Appl. Optics 23, 3010–3013 (1984). [DOI] [PubMed] [Google Scholar]
- Liu Y. & Daum P. H. Relationship of refractive index to mass density and self-consistency of mixing rules for multicomponent mixtures like ambient aerosols. J. Aerosol Sci. 39, 974–986 (2008). [Google Scholar]
- Malloy Q. G. J. et al. Real-time aerosol density determination utilizing a modified scanning mobility particle sizer—aerosol particle mass analyzer system. Aerosol Sci. Tech. 43, 673–678 (2009). [Google Scholar]
- Qi L., Nakao S., Tang P. & Cocker D. R. III Temperature effect on physical and chemical properties of secondary organic aerosol from m-xylene photooxidation. Atmos. Chem. Phys. 10, 3847–3854 (2010). [Google Scholar]
- Smith D. F., Kleindienst T. E. & McIver C. D. Primary product distributions from the reaction of OH with m-, p-xylene, 1,2,4- and 1,3,5-trimethylbenzene. J. Atmos. Chem. 34, 339–364 (1999). [Google Scholar]
- Cao G. & Jang M. Effects of particle acidity and UV light on secondary organic aerosol formation from oxidation of aromatics in the absence of NOx. Atmos. Environ. 41, 7603–7613 (2007). [Google Scholar]
- Lide R. ed., CRC Handbook of Chemistry and Physics, Internet Version 2005. <http://www.hbcpnetbase.com>, (CRC Press, 2005) Date of access: 09/01/2014. [Google Scholar]
- Kinne S. et al. Monthly averages of aerosol properties: A global comparison among models, satellite data, and AERONET ground data. J. Geophys. Res. 108, D20 (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information